Abstract
Objective
To study the role of autophagy in angiotensin II-induced cardiac hypertrophy in C57BL/6 mice.
Methods
We randomly assigned 10 C57BL/6 mice into the control and angiotensin II (Ang II) groups (n = 5 in each group). Ang II group mice were injected with Ang II (3 mg/kg/day). Cardiac structure, myocardial pathological changes, mitochondrial structure, autophagosomes, mitochondrial membrane potential (MMP), and myocardial apoptosis were examined. Phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1), Parkin, and microtubule-associated protein1A/1B-light chain 3 (LC3) II protein expression levels and mRNA expression of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) were examined.
Results
The heart weight/body weight ratio, posterior wall of the left ventricle, myocardial apoptosis (%), relative number of autophagosomes, ANP and BNP mRNA levels, and PINK1, Parkin, and LC3 II protein levels were significantly higher in the Ang II group than in the control group. The MMP and left ventricular ejection fraction were significantly lower in the Ang II group than in the control group. There was disordered arrangement of cardiomyocytes and mitochondria, and obvious mitochondrial swelling, cardiomyocyte hypertrophy, and fibrosis in the Ang II group.
Conclusion
PINK1/PARKIN-mediated autophagy is involved in Ang II-induced cardiac hypertrophy by affecting myocardial apoptosis and mitochondrial function.
Keywords: Cardiac hypertrophy, angiotensin II, autophagy, phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1), Parkin, apoptosis
Introduction
Pathological cardiac hypertrophy is the most important predictor of morbidity and mortality of cardiovascular disease.1 Pathological hypertrophy is associated with increased death of cardiomyocytes, re-expression of fetal genes, and fibrotic heart remodeling, and is characterized by reduced cardiac function that often progresses towards heart failure.2 The mechanism of pathological hypertrophy is complex and multifactorial. Although numerous studies have attempted to determine the exact mechanism of pathological hypertrophy, the efficacy of treatments based on recent studies is not sufficient. Further understanding of the mechanism underlying pathological hypertrophy is important for providing novel therapeutic strategies for hypertrophy and heart disease.
Autophagy is a conserved catabolic process from lower eukaryotes to mammals, by which cellular components are transported to and degraded in lysosomes.3 Dysregulation of autophagy is associated with multiple clinical disease, including cancer, neurodegeneration, and metabolic syndrome.4 In recent years, many studies have focused on the mechanism of autophagy in cardiac hypertrophy.5 However, the beneficial or detrimental role of autophagy in hypertrophy remains controversial. Mitochondrial autophagy is selective autophagy that specifically degrades dysfunctional mitochondria and is one of the most important mitochondrial quality control mechanisms. In mammals, mitophagy is mainly regulated by the phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1)/Parkin signaling pathway.6 In a previous study, PINK1 knockout mice developed hypertrophy within 2 months.7
Because autophagy serves an important and controversial role in pathological hypertrophy, the present study aimed to investigate whether PINK1/Parkin-mediated autophagy is involved in angiotensin II (Ang II)-induced hypertrophy and its exact mechanism.
Materials and methods
Materials
C57BL/6 mice (8 weeks old) were purchased from the Animal Center of Xi’an Jiaotong University. All of the chemicals and reagents were purchased from Sigma-Aldrich (St Louis, MO, USA), unless otherwise mentioned.
Animal model
A model of pathological hypertrophy was generated in C57BL/6 mice by injection of angiotensin II (Ang II) (3 mg/kg/day) during 14 days through an Alzet micro-pump (Durect Corporation, Cupertino, CA, USA), which was placed in hypodermal tissue on the back of mice in the Ang II group (n = 5).8 Mice in the control group were injected with normal saline (n = 5). All animal study protocols were approved by the Animal Research and Ethics Committee of Xi’an Jiaotong University.
