Abstract
Objective
To explore whether the plasma total β-amyloid (Aβ) Aβ42/Aβ40 ratio is a reliable predictor of the amyloid-PET status by exploring the association between these 2 variables in a subset of the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging cohort.
Methods
Taking plasma samples at 3 separate time points, month 18 (n = 176), month 36 (n = 169), and month 54 (n = 135), we assessed the total Aβ42/Aβ40 ratio in plasma (TP42/40) with regard to neocortical Aβ burden via PET standardized uptake value ratio (SUVR) and investigated both association with Aβ-PET status and correlation (and agreement) with SUVR.
Results
The TP42/40 plasma ratio was significantly reduced in amyloid-PET–positive participants at all time points (p < 0.0001). Adjusting for covariates age, gender, APOE ε4 allele status, and clinical classification clearly affects the significance, with p values reduced and only comparisons at 54 months retaining significance (p = 0.006). Correlations with SUVR were similar across each time point, with Spearman ρ reaching −0.64 (p < 0.0001). Area under the curve values were highly reproducible over time points, with values ranging from 0.880 at 36 months to 0.913 at 54 months. In assessments of the healthy control group only, the same relationships were found.
Conclusions
The current study demonstrates reproducibility of the plasma assay to discriminate between amyloid-PET positive and negative over 3 time points, which can help to substantially reducing the screening rate of failure for clinical trials targeting preclinical or prodromal disease.
Classification of evidence
This study provides Class II evidence that plasma total Aβ42/Aβ40 ratio is associated with neocortical amyloid burden as measured by PET SUVR.
The current shift in the Alzheimer disease (AD) paradigm is transforming the therapeutic target population for clinical trials from people with dementia or mild cognitive impairment (MCI) to cognitively healthy people at risk. These people are not easy to find in a community setting when β-amyloid (Aβ) positivity is a criterion for eligibility and the screening rate of failure (SRF) rises to >70%.
Under these conditions, the use of Aβ-PET scans for screening represents a huge burden on the budget of any clinical trial, hampering its feasibility. Furthermore, the complex logistic handling of radiotracers coupled with the low availability of PET scanners can seriously limit the follow-up of a population, to determine when and to whom to administer a preventive treatment, once one becomes available. CSF analysis may be significantly less expensive, but a lumbar puncture reduces its suitability for periodic population assessment.
Thus, the discovery and development of accessible and inexpensive biomarkers that may help to enrich a population-based sample, reducing the SRF for prevention trials, has been noted as a top research priority to prevent and to effectively treat AD in the shortest possible time frame.1,2
In the last decade, accruing experimental results have shown that lower Aβ42/Aβ40 (Aβ42/40) plasma ratio is associated with higher amyloid cortical burden and steeper accumulation trajectories,3–11 greater cognitive decline,12 or increased risk of developing AD dementia at follow-up.13–17 However, some studies were unable to replicate these findings, introducing controversy and casting doubts on the reliability of blood-based biomarkers.18–21 Nevertheless, this issue is being currently elucidated by a better understanding of the interactions of Aβ peptides within the complex plasma matrix and the use of the cortical amyloid status instead of the clinical diagnosis as the gold standard to assess Aβ blood-based biomarkers.22
In line with this, Araclon's team developed an ELISA assay (ABtest, Araclon Biotech Ltd, Zaragoza, Spain) to assess the free (FP), total (TP), and bound (BP) Aβ40 and Aβ42 levels in plasma.23 Recently, we used the ABtest to determine the TP42/40, FP42/40, and BP42/40 (the difference between TP and FP) in a subcohort of healthy controls (HCs) from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study.3 In that study, we found that lower Aβ42/40 plasma ratios (particularly TP42/40) were associated with higher cortical Aβ burden and faster Aβ accumulation rates. In the present study, we aim to assess the reproducibility of these ratios, particularly TP42/40, which had previously shown a better performance, in separating people with or without PET-confirmed amyloid cortical pathology over 3 time points in a larger population sample spanning the disease continuum.
Methods
Standard protocol approvals, registrations, and patient consents
The AIBL study was approved by the institutional ethics committees of Austin Health, St. Vincent's Health, Hollywood Private Hospital, and Edith Cowan University, and all volunteers gave written informed consent before participating in the study (further information is available in reference 24).
Study population
A subset of samples from AIBL with information pertaining to neocortical amyloid burden from PET imaging over 3 time points (18, 36, and 54 months) were selected. The AIBL study was initiated in 2006 with the express aim to identify those biomarkers that were both associated with and predictive of AD pathology and clinical disease. To this end, the current study used plasma from a subselection of participants who were followed up over at least 54 months. Other information collected and used in this study includes results from cognitive assessments to derive the AIBL Preclinical Alzheimer's Cognitive Composite score, the Mini-Mental State Examination, the Clinical Dementia Rating score, APOE ε4 allele status, age, sex, and relative information pertaining to the PET imaging.
The primary research question of the present work is to explore the association between plasma TP42/40 ratio and neocortical amyloid burden as measured by PET standardized uptake value ratio (SUVR), which has received a Class II classification of evidence.
Amyloid PET imaging
PET information using the Pittsburgh compound B radiotracer was collected for the cohort for at least 1 of the 3 time points. When PET measurements were not collected at a corresponding time point, data from the last known PET measurement were used. In brief, the quantitative representation of neocortical amyloid plaques was determined by taking the sum of the spatially normalized PET images to create a standardized update value. Standardized uptake values were normalized to the cerebellar cortex25 to form the SUVR. SUVR values were then transformed to a binary scale (Aβ-PET+ve/Aβ-PET−ve) via the precalculated threshold (1.4).
