The scientific world has brought to the fore angiotensin-converting enzyme 2 (ACE2) for its pivotal role in the pathogenesis of novel coronavirus infectious disease (COVID-19). ACE2 is part of the renin-angiotensin system (RAS), an intricate interlinked system of regulatory mediators involved in the control of blood pressure and hemostasis of several organs. RAS has been implicated in the regulation of inflammatory pathways in models of lung injury and pulmonary vascular disease. Recently, physicians have suggested a possible concerning effect of ace inhibitors and angiotensin II (Ang II)-receptor blockers with the COVID-9 outbreak, as ACE2 is the host receptor required for cellular entry of severe acute respiratory syndrome (SARS)-CoV-2, the etiological agent of the current SARS.
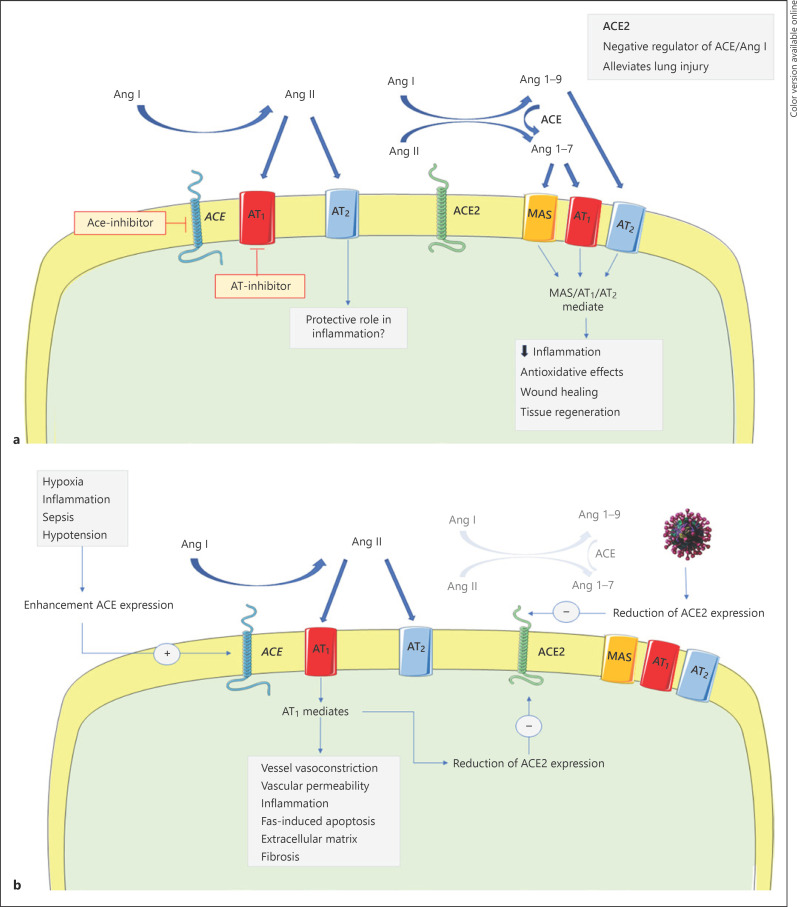
ACE2 was discovered 46 years after [1] the first description of this enzyme by Skeggs et al. [2]. ACE is a protein produced in the endothelium of somatic tissues that catalyzes the conversion from angiotensin I (Ang I) to Ang II, a potent vasoconstrictor, and degrades bradykinin, an inflammatory mediator with vasodilator propriety (Fig. 1a) [3].
Fig. 1.
a Representation of the RAS homeostasis in the lung. b Pathologic modulation of the RAS in SARS-CoV-2 infection. ACE, angiotensin-converting enzyme; Ang I, angiotensin I; Ang II, angiotensin II.
ACE2 is both a membrane associated and secreted enzyme able to cleavage Ang I to 1–9 and Ang II to Ang 1–7. ACE2 has partial analogy with ACE. Analysis of the human genomic sequence shows that the metalloprotease catalytic domain of ACE2 is identical to ACE for 42% [1]. This evidence supports the hypothesis that the 2 genes may derivate from a common ancestor. Similar to ACE, the expression of ACE2 has been found in many organs including the lung, heart, kidney, and testis [1]. However, vasopressor activity and response to commercially available antihypertensive drugs appear different in these two enzymes, despite their homologous catalytic domain. ACE2 is a counter-regulator of ACE and participates in the regulation of blood pressure decrease with the formation of non-vasoactive Ang 1–9 and vasodilator Ang 1–7. In addition, ACE2 activity is not inhibited by ACE inhibitors [1, 4].