Echocardiographic measurements
Mice were anesthetized with an intraperitoneal injection of 2% pentobarbital at a concentration of 20 mg/kg/body weight based on the method previously described by Ma et al, with slight modification.9 When breathing slowed down, the light reflex was insensitive, pupils were slightly bigger, and there was no reaction to pain stimulation in mice, further operation and measurement were considered. The chest of mice was cleaned, and six cardiac cycle values were measured with a small animal ultrasound probe before calculating the average value. These measurements included left ventricular end-diastolic diameter, left ventricular end-systolic diameter, left ventricular posterior wall thickness (LVPWD), and left ventricular ejection fraction (LVEF).
Heart weight/body weight ratio
Upon anesthetizing the mice by intraperitoneal injection of pentobarbital, the mice were sacrificed by cervical dislocation and weighed. The chest was opened and the heart was extracted. The heart was placed in 10% potassium chloride solution and then in iced phosphate-buffered saline to remove any remnants of blood, followed by drying the water with filter paper. The hearts were weighed and the heart weight/body weight (HW/BW) ratio was calculated.
Histomorphology
Partial left ventricular myocardium was placed in 4% paraformaldehyde for 24 hours, embedded in paraffin, and sliced. The slices were stained with hematoxylin and eosin and Masson’s trichrome. Pathological changes in mouse myocardial tissue were observed under an optical microscope.
Transmission electron microscopy
Myocardial tissue of the left ventricular anterior wall was dissected, and the myocardial tissue was cut into 1-mm3 pieces and fixed in 2.5% glutaraldehyde for ≥24 hours. This was followed by rinsing in 0.1 M phosphoric acid and washing (3 times, 15 minutes each time). After the dehydration, embedding, and curing process, ultra-thin slices of 50 to 60 nm were obtained. The slices were stained by 3% uranyl acetate and lead citrate, and observed under transmission electron microscopy to evaluate mitochondrial structure and autophagosomes.
Mitochondrial isolation and mitochondrial membrane potential
Cardiac mitochondria were isolated from mouse hearts. Trypsin digestion liquid was added to the myocardial tissue in a cold ice bath for 20 minutes and then centrifuged at 600 × g for 10 to 20 seconds at 4 °C. Next, two volumes of the corresponding mitochondrial separation reagents were added to the precipitate, which was then centrifuged at 600 × g for 10 to 20 seconds at 4 °C. Subsequently, eight volumes of the corresponding mitochondrial separation reagents were added to the precipitate and were homogenized on ice. The homogenate was then centrifuged at 600 × g for 5 minutes at 4 °C. Mitochondria were obtained by centrifuging the supernatant at 11,000 × g for 10 minutes at 4 °C. Finally, 1 ug/mL rhodamine 123 (10 µL) was added to the extracted mitochondria, and the mitochondrial membrane potential (MMP) was assessed by flow cytometry, with excitation and emission wavelengths of 507 and 530 nm, respectively.
Western blotting
Protein concentrations were determined by the bicinchoninic acid method. Equal amounts of protein were then electrophoresed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a 0.22-µm polyvinylidene fluoride membrane. The membrane was blocked in 5% skimmed milk for 2 hours and then incubated with the corresponding specific antibody against Parkin (1: 1000), PINK1 (1: 500), microtubule-associated protein 1 A/1B-light chain 3 (LC3) II (1: 1,000), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1: 1000) at 4 °C overnight. The corresponding peroxidase-conjugated secondary antibody was incubated at room temperature for 2 hours, followed by washes with TBST for three times (10 minutes each time). The blots were visualized with an enhanced chemiluminescence detection system (Pierce Biotechnology, Shaanxi, China). GAPDH was used as a loading control.