Plasma Aβ40 and Aβ42 quantification
Plasma samples were collected with ethylene-diamine-tetra-acetic acid used as the anticoagulant and conserved at −70°C until analysis, following AIBL procedures.26 Levels of Aβ40 and Aβ42 were quantified with the ABtest40 and ABtest42, respectively (Araclon Biotech Ltd), 2 validated colorimetric assays based on the sandwich ELISA technique, as described elsewhere.23 Each plasma sample was analyzed both undiluted and diluted one-third in a proprietary sample/standard diluent specifically formulated to disrupt the interactions between Aβ and other plasma components. As a result, FP and TP levels of Aβ40 and Aβ42 were determined. The difference between the concentrations of TP and FP corresponds to the amyloid peptide bound to plasma components (BP). The Aβ42/40 ratios in each of these plasma fractions (FP42/40, TP42/40, and BP42/40) were calculated, with the TP42/40 ratio being the target plasma biomarker assessed in this study. Free and total Aβ plasma levels were always analyzed in duplicates, and the 4 determinations (FP40, TP40, FP42, and TP42) from 1 sample were measured intra-assay to reduce variability and to avoid extra freeze/thaw cycles. The analyses were always performed in a coded manner to ensure blindness of the operator.
Plasma Aβ data used in this study come from 2 set of assays carried out in June to July 20143 and January to February 2016, respectively; 98 samples from 33 individuals were included in both sets of ELISAs and were used to assess test-retest reproducibility. Different batches of these kits were used in each ELISA set. The average coefficient of variation (CV), the average relative difference (percent), and the intraclass correlation coefficient (ICC) between both sets of analyses were calculated. An ICC >0.75 indicates excellent reproducibility; 0.4 ≤ ICC ≤0.75 indicates fair to good reproducibility. In addition, the percentage difference of the results in each pair of repeated samples was calculated as 100 × (repeated − original)/original. Following regulatory guidelines for validation of bioassays,27 the acceptability criteria for incurred sample reanalysis (4-6-30) is that at least 67% of the repeated samples results should be within 30% of the original results.
Statistical analysis
Sample demographic and clinical characteristics were investigated with a range of statistical methods, including independent-samples t test, Kruskal-Wallis ranks test, and the χ2 test with the Fisher exact approximation when necessary. Plasma TP42/40 means were compared between Aβ-PET groups with generalized linear models. Random effects due to the 2 assay sets were assessing with generalized linear mixed models (GLMMs). Assessment of potential confounders age, sex, and APOE ε4 allele status was performed in separate analyses (within the GLMM), with clinical classification added as a surrogate for cognitive staging. Receiver operating characteristic (ROC) analyses was used to calculate thresholds from fitted GLMM via the Youden28 method (Youden maximum). Ensuing sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using the chosen thresholds without adjustment for population prevalence. To ensure that the calculated thresholds had utility, we let the ROC analyses find the optimum threshold given the Youden index at each time point and then used the average threshold for plotting correlation (Spearman ρ) and agreement results (for both unadjusted and adjusted analyses). GLMM comparisons were performed with analysis of variance analyses of deviance, with p values determined with χ2 distribution with 1 df.29 Predictive ROC models were compared with the DeLong method.30 Values of p from comparisons of biomarker means were compared with a Bonferroni-adjusted α value (α = 0.05/k number of tests; the main biomarker, TP42/40, was assessed at 3 time points, k = 3: 0.0167). All statistical analyses were performed with the R Statistical Environment (R Foundation for Statistical Computing, Vienna, Austria).31
Data availability
Deidentified participant data used for this article, together with the study protocol and statistical methods used, will be made available, after the article publication date and for 5 years, for any scientist by request to the authors for the only purpose of assessing replicability of the results published in the present article.
Results
Sample demographics
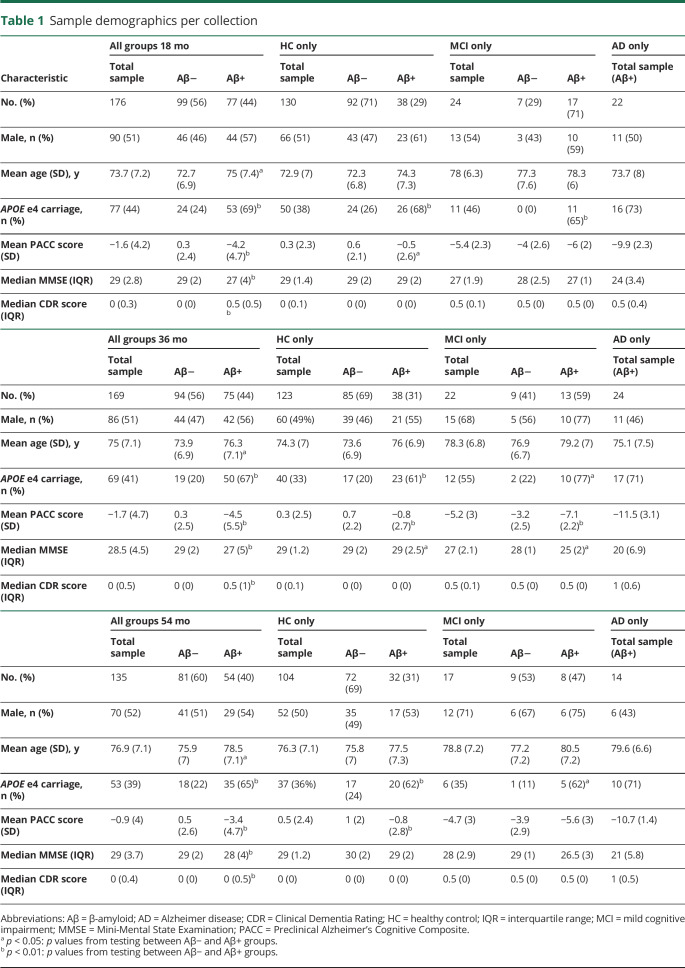
Clinical characteristics, including APOE ε4 allele status, Preclinical Alzheimer's Cognitive Composite score, Mini-Mental State Examination, and Clinical Dementia Rating score, along with sample demographics age and sex, were assessed between Aβ-PET+ve and Aβ-PET−ve groups at the 18-, 36-, and 54-month time points, stratified for clinical classification. Because there were no Aβ-PET−ve participants in the AD group, no comparisons were made. At all time points, there were more APOE ε4–positive participants in the Aβ-PET+ve groups compared with the Aβ-PET−ve groups (table 1). Compared to Aβ-PET−ve participants, those in the Aβ-PET+ve group were older and had lower cognitive performance at all time points; however, this varied between clinical classifications. There were no sex differences between Aβ-PET groups.
