Abstract
Background
Defining clinical and subclinical progression in multiple sclerosis (MS) is challenging. Patient history, expanded disability status scale (EDSS), and magnetic resonance imaging (MRI) all have shortcomings and may underestimate disease dynamics. Emerging serum biomarkers such as glial fibrillary acidic protein (GFAP) and neurofilament light chain (NfL) proved useful in many cross-sectional studies. However, longitudinal data on patients with progressive MS is scarce.
Objectives
To assess whether the serum biomarkers GFAP and NfL might differentiate between patients with progressive vs. non-progressive disease stages and predict the disease course according to the Lublin criteria.
Methods
EmBioProMS is a pilot, observational, prospective, multicentric study funded by the German Multiple Sclerosis Society (DMSG). 200 patients with MS according to the 2017 McDonald criteria and history of relapse-independent progression at any time (progressive MS, PMS), younger than 65 years, and with EDSS ≤ 6.5 will be recruited in 6 centres in Germany. At baseline, month 6, and 18, medical history, EDSS, Nine-Hole-Peg-Test (9-HPT), Timed-25-Foot-Walk-Test (T-25FW), Symbol-Digit-Modalities-Test (SDMT), serum GFAP, and NfL, MRI (at least baseline and month 18) and optional optical coherence tomography (OCT) will be performed. Disease progression before and during the study is defined by confirmed EDSS progression, increase by ≥ 20% in 9-HPT or T-25FW time.
Conclusions
This longitudinal multicentre study will reveal to what extent the prediction of disease progression in patients with PMS will be improved by the analysis of serum biomarkers in conjunction with routine clinical data and neuroimaging measures.
Trial registration
German Clinical Trials Register (Deutsches Register Klinischer Studien - DRKS), DRKS00020132, Registered on 19 December 2019– Retrospectively registered.
1. Background
The detection of disease progression in multiple sclerosis (MS) is a challenging task, but one with significant therapeutic consequences. Clinical symptoms, such as fatigue, cognitive dysfunction, and subtle changes in mobility, can be challenging to measure, and deterioration cannot be easily quantified in the routine clinical setting. Depending solely on clinical scores like the Expanded Disability Status Scale (EDSS) has its shortcomings. The EDSS strongly relies on the walking distance, underestimates upper limb function, and cannot assess cognitive deficits adequately [1]. Moreover, the EDSS has intra- and interrater variability, and EDSS-based confirmed disability progression assessment can overestimate the long-term disability accumulation [2]. Other progression assessment modalities, such as MRI atrophy parameters, can deliver valuable information regarding long-term disability outcomes. However, the substantial variation among devices, scan parameters, and rendering software precludes their application in the clinical care of MS patients [3,4].
Body fluid biomarkers might offer additional information for an accurate evaluation of the clinical course of the disease and a better reflection of the disease dynamics. The introduction of highly sensitive methods, like the single molecular array (SIMOA), enables the measurement of very low concentrations of brain-derived proteins in serum [5]. Evidence for the relevance of neurofilament light chain (NfL) in MS is accumulating; serum NfL has correlated with the EDSS and MRI brain atrophy measurements and reflected the effect of the disease-modifying treatment (DMT) in relapsing-remitting MS (RRMS) [[6], [7], [8], [9], [10], [11]]. Data regarding NfL levels in progressive MS (PMS) are less clear; levels of NfL were higher in PMS compared to RRMS in some [12], but not all studies [13], and the correlation with the EDSS score in PMS patients was not always reproducible [13,14]. Another serum marker, glial fibrillary acidic protein (GFAP), has consistently correlated with disease severity, making it a promising candidate to reflect the subclinical, relapse-independent disease progression [[13], [14], [15], [16]]. Nevertheless, longitudinal multicentre validation studies are still needed.
Similarly, retinal optic coherence tomography (OCT) is a promising structural marker, using a non-invasive diagnostic method with high inter-rater reliability, making it a useful tool in multicentric studies [17]. It provides measures of axonal and neuronal degeneration [18]. Recent studies have shown reproducibly on a group level that the measurement of axonal degeneration (peripapillary retinal nerve fiber layer (pRNFL) thickness) in eyes without optic neuritis (ON) correlates with disability worsening [19], brain atrophy [20], and cognitive impairment [21]. Further measurements of neuronal degeneration (like combined ganglion cell inner plexiform layer (GCIPL) thickness) correlate with EDSS, the progression of disability, and future clinical and radiological disease activity [22,23]. Data regarding the correlation between axonal and neuronal degeneration and the serum biomarkers are scarce [24], and longitudinal studies are still needed.
