Abstract
Aim
The purpose of this study was to evaluate the prognostic impact of red-cell distribution width (RDW) on the overall survival (OS) of patients with squamous cell carcinoma (SCC) of the tongue.
Background
Development of cancer is connected with an ongoing inflammatory process which is reflected by laboratory indices, such as RDW that can be used as prognostic tools.
Material and methods
The study group consists of 74 consecutive patients treated with radical radiotherapy or chemo-radiotherapy for SSC of the tongue at one institution between 2005−2014. RDW was assessed based on routine blood tests done before the start of the treatment. ROC curve was applied to assess value of RDW in prediction of OS, and a cut-off value for further tests was obtained using the Younden index. The survival analysis was performed using the Kaplan-Meier method, log-rank testing and Cox regression model.
Results
The AUC for RDW in ROC analysis was 0.703, and the optimal cut-off value was 13.5%. 5-year OS was significantly lower in patients with RDW ≥ 13.5% compared with patients with RDW < 13.5% (67% vs. 26%, p-value = 0.0005). Additionally, high RDW was associated with a greater odds ratio for 5-year OS in a multivariate Cox proportional hazards regression analysis (3.43, 1.62–7.25; p = 0.001).
Conclusion
Our study demonstrated that pre-treatment RDW ≥ 13,5% is an indicator of poor overall survival in patients with SCC of the tongue. Since RDW is a cheap and convenient marker, usually routinely assessed during complete blood count tests, it could be further used as an additional prognostic tool in patients with tongue cancers.
Keywords: Tongue cancer, Radiotherapy, Red cell distribution width, RDW
1. Introduction
Cancers located in the base and anterior tongue (C01-C02) account for 0.6% (males) and 0.2% (females) cancer incidence in Poland, which adds up to approximately 400–600 new cases per year.1 Infrequent as it is, this disease is connected with high mortality and 5-year overall survival (OS) below 30% for males and around 45% for females.2 The main risk factors include tobacco, alcohol consumption, HPV infection, poor oral hygiene and chewing of betel and areca nuts, the latter being rather uncommon in central Europe.3,4
According to NCCN guidelines, the primary treatment modalities include standalone surgery with or without lymphadenectomy, radiotherapy, chemo-radiotherapy and multimodality approaches.5 Anterior tongue lesions are preferably treated with surgical approach while radiotherapy and chemo-radiotherapy is better suited for base-of-tongue cancers.4
Red blood cell distribution width (RDW) is defined as the quotient of the standard deviation of red blood cells volume and their mean volume. In practical terms, higher RDW values reflect greater size variations of red blood cells, which might be associated with multiple interrelated factors, such as chronic inflammation, prolonged bleeding or oxidative stress.6,7 Inflammation, in particular, can be associated with poorer prognosis8 due to cytokines and other mediators stimulating increased angiogenesis, tumor proliferation and metastasis.9 Up to now, the prognostic value of RDW has been confirmed in numerous malignancies.8,10,19,11, 12, 13, 14, 15, 16, 17, 18 However, in certain publications the correlation was not apparent20,21 or a variation of RDW index had to be used.22 The primary aim of our paper was to evaluate the RDW as a prognostic factor in a cohort of patients with squamous cell tongue cancers subjected to primary radio- or chemo-radiotherapy.
2. Material and methods
2.1. Patients
Out of 2156 patients treated for oropharyngeal and oral cavity cancers between January 2005 and December 2014 in our institution, 80 patients met the following inclusion criteria:
-
-
histopathologically proven squamous cell cancer primarily localized in the anterior tongue or base of tongue.
-
-
radiotherapy or chemo-radiotherapy with radical intent as the only method of treatment.
-
-
no prior tumorectomy or lymphadenectomy
-
-
no other oncological treatment within 5 years prior to treatment excluding treatment of non-melanoma skin cancers.
The blood tests were performed prior to the first fraction of radiotherapy or first dose of chemotherapy in patients treated with inclusion of neoadjuvant chemotherapy. Six cases had to be removed due to missing data. The final cohort included 74 retrospectively collected cases of patients treated with radical radiotherapy (54 patients) or chemo-radiotherapy (20 patients) for squamous cell tongue cancers (Table 1). 80% of the patients were male and 20% female. The median age was 59 (mean – 61). Data regarding concomitant diseases, tobacco smoking and alcohol drinking history was gathered. Since HPV status tests were not routinely performed before 2014 in our institution, the TNM classification was assessed in compliance with the American Joint Committee on Cancer, 7th edition (2010) for all of the patients. The main endpoint was 5-year OS.