The key role of ACE2 in SARS-CoV infection was established in 2003 during the first coronavirus outbreak in China. S1 domain of the SARS-CoV (protein S1) mediates the entry of the virus into target host cells after binding with the transmembrane ACE2 receptor [5]. The virus has marked tropism for the respiratory tract, bowel, and heart cells, where the ACE2 expression is well-represented.
Data show that the severity of SARS-CoV-2 is unpredictable, and the clinical manifestations, especially respiratory symptoms, can quickly worsen even in paucisymptomatic subjects [6]. Initial data from Italy document that the infection is severe and carries high morbidity and mortality [7]. Lung involvement is the most severe complication, often evolving in acute respiratory distress syndrome (ARDS), a devastating clinical syndrome with a high mortality rate (30–60%) [8]. There is a consensus that ACE2 is a key protective molecule in the development and maintenance of lung injury (Fig. 1b) [9]. An elegant experiment in mice showed that the loss of ACE2 expression resulted in lung edema, inflammatory cell infiltration, and hypoxia in different models of acute lung injury. Damage of the lungs led to an increase in the level of AngII that, in turn, drove downregulation of ACE2 through receptor AT1a. The authors also demonstrated that infusion of recombinant ACE2 significantly ameliorated lung injuries, findings that emphasized the protective role of ACE2 in the lungs [10].
We believe that multiple factors are implicated in the battle of the host against SARS-Cov-2 including immune response, comorbidities, early respiratory support, and modulation of inflammation. Regarding this latter variable, understanding the physiological regulation of the ACE2 pathway can help physicians for the management of SARS-CoV-2 infection. Variability of RAS activation in patient subgroups might explain the enormous variability in clinical manifestations.
ACE2 plays a twofold role in SARS-CoV-2 infection. The protective role of ACE2 from acute lung failure is antagonized by its crucial role in virus entry. Hence, the reduced expression of transmembrane ACE2, which theoretically minimizes the spread of the virus among cells, could negatively balance an augmented risk of ARDS in the infected people. It is not surprising that the disruption of ACE2 signaling pathway in lung cells is the main determinant of SARS-CoV-2 virulence. The targeted injury of fundamental respiratory epithelial cells involved in immunomodulation (Clara cells) and maintaining of a functional environment and tissue regeneration (type II alveolar cells) probably supports the high prevalence of severe lung involvement complicated by ARDS.
Animal studies have demonstrated that SARS-CoV infection downregulates the expression of ACE2 in the lungs. The binding of circulating S protein to ACE2-expressing cells aggravates the model of lung injury resulting in severe acute respiratory failure [11]. Likewise, the SARS-CoV-2-mediated downregulation of ACE2 in humans could be the causative factor of the severe lung pathology. The evidence coming from clinical practice reports that the duration of illness in patients with lung involvement lasts much longer than the time necessary to mount an adequate host immune response against the virus. In this way, an excessive inflammatory respiratory response triggered by SARS-CoV-2 and maintained by dysregulation of the RAS system may be the principal pathologic mechanism in this infection and partly account for the disparity in the rate of morbidity and mortality of subjects affected by SARS-CoV-2. The high prevalence of elderly and the slightly higher incidence in males than women among subjects with worse outcomes [12] may be explained by dysregulation of ACE/ACE2 secondary to an imbalance in sex steroid hormones with age. Experiments conducted in organs other than lungs reported that a low level of testosterone has been associated with low ACE2 activity in heart tissue in rats [13, 14] and vice versa and estrogen increased myocardium expression of ACE2 in humans [15]. The high prevalence of diabetes status, constantly present among severely affected subjects [6, 16], could be determined by the imbalance in ACE and ACE2 production in the lungs [17]. Studies on diabetic animal models documented a decrease in ACE2 expression in the lungs and a reduced ACE2 to ACE ratio, which lowers Ang1–7 concentration and enhances AngII accumulation. Besides the age- and gender-associated variability, genomic variants of ACE/ACE2 may further explain the different outcomes seen in COVID-19 outbreak [18].
