Abstract
Coronavirus disease 2019 (COVID-19) is a global pandemic affecting more than 200 countries and 180,000 cases in the United States. While the outbreak began in China, the number of cases outside of China exceeded those in China on March 15, 2020 and are currently rising at an exponential rate. The number of fatalities in the United States are expected to exceed more than Italy and China. The disease is characterized predominantly as an acute respiratory illness. However, preliminary data suggests that kidney is a target for the virus and deterioration of renal function was associated with poor outcomes including in-hospital mortality. We present a report of a patient with COVID-19 who presented with acute onset of symptoms and normal renal function at baseline but rapidly deteriorated resulting in death. The timing of decline in renal function correlated with his worsening clinical status. He was started on continuous veno-venous hemofiltration without signs of clinical benefit. We also present the possible mechanisms for acute kidney injury in these patients. We performed a review of the emerging literature by searching PubMed, Google Scholar, and EMBASE for studies and/or case series published on this topic. Acute kidney injury might help risk stratify critically ill patients on a fatal course of COVID-19.
Keywords: Coronavirus disease 2019, Acute kidney injury, ACE2 receptors, Continuous veno-venous hemofiltration
Introduction
Coronaviruses (CoVs) are a family of enveloped, single-stranded RNA viruses that are extensively present in bats but also seen in many other birds and mammals including humans [1]. CoVs tend to cause mild to moderate upper respiratory tract infections such as the common cold. However, there have been outbreaks of severe acute respiratory syndrome (SARS) caused by distinct CoV strains, which were determined to be phylogenetically distinct from the common human CoVs, A similar severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) was reported recently from Wuhan, China [2]. The outbreak has now extended to more than 200 countries from human-to-human transmission affecting more than 690'000 patients and 30'000 deaths globally, and the World Health Organization (WHO) declared it as a global pandemic. Preliminary reports have suggested that the CoVID-19 virus originated in bats and was transmitted to human beings through an intermediate host [3]. The SARS-CoV-2 virus enters the cell by endocytosis through ACE2 receptors which are predominantly present in type 2 alveolar cells of the lung, upper esophagus and stratified epithelial cells, myocardial cells, proximal renal tubules [4, 5, 6, 7]. It is characterized predominantly as an acute respiratory illness with diffuse alveolar hemorrhage and acute respiratory failure [8]. However, the virus may enter the blood stream and accumulate in other organ systems such as the heart, GI tract, and kidneys causing damage. Preliminary data on extrapulmonary manifestations, specifically the renal system, are very limited. A single large prospective study of 701 COVID-19 patients from China showed that serum creatinine and blood urea nitrogen were elevated in 14.4 and 13.1% of the patients on admission [9]. Additionally, hematuria and proteinuria have been described. Patients with elevated baseline serum creatinine demonstrated a higher leukocyte count, lower lymphocyte and platelet counts, had a higher prevalence of comorbidities, and were more likely to be admitted to intensive care units requiring mechanical ventilation compared to patients with normal renal function on admission. During hospitalization, acute kidney injury (AKI) occurred in 5.1% of patients and was significantly higher in patients with elevated baseline serum creatinine (11.9%) than in patients with normal baseline values (4%). Patients with AKI and proteinuria of any degree, hematuria, elevated baseline blood urea nitrogen, serum creatinine, peak serum creatinine >1.5 mg/dL, and AKI over stage 2 were independently associated with increased mortality even after adjusting for other risk factors. We describe a COVID-19 patient who presented with normal serum creatinine at baseline but developed acute and rapid deterioration of kidney function, with an attempt of renal replacement therapy on the pathway to death.