Apoptosis analysis by flow cytometry
The effect of Ang II on myocardial apoptosis in mice was determined by using the Annexin V-FITC/PI cell apoptosis detection kit (KeyGEN BioTECH, Nanjing, China) according to a protocol used previously.10
Quantitative real-time PCR analysis
Myocardial tissue was cut into pieces and then placed into 2-mL Eppendorf micro test tubes. Total RNA was extracted in accordance with the specification of the TRIzol kit (Life Technologies, Carlsbad, CA, USA). RNA levels were determined and RNA (1 μg) was reverse transcribed into cDNA. Real-time PCR was performed and mRNA expression levels of hypertrophic markers, including atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), were evaluated. GAPDH was used as the internal control. The primer sequences were as follows: ANP forward, 5′-GATTTCAAGAACCTGCTAG ACCACC-3′ and reverse, 5′-GCGAGCAGAGCCCTA GTTT-3′; BNP forward, 5′-GATGATTCTGCTCCTGCTTTTCC-3′ and reverse, 5′-CAGCTTCTGCATCGTGGATT-3′; and GAPDH forward, 5′-CTGGAGAAACCTGCCAAGTATG-3′ and reverse, 5′-GGTGGAAGAATGGGAGTTGCT-3′.
Statistical analysis
Data are expressed as mean ± standard deviation of three independent experiments and were statistically analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The independent t-test for continuous variables was used to assess mean differences between the control and Ang II groups. P < 0.05 was considered to indicate statistical significance.
Results
Mouse heart
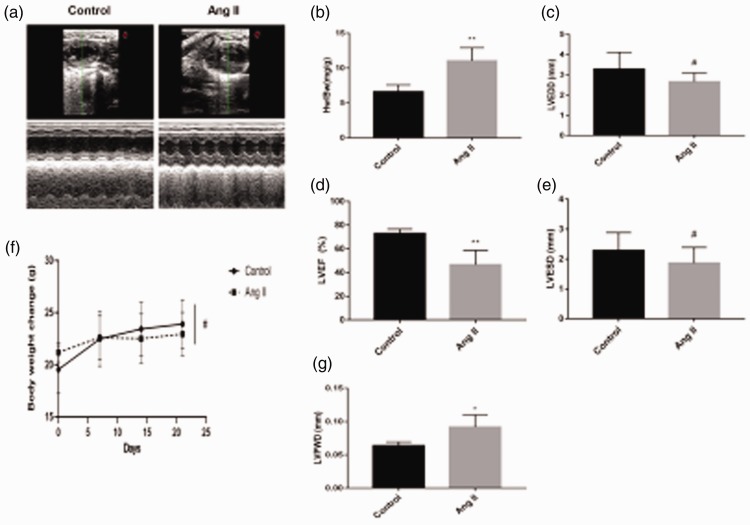
Echocardiography showed that the LVPWD in the Ang II group was significantly higher than that in the control group (P < 0.05, Figure 1a and g), but the LVEF was less than that in the control group (P < 0.01) (Figure 1d). There were no significant differences in LVEDD and LVESD between the Ang II and control groups. The weight of mice in the AngII group was significantly lower than that of mice in the control group, although this difference was not significant (Figure 1f). The HW/BW ratio in the AngII group was significantly higher than that in the control group (P < 0.05, Figure 1b).
Figure 1.
(a) Cardiac structure detected by echocardiography. (b) Heart weight/body weight ratio of mice (**P < 0.01 versus the control group). (c) Left ventricular end-diastolic diameter (LVEDD) (#P > 0.05 versus the control group). (d) Left ventricular ejection fraction (LVEF) (%) of mice detected by echocardiography (**P < 0.01 versus the control group). (e) Left ventricular end-systolic diameter (LVESD) (#P > 0.05 versus the control group). (f) Changes in weight of mice were measured every week for 3 weeks (#P > 0.05). (g) Left ventricular posterior wall thickness (LVPWD) of mice detected by echocardiography (*P < 0.05 versus the control group). Ang II, angiotensin II.