Table 1.
Baseline characteristics, pathological findings and outcomes in the whole population of patients stratified by RDW level and their correlation with RDW.
| Whole population n = 74 |
RDW |
|||
|---|---|---|---|---|
| Low (<13.5%) n = 28 |
High (≥13.5%) n = 46 |
p-value | ||
| Sex (% of males) | 79,73% | 82,14% | 78,26% | 0.69 |
| Age [years] | 60,7 (40.7−83.0) | 59,8 (49.6−76.4) | 61,2 (40.7−83.0) | 0.47 |
| RDW [%] | 13.9 (12.0−16.3) | 12,8 (12,0−13.4) | 14,5 (13.5−16.3) | |
| Hemoglobin [g/dl] | 13.8 (10.3−18.5) | 14.0 (11.0−15.9) | 13.7 (10.3−18.5) | 0.23 |
| Hematocrit [%] | 40,3 (30.8−54.6) | 41,2 (33.1−47.2) | 39.8 (30.8−54.6) | 0.1 |
| RBC (106/mm3) | 4.38 (3.56−5.64) | 4.43 (3.68−5.21) | 4.35 (3.56−5.64) | 0.31 |
| WBC (103/mm3) | 8.3 (5−15) | 8.2 (5.4−14.5) | 8.4 (5−15) | 0.86 |
| TNM T stage | 0.84 | |||
| 1 | 2.7% | 3.6% | 2.2% | |
| 2 | 24.3% | 17.8% | 28.3% | |
| 3 | 18.9% | 25% | 15.2% | |
| 4 | 54.1% | 53.6% | 54.3% | |
| TNM N stage | 0.37 | |||
| 0 | 25.7% | 28.6% | 23.9% | |
| 1 | 18.9% | 25% | 15.2% | |
| 2 | 47.3% | 39.3% | 52.2% | |
| 3 | 8.1% | 7.1% | 8.7% | |
| Tumor volume | 0.5 | |||
| <15cc | 28.4% | 28.6% | 28.3% | |
| 15−30cc | 31.1% | 35.7% | 28.3% | |
| >30cc | 40.5% | 35.7% | 43.4% | |
| % base of tongue | 75.7% | 78.6% | 73.9% | 0.65 |
| Concomitant diseases | 58.1% | 50% | 62% | 0.30 |
| History of smoking | 0.29 | |||
| Non-smoker | 11% | 17.9% | 6.7% | |
| Former smoker | 6.8% | 7.1% | 6.7% | |
| Active smoker | 82.2% | 75% | 86.6% | |
| Pack-years | 32.5 (0−105) | 27.6 (0−90) | 35.6 (0−105) | 0.07 |
| History of alcohol abuse | 17.8% | 17.9% | 17.8% | 0.99 |
| ZUBROD | 0.97 | |||
| 0−1 | 93,2 | 89,3% | 95,6% | |
| 2 | 5.4% | 10.7% | 2.2% | |
| 3+ | 1.4% | 0% | 2.2% | |
| Fractionation regimen: | 0.01 | |||
| Conventional | 48.6% | 64.3% | 39.1% | |
| CAIR31 | 27% | 28.6% | 26.1% | |
| CHA-CHA32 | 19% | 7.1% | 26.1% | |
| EBRT + BT | 5.4% | 0% | 8.7% | |
| Addition of chemotherapy | 27% | 36% | 22% | 0.19 |
| Type of chemotherapy | 0.12 | |||
| Neoadjuvant | 25% | 20% | 30% | |
| Concomitant | 40% | 40% | 40% | |
| Both | 35% | 40% | 30% | |
| 5-year OSa | 42% | 67% | 26% | 0,0005 |
Continuous variables are presented as mean (min-max range) unless indicated otherwise. Dichotomous variables are presented as percentages.
log-rank.