2. Design
The explorative study of Emerging blood Biomarkers in Progressive Multiple Sclerosis (EmBioProMS) is a prospective observational multicentre pilot study. We will recruit patients with either primary or secondary PMS (PPMS, SPMS) visiting the MS centres of the university hospitals of Ulm, Tübingen, Munich (Ludwig-Maximilians university hospital), and Rostock as well as the MS centres; Fachklinik für Neurologie Dietenbronn and Marianne-Strauss-Klinik.
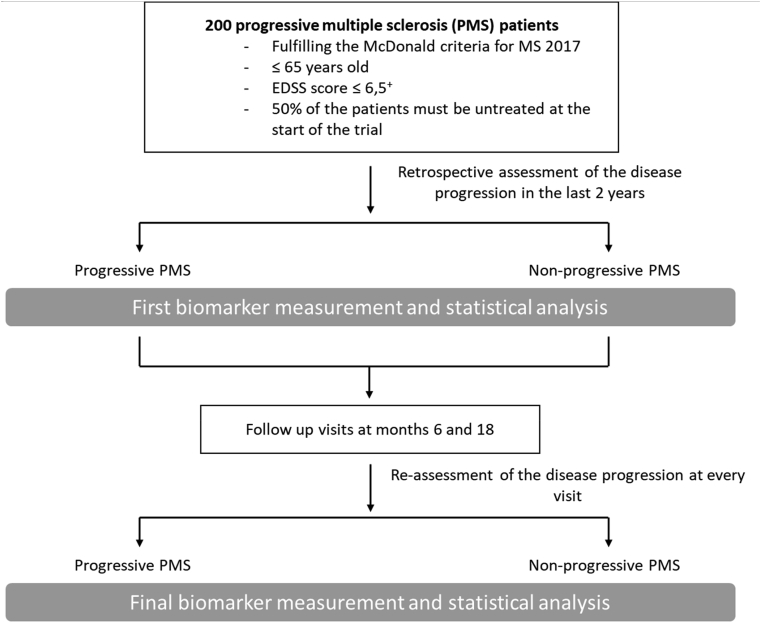
Patients will undergo two mandatory follow-ups (month 6 and month 18) after the baseline visit. Additional unscheduled visits are allowed (e.g., after having a clinical relapse; Fig. 1).
Fig. 1.
Study flowchart.
+ only 25% of the patients are allowed to have EDSS scores above 5.5.
3. Study outcome measures
3.1. Evaluation of the disease progression
Disease progression (progressive versus non-progressive) will be determined according to the results of the EDSS, Nine-Hole Peg test (9HPT) OR of the timed 25-foot walk test (T25FWT). The disease course will be considered ‘progressive’ in the following cases: an increase of the EDSS score by 1 point in case of a baseline or previous EDSS score below 5.5 and by ≥ 0.5 point in case of a baseline or previous EDSS score ≥5.5 OR an increase of the 9HPT OR of the T25FWT by 20% or more [25]. The disease progression at the baseline visit will be retrospectively assessed according to the available clinical data from the last two years, while prospective assessment of disease progression will be evaluated at the last mandatory visit (follow-up visit at month 18) in comparison to the baseline visit. To assess disease progression at other visits (follow-up at month 6 or unscheduled follow-up visits) and in the case of the continuation of the study beyond 18 months, the previous visit scores will be used as the reference.
3.2. Determination of disease activity
The evaluation of disease activity is one of the secondary outcome measures. Disease activity will be determined either clinically (i.e., relapses) or based on the available ambulatory MRI scans, which will be performed in the clinical setting. The active disease course will be defined by new T2-weighted (T2w) hyperintensities, enlarging T2w lesions, or gadolinium-enhancing lesions in the brain or spinal MRI [26].
Similar to the evaluation of disease progression, disease activity at baseline will be retrospectively assessed according to the available clinical data and MRI scans from the last two years. For prospective assessment, disease activity will be evaluated at the last mandatory visit (follow-up at month 18) in relation to the baseline visit. To determine disease activity at all other visits (follow-up at month 6 or additional or unscheduled follow-up visits), and in the case of the continuation of the study beyond 18 months, the previous visit will be used as the reference.
