Abstract
Metastasis remains the overwhelming cause of death for cancer patients. During metastasis, cancer cells will leave the primary tumor, intravasate into the bloodstream, arrest at a distant organ, and eventually develop into gross lesions at the secondary sites. This intricate process is influenced by innumerable factors and complex cellular interactions described in 1889 by Stephen Paget as the seed and soil hypothesis. In this review, we revisit this seed and soil hypothesis with an emerging understanding of the cancer cell (i.e. seed) and its microenvironment (i.e. soil). We will provide background to suggest that a critical outcome of the seed–soil interaction is resistance of the stresses that would otherwise impede metastasis.
Keywords: Metastasis, Microenvironment, Stress, Seed, Soil
1. Introduction
1.1. Problem of metastasis
The cause of death for the vast majority of cancer patients is the development of metastatic lesions at sites distant from that of the primary tumor. Metastasis describes both the process of cancer spread (i.e. the verb, describing the events that characterize the spread of cancer) and the resultant secondary cancer (i.e. noun, describing the actual metastatic lesion). Since most cancer patients present with localized disease that is effectively managed with multimodality therapies including surgery, radiation, and chemotherapy, the development of metastasis at distant secondary organs must involve the dissemination of metastatic cells before patients present with a primary tumor. Based on the work of several groups, it is believed that the process of metastasis (i.e. the verb) involves tumor cells leaving the primary tumor through a well-regulated lysis of surrounding stroma. These cells must pass through the tumor basement membrane and then through or between endothelial cells in order to enter the circulation. While in the circulation, a tumor cells must resist the process of anoikis (programmed cell death associated with loss of cellular contact), evade immune recognition, cope with the sheer physical stress of the circulatory system, and eventually arrest at a distant organ. At the distant site, the cell must exit the circulation, survive the stresses of a new and likely hostile microenvironment, proliferate, induce angiogenesis and/or co-opt existing blood vessels, and then successfully grow into a measurable metastatic lesion (Steeg and Theodorescu, 2008).
1.2. Clinical features/description
The timing, pattern and sites for the spread of cancer are in part defined by the specific cancer type. The route of spread of cancer may include blood stream (hematogenous), lymphatic vessels, or third space extension (i.e. ascitic fluid dissemination as seen in ovarian cancer). The site of distant metastasis may include regional lymph nodes, or visceral organs such as lungs, liver, brain, and bone. While Weiss et al. (1988) have shown that the primary site of metastases tends to occur at the first capillary bed encountered, it is increasingly believed that the specific site of distant metastasis is not simply to be due to anatomic location of the primary tumor or proximity to secondary sites but rather, involves interactions between tumor cells and the local microenvironment at the secondary site.
For many reasons, metastatic lesions are often not amenable to the surgical cures achieved in the management of the primary tumor. First, the development of metastases at distant secondary organs is often so widespread that surgery is not possible. In addition, the organs in which metastases develop may not be able to accommodate the necessary wide surgical margins needed for cure (i.e. brain). For many cancers, metastatic lesions themselves demonstrate increased resistance to conventional treatment modalities (i.e. chemotherapy). This resistance to therapy may be acquired as a result of past treatment of the patient or may be an innate feature of cells that have successfully negotiated the metastatic process. Based on the challenges that metastatic disease presents, new treatment options are needed in order to decrease morbidity and mortality.
1.3. Revisiting the seed and the soil
Stephen Paget’s seed and soil hypothesis suggested that the sites where metastases occur are defined not only by the tumor cell (“seed”) but also the microenvironment of the secondary metastatic site (“soil”). Recent data have shed new light on the acquisition of the “seeds” of metastasis, suggesting that some of the features of this tumor cell phenotype are conveyed early in the process of oncogenesis, whereas others are selected for during cancer progression (Scheel et al., 2007; Talmadge, 2007). Indeed, many of the features of the tumor cells (“seed”) now appear to be shared with primitive stem-like cells capable of not only tumor-initiation but also metastasis (Mehlen and Puisieux, 2006; Croker and Allan, 2008; Vermeulen et al., 2008). An attractive link between the tumor-initiating cell (cancer stem cell) hypothesis and metastasis is the programming and ability of these stem-like, self-renewing and multipotent cells to resist stress, perhaps facilitated by their abilities to engage and develop connections with specific microenvironments (“soil”). In this review we will consider new data that describe the tumor cell, its microenvironment, and connections between the two as a critical means to resist the stresses that cells encounter during metastatic progression.
2. Pathogenesis
2.1. Characteristics and emergence of the tumor cell (the “seed”)
The emergence of the metastatic phenotype within a primary tumor has been explained as a process that happens late in carcinogenesis. This hypothesis holds that tumor cells possessing the metastatic phenotype represent a very small fraction of cells within a heterogeneous primary tumor and that over time, a small proportion of tumor cells gain the attributes necessary for metastasis (Fidler and Kripke, 1977). In testing of this hypothesis, Varmus and colleagues were able to show untransformed mammary cells that had been delivered to mice by tail-vein injection and were able to accomplish many of the steps required for metastasis. Induction of MYC and mutant Kras oncogenes in these cells after their arrival and establishment in the lung yielded tumor after three to four weeks (Podsypanina et al., 2008). Additionally, another possibility has suggested that the emergence of the metastatic phenotype may not only occur as a late event in metastatic progression but rather may be linked to early oncogenic events that also drive primary tumor formation (Scheel et al., 2007). To further complicate this analysis, work by Hunter et al. (2003) has further suggested that the propensity for a primary tumor to metastasize may even precede primary tumor formation and is in fact related to the patient’s germline genetics. In a sense, these data return us to Paget’s hypothesis by suggesting that specific genetic determinants of the host (i.e. soil) contribute to the success of the metastatic process (i.e. seed). Taken together the work by several groups suggests that the risk for metastatic progression is in part defined by the genetics of the patient, genetic changes that develop early in the process of tumor development, and the subsequent and incremental emergence of cells within the tumor that possess the cellular armamentarium needed for metastasis.