Table 1.
Sample demographics per collection
Mean biomarker differences between Aβ-PET groups
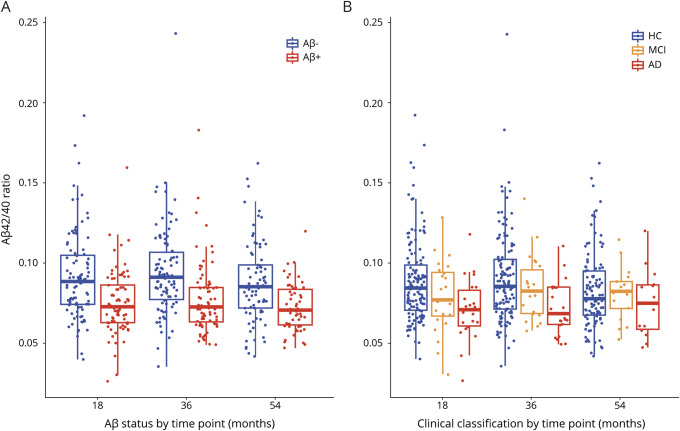
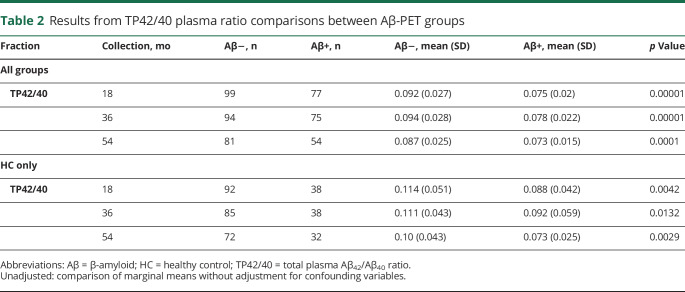
Figure 1 shows that the TP42/40 ratio is consistently lower in both Aβ-PET+ve participants and those with AD compared with Aβ-PET−ve participants and HCs across all time points. Comparisons of mean TP42/40 biomarker values (mean ± SD) between Aβ-PET groups with unadjusted p values are shown in table 2 (p values from adjusted models are presented in table e-1, available from Dryad, doi.org/10.5061/dryad.f300d56). For both the complete study population and HC group only, it is clear that at all time points the TP42/40 ratio is significantly lower in the Aβ-PET+ve group compared with the Aβ-PET−ve group (unadjusted marginal means). The degree of these differences, however, is clearly affected by the confounding factors, and significance is diminished in adjusted models (18-month unadjusted p = 0.00001, adjusted p = 0.057, table e-1). The confounders APOE ε4 allele status and clinical classification were associated with PET-Aβ status in all models (p < 0.02). Assessing the HC group specifically, we saw similar relationships, indicating that the TP42/40 ratio in plasma is consistent with cerebral amyloid pathology, not with the clinical stage, although a tendency to lower levels in patients with AD is appreciated for the plasma ratio (figure 1). A comparison of the base model, including only the covariates age, sex, APOE ε4 allele status, and clinical classification, with the base model plus the TP42/40 biomarker showed that adding the biomarker performed significantly better than the base model alone at all time points (18 months p = 0.00068, 36 months p = 0.016, 54 months p < 0.0001).
Figure 1. TP42/40 plots for Aβ-PET and clinical classification groups.
Box and whisker plots of total plasma β-amyloid (Aβ)42/Aβ40 (TP42/40) ratio between the 3 time points and (A) PET Aβ groups or (B) clinical classification. Raw data are presented on a box-and-whisker plot background. Middle line of the box represents the median; lower and upper lines represent first and third quartiles, respectively. In panel A, blue represents those participants who are PET-Aβ−ve, and red represents those participants who are PET-Aβ+ve. In panel B, blue represents those participants who are in the healthy control (HC) group, orange represents those participants who are in the mild cognitive impairment (MCI) group, and red represents those participants who are in the Alzheimer disease (AD) group.
Table 2.
Results from TP42/40 plasma ratio comparisons between Aβ-PET groups
Correlation and agreement between plasma TP42/40 ratio and SUVR/Aβ-PET
We approached the correlation and agreement between the plasma biomarker and PET biomarker in 2 separate ways; (1) given the p value attenuation due to confounders (mainly APOE ε4 allele status and clinical classification), we used the fitted values from each model (fitted biomarker) to assess both the correlation with SUVR and the agreement with Aβ-PET after threshold derivation (figure 2), and (2) we assessed the raw plasma biomarker data against SUVR (figure e-1, available from Dryad, doi.org/10.5061/dryad.f300d56).