3.3. Hypotheses and objectives
The main objective of the study is to determine the ability of the serum biomarkers GFAP to differentiate between PMS patients with vs. without measurable disease progression over 18 months, as defined by the combined clinical outcome measure (see above).
The secondary objectives will address the same question by using other progression assessment tools (including NfL, OCT data) over various follow-up periods. Correlation between axonal and neuronal OCT measurements and the biomarkers (GFAP and NfL) will be investigated. In a second step, serum GFAP, serum NfL, as well as OCT data will be tested to assess whether they can differentiate alone or in combination between disease courses according to the Lublin classifications: progressive active, progressive non-active, non-progressive active, and non-progressive non-active [26]. Finally, our study will enable assessment of the dynamics of the serum biomarker concentrations after initiation of disease-modifying treatment (DMT).
We postulate higher levels of GFAP and/or NfL in progressive versus non-progressive PMS patients according to the Lublin classification. Furthermore, we expect patients with high levels of GFAP or NfL or both at the time of recruitment to have an increased probability of exhibiting disease progression after 18 months.
3.4. Study population
PPMS will be defined according to the 2017 McDonald criteria, and SPMS will be defined as patients with previous RRMS (fulfilling the 2017 McDonald criteria) who develop relapse-independent disability accumulation for at least one year. For inclusion in the trial, EDSS scores must not exceed 6.5 (only 25% of patients are allowed to exceed 5.5). The upper age limit should not exceed 65 years to minimise the effect of contributing factors to astrocytic activation and neurodegeneration, such as minor or major cerebrovascular diseases and other undiagnosed neurodegenerative diseases.
The following patients are excluded from the study: patients with RRMS or PMS who had clinical relapses in the last three months (before baseline), and patients with other inflammatory or non-inflammatory diseases of the central nervous system. At least 50% of the patients must be untreated at the start of the trial (as defined in Table 1).
Table 1.
Definition of treated patients in the EmBioProMS study.
| Patients will be considered ‘treated’ if they have received: a) treatment with corticosteroids in the last 30 days; b) treatment with interferon, glatiramer acetate, natalizumab, dimethyl fumarate, or teriflunomide in the last three months; c) treatment with rituximab, ocrelizumab, or mitoxantrone in the previous 12 months; d) or treatment with cladribine or alemtuzumab in the previous 24 months. |
4. Clinical assessment
4.1. Clinical data to be documented
Data regarding the disease onset, date of the first manifestation, symptoms of the first manifestation, date of the diagnosis, number of documented relapses, duration of the progressive phase, number of documented relapses in the last two years, date of the most recent relapse, current and previous treatments, and concomitant diseases are to be recorded at the baseline visit. At the follow-up visits, relapses, and changes in DMT or concomitant treatment will be documented.
4.2. Clinical examinations
The EDSS, 9HPT, T25FWT, and Symbol Digit Modalities Test (SDMT) will be performed. Furthermore, the following questionnaires are to be completed: Beck Depression Inventory-II (BDI-II), Fatigue Scale for Motor and Cognition (FSMC), and Multiple Sclerosis Impact Scale-29 (MSIS-29).
5. Paraclinical parameters
5.1. Biosamples
Serum samples from the study participants will be stored, according to predefined Standard Operating Procedure (SOP), at a local biobank at minus 80 °C and transferred for measurement on dry ice to the biobank of the coordinating centre in Ulm for further analysis.
5.2. Cranial and spinal MRI scans
Regular MRI scans are not mandatory in the framework of the EmBioProMS. However, the results of routine ambulatory scans will be standardised according to a unified assessment protocol. The scans will be stored in the local archive of the participating centre. The MRIs will be accepted as long as they are of sufficient quality to quantify T2w-lesions (in T2w fluid-attenuated inversion recovery (FLAIR) scans), assess the presence of black holes (in T1w scans), and reveal signs of disease activity, such as enlarging lesions (in T2w FLAIR scans) or pathological gadolinium enhancement (gadolinium contrast injection will be considered not necessary if no new T2 lesions are reported [27]). Available MRIs can be used to assess disease activity, as long as they were performed within three months before the clinical visit. Beyond assessment of radiological disease activity, changes in the normalized brain volume (nBV) will be assessed, if the required T1-weighted, high-resolution three-dimensional (3D) magnetization-prepared rapid gradient echo (MP-RAGE) sequences are available.