ACE-inhibitors and Ang II-receptor blockers have been associated with a high risk of infection through upregulation of ACE2 expression that, in turn, would facilitate SARS-CoV-2 infection [19]. The lack of clinical data in support of this hypothesis has led the most representative cardiologist groups to declare to continue ACE inhibitors or angiotensin-receptor blockers. Moreover, some evidence in animals shows that these drugs might be rather protective against serious lung complications. Indeed, ACE inhibitors reduce the level of deleterious AngII and increase expression of the protective of ACE2, whereas Ang II-receptor blockers may attenuate the inflammatory response driven by the AT1a pathway [10, 20].
It is worth noting that the management of hypertension raises questions about the appropriate treatment in symptomatic patients with SARS-CoV-2 infection. Based on reports from clinical practice, blood pressure is notably variable and showed a tendency to difficult-to-control hypertension after withdrawing antihypertensive drugs. Patients with fever, hyperventilation, diarrhea, loss of appetite, and a reduction of fluid intake due to continuous oxygen therapy (mask, helmet) are prone to become severely dehydrated. In this setting, antihypertensive drugs such as diuretics, ace inhibitors, and Ang II-receptor blockers must be withdrawn to prevent acute kidney injury [21]. Treatment with beta-blockers should be accurately assessed in patients with severe hypoxemia and hemodynamic instability due to sepsis. The beneficial effects of beta blockers in protecting against myocardial ischemia and hypoxia-induced arrhythmia should be balanced with a concrete risk of worsening of lung function, especially with nonselective beta blocker (propranolol, carvedilol) [22]. Therapy with nonselective beta blocker needs to be converted to the minimum effective dose of more selective agents (nebivolol, bisoprolol), and dose should be modulated on heart rate and respiratory performance. To note, tachycardia should not be completely corrected, as it acts as a compensatory mechanism for maintaining hemodynamics and peripheral oxygenation. Instead, treatment with calcium channel blockers, peripheral alpha antagonist, and central agonists of alpha 2-adrenergic receptors appears to have a good safety profile in this cohort of patients.
In summary, there is no scientific evidence supporting an aggravation of lung injury in patients treated with RAS blockers who develop pneumonia. The apparent beneficial effect of RAS modulation and the use of ace inhibitor and Ang II-receptor blockers in these patients warrant the conduction of further clinical trials [23], to elucidate the underlying pathophysiological mechanism of this concerning infectious disease and provide information on its best management.
Statement of Ethics
This article does not contain any studies or data on human participants or animals performed by any of the authors.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not funded.
Author Contributions
G.A.: conceptualization, methodology, writing – original draft, writing – data curation, review, and editing. G.G.: conceptualization. A.F.: data curation, review, and editing. F.F.: writing, review, and editing. C.M. and G.C. supervision, review, and editing. R.M.: supervision.
Appendix
Modena Covid-19 Working Group
Cristina Mussini, Giovanni Guaraldi, Erica Bacca, Andrea Bedini, Vanni Borghi, Giulia Burastero, Federica Carli, Giacomo Ciusa, Luca Corradi, Gianluca Cuomo, Margherita Digaetano, Giovanni Dolci, Matteo Faltoni, Riccardo Fantini, Giacomo Franceschi, Erica Franceschini, Vittorio Iadisernia, Damiano Larné, Marianna Menozzi, Marianna Meschiari, Jovana Milic, Gabriella Orlando, Francesco Pellegrino, Alessandro Raimondi, Carlotta Rogati, Antonella Santoro, Roberto Tonelli, Marco Tutone, Sara Volpi, and Dina Yaacoub (Infectious Diseases Clinics, University Hospital, via del Pozzo 71, 41124 Modena, Italy); Gianni Cappelli, Riccardo Magistroni, Gaetano Alfano, Francesco Fontana, Ballestri Marco, Giacomo Mori, Ferrari Annachiara (Nephrology Dialysis and Transplant Unit, University Hospital of Modena, Modena, Italy); Massimo Girardis, Alberto Andreotti, Emanuela Biagioni, Filippo Bondi, Stefano Busani, Giovanni Chierego, Marzia Scotti, and Lucia Serio (Department of Anesthesia and Intensive Care, University Hospital, via del Pozzo 71, 41124 Modena, Italy); Andrea Cossarizza, Caterina Bellinazzi, Rebecca Borella, Sara De Biasi, Anna De Gaetano, Lucia Fidanza, Lara Gibellini, Anna Iannone, Domenico Lo Tartaro, Marco Mattioli, Milena Nasi, Annamaria Paolini, and Marcello Pinti (Chair of Pathology and Immunology, University of Modena and Reggio Emilia, Via Campi, 287, 41125 Modena, Italy).