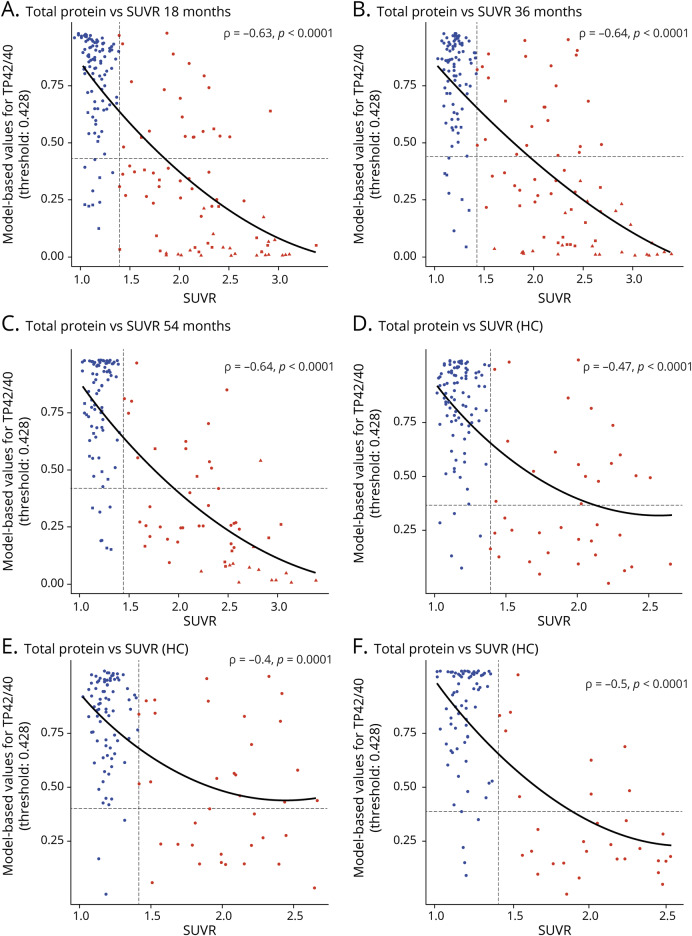
Figure 2. Correlation and agreement between model fitted values including TP42/40 and amyloid-PET SUVR.
Correlation and threshold plot for the fitted values from the amyloid Aβ42/Aβ40 (TP42/40) model (generalized linear mixed model [GLMM]) including covariates as represented in table e-1 (available from Dryad, doi.org/10.5061/dryad.f300d56) (y-axis, inverse with values close to 1 indicating high probability that they are plasma Aβ-ve, and values close to 0 indicating high probability that they are plasma Aβ+ ve) vs standardized uptake value ratio (SUVR) (x-axis). (A– C) Relationships for all participants, and (D–F) relationships for healthy control (HC) participants only. (A and D) Relationships at 18 months, (B and E) relationships at 36 months, and (C and F) relationships at 54 months. Shown on each are the quadratic fit lines representing the relationship between fitted values from the TP42/40 model (from a full GLMM including adjustment for confounders) and SUVR. Spearman ρ value with associated p value from the same data is shown in the top right of each plot. Circles represent those participants from the HC group; squares represent those participants from the mild cognitive impairment group; triangles represent those participants from the Alzheimer disease group. Blue points represent those participants who are PET-Aβ-ve, and red points represent those participants who are PET-Aβ+ve.
The correlation between fitted model values, including TP42/40 and SUVR for the whole study population (figure 2, A–C), was similarly strong at the 3 time points (ρ = ≈−0.63, −0.64, −0.64, p < 0.0001 at 18, 36, and 54 months, respectively). Similar correlations, albeit slightly weaker (ρ = ≈−0.47, −0.40, −0.50, p < 0.0001 at 18, 36, and 54 months, respectively), were seen for the HC group only (figure 2, D–F). Derived thresholds from the adjusted model were 0.433, 0.488, and 0.361. Taking the mean of these, we plotted the overall threshold (0.428) on both the complete and HC data for agreement statistics calculations. Correlation between the raw plasma biomarker and SUVR was weaker, but still highly significant, across all time points for all participants and the HC-only sample (figure e-1, available from Dryad, doi.org/10.5061/dryad.f300d56).
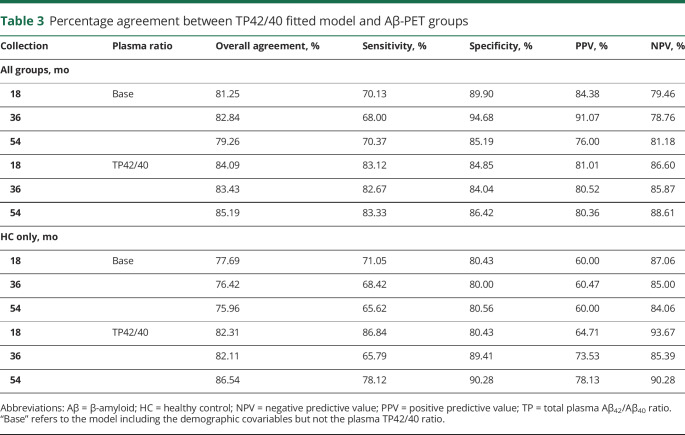
Table 3 shows the agreement statistics that align with the quartiles seen in figure 2. Overall agreement for TP42/40 was quite consistent throughout the 3 time points, ranging between 83% and 85% for the complete group and between 82% and 86% in the HC group. Regarding discriminating capability, each test had a better capability to predict those with subthreshold cortical amyloid burden, particularly in the HC group, with NPVs ranging from 85% to 93%, compared with the PPVs, which were lower (64%–78%). Agreement results for the plasma biomarker alone were stronger in the HC-only group than in the all-participants group, with overall percent agreement higher by at least 2% at each time point (figure e-1 and table e-2, available from Dryad, doi.org/10.5061/dryad.f300d56).
Table 3.