5.3. Optical coherence tomography (OCT)
If the technical requirements are available at the centre (expected in up to 40% of the cases), OCT scans will be performed using the spectral domain (SD)-OCT (Spectralis platform, Heidelberg Engineering, Heidelberg, Germany) at every visit. Two scans will be performed: a ring scan of the optic nerve head of both sides (12°, 100 ART) and a macular scan with a diameter of at least 6 mm on both sides (20° × 20°, 49 ART). The scans will be stored electronically at the participating centre and transferred to be centrally assessed for quality according to the OSCAR-IB criteria [28]. Peripapillary retinal nerve fiber layer thickness (pRNFL), volumes of macular RNFL, combined ganglion cell, inner plexiform layer (GCIP), inner nuclear layer (INL), macular volume (MV) as well as the combined outer plexiform and outer nuclear layer (OPONL) will be acquired. The results will be reported according to the Advised Protocol for OCT Study Terminology and Elements (APOSTEL) recommendations [29].
6. Statistical analysis
Appropriate summary statistics will describe the baseline characteristics of the cohort, i.e., mean with standard deviation or median with interquartile range for continuous variables and frequencies (percentages) for categorical variables, respectively. The analyses are structured in two parts: 1) a cross-sectional study comparing the concentration of GFAP and NfL between patients with progressive vs. non-progressive PMS and analysing GFAP and NfL in correlation with clinical and imaging data 2) a prediction model using the baseline biomarker data to predict disease progression.
Pearson or nonparametric Spearman correlation coefficients, depending on the measurement scale, will be used to assess the statistical relationships between the concentrations of biomarkers, clinical parameters, and retinal layer thickness. These will be reported with 95% CI. Regression models appropriate for the outcome scale, e.g., logistic regression for binary outcomes and linear regressions for continuous outcomes, will be used to assess various markers and clinical characteristics simultaneously. The AUC of the receiver operating characteristic (ROC) of the GFAP levels to differentiate between subjects with and without disease progression will be calculated with 95% confidence interval (CI). In logistic regression models, the discriminative nature of GFAP will be further explored, including additional baseline variables such as NfL concentrations and clinical characteristics into the model. The resulting AUC will be adjusted using cross-validation and will be reported with 95% CI. Furthermore, the AUC of the ROC of the NfL levels and retinal layer diameters will be analysed to meet the secondary objectives. Logistic regression models will be used to explore the discriminative nature of these markers simultaneously and adjusted for relevant baseline variables (including the initial assessment of the progression).
With a fixed follow-up of 18 months, the prognostic value of the biomarkers will be assessed in logistic regression models with progression as an independent variable. As longer follow-up times become available, time to progression will be used as an endpoint, which will be analysed by Cox proportional hazards regressions. Regression models will be utilised to assess the prognostic value of individual markers and their combinations. An interim analysis is planned after the inclusion of 50% of the patients.
6.1. Determination of sample size
With 200 PMS patients, correlation coefficients can be estimated with a precision (half-width of the 95% confidence interval) of 0.14 in the worst case (i.e., independence). For instance, with a correlation of 0.7, the precision is 0.08. As these are baseline assessments, no adjustment for dropout is required.
Based on the patient characteristics reported for previous phase 3 clinical trials for PMS: PROMiSe, OLYMPUS, ORATORIO, and EXPAND [[30], [31], [32], [33], [34]], we expect that patients with progression will consist 20–40% of our sample. Based on epidemiological and histological findings as well as our previous results, we do not assume that SPMS patients will differ significantly from PPMS patients. In sum, we expect to see about 65 patients with progressions in 200 PMS patients over the follow-up period of up to 18 months. This number of events yields a power of 80% comparing two equally sized groups with a hazard ratio of 2 (Schoenfeld formula). The sample size calculations were performed using nQuery 8 (Statistical Solutions Ltd) and SAS version 9.4.
6.2. Biomarkers assessment
The serum samples will be analysed using commercially available kits for GFAP and NfL using the Simoa technology (Quanterix Cooperation, Massachusetts, USA).