References
- 1.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000 Sep;87((5)):E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 2.Skeggs LT, Jr, Marsh WH, Kahn JR, Shumway NP. The purification of hypertensin I. J Exp Med. 1954 Oct;100((4)):363–70. doi: 10.1084/jem.100.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehlers MR, Chen YN, Riordan JF. Purification and characterization of recombinant human testis angiotensin-converting enzyme expressed in Chinese hamster ovary cells. Protein Expr Purif. 1991 Feb;2((1)):1–9. doi: 10.1016/1046-5928(91)90001-y. [DOI] [PubMed] [Google Scholar]
- 4.Turner AJ, Tipnis SR, Guy JL, Rice G, Hooper NM. ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can J Physiol Pharmacol. 2002 Apr;80((4)):346–53. doi: 10.1139/y02-021. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003 Nov;426((6965)):450–4. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China a retrospective cohort study. Lancet. 2020 Mar;395((10229)):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Civil Protection Department - Presidency of the Council of Ministers Risks prevision and prevention activities, emergency management. [Internet] Coronavirus - Press Release Detail. [cited 2020 Mar 21]. Available from: http://www.protezionecivile.gov.it/mediacommunication/press-release/detail/-/asset_publisher/default/content/coronavirus-sono-42-681-i-positivi.
- 8.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000 May;342((18)):1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Baker A. Recombinant human ACE2 acing out angiotensin II in ARDS therapy. Crit Care. 2017 Dec;21((1)):305. doi: 10.1186/s13054-017-1882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005 Jul;436((7047)):112–6. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005 Aug;11((8)):875–9. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalpiaz PLM, Lamas AZ, Caliman IF, Ribeiro RF, Abreu GR, Moyses MR, et al. Sex Hormones Promote Opposite Effects on ACE and ACE2 Activity Hypertrophy and Cardiac Contractility in Spontaneously Hypertensive Rats. PLoS One. 2015 May;10((5)):e0127515. doi: 10.1371/journal.pone.0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heffernan DS, Dossett LA, Lightfoot MA, Fremont RD, Ware LB, Sawyer RG, et al. Gender and ARDS in Critically Injured Adults A Prospective Study. J Trauma. 2011 Oct;71((4)):878–85. doi: 10.1097/TA.0b013e31822c0d31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bukowska A, Spiller L, Wolke C, Lendeckel U, Weinert S, Hoffmann J, et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp Biol Med (Maywood) 2017 Aug;242((14)):1412–23. doi: 10.1177/1535370217718808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan China. JAMA. 2020 Feb;323((11)):1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM, Santisteban P, González-Matías LC, Vigo E, et al. Activation of the GLP-1 Receptor by Liraglutide Increases ACE2 Expression Reversing Right Ventricle Hypertrophy and Improving the Production of SP-A and SP-B in the Lungs of Type 1 Diabetes Rats. Endocrinology. 2015 Oct;156((10)):3559–69. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- 18.Marshall RP, Webb S, Bellingan GJ, Montgomery HE, Chaudhari B, McAnulty RJ, et al. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002 Sep;166((5)):646–50. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- 19.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 Apr;8((4)):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong JC, Ye JY, Jin HY, Yu X, Yu HM, Zhu DL, et al. Telmisartan attenuates aortic hypertrophy in hypertensive rats by the modulation of ACE2 and profilin-1 expression. Regul Pept. 2011 Jan;166((1-3)):90–7. doi: 10.1016/j.regpep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Tomson C, Tomlinson LA. Stopping RAS Inhibitors to Minimize AKI More Harm than Good? Clin J Am Soc Nephrol. 2019 Apr;14((4)):617–9. doi: 10.2215/CJN.14021118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipworth B, Wedzicha J, Devereux G, Vestbo J, Dransfield MT. Beta-blockers in COPD time for reappraisal. Eur Respir J. 2016 Sep;48((3)):880–8. doi: 10.1183/13993003.01847-2015. [DOI] [PubMed] [Google Scholar]
- 23.Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017 Sep;21((1)):234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]