Histopathological changes
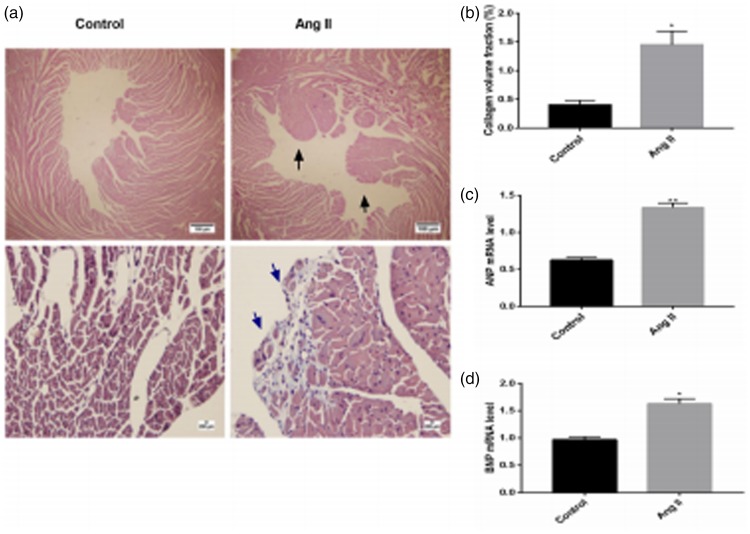
Hematoxylin and eosin staining of mouse myocardial tissue under low magnification (×40) showed that the papillary muscle of mice and infiltration of inflammatory cells in heart tissue were greater in the Ang II group compared with the control group (Figure 2a). Masson’s trichrome staining of mouse myocardial tissue under high magnification (×400) indicated that myocytes of mice were obviously hypertrophic and arranged in a disordered manner, and showed more obvious fibrosis in the Ang II group than in the control group (P < 0.05, Figure 2a and b).
Figure 2.
(a) Hematoxylin and eosin staining of mouse myocardial tissue. (b) Collagen volume fraction of mouse myocardial tissue (*P < 0.05 versus the control group). Black arrows indicate increased papillary muscle of mice and blue arrows indicate fibrosis. (c) Expression of the hypertrophic marker atrial natriuretic peptide (ANP) (**P < 0.01 versus the control group). (D) Expression of the hypertrophic marker brain natriuretic peptide (BNP) (*P < 0.05 versus the control group). Ang II, angiotensin II.
Relative ANP and BNP mRNA expression levels
We assessed mRNA expression of the hypertrophic markers ANP and BNP in the two groups. Expression of ANP and BNP mRNA was significantly higher in the Ang II group than in the control group (both P < 0.05, Figure 2c and d).
Mitochondrial structure, autophagosomes, and MMP
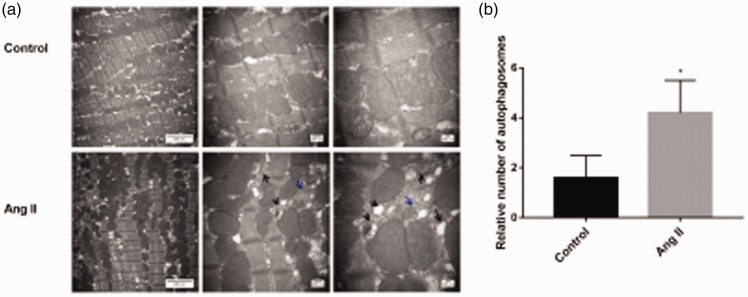
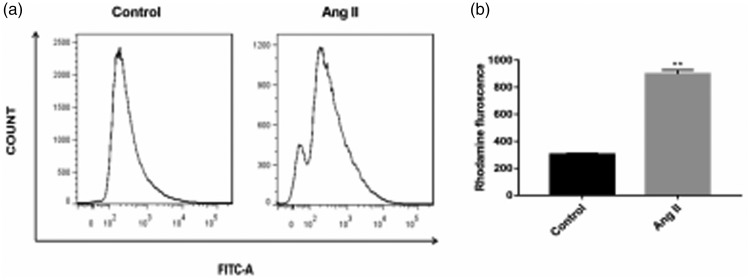
Transmission electron microscopy showed that myocardial mitochondria of mice were closely arranged, with no disordered arrangement of mitochondria or morphological or structural abnormalities in the control group (Figure 3a). However, the mitochondria of mouse myocardial tissue in the Ang II group were irregular, disordered, showed obvious swelling, mitochondrial cristae were broken or had even disappeared, and a number of cristae were vacuolated (Figure 3a). Therefore, mitochondria in the Ang II group were evidently damaged. Additionally, the number of autophagosomes in mouse myocardium in the Ang II group was significantly higher than that in the control group, and residual mitochondria were observed in the double membrane structure (P < 0.05, Figure 3a and b). When the mitochondrial membrane is intact, the MMP is relatively normal and the fluorescence intensity of rhodamine is low. The fluorescence intensity of rhodamine increases as the MMP decreases. The fluorescence intensity of rhodamine in the Ang II group was significantly higher than that in the control group (P < 0.01). Therefore, the MMP in the Ang II group was lower than that in the control group (Figure 4a and b).