6.3. Data management
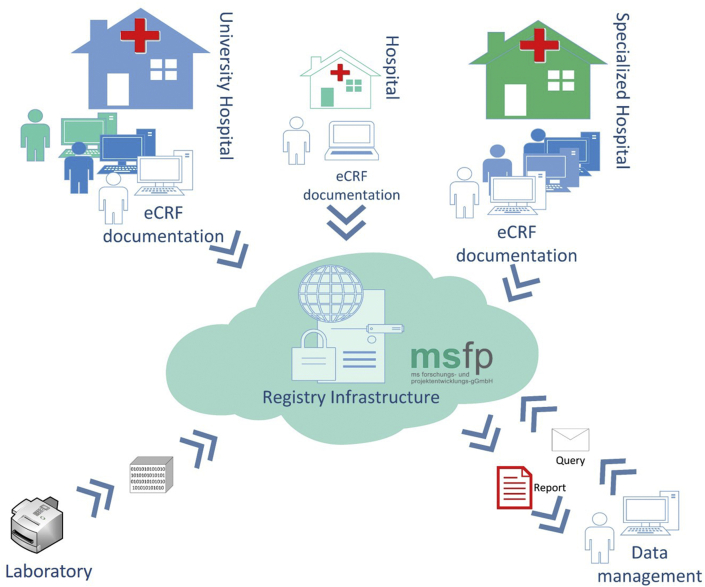
Data collection for this study is conducted through the electronic data capture system (EDC) of the German MS-Register [35], established by the Deutsche Multiple Sklerose Gesellschaft Bundesverband e.V. (GMSR) (Fig. 2). A link between the pseudonymised medical (study) and identifying data is only accessible to treatment centres to which the patient has given his or her informed consent to register. For centres in the study participating already in the data collection for the GMSR, the study documentation follows the same workflows as for the registry. For patients participating in both the study and the registry, IDs can be linked, and double data entry efforts reduced. Completeness and validity of data entered are assured through a broad ruleset, which is complemented by manual queries from the data management team. Data from the central biomarker assessment (Ulm) is imported directly to the study database to perform the statistical analysis but will not be available to the treating physician and patients until the end of the study.
Fig. 2.
Electronic data capture structure applied in the EmBioProMS study.
The clinical data will be documented in the central electronic data registry under pseudonymised ID. After the completion of the biomarker analysis, the levels of GFAP and NfL can be linked with the available clinical data using patients’ specific IDs. Identifying data is only accessible to treatment centres to which the patient has given his or her informed consent.
7. Discussion
With the increasing number of approved medications for PMS, the accurate characterisation of the clinical phenotype in individual patients has important therapeutic consequences. Considering the shortcomings of the medical history, EDSS, and routine MRI, a biomarker-based approach sounds promising and clinically feasible. We expect due to the multimodality of the study concept with blood biomarkers in combination with detailed clinical data and MRI imaging as well as modern retinal structure imaging (i.e., OCT), to have the opportunity to develop a prediction model for progressive MS based on the combined data. The detailed clinical and imaging-based characterization, longitudinal design, and available biomaterials establish a reliable and efficient platform for further biomarker discovery and validation studies in the future.
However, data regarding the prevalence of the sub-courses of PMS is scarce; thus, our power calculation was based on our previous results [13,14] as well as the published results from phase 2 and 3 treatment trials. Nevertheless, our study shall still be considered an exploratory pilot trial, and depending on our findings, other validation cohorts with adapted sample sizes should be conducted in the future.
Despite the novelty of the applied markers, our study design has some limitations; the inclusion of both treated and untreated patients might result in a heterogeneous population, which might be addressed through additional subgroup analysis. The retrospective evaluation of the disease progression at baseline shall also be considered as one of the limitations of the study. Moreover, the lack of standardised MRI protocols and centralized analysis strategy might lead to underestimation of the disease activity, which can affect the levels of the biomarkers (mainly NfL). However, the correlation of MRI parameters of disease activity with levels of GFAP was modest in our previous data [13]. Thus, we expect the cofounding effect of MRI activity while assessing the discriminating value of GFAP regarding the disease progression, the primary outcome measure of our study, to be marginal.
Availability of data and materials
The data that support the findings of the study can be made available from the corresponding author upon reasonable request.
Ethical approval
Ethical approval for this study was obtained from the ethics committee of the University of Ulm (270/17).
Patient involvement
Patients were not involved in the development of the research question, design, or conduct of this study. However, this study is founded by a research grant from the German MS Society, after independent scientific review, the decision on funding by the DMSG is always subject to the decision of their executive board (includes MS patients). Patients can be informed about the biomarker analysis results at their request at the end of the study.