Among the patients receiving chemotherapy – Cisplatin was used as a sole agent in concurrent chemotherapy in all cases. The neoadjuvant regimens varied from Docetaxel/Cisplatin/5-fluorouracil in 7 cases, Docetaxel/Cisplatin in 4 cases, and 5-fluorouracil/Cisplatin in one case. A detailed description of radiotherapy fractionation schemes is presented in Table 2. The treatment was delivered using IMRT in vast majority of the cases; however, there were a few cases where 3D conformal radiotherapy was used due to superior dose distribution achieved with such a method.
Table 2.
Description of radiotherapy modalities.
| Fractionation schedule: | Description: | Number of patients: | Complete treatment time (days) | Total dose: | Fraction dose: |
|---|---|---|---|---|---|
| Conventional Fractionation | One fraction per day, 5 days a week | 36 | 51 | 70Gy | 2Gy |
| CAIR | One fraction per day, 7 days a week | 20 | 40 | 72Gy | 1.8Gy |
| CHA-CHA | Two fractions a day, 7 days a week. 8 days break midterm. | 14 | 28 | 64Gy | 1.6Gy |
| External beam radiotherapy + brachytherapy boost (EBRT + BT) |
One fraction per day, 5 or 7 days a week, then BT. | 4 | 63 | 60 Gy + 18 Gy | 2Gy/3 Gy |
Data presented as median value in applicable cases.
The follow-up was based on data available from the patients’ medical history, Polish National Cancer Registry and the National Health Fund. The information about the date of patients’ death was available in all applicable cases. OS was defined as the time from the completion of radiotherapy to the date of death in all applicable cases or end of follow-up data in patients alive at the time of completing database.
Due to the fact that the study was retrospective, in accordance with our institutional policy, the Bioethical Committee approval was not necessary.
2.2. Statistical analysis
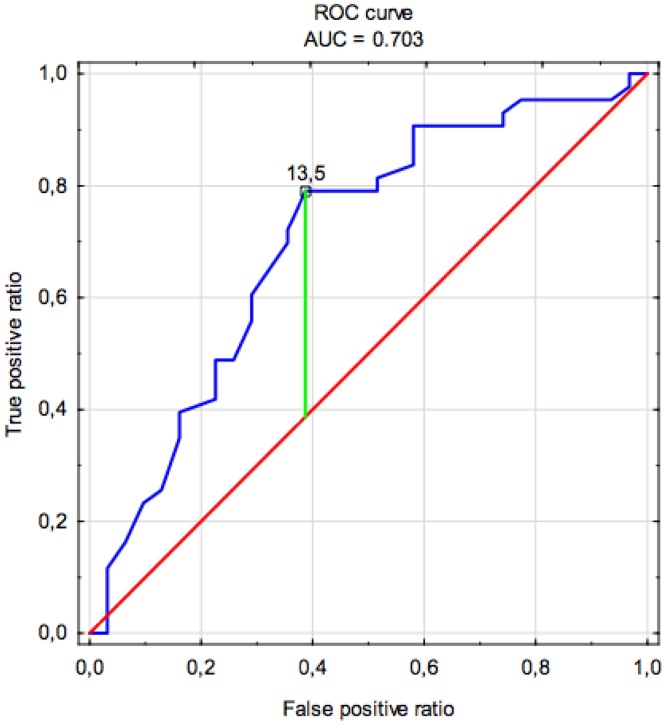
Receiver operating characteristics (ROC) curve was used to evaluate the accuracy of RDW as a prognostic factor. The cut-off value was determined using a method based on the Younden index and the patients were divided into two groups based on the RDW score (Fig. 1). The distribution of continuous variables was tested with the Shapiro-Wilk test. The statistical analysis employed the Chi-Square test for dichotomous categorical variables and Mann-Whitney U test for continuous variables. The survival analysis was performed using the Kaplan-Meier method and log-rank testing. The Cox proportional hazards regression model was applied to perform uni- and multivariate analysis including variables that reached a p-value lower than 0.05 in univariate analysis.
Fig. 1.
Receiver operating characteristic curve of red-cell distribution width for overall survival prediction.
The statistical analysis was performed using the STATISTICA 13 software with Medical Bundle (StatSoft INC., Tulsa Oklahoma, USA).