Figure 3.
(a) Mitochondrial structure and autophagosomes of mouse cardiac tissue detected by transmission electron microscopy. (b) Relative number of autophagosomes. Black arrows indicate autophagosomes and blue arrows indicate abnormal or damaged mitochondria. Ang II, angiotensin II.
Figure 4.
Fluorescence intensity of rhodamine. The mitochondrial membrane potential was lower in the angiotensin II group than in the control group. **P < 0.01 versus the control group. Ang II, angiotensin II.
Protein expression
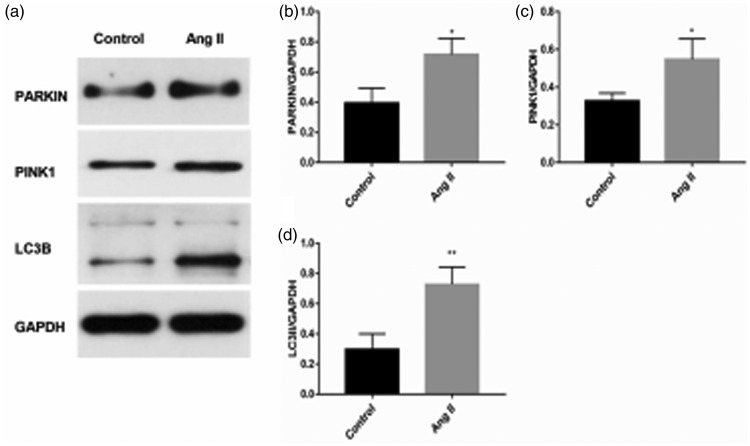
Western blot analysis showed that protein expression levels of PINK1, Parkin, and LC3 II were significantly higher in the AngII group compared with the control group (all P < 0.05, Figure 5a, b, c, and d).
Figure 5.
(a–d) Western blot analysis of PINK1, Parkin, and LC3 II protein levels of mice myocardium. *P < 0.05, **P < 0.01. Ang II, angiotensin II; PINK1, phosphatase and tensin homolog-induced putative kinase 1; LC3, microtubule-associated protein 1A/1B-light chain 3, GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
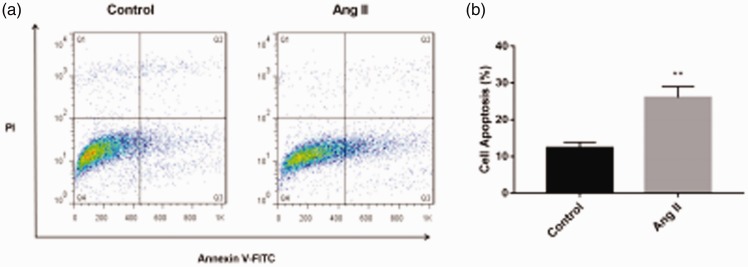
Analysis of apoptosis
Myocardial apoptosis was detected by flow cytometry. Ang II-induced cardiac hypertrophy can increase autophagosomes and apoptosis. The percentage of apoptosis was significantly higher in the Ang II group compared with the control group (P < 0.01) (Figure 6a and b).
Figure 6.
Myocardial apoptosis as determined by flow cytometry by using the Annexin V-FITC/PI cell apoptosis detection kit. **P < 0.01 versus the control group. Ang II, angiotensin II.