Percentage agreement between TP42/40 fitted model and Aβ-PET groups
ROC analyses
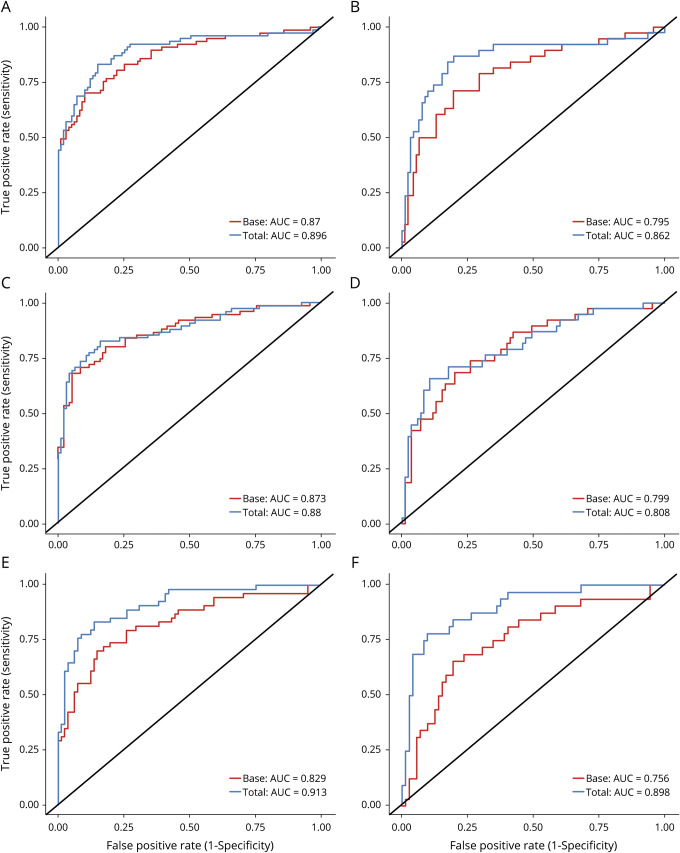
ROC analyses for the TP42/40 biomarker adjusted for confounders (age, sex, APOE ε4 allele status, and clinical classification) are shown in figure 3. Results are consistent at each time point, with the adjusted area under the curve (AUC) for the TP42/40 plasma ratio ranging from 0.88 to 0.913 for all groups and from 0.808 to 0.898 for the HC group. The adjusted ROC models (DeLong method) containing the TP42/40 biomarker were significantly stronger than the base model (age, sex, APOE ε4 allele status, and clinical classification) at both 18 and 54 months (p = 0.043 and p = 0.002, respectively), but not at 36 months (p = 0.497) (table e-3, available from Dryad, doi.org/10.5061/dryad.f300d56). Results for the HC group analyses were similar (18 months p = 0.02, 36 months p = 0.686, 54 months p = 0.0007).
Figure 3. ROC curves for the TP42/40 plasma ratio vs the base model.
Plots show the receiver operating characteristic (ROC) curves from 2 models to predict PET β-amyloid (Aβ) status, the base model with covariates only, and the base model plus the total plasma Aβ42/Aβ40 (TP42/40) biomarker. (A, C, and E) ROC curves from all participants at 18, 36, and 53 months, respectively. (B, D and F) ROC curves from healthy control (HC) participants only at 18, 36, and 53 months. Shown on each panel are the ROC curves with the calculated area under the curve (AUC) value in the bottom right corner at 18 months (A and B) 36 months (C and D), and 54 months (E and F).
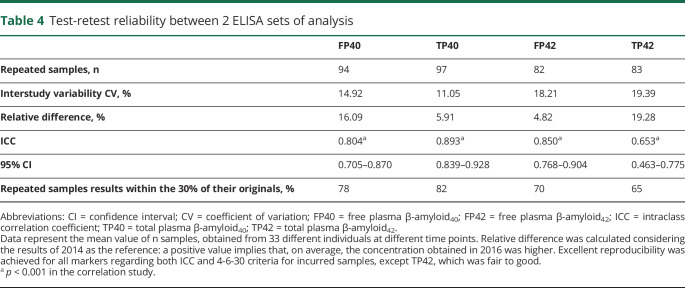
Test-retest study
Because 98 samples of this study had already been analyzed in a previous study, we could evaluate the test-retest reproducibility of our assay between both studies. The results of the test-retest reproducibility study are summarized in table 4. On average, the CV between the 2 ELISA sets, which were separated by 18 months, was <20% for the 4 determinations assayed (FP40, TP40, FP42, and TP42). The relative difference between determinations in the 2 set of assays was also <20% for all of these determinations. The ICC was >0.8 for FP40, TP40, and FP42, exhibiting an excellent reproducibility. TP42 presented an ICC = 0.653, which is also considered fair to good. In addition, following regulatory guidelines for the validation of ligand binding assays, 3 of the Aβ determinations (table 4) met acceptability criteria (4-6-30 criteria) for incurred samples reanalysis, while TP42 results were borderline.
Table 4.
Test-retest reliability between 2 ELISA sets of analysis
Discussion
In this study, we have found that the TP42/40 plasma ratio is consistently associated with Aβ-PET status over the 3 time points assayed. Results from all time points show highly significant lower plasma TP42/40 ratio in the Aβ-PET+ve group than in the Aβ-PET−ve group, before adjustment for covariates (table e-1, available from Dryad, doi.org/10.5061/dryad.f300d56). We observed that significance was markedly reduced due to the strong association between both APOE ε4 allele status and clinical classification with Aβ-PET. The role of the confounders in the association between the plasma biomarker and Aβ-PET status could be a concern if changes in both variables were due to common causes. However, assuming that TP42/40 depends exclusively on levels of brain Aβ burden (not from other sources) and that confounders such as clinical classification are upstream to brain amyloid accumulation and consequently to both biomarkers, we considered that presentation of results without adjustment for confounding factors was necessary.