Funding
This study is funded by a grant from the German Multiple Sclerosis Society Federal Association (DMSG).
Authors' contributions
AA, HT, AS, MS, AH, JH, TK, UZ, IK: study design. TF: statistical analysis. AS: Data management. AH: biomarker analysis, JH, TK: OCT data analysis. All authors contributed equally to the finalisation of the protocol, recruitment of the patients, writing, and revision of the manuscript.
Declaration of competing interest
AA received research funding from DMSG and travel grants from Biogen, AS has received institutional research grants from Merck and Novartis, all outside the submitted work. MS received consulting and/or speaker honoraria as well as travel reimbursements from Bayer, Biogen, Celgene, Roche, Sanofi Genzyme and TEVA and research funding from the Hertha-Nathorff-Program and the University of Ulm. MK received financial support from Biogen, Celgene, Merck, Novartis, Roche, and Sanofi-Genzyme. MCK receives financial support from Merck, Novartis, Biogen, Celgene and Roche. JH reports personal fees and non-financial support from Merck, Novartis, Roche, Santhera, Biogen, Alexion, Sanofi Genzyme, and non-financial support of the Guthy-Jackson Charitable Foundation, all outside the submitted work. JH is (partially) funded by the German Federal Ministry of Education and Research (Grant Numbers 01ZZ1603[A-D] and 01ZZ1804[A-H] (DIFUTURE)). TK has received speaker honoraria including advisory boards from Bayer Healthcare, Teva Pharma, Merck, Novartis Pharma, Sanofi-Aventis/Genzyme, Roche Pharma and Biogen as well as grant support from Novartis and Chugai Pharma in the past. IK has received speaker honoraria and travel funding from Bayer, Biogen, Novartis, Merck, Sanofi Genzyme, Roche; speaker honoraria from Mylan; travel funding from the Guthy-Jackson Charitable Foundation; consulted for Alexion, Bayer, Biogen, Celgene, Chugai, IQVIA, Novartis, Merck, Roche; received research support from Chugai, Diamed; all outside the submitted work. UKZ received travel compensation for research meetings from Aventis, Bayer, Biogen, Celgene as well as speakers fee from Almirall, Alexion, Bayer, Biogen, Merck, Novartis, Roche and Teva. TF reports personal fees for consultancies (including data monitoring committees) in the past three years from Bayer, BiosenseWebster, Boehringer Ingelheim, Cardialysis, CSL Behring, Sankyo, Enanta, Feldmann Patent Attorneys, Fresenius Kabi, Galapagos, IQVIA, Janssen, Mediconomics, Novartis, Penumbra, Roche, SGS, and Vifor; all outside the submitted work. ACL received personal fees from Hoffmann-La Roche, Novartis, Desitin Pharma, Syneos Health, Teva Pharmaceutical Industries, Boehringer Ingelheim, Biogen, and Mitsubishi Pharma for consultancy services. UZ received financial support from Biogen Idec GmbH, Bayer Vital GmbH, Bristol Myers Squibb GmbH, Pfizer, CorTec GmbH, Medtronic GmbH, and research support from European Research Council, DFG, BMBF, Servier, and Janssen Pharmaceuticals. HT reports personal fees and/or research grants from Fresenius Medical Care GmbH and Fresenius Medical Care Deutschland GmbH, Bayer, Biogen, Merck, Mylan, Novartis, Roche, Sanofi-Genzyme, Teva, DMSG, and BMBF. All other authors declare no competing interests.
Acknowledgments
Without the passion and cooperation of our patients, our study will not be achievable. We want to thank them for their engagement. We also thank our study nurses, technicians, and physicians of the participating centres for actively supporting this project. We want to thank Prof. Martin Kerschensteiner and Prof. Reinhard Hohlfeld for their support during the planning and designing of the study. Moreover, we want to thank the DMSG for generous funding.