Discussion
To the best of our knowledge, this is the first study to show mitochondrial changes and PINK1/Parkin-mediated autophagy in Ang II-induced cardiac hypertrophy. We found that mitochondria were obviously damaged and PINK1/Parkin-mediated autophagy may be upregulated in Ang II-induced cardiac hypertrophy.
Renin–angiotensin–aldosterone system activation is one of the most important mechanisms for development of cardiac hypertrophy. Ang II can induce cardiac hypertrophy through multiple signaling pathways.11–13 The present study successfully established Ang II-induced cardiac hypertrophy in mice. Mitochondria are “power plants” for the heart, and mitochondrial structure and function are often abnormal during hypertrophy of the myocardium.14–16 Our study showed that mitochondria were dysfunctional, disordered, showed swelling, their cristae were broken or had even disappeared, and a number of cristae appeared vacuolated in the Ang II group. Additionally, the MMP was lower in the Ang II group than in the control group.
The role of autophagy in hypertrophy remains controversial and mitochondrial autophagy (mitophagy) has been rarely reported.17,18 To date, the mitophagy pathway mainly includes PINK1/Parkin,19 Bnip3/Nix,20 and FUN14 domain containing 121 signaling. In mammals, mitophagy is mainly regulated by the PINK1/Parkin signaling pathway.
Normally, PINK1 undergoes rapid degradation at the mitochondria inner membrane. When mitochondria are damaged, especially with a decrease in the membrane potential, PINK1 accumulates on the outer membrane and phosphorylates ubiquitin and other mitochondrial outer membrane proteins, facilitating cytosolic PARKIN translocation to mitochondria.6,22 Parkin, which is an E3 ubiquitin ligase, ubiquitinates mitochondrial outer membrane proteins and facilitates recruitment of P62/SQSTM1. P62 is an adaptor that interacts with ubiquitinated proteins and LC3, thereby recruiting a phagophore to engulf the damaged mitochondria.23 LC3 II is located in the outer and inner membranes of autophagosome, and its level is proportional to the number of autophagosomes.24 P62 is combined with LC3 II and is selectively degraded by autophagy, and its expression level is inversely proportional to autophagic activity.25 In the present study, PINK1, Parkin and LC3 II protein levels were higher in the Ang II group than in the control group. Additionally, the number of autophagosomes was higher in the Ang II group than in the control group. The above-mentioned results indicate that PINK1/Parkin-mediated mitophagy may be upregulated in Ang II-induced cardiac hypertrophy. Wei et al reported the relationship between SIRT3-mediated angiogenesis and cardiac remodeling and they showed that PINK1/Parkin-mediated mitophagy may be involved in the mechanism of angiogenesis.26 However, Wei et al’s study mainly focused on PINK1/Parkin-mediated mitophagy in angiogenesis. The present study directly investigated the role of mitophagy in Ang II-induced cardiac hypertrophy. We provide important and supplementary evidence for the mechanism of PINK1/Parkin-mediated autophagy (mitophagy) in hypertrophy. In our study, we observed that, in the Ang II group, mitochondria were damaged, the MMP was decreased, autophagosomes were accumulated, apoptosis was increased, and PINK1/Parkin-mediated autophagy was upregulated. These factors contributed to the abnormal cardiac function of Ang II-induced cardiac hypertrophy.
In conclusion, the present study indicates that PINK1/Parkin-mediated autophagy may be an important mechanism of Ang II-induced cardiac hypertrophy. PINK1/Parkin-mediated autophagy is involved in Ang II-induced cardiac hypertrophy by affecting myocardial apoptosis and mitochondrial function. However, the specific role and mechanism of PINK1/Parkin-mediated autophagy in hypertrophy need to be further studied.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81870298).