On the other hand, concerning the correlation and agreement of TP42/40 with Aβ-PET and its performance for discriminating Aβ-PET status, results are presented both adjusted and unadjusted for the relevant covariates. In this case, we included the clinical classification, such that any person with normal cognition, MCI, or AD can be tested for cortical amyloid positivity with our test in plasma. Nevertheless, given the interest in blood-based biomarkers for secondary prevention clinical trials and management of preclinical individuals, we explored those variables in the HC group alone, in which, obviously, clinical classification was not used.
Along these lines, the result of the adjusted model containing TP42/40 showed a consistent inverse correlation with the SUVR at the 3 time points (ρ =≈−0.63, p < 0.0001) and an overall agreement with Aβ-PET status ranging from 83% to 85%, with PPVs of 80% to 81% and NPVs of 86% to 88%. The results for the HC group were similar at each time point (overall agreement with Aβ-PET status ranging from 82%–86%), even outperforming the NPV (85%–93%) with regard to all participants (despite the reduction in the sample size), which is very relevant for any screening test. The agreement of the unadjusted plasma ratio (table e-2 and figure e-1, available from Dryad, doi.org/10.5061/dryad.f300d56) with the Aβ-PET status was poorer, ranging from 63% to 65% in the all-participants group. Nevertheless, sensitivity (81%–89%) and NPV (64%–84%) in the HC group could still lead to a substantial reduction in the number of amyloid-PET scans in a screening scenario (see below).
These results are consistent with our previous findings showing an inverse association between the Aβ42/40 plasma ratios, particularly TP42/40, and cortical Aβ burden in an AIBL subcohort of cognitively normal controls3 and other independent cohorts.10,32 In agreement with this, it has previously been reported that the plasma Aβ42/40 ratio correlated directly with Aβ42 CSF levels and inversely with Aβ–PET SUVR.4–9 Moreover, our present results support previous community-based studies in healthy people reporting an association between lower plasma Aβ42/40 ratio and greater cognitive decline12 or increased risk of developing AD dementia at follow-up.13–17 More recently, an association between cortical Aβ burden and the Aβ42/40 plasma ratio, as determined by liquid chromatography tandem mass spectrometry, has also been found in HCs and patients with MCI and AD.33,34 Several studies have provided mechanistic descriptions supporting this association by demonstrating the existence of an Aβ-specific molecular transporter at the blood-brain barrier35–37 and positive clearance of Aβ peptides from the brain to the peripheral vasculature in humans.38,39 Thus, mounting evidence coming from varied experimental designs and different analytical methods supports the existence of an association between the Aβ42/40 plasma ratio and the cortical amyloid burden. Furthermore, the ROC curve analysis in the present work demonstrated the reproducibility of our plasma assay to separate Aβ-PET groups over 3 time points, with an AUC for the TP42/40–adjusted model ranging from 0.880 to 0.913 in the all-participants group and from 0.808 to 0.898 in the HC-only group. Thus, the consistency of our assay, demonstrated through follow-up, confers reliability to TP42/40 as a biomarker for cortical amyloidosis, which is additionally supported by the test-retest results obtained with the 98 samples assayed in the 2 sets of ELISAs carried out 1.5 years apart. The CV (<20%) for these test-retests was within the criteria recommended for interassay reproducibility and confirm previous ABtest validation results following regulatory agencies guidelines.27
Various studies from other groups have failed to replicate this association between plasma Aβ levels and clinical or pathologic aspects of AD.18–21 This disparity in results can be explained at least in part by the use of the clinical diagnosis (instead of brain Aβ burden) as the gold standard to assess performance of Aβ blood-based tests. Our results show that the TP42/40 plasma ratio is consistent with cortical amyloid pathology as visualized by PET and less so with the clinical diagnosis, which itself has shown sensitivities ranging from 70.9% to 87.3% and specificities from 44.3% to 70.8%.11,40 This relatively poor performance for a gold standard can seriously skew the results of any testing and is almost certainly a relevant source of variability between studies.
Concordance of Aβ blood tests among different studies may also be hindered by the relatively small difference in the Aβ42/40 plasma ratio among Aβ-PET groups. In the present study, the TP42/40 was on average ≈17% lower in the Aβ-PET+ve participants than in the Aβ-PET−ve (≈22% lower among the HCs; table 2) whereas in the CSF, Aβ42/40 ratio differences between those 2 groups are ≈50%.33 Thus, stringent adherence to the protocols, including preassay handling of the samples, is of maximum relevance to minimize the variability of determinations in the highly complex plasma matrix that may blur relatively small, but meaningful, differences. In this regard, it deserves to be underlined that correlations between TP42/40 plasma levels and Aβ-PET SUVR found in the present study (ρ = ≈−0.63; p < 0.0001) were stronger than those found in a recent study using an ultrasensitive single-molecule assay (Aβ42/40 vs [18F]flutemetamol SUVR, ρ = −0.167, p = 0.002).4 On the other hand, the performance of the TP42/40–adjusted model to discriminate Aβ-PET status in the present work (AUC ranging from 0.88–0.91) was very similar to that obtained by liquid chromatography tandem mass spectrometry (AUC 0.88).33,34 The concordance of our results with these studies using 2 technically different modes of assessments again strongly supports the reliability of the plasma Aβ42/40 ratio measurement as a biomarker for predicting amyloid-PET results.
Furthermore, we have reported that in a recruitment scenario targeting cognitively normal Aβ-PET+ve participants, the TP42/40 ratio could be used as a prescreening tool able to reduce the number of individuals undergoing Aβ-PET scans by ≈50%.3 Assuming a 30% prevalence of Aβ-PET in the HC group of this particular study, a recruitment based exclusively on amyloid-PET scans would need to test 500 individuals to recruit 150 Aβ-PET+ve with an SRF of ≈70% (150 chosen for the sake of simplicity in the calculation; any pivotal secondary prevention clinical trial would most probably have a sample size closer to an order of magnitude greater).