References
- 1.Hyland M., Rudick R.A. Challenges to clinical trials in multiple sclerosis: outcome measures in the era of disease-modifying drugs. Curr. Opin. Neurol. 2011;24 3:255–261. doi: 10.1097/WCO.0b013e3283460542. [DOI] [PubMed] [Google Scholar]
- 2.Tur C., Moccia M., Barkhof F., Chataway J., Sastre-Garriga J., Thompson A.J. Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting. Nat. Rev. Neurol. 2018;14 2:75–93. doi: 10.1038/nrneurol.2017.171. [DOI] [PubMed] [Google Scholar]
- 3.Rocca M.A., Battaglini M., Benedict R.H., De Stefano N., Geurts J.J., Henry R.G. Brain MRI atrophy quantification in MS: from methods to clinical application. Neurology. 2017;88 4:403–413. doi: 10.1212/WNL.0000000000003542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amiri H., de Sitter A., Bendfeldt K., Battaglini M., Gandini Wheeler-Kingshott C.A.M., Calabrese M. Urgent challenges in quantification and interpretation of brain grey matter atrophy in individual MS patients using MRI. NeuroImage Clin. 2018;19:466–475. doi: 10.1016/j.nicl.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rissin D.M., Kan C.W., Campbell T.G., Howes S.C., Fournier D.R., Song L. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010;28 6:595–599. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakansson I., Tisell A., Cassel P., Blennow K., Zetterberg H., Lundberg P. Neurofilament levels, disease activity and brain volume during follow-up in multiple sclerosis. J. Neuroinflammation. 2018;15:209. doi: 10.1186/s12974-018-1249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chitnis T., Gonzalez C., Healy B.C., Saxena S., Rosso M., Barro C. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol. 2018;5 12:1478–1491. doi: 10.1002/acn3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhle J., Plavina T., Barro C., Disanto G., Sangurdekar D., Singh C.M. Neurofilament light levels are associated with long-term outcomes in multiple sclerosis. Mult. Scler. 2019 doi: 10.1177/1352458519885613. 1352458519885613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sejbaek T., Nielsen H.H., Penner N., Plavina T., Mendoza J.P., Martin N.A. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naive relapsing MS patients. J. Neurol. Neurosurg. Psychiatr. 2019 doi: 10.1136/jnnp-2019-321321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferraro D., Guicciardi C., De Biasi S., Pinti M., Bedin R., Camera V. Plasma neurofilaments correlate with disability in progressive multiple sclerosis patients. Acta Neurol. Scand. 2019 doi: 10.1111/ane.13152. [DOI] [PubMed] [Google Scholar]
- 11.Akgun K., Kretschmann N., Haase R., Proschmann U., Kitzler H.H., Reichmann H. Profiling individual clinical responses by high-frequency serum neurofilament assessment in MS. Neurol. Neuroimmunol. Neuroinflamm. 2019;6(3):e555. doi: 10.1212/NXI.0000000000000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Disanto G., Barro C., Benkert P., Naegelin Y., Schadelin S., Giardiello A. Serum Neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017;81 6:857–870. doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdelhak A., Huss A., Kassubek J., Tumani H., Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci. Rep. 2018;8(1):14798. doi: 10.1038/s41598-018-33158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelhak A., Hottenrott T., Morenas-Rodriguez E., Suarez-Calvet M., Zettl U.K., Haass C. Glial activation markers in CSF and serum from patients with primary progressive multiple sclerosis: potential of serum GFAP as disease severity marker? Front. Neurol. 2019;10:280. doi: 10.3389/fneur.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogel H., Rissanen E., Barro C., Matilainen M., Nylund M., Kuhle J. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult. Scler. 2018 doi: 10.1177/1352458518819380. 1352458518819380. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe M., Nakamura Y., Michalak Z., Isobe N., Barro C., Leppert D. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology. 2019;93 13:e1299–e1311. doi: 10.1212/WNL.0000000000008160. [DOI] [PubMed] [Google Scholar]
- 17.Oberwahrenbrock T., Traber G.L., Lukas S., Gabilondo I., Nolan R., Songster C. Multicenter reliability of semiautomatic retinal layer segmentation using OCT. Neurol. Neuroimmunol. Neuroinflamm. 2018;5(3):e449. doi: 10.1212/NXI.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petzold A., Balcer L.J., Calabresi P.A., Costello F., Frohman T.C., Frohman E.M. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16 10:797–812. doi: 10.1016/S1474-4422(17)30278-8. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Lapiscina E.H., Arnow S., Wilson J.A., Saidha S., Preiningerova J.L., Oberwahrenbrock T. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15 6:574–584. doi: 10.1016/S1474-4422(16)00068-5. [DOI] [PubMed] [Google Scholar]