3. Results
The mean and median follow-up were 38.8 and 22.4 months, respectively. Death occurred in 58.1% of the cases within the first 5 years after treatment. The optimal cut-off value of RDW for 5-year OS calculated using ROC curve analysis was 13.5% (79% sensitivity and 39% specificity, Fig. 1). The AUC (area under curve) for 5-year OS was 0.703.
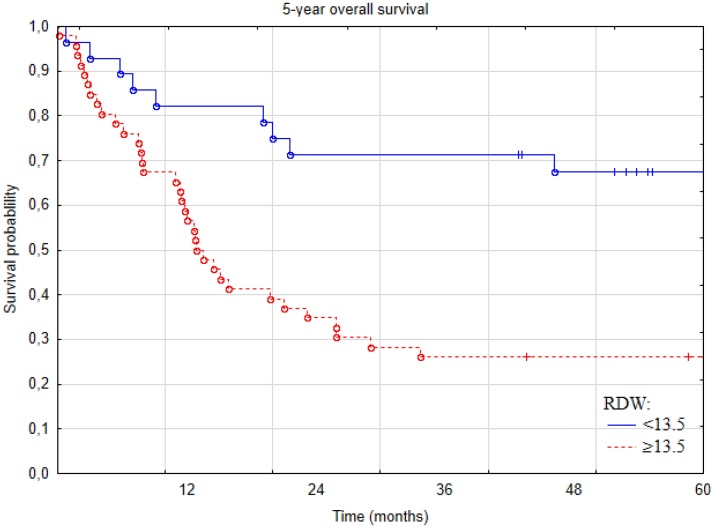
5-year overall survival was significantly higher in patients with RDW < 13.5% compared to patients with RDW ≥ 13.5%, and was 67% at 5 years compared to 26% in the high-RDW group (p-value = 0.0005, Fig. 2).
Fig. 2.
Kaplan-Meier curves for overall survival of patients with squamous cell carcinoma of the tongue stratified according to red-cell distribution width (RDW) level.
The univariate Cox regression analysis included sex, age, concomitant diseases’ burden, smoking, pack-years, history of alcohol abuse, ZUBROD score, location of the tumor (base/anterior), addition of chemotherapy, RT fractionation scheme and dose, total volume of the tumor, TNM stage groups, lymph node involvement, and following hematological indices: white blood cell, red blood cell, neutrophil, lymphocyte, and platelet count and hemoglobin concentration.
The final model included parameters with p-value <0.05: RDW, location of the tumor (base/anterior), addition of chemotherapy, RBC and HGB. RDW ≥ 13.5% was associated with a greater hazard ratio for 5-year OS in multivariate analysis (3.43, 1.62–7.25; p = 0.001) and addition of chemotherapy was associated with lower HR (0.35, 0.15−0.84, p = 0.02) after adjustment for significant covariates in Cox proportional hazards regression analysis (Table 3).
Table 3.
Multivariate Cox Regression analysis of HR for OS in patients with squamous cell carcinoma of the tongue.
| 95% CI |
||||
|---|---|---|---|---|
| Covariatesa | HR | Lower | Upper | P value |
| RDW (<13.5% vs. ≥ 13.5%) | 3,43 | 1,62 | 7,25 | 0,001 |
| Addition of chemotherapy | 0,35 | 0,15 | 0,84 | 0,018 |
| Tumor location (base vs. anterior) | 1,79 | 0,92 | 3,43 | 0,086 |
| RBC (mln/μl) | 0,41 | 0,13 | 1,27 | 0,121 |
| HGB (g/dl) | 1,03 | 0,69 | 1,55 | 0,871 |
The analysis includes parameters that were significantly correlated with OS in a univariate analysis at a p < 0.05 level of significance. The complete list of variables included in the univariate analysis is presented in the 3rd paragraph of the Results section.
We did not find any significant differences between the groups with low and high RDW in terms of sex, age, TNM stage group, tumor volume, lymph node involvement, concomitant diseases, history of smoking or alcohol abuse, ZUBROD performance scale score prior to treatment or addition of chemotherapy as is depicted in Table 1. Although red blood cell count, hemoglobin count and hematocrit were slightly lower in the high RDW group – the differences were not statistically significant. The groups were significantly different regarding applied modality of radiotherapy (p = 0.01).