In the present study, the average values for sensitivity and PPV from the 3 time points analyzed within the HC group were 77% and 72%, respectively. Thus, to find those 150 individuals using our plasma prescreening tool (sensitivity 77%), we would need a population containing 195 Aβ-PET+ve (150 × 100/77) which at a 30% prevalence would mean 650 HCs (195 × 100/30). Because our plasma marker model had a 72% PPV, we would have 58 plasma false positives (150 × 100/72) together with the pursued 150. Thus, the total number of amyloid-PET required to recruit those 150 individuals would be reduced from 500 to 208. In addition, this 2-step screening strategy would reduce the SRF at the amyloid-PET scan visit from 70% to ≈28%, reducing substantially the patients' burden and overall budget for secondary prevention trials for amyloid-targeting therapies. Moreover, the high NPV (average for the 3 time points ≈90% in the HC group) indicates that only a small fraction of the suitable Aβ-PET+ve individuals will be missed during prescreening as plasma test false negative, contributing to shortening of the recruitment period.
A strength of the present study is the large proportion of HC participants across all time points (≈75%). The Aβ-PET positivity rate for this group across each of the 3 time points is ≈30%, which is higher than previously published for other cohorts such as BioFinder and the Alzheimer's Disease Neuroimaging Initiative.41 Given this higher prevalence of Aβ-PET positivity, it is possible that the PPV could have been overestimated and the NPV underestimated. However, we used the PPV and NPV calculations without the addition of the sample or population prevalence such that it would be an unbiased calculation. Furthermore, the strong NPV values for the plasma ratio in the HC-only group provide strong evidence for real-life inference across healthy and clinically impaired people >65 years of age.
In addition, we demonstrate the strength of the differences in TP42/40 between Aβ-PET groups both with and without potential confounders. The presence of an APOE ε4 allele within the modeling appeared to play quite a strong role in explaining variance in Aβ-PET groups, with higher prevalence in the Aβ-PET+ groups; however, its importance was decreased in the later time point for both complete and HC-only samples. Regulating Aβ aggregation and clearance in the brain,42 variants in the APOE gene are important to account for in biomarker analyses, especially so when considering the age of participants. Given these underlying associations with age, APOE, and amyloid, it is important then to account for these factors when looking at blood-based biomarkers, helping to better understand a pathologic picture of the disease. Adding a representative of the clinical form of the disease (i.e., clinical classification) improves our estimates of where a person lies on the disease trajectory, which is very useful in both clinical trial design and the clinic.
These results show that Aβ peptides can be measured in plasma with enough reproducibility and consistency to implement TP42/40 as a reliable biomarker discriminating Aβ-PET status. The use of TP42/40 could facilitate the screening process for preventive clinical trials in AD, avoiding invasive testing in a significant number of clinically healthy volunteers and saving substantial amounts of money and time.
Nevertheless, we acknowledge the potential weakness of this study due to the relatively small sample size and the variability of determinations of Aβ in plasma, largely related to the characteristics of the plasma matrix in each individual. This variability, together with the differences and overlapping levels of TP42/40 between the Aβ-PET+ve and Aβ-PET−ve groups, still hampers the use of markers in plasma. Larger population studies should be addressed to overcome such weakness and to demonstrate sufficient precision to be prospectively used in secondary prevention clinical trials.
Glossary
- Aβ
β-amyloid
- Aβ42/40
Aβ42/Aβ40 ratio
- AD
Alzheimer disease
- AIBL
Australian Imaging, Biomarkers and Lifestyle
- AUC
area under the curve
- BP42/20
bound plasma Aβ42/Aβ40 ratio
- CV
coefficient of variation
- FP42/20
free plasma Aβ42/Aβ40 ratio
- GLMM
generalized linear mixed models
- HC
healthy control
- ICC
intraclass correlation coefficient
- MCI
mild cognitive impairment
- NPV
negative predictive value
- PPV
positive predictive value
- ROC
receiver-operating characteristic
- SRF
screening rate of failure
- SUVR
standardized uptake value ratio
- TP42/20
total plasma Aβ42/Aβ40 ratio
Appendix 1. Authors
Appendix 2. Coinvestigators
Footnotes
Class of Evidence: NPub.org/coe
Study funding
This work has been financed by Araclon Biotech Ltd.
Disclosure
J. Doecke reports no disclosures relevant to the manuscript. V. Pérez-Grijalba and N. Fandos are full-time employees at Araclon Biotech Ltd. C. Fowler reports no disclosures relevant to the manuscript. V. Villemagne received funding for a trip to attend the CTAD 2018 congress. C. Masters reports no disclosures relevant to the manuscript. P. Pesini is a full-time employee at Araclon Biotech Ltd. M. Sarasa is a full-time employee at and a shareholder of Araclon Biotech Ltd. Go to Neurology.org/N for full disclosures.