ORCID iD
References
- 1.Kavey RE. Left ventricular hypertrophy in hypertensive children and adolescents: predictors and prevalence. Curr Hypertens Rep 2013; 15: 453–457. [DOI] [PubMed] [Google Scholar]
- 2.Crozatier B, Ventura-Clapier R. Inhibition of hypertrophy, per se, may not be a good therapeutic strategy in ventricular pressure overload: other approaches could be more beneficial. Circulation 2015; 131: 1448–1457. [DOI] [PubMed] [Google Scholar]
- 3.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000; 290: 1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008; 132: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou L, Ma B, Han Xet al. The role of autophagy in angiotensin II -induced pathological cardiac hypertrophy. J Mol Endocrinol 2016; 57: R143–R152. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda N, Sato S, Shiba Ket al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 2010; 189: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billia F, Hauck L, Konecny Fet al. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci U S A 2011; 108: 9572–9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera-Torres J, Guzman-Martinez G, Villa-Bellosta Ret al. Targeting gamma-secretases protect against angiotensin II-induced cardiac hypertrophy. J Hypertens 2015; 33: 843–850. [DOI] [PubMed] [Google Scholar]
- 9.Ma H, Kim CS, Ma Yet al. Magnolol enhances pentobarbital-induced sleeping behaviors: possible involvement of GABAergic systems. Phytother Res 2009; 23: 1340–1344. [DOI] [PubMed] [Google Scholar]
- 10.Lu L, Liu Q, Wang Pet al. MicroRNA-148b regulates tumor growth of non-small cell lung cancer through targeting MAPK/JNK pathway. BMC Cancer 2019; 19: 209. doi: 10.1186/s12885-019-5400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrario CM. Cardiac remodelling and RAS inhibition. Ther Adv Cardiovasc Dis 2016; 10: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Zhang YL, Lin QYet al. CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J 2018; 20: 1818–1831. [DOI] [PubMed] [Google Scholar]
- 13.Qian LB, Jiang SZ, Tang XQet al. Exacerbation of diabetic cardiac hypertrophy in OVE26 mice by angiotensin II is associated with JNK/c-Jun/miR-221-mediated autophagy inhibition. Oncotarget 2017; 8: 106661–106671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Bilsen M, van Nieuwenhoven FA, van der Vusse GJ. Metabolic remodelling of the failing heart: beneficial or detrimental. Cardiovasc Res 2009; 81: 420–428. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Zhou J, An Wet al. Apocynin attenuates pressure overload-induced cardiac hypertrophy in rats by reducing levels of reactive oxygen species. Can J Physiol Pharmacol 2010; 88: 745–752. [DOI] [PubMed] [Google Scholar]
- 16.Lingan JV, Alanzalon RE, Porter GA., Jr. Preventing permeability transition pore opening increases mitochondrial maturation, myocyte differentiation and cardiac function in the neonatal mouse heart. Pediatr Res 2017; 81: 932–941. [DOI] [PubMed] [Google Scholar]
- 17.Nakai A, Yamaguchi O, Takeda Tet al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007; 13: 619–624. [DOI] [PubMed] [Google Scholar]
- 18.Jaber N, Dou Z, Chen JSet al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A 2012; 109: 2003–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shires SE, Gustafsson AB. Regulating renewable energy: connecting AMPKalpha2 to PINK1/Parkin-mediated mitophagy in the heart. Circ Res 2018; 122: 649–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandoval H, Thiagarajan P, Dasgupta SKet al. Essential role for Nix in autophagic maturation of erythroid cells. Nature 2008; 454: 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Feng D, Chen Get al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol 2012; 14: 177–185. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Dorn GW., 2nd. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 2013; 340: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito T, Sadoshima J. Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ Res 2015; 116: 1477–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Arribas M, Yakhine-Diop SM, Gonzalez-Polo RAet al. Fuentes, turnover of lipidated LC3 and autophagic cargoes in mammalian cells. Methods Enzymol 2017; 587: 55–70. [DOI] [PubMed] [Google Scholar]
- 25.Pankiv S, Clausen TH, Lamark Tet al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 2007; 282: 24131–24145. [DOI] [PubMed] [Google Scholar]
- 26.Wei T, Huang G, Gao Jet al. Sirtuin 3 deficiency accelerates hypertensive cardiac remodeling by impairing angiogenesis. J Am Heart Assoc 2017; 6: 2047–9980. [DOI] [PMC free article] [PubMed] [Google Scholar]