4. Discussion
Squamous cell carcinoma of the tongue is an aggressive disease characterized by a poor prognosis. The local advancement of the tumor often results in an ongoing inflammatory process and subclinical bleeding. RDW is an indicator allowing differentiation of anemia. High RDW values are associated with a chronic inflammatory state and disrupted erythropoiesis.23,24 On top of that, RDW also serves as a marker reflecting systemic inflammation, which is regarded as the 7th hallmark of cancer.25 Due to the fact that this disease is often diagnosed at a late stage when subclinical bleeding and ongoing inflammatory process are chronically present and heavily pronounced, markers such as RDW can reflect the prognosis of the patients and present a cheap addition (or alternative) to common diagnostic tools, especially important considering much higher oral cavity cancers incidence in less wealthy Asian countries with betel and areca nut chewing traditions.26
In the study conducted by Lippi et al., the authors discovered that RDW correlated with one of the most commonly used markers of inflammation, hs-CRP and erythrocyte sedimentation rate test.27 The association between RDW and cancer patients prognosis can also be explained by the relationship between the red blood cell distribution width and factors like: interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), hepcidin and other circulating cytokines that may support the development of cancer.28,29 As to tongue cancer patients, who very often suffer from malnutrition, it is proven than RDW correlates with a low nutritional status.30
In this study, 5-year overall survival was significantly higher in patients with lower RDW. Similar results have been reported by other authors. Wang et al. performed a retrospective study concerning non-metastatic nasopharyngeal cancer on 2318 patients, and found a correlation between higher RDW level and lower OS.12 Hsueh et al. demonstrated a significant (p-value 0001) correlation between higher RDW quartiles and worse overall survival in a retrospective analysis of 809 male patients with laryngeal squamous cell carcinoma.15 Regarding oral cavity cancer, Tangthongkum et al. reported no significant differences in OS between the high and low RDW groups in a retrospective study on 374 patients.20 A retrospective analysis by Ge et al. on 236 patients with oral squamous cell carcinoma demonstrated that a high RDW was connected with poor overall survival.16
Koma et al. carried out a retrospective study on 332 patients with lung cancer, and reported that elevated RDW was associated with poorer survival. Patients were divided into four groups according to the TNM staging system, and for stage III and IV, RDW did not significantly correlate with prognosis.13 Kiriu et al. performed a retrospective analysis of 47 patients with non-small cell lung cancer dividing them into three groups, which corresponded to RDW levels. Higher RDW was correlated with shorter OS.17 Also Hirahara et al. reported a significant association between high RDW and poorer prognosis in the matter of overall survival in a retrospective study of 366 patients with gastric adenocarcinoma.18 Beltran et al. showed that in a cohort of 121 DLBCL patients, 5-year OS was 51% for patients with elevated RDW, compared to 79% for the rest of the group (p-value 0001) (19). Życzkowski et al. reported that higher RDW is correlated with poor overall survival in a retrospective study of 434 patients with renal cell carcinoma.10 Hu et al. performed meta-analysis that included 15 retrospective studies, and one article with both retrospective and prospective design in two independent patients groups, and reported that elevated RDW is associated with poor OS.8 However, Xu et al. in meta-analysis regarding esophageal cancer showed that higher RDW values are not associated with a better prognosis.11
Since the study was conducted retrospectively on patients treated between 2005 and 2014, and the 8th edition of TNM staging system that includes the assessment of HPV status came into effect between 2017–2018, practically no patients had their HPV status assessed at the time of the treatment. We have tried contacting respective hospitals for access to the histopathological material, but we have received responses in less than 10 cases. It is an important limitation of the study since the HPV significantly impacts the survival of patients with oropharyngeal cancers. The study group was also relatively heterogeneous in terms of applied treatment modality (radiotherapy fractionation schemes and addition of chemotherapy), which influences the treatment results and, therefore, limits the value of the analysis.