Case Presentation
A 49-year-old Hispanic male with a past medical history significant for non-insulin-dependent well-controlled diabetes mellitus, hypertension, morbid obesity, OSA on CPAP presented with acute-onset fever associated with shortness of breath, myalgia, and nausea. He endorsed these symptoms for 3 days but worsened the night before the presentation. A comprehensive review of system was otherwise negative including chest pain, cough, urinary symptoms (frequency, dysuria, hematuria), vomiting, diarrhea, and headache. He denied any sick contacts or recent travel. His vital signs on presentation were significant for a temperature of 100.9°F and blood pressure of 173/85 mm Hg. He was mildly tachypneic with oxygen saturation of 96% on room air. Examination revealed mildly decreased air entry in bilateral lower lung bases with no audible wheeze. Initial laboratory data revealed lymphopenia (19.1%) with normal white blood cell count and basic metabolic panel. Renal function was normal at the time of admission including serum creatinine (1.0 mg/dL), blood urea nitrogen (10 mg/dL), and eGFR (79 mL/min/1.73 m2). Urine analysis showed mild proteinuria (30 mg/dL) with no hematuria, myoglobin, casts. Additionally, C-reactive protein (34 mg/L) was elevated on admission; however, BNP (<10), troponin I (0.01), LDH (193 U/L), aspartate aminotransferase (AST; 23 U/L), and alanine aminotransferase (ALT; 28 U/L) were within normal limits. Chest X-ray revealed peribronchial cuffing with increased perihilar markings concerning for viral pneumonia. CT chest was not performed due to morbid obesity with body weight above the cutoff for CT scan. EKG revealed normal sinus rhythm with QTc of 469 ms. Blood cultures were obtained and the patient was started on empiric antibiotics (doxycycline and ceftriaxone). A nasopharyngeal swab testing was also done to rule out COVID-19. He was then admitted to the hospital on droplet and airborne precautions and was prescribed oxygen through nasal cannula to maintain saturation over 92%. The patient was on lisinopril at home and was discontinued on admission.
The patient had fever spikes on and off during the hospitalization and started developing additional symptoms such as diarrhea. Urine culture, blood culture, and respiratory viral panel for influenza A/B, respiratory syncytial virus, parainfluenza, rhinovirus, and adenovirus were negative.
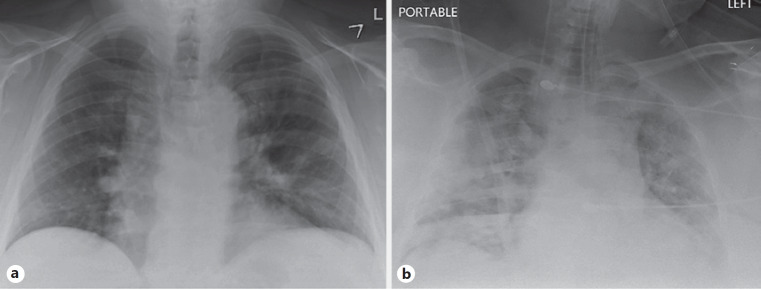
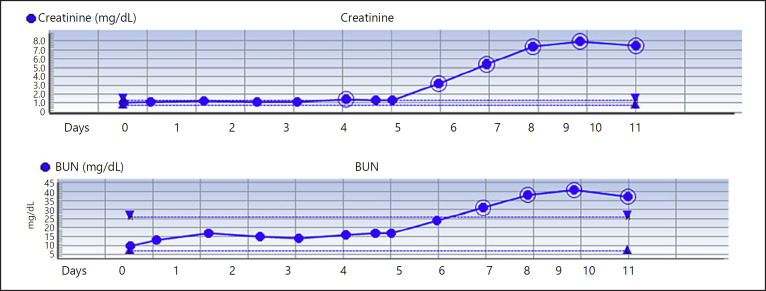
The positive COVID-19 result was received on day 5 of hospitalization. He developed respiratory deterioration on day 6 of hospitalization with increased work of breathing and hypoxia requiring high-flow nasal cannula. Chest X-ray revealed worsening multifocal air opacities concerning for multifocal pneumonia (Fig. 1a, b). His arterial blood gas revealed severe hypoxia with PaO2 of 43. He was transferred to the intensive care unit and was intubated due to worsening hypoxia from acute respiratory distress syndrome. He was started on norepinephrine infusion to maintain MAP >65 mm Hg. Doxycycline and ceftriaxone were discontinued and treatment with hydroxychloroquine, azithromycin, and vancomycin