References
- 1.Blennow K. A review of fluid biomarkers for Alzheimer's disease: moving from CSF to blood. Neurol Ther 2017;6:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alber J, Lee AKW, Menard W, Monast D, Salloway SP. Recruitment of at-risk participants for clinical trials: a major paradigm shift for Alzheimer's disease prevention. J Prev Alzheimers Dis 2017;4:213–214. [DOI] [PubMed] [Google Scholar]
- 3.Fandos N, Perez-Grijalba V, Pesini P, et al. Plasma amyloid beta 42/40 ratios as biomarkers for amyloid beta cerebral deposition in cognitively normal individuals. Alzheimers Dement (Amst) 2017;8:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janelidze S, Stomrud E, Palmqvist S, et al. Plasma beta-amyloid in Alzheimer's disease and vascular disease. Sci Rep 2016;6:26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rembach A, Faux NG, Watt AD, et al. Changes in plasma amyloid beta in a longitudinal study of aging and Alzheimer's disease. Alzheimers Dement 2014;10:53–61. [DOI] [PubMed] [Google Scholar]
- 6.Toledo JB, Vanderstichele H, Figurski M, et al. Factors affecting Abeta plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol 2011;122:401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devanand DP, Schupf N, Stern Y, et al. Plasma Abeta and PET PiB binding are inversely related in mild cognitive impairment. Neurology 2011;77:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lui JK, Laws SM, Li QX, et al. Plasma amyloid-beta as a biomarker in Alzheimer's disease: the AIBL study of aging. J Alzheimers Dis 2010;20:1233–1242. [DOI] [PubMed] [Google Scholar]
- 9.Swaminathan S, Risacher SL, Yoder KK, et al. Association of plasma and cortical amyloid beta is modulated by APOE epsilon4 status. Alzheimers Dement 2014;10:e9–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Grijalba V, Romero J, Pesini P, et al. Plasma AB42/40 ratio detects early stages of Alzheimer's disease and correlates with CSF and neuroimaging biomarkers in the AB255 study. JPAD 2019;6:34–41. [DOI] [PubMed] [Google Scholar]
- 11.Palmqvist S, Janelidze S, Stomrud E, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related beta-amyloid status. JAMA Neurol Epub 2019 June 24. [DOI] [PMC free article] [PubMed]
- 12.Yaffe K, Weston A, Graff-Radford NR, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA 2011;305:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullah L, Luis C, Paris D, et al. Serum Abeta levels as predictors of conversion to mild cognitive impairment/Alzheimer disease in an ADAPT subcohort. Mol Med 2009;15:432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chouraki V, Beiser A, Younkin L, et al. Plasma amyloid-beta and risk of Alzheimer's disease in the Framingham Heart Study. Alzheimers Dement 2015;11:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graff-Radford NR, Crook JE, Lucas J, et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol 2007;64:354–362. [DOI] [PubMed] [Google Scholar]
- 16.Lambert JC, Schraen-Maschke S, Richard F, et al. Association of plasma amyloid beta with risk of dementia: the prospective Three-City Study. Neurology 2009;73:847–853. [DOI] [PubMed] [Google Scholar]
- 17.van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Abeta(1-40) and Abeta(1-42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol 2006;5:655–660. [DOI] [PubMed] [Google Scholar]
- 18.Hansson O, Zetterberg H, Vanmechelen E, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer's disease in patients with mild cognitive impairment. Neurobiol Aging 2010;31:357–367. [DOI] [PubMed] [Google Scholar]
- 19.Le Bastard N, Aerts L, Leurs J, Blomme W, De Deyn PP, Engelborghs S. No correlation between time-linked plasma and CSF Abeta levels. Neurochem Int 2009;55:820–825. [DOI] [PubMed] [Google Scholar]
- 20.Lopez OL, Kuller LH, Mehta PD, et al. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology 2008;70:1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovheim H, Elgh F, Johansson A, et al. Plasma concentrations of free amyloid-beta cannot predict the development of Alzheimer's disease. Alzheimers Dement 2017;13:778–782. [DOI] [PubMed] [Google Scholar]
- 22.Pesini P, Sarasa M. Beyond the controversy on Aß blood-based biomarkers. J Prev Alz Dis 2015;2:51–55. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Grijalba V, Fandos N, Canudas J, et al. Validation of immunoassay-based tools for the comprehensive quantification of Abeta40 and Abeta42 peptides in plasma. J Alzheimers Dis 2016;54:751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis KA, Bush AI, Darby D, et al. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr 2009;21:672–687. [DOI] [PubMed] [Google Scholar]
- 25.Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med 2005;46:1959–1972. [PubMed] [Google Scholar]
- 26.Rembach A, Watt AD, Wilson WJ, et al. Plasma amyloid-beta levels are significantly associated with a transition toward Alzheimer's disease as measured by cognitive decline and change in neocortical amyloid burden. J Alzheimers Dis 2014;40:95–104. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman D. Statistical considerations for assessment of bioanalytical incurred sample reproducibility. AAPS J 2009;11:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 29.Hastie TJ, Pregibon D. Generalized linear models. In: Chambers JM, Hastie TJ, editors. Statistical Models in S. Wadsworth & Brooks/Cole; 1992:chapter 6. [Google Scholar]
- 30.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 31.R Computing Team. A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 32.De Rojas I, Romero J, Rodriguez-Gomez O, et al. Correlations between plasma and PET beta-amyloid levels in individuals with subjective cognitive decline: the Fundació ACE Healthy Brain Initiative (FACEHBI). Alzheimer's Res Ther 2018;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ovod V, Ramsey KN, Mawuenyega KG. et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimer's Demen 2017;13:841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature 2018;554:249–254. [DOI] [PubMed] [Google Scholar]
- 35.Cirrito JR, Deane R, Fagan AM, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest 2005;115:3285–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sagare A, Deane R, Bell RD, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med 2007;13:1029–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata M, Yamada S, Kumar SR, et al. Clearance of Alzheimer's amyloid-Ab(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 2000;106:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS {beta}-amyloid in Alzheimer's disease. Science 2010;330:1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts KF, Elbert DL, Kasten TP, et al. Amyloid-beta efflux from the CNS into the plasma. Ann Neurol 2014;76:837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease centers. J Neuropathol Exp Neurol 2012;71:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid-beta PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 2018;14:1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong N, Weisgraber KH. Understanding the association of apolipoprotein E4 with Alzheimer disease: clues from its structure. J Biol Chem 2009;284:6027–6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data used for this article, together with the study protocol and statistical methods used, will be made available, after the article publication date and for 5 years, for any scientist by request to the authors for the only purpose of assessing replicability of the results published in the present article.