We did not find significant differences between the groups with low and high RDW in terms of sex and age, which is similar to other studies regarding sex,10,16,20 however, certain publications suggest that RDW is correlated with age.13,18 We did not find any significant correlation between RDW value and TNM stage groups, tumor volume, lymph node involvement or concomitant diseases. Such correlations have been reported in certain studies.13,16,18 We have found a significant difference between the choice of fractionation schemes in RDW groups. Patients with high RDW were more likely to be treated with CHA-CHA, and less likely to be treated with conventional radiotherapy. It can be attributed to the fact that the CHA-CHA trial recruited patients with advanced stage disease (T2 N3, T3 N0-3 and T4 N0-3), that had worse prognosis and higher RDW due to the more locally advanced disease in comparison with the patients recruited for CAIR trial (T2-4 N0-1). It is important to note that the cut-off values for RDW, in a vast majority of the studies, including ours, were determined a posteriori using tests such as ROC analysis. Therefore, the differences are significant between studies, and the values ranged from 13,55% to 16%.
5. Conclusion
The results of our study support the evidence of the clinical utility of simple blood-based indicators. Our findings suggest that inexpensive, convenient and routinely assessed markers, such like RDW, could be a useful prognostic tool for patients treated with radiotherapy or radio-chemotherapy, and may provide additional information about possible treatment outcome for no additional cost.
Conflict of interest
None.
Financial disclosure
None.
References
- 1.Nowotwory języka (C01-02) | KRN. http://onkologia.org.pl/nowotwory-jezyka-c01-02/; 2013 [accessed 29 February 2020].
- 2.Didkowska Joanna WU. Zachorowania i zgony na nowotwory złośliwe w Polsce. Krajowy Rejestr Nowotworów, Centrum Onkologii - Instytut im. Marii Skłodowskiej - Curie. http://onkologia.org.pl/raporty/; 2019 [accessed 29 February 2020].
- 3.Gillison M.L., Chaturvedi A.K., Anderson W.F., Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(27):3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman C.H., Garsa A. Cancer of the lip and Oral cavity. In: Hansen E.K., Roach I.I.I.M., editors. Handbook of evidence-based radiation oncology. Springer International Publishing; 2018. pp. 193–207. [Google Scholar]
- 5.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology (NCCN guidelines®) head and neck. Version 1.2020. 2020. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf [accessed 28 February 2020]
- 6.Karnad A., Poskitt T.R. The automated complete blood cell count. Use of the red blood cell volume distribution width and mean platelet volume in evaluating anemia and thrombocytopenia. Arch Intern Med. 1985;145(7):1270–1272. doi: 10.1001/archinte.145.7.1270. [DOI] [PubMed] [Google Scholar]
- 7.Bujak K., Wasilewski J., Osadnik T. The prognostic role of red blood cell distribution width in coronary artery disease: A review of the pathophysiology. Dis Markers. 2015;2015 doi: 10.1155/2015/824624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu L., Li M., Ding Y. Prognostic value of RDW in cancers: A systematic review and meta-analysis. Oncotarget. 2017;8(9):16027–16035. doi: 10.18632/oncotarget.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation : Abstract. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 10.Życzkowski M., Rajwa P., Gabrys E., Jakubowska K., Jantos E. Paradysz a. The relationship between red cell distribution width and cancer-specific survival in patients with renal cell carcinoma treated with partial and radical nephrectomy. Clin Genitourin Cancer Clin Genitourin Cancer. 2018;16(3):e677–e683. doi: 10.1016/j.clgc.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Xu W.-Y., Yang X.-B., Wang W.-Q. Prognostic impact of the red cell distribution width in esophageal cancer patients: A systematic review and meta-analysis. World J Gastroenterol. 2018;24(19):2120–2129. doi: 10.3748/wjg.v24.i19.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., He S.-S., Cai X. The novel prognostic score combining red blood cell distribution width and body mass index (COR-BMI) has prognostic impact for survival outcomes in nasopharyngeal carcinoma. J Cancer. 2018;9(13):2295–2301. doi: 10.7150/jca.24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koma Y., Onishi A., Matsuoka H. Increased red blood cell distribution width associates with Cancer stage and prognosis in patients with lung Cancer. PLoS One. 2013;8(11):e80240. doi: 10.1371/journal.pone.0080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirahara N., Matsubara T., Kawahara D., Mizota Y., Ishibashi S., Tajima Y. Prognostic value of hematological parameters in patients undergoing esophagectomy for esophageal squamous cell carcinoma. Int J Clin Oncol. 