was started. His renal function including serum creatinine, blood urea nitrogen was normal until day 6 of hospitalization and started to worsen with his acute respiratory distress. Serum creatinine increased from 1.3 to 3.2 to 5.4 to 7.3 to 7.9 mg/dL. Trends in serum creatinine and blood urea nitrogen are displayed in Figure 2. Repeat urine analysis showed worsening proteinuria (317 mg/dL), urine creatinine (233 mg/dL), and granular casts with no evidence of hematuria. Urine sodium was 23 mEq/L, urine chloride was 23 mEq/L, and fractional excretion of sodium was 0.6%. Renal ultrasound showed slightly echogenic kidneys suggestive of mild medical renal disease with no evidence of hydronephrosis or renal calculi. The patient was started on continuous veno-venous hemofiltration on day 9 of hospitalization due to oliguria and worsening renal function. Other inflammatory markers trended up including C-reactive protein (121 mg/L), LDH (815 U/L), and total creatinine kinase (1,787 U/L to 2,464 U/L). Other significant laboratory data that worsened include lymphopenia (19% to 16.4% to 12.1% to 6.7%), AST (131 U/L), ALT (74 U/L). The patient unfortunately died on day 11 of hospitalization.
Fig. 1.
a Portable chest X-ray on admission. b Chest X-ray at the time of respiratory deterioration.
Fig. 2.
Trends in serum creatinine and blood urea nitrogen (BUN) since admission.
Discussion
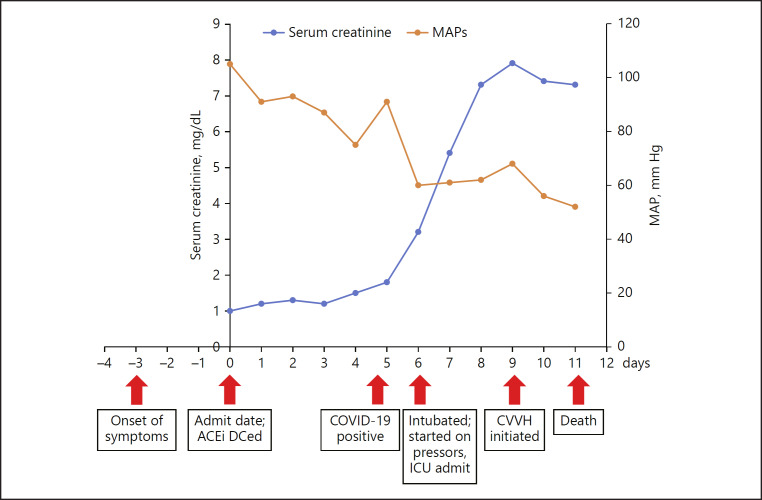
We present a case of severe COVID-19 infection in a young patient with no history of chronic kidney disease and normal creatinine at presentation but later developed rapid and acute decline in renal function (AKI stage 3) coinciding with acute respiratory distress. A timeline of significant clinical events along with trends in serum creatinine levels is shown in Figure 3. Our case report is similar to the recently published prospective cohort where patients with AKI are likely to be admitted to the intensive care unit and had any degree of proteinuria at baseline. The etiology for kidney injury in these patients could be multifactorial. Firstly, it could be due to the direct effects of the coronavirus on renal parenchyma as ACE2 expression in urinary organs (kidney) was nearly 100-fold higher than in respiratory organs demonstrated by human tissue RNA sequencing [10]. A recent study of the world's largest human kidney cell atlas found that ACE2 genes were significantly expressed in podocytes and proximal convoluted tubules as potential hosts cells targeted by SARS-CoV-2 and were no less than that in lung, esophagus, colon, and intestine, suggesting that the kidney may be an important target organ for SARS-CoV-2 [11]. Secondly, virus-mediated cytokine storm might impact the kidney function either directly or indirectly by causing hypoxia, shock, and rhabdomyolysis as seen in our patient. Elevation in serum creatine kinase was also seen in patients with H1N1 [12]. Thirdly, there is evidence of microthrombi formation in patients with COVID-19. Microthrombi can cause acute ischemia and result in acute kidney injury [13]. Finally, immune-mediated mechanism resulting in immune complex deposition of viral antigens could damage the kidneys.