- 20.Saidha S., Al-Louzi O., Ratchford J.N., Bhargava P., Oh J., Newsome S.D. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann. Neurol. 2015;78 5:801–813. doi: 10.1002/ana.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birkeldh U., Manouchehrinia A., Hietala M.A., Hillert J., Olsson T., Piehl F. Retinal nerve fiber layer thickness associates with cognitive impairment and physical disability in multiple sclerosis. Mult. Scler. Relat. Disord. 2019;36:101414. doi: 10.1016/j.msard.2019.101414. [DOI] [PubMed] [Google Scholar]
- 22.Alonso R., Gonzalez-Moron D., Garcea O. Optical coherence tomography as a biomarker of neurodegeneration in multiple sclerosis: a review. Mult. Scler. Relat. Disord. 2018;22:77–82. doi: 10.1016/j.msard.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann H.G., Knier B., Oberwahrenbrock T., Behrens J., Pfuhl C., Aly L. Association of retinal ganglion cell layer thickness with future disease activity in patients with clinically isolated syndrome. JAMA Neurol. 2018;75 9:1071–1079. doi: 10.1001/jamaneurol.2018.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bsteh G., Berek K., Hegen H., Teuchner B., Buchmann A., Voortman M.M. Serum neurofilament levels correlate with retinal nerve fiber layer thinning in multiple sclerosis. Mult. Scler. 2019 doi: 10.1177/1352458519882279. 1352458519882279. [DOI] [PubMed] [Google Scholar]
- 25.Koch M.W., Cutter G.R., Giovannoni G., Uitdehaag B.M.J., Wolinsky J.S., Davis M.D. Comparative utility of disability progression measures in PPMS: analysis of the PROMiSe data set. Neurol. Neuroimmunol. Neuroinflamm. 2017;4(4):e358. doi: 10.1212/NXI.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lublin F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014;72(Suppl 1):1–5. doi: 10.1159/000367614. [DOI] [PubMed] [Google Scholar]
- 27.Karimian-Jazi K., Wildemann B., Diem R., Schwarz D., Hielscher T., Wick W. Gd contrast administration is dispensable in patients with MS without new T2 lesions on follow-up MRI. Neurol. Neuroimmunol. Neuroinflamm. 2018;5(5):e480. doi: 10.1212/NXI.0000000000000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schippling S., Balk L.J., Costello F., Albrecht P., Balcer L., Calabresi P.A. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult. Scler. 2015;21 2:163–170. doi: 10.1177/1352458514538110. [DOI] [PubMed] [Google Scholar]
- 29.Cruz-Herranz A., Balk L.J., Oberwahrenbrock T., Saidha S., Martinez-Lapiscina E.H., Lagreze W.A. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86 24:2303–2309. doi: 10.1212/WNL.0000000000002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolinsky J.S., Narayana P.A., O'Connor P., Coyle P.K., Ford C., Johnson K. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann. Neurol. 2007;61 1:14–24. doi: 10.1002/ana.21079. [DOI] [PubMed] [Google Scholar]
- 31.Hawker K., O'Connor P., Freedman M.S., Calabresi P.A., Antel J., Simon J. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 2009;66 4:460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 32.Montalban X., Hauser S.L., Kappos L., Arnold D.L., Bar-Or A., Comi G. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 2017;376 3:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 33.Kappos L., Bar-Or A., Cree B.A.C., Fox R.J., Giovannoni G., Gold R. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391 10127:1263–1273. doi: 10.1016/S0140-6736(18)30475-6. [DOI] [PubMed] [Google Scholar]
- 34.Nicholas R.S., Han E., Raffel J., Chataway J., Friede T. Over three decades study populations in progressive multiple sclerosis have become older and more disabled, but have lower on-trial progression rates: a systematic review and meta-analysis of 43 randomised placebo-controlled trials. Mult. Scler. 2019;25 11:1462–1471. doi: 10.1177/1352458518794063. [DOI] [PubMed] [Google Scholar]
- 35.Thiel Fl S., Röpke L., Wandinger K.P., Kümpfel T., Aktas O., von Bismarck O., Salmen A., Ambrosius B., Ellrichmann G., Antony G., Dankowski T., Ziegler A., Stahmann A., Meyer C., Eichstädt K., Buckow K., Meißner T., Thibaut J., Khil L., Berger K., Gold R., Hellwig K. Neuroimmunological registries in Germany. Neurol. Int. Open. 2018 doi: 10.1055/s-0043-108830. 2018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of the study can be made available from the corresponding author upon reasonable request.