2016;21(5):909–919. doi: 10.1007/s10147-016-0986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsueh C.Y., Lau H.C., Li S. Pretreatment level of red cell distribution width as a prognostic indicator for survival in a large cohort study of male laryngeal squamous carcinoma. Front Oncol. 2019;9:271. doi: 10.3389/fonc.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge W., Xie J., Chang L. Elevated red blood cell distribution width predicts poor prognosis in patients with oral squamous cell carcinoma. Cancer Manag Res. 2018;10:3611–3618. doi: 10.2147/CMAR.S176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiriu T., Yamamoto M., Nagano T. Prognostic value of red blood cell distribution width in non-small cell lung cancer treated with anti-programmed cell death-1 antibody. In Vivo (Brooklyn) 2019;33(1):213–220. doi: 10.21873/invivo.11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tajima Y., Fujii Y., Kaji S. Comprehensive analysis of red blood cell distribution width as a preoperative prognostic predictor in gastric cancer. Anticancer Res. 2019;39(6):3121–3130. doi: 10.21873/anticanres.13448. [DOI] [PubMed] [Google Scholar]
- 19.Beltran B.E., Paredes S., Castro D., Cotrina E., Sotomayor E.M., Castillo J.J. High red cell distribution width is an adverse predictive and prognostic factor in patients with diffuse large B-Cell lymphoma treated with chemoimmunotherapy. Clin Lymphoma Myeloma Leuk. 2019;19(9):e551–e557. doi: 10.1016/j.clml.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Tangthongkum M., Tiyanuchit S., Kirtsreesakul V., Supanimitjaroenporn P., Sinkitjaroenchai W. Platelet to lymphocyte ratio and red cell distribution width as prognostic factors for survival and recurrence in patients with oral cancer. Eur Arch Otorhinolaryngol. 2017;274(11):3985–3992. doi: 10.1007/s00405-017-4734-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhang F., Chen Z., Wang P., Hu X., Gao Y., He J. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol. 2016;37(7):9323–9331. doi: 10.1007/s13277-015-4774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun P., Zhang F., Chen C. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: a retrospective study from southern China. Oncotarget. 2016;7(27):42650–42660. doi: 10.18632/oncotarget.9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrucci L., Guralnik J.M., Woodman R.C. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am J Med. 2005;118(11):1288. doi: 10.1016/j.amjmed.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Patel K.V., Ferrucci L., Ershler W.B., Longo D.L., Gurainik J.M. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169(5):515–523. doi: 10.1001/archinternmed.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 26.Mallath M.K., Taylor D.G., Badwe R.A. The growing burden of cancer in India: Epidemiology and social context. Lancet Oncol. 2014;15(6):e205–12. doi: 10.1016/S1470-2045(14)70115-9. [DOI] [PubMed] [Google Scholar]
- 27.Lippi G., Targher G., Montagnana M., Salvagno G.L., Zoppini G., Guidi G.C. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133(4):628–632. doi: 10.1043/1543-2165-133.4.628. [DOI] [PubMed] [Google Scholar]
- 28.de Gonzalo-Calvo D., de Luxan-Delgado B., Rodriguez-Gonzalez S. Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: A translational approach. Cytokine. 2012;58(2):193–198. doi: 10.1016/j.cyto.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes C.J., Howard L.S., Busbridge M. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: Clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol. 2011;58(3):300–309. doi: 10.1016/j.jacc.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 30.Tete S., Nicoletti M., Saggini A. Nutrition and cancer prevention. Int J Immunopathol Pharmacol. 2012;25(3):573–581. doi: 10.1177/039463201202500303. [DOI] [PubMed] [Google Scholar]
- 31.Miszczyk L., Maciejewski B., Tukiendorf A. Split-course accelerated hyperfractionated irradiation (CHA-CHA) as a sole treatment for advanced head and neck cancer patients—Final results of a randomized clinical trial. Br J Radiol. 2014;87(1041) doi: 10.1259/bjr.20140212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skladowski K., Maciejewski B., Golen M. Continuous accelerated 7-days-a-week radiotherapy for head-and-neck cancer: Long-term results of Phase III clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(3):706–713. doi: 10.1016/j.ijrobp.2006.05.026. [DOI] [PubMed] [Google Scholar]