Fig. 3.
Timeline of clinical events in correlation with serum creatinine. CVVH, continuous veno-venous hemofiltration.
Our case has several teaching points for clinicians facing this rapidly moving pandemic. Firstly, recent data suggest the baseline use of ACE or ARB are associated with a reduced risk of mortality [14, 15]. Our case like so many fatal cases developed hypotension and would not have been able to maintain lisinopril administration. However, going forward, it would be advisable to continue RAASi unless not tolerated due to hypotension or refractory hyperkalemia. Additionally, we started HCQ and azithromycin late into the course of COVID-19. A small French study suggests if these drugs work to reduce viral shedding, they do so early in the course of disease [16]. Our patient did not have hematuria at baseline, but presence of hematuria at baseline, even with normal creatinine and blood urea nitrogen, could be a harbinger of acute kidney injury in patients with COVID-19.
In conclusion, clinicians should be aware of the impact of acute kidney injury while taking care of patients with COVID-19 as it is associated with mortality and poor outcomes despite mechanical ventilation and attempts at renal replacement therapy. Worsening renal function may be an early identifier of fatal outcomes on COVID-19 despite maximally provided supportive care.
Statement of Ethics
Written consent from the patient's immediate family member (spouse) was obtained to publish the case report. Institutional review board approval was also obtained for this publication.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
None.
References
- 1.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb;395((10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579((7798)):270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou X, et al. Front. Med. 2020. The single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to Wuhan 2019-nCoV infection. http://journal.hep.com.cn/fmd/EN/10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y, et al. bioRxiv. 2020. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. Preprint available at https://www.biorxiv.org/content/10.1101/2020.01.26.919985v1. [Google Scholar]
- 6.Zhang H, et al. bioRxiv. 2020. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. Preprint available at https://www.biorxiv.org/content/10.1101/2020.01.30.927806v1. [Google Scholar]
- 7.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020 Feb;12((1)):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Peng F, Wang R, Guan K, Jiang T, Xu G, et al. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020 May;109:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y, Luo R, Wang K., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 May;97((5)):829–38. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu D, Zhang H, Gong H, et al. Identification of a Potential Mechanism of Acute Kidney Injury During the Covid-19 Outbreak: A Study Based on Single-Cell Transcriptome Analysis. Preprints; 2020. p. p. 2020020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Wu M, Guo J., et al. medRxiv. 2020. Caution on kidney dysfunctions of COVID-2019 patients. Preprint available at: [DOI] [Google Scholar]
- 12.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Canadian Critical Care Trials Group H1N1 Collaborative Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009 Nov;302((17)):1872–9. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 13.Mallidi J. Cardiology in the Time of COVID-19. Medscape. 2020 Mar;26 https://www.medscape.com/viewarticle/927522. [Google Scholar]
- 14.Liu Y, Huang F, Xu J, et al. medRxiv. 2020. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. Preprint available at: [DOI] [Google Scholar]
- 15.Lo KB, McCullough PA, Rangaswami J. Antihypertensive drugs and risk of COVID-19? Lancet Respir Med. 2020 Mar;:S2213-2600(20)30156-9. doi: 10.1016/S2213-2600(20)30156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautret P, Lagier JC, Parola P., et al. International Journal of Antimicrobial Agents - In Press 17 March. 2020. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open label non-randomized clinical trial. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]