2.2. Cancer stem cell/tumor-initiating cell hypothesis
A re-emerging hypothesis in the field of cancer biology is that cancers emerge from cells with primitive or stem-like features (see recent review (Lobo et al., 2007)). The basis of this hypothesis is that only a very small population of tumor cells is capable of tumor-initiation and self-renewal (stem-like). Although these tumor cells are not discretely identifiable, the population can be enriched using markers commonly found on primitive or stem-like cells (Singh et al., 2004b; Prince et al., 2007; Ricci-Vitiani et al., 2007; Cho et al., 2008). The recent finding of stem cell markers in many adult solid tumors has refueled an interest in this hypothesis that extends beyond pediatric and blood borne cancers where this model was first suggested. Interestingly, cells with stem-like properties include many of the features of the metastatic cell (“seed”). For example, embryonic and adult stem cells are capable of motility, invasion, survival during circulation, extravasation at secondary sites, dormancy and perhaps most importantly an ability to engage and interact with appropriate cellular and non-cellular partners at a secondary location to ensure their continued survival and eventual proliferation (seed–soil) (Ozturk et al., 2004). Since primary tumor-initiating cells may exhibit many of the features of the metastatic phenotype, these cells may be amenable to ‘reprogramming’ through secondary genetic or epigenetic events (acquired or selected). The result of this reprogramming may be selection of an optimal metastatic phenotype. These cells are thought to be crucial to the initiation and dissemination of several cancer types and, with their ability to resist conventional therapies, are likely to play a significant role in the metastatic process (Barnhart and Simon, 2007).
2.3. Stress and metastatic success
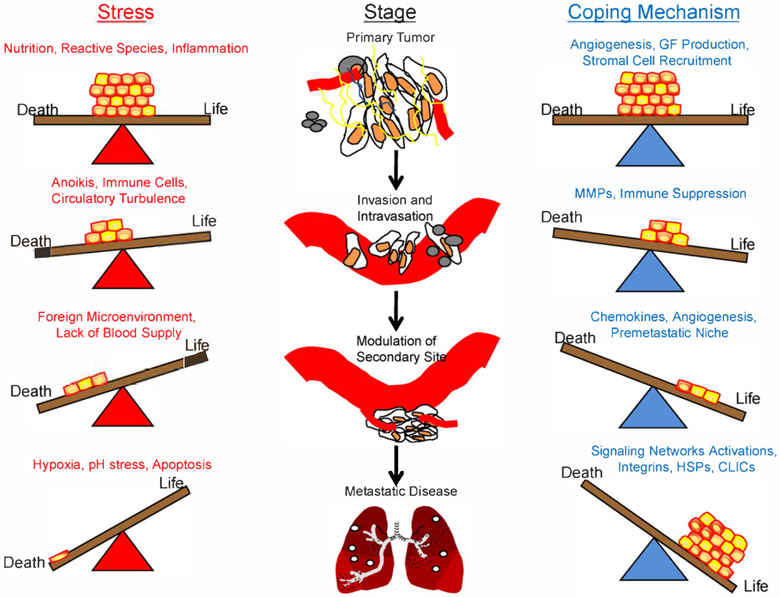
Of the determinants of success in metastasis, the tumor cell’s ability to resist the stresses associated with the multiple steps in the metastatic cascade and survive at distant sites may be paramount (Hedley et al., 2008). Indeed, this resistance of stress may be a feature common to stem-like cells, tumor-initiating cells, and metastatic cells. Stresses such as hypoxia, reactive species (RS), inflammation, nutrient deprivation, and pH can be a challenge to the growth and progression of a cancer and are usually and initially detrimental to the tumor’s survival. However, such stresses may also provide a selective pressure favoring growth of more metastatically ‘fit’ cells (Witz and Levy-Nissenbaum, 2006). These stresses are likely to be felt within the primary tumor, during the process of metastasis, and at the distant secondary metastatic sites (Fig. 1). The following section will summarize some of the stresses listed above that are faced by metastatic cells and the mechanisms that allow for accommodation to these stresses by successful metastatic cells.
Fig. 1.
Tumor cells must resist stress in order to metastasize. Metastasis is thought to be a very inefficient process, in part, due to the number of stresses tumor cells must overcome in order to reach secondary sites and develop into gross metastatic lesions. Throughout each stage, tumor cells are confronted with various stresses, any of which may kill the cell. This results in a fragile balance between life and death for the cell. Only those tumor cells which can successfully manage the stress will survive. Depicted are examples of the various stresses tumor cells face during each stage of the metastatic process and some of the mechanisms the cell may use to deal with those stresses. Note that each stress and coping mechanism listed above are not exclusive to a particular stage of metastasis and likely apply to more than one of the stages. Cancer stem cells, those cells which are thought most able to resist the stresses of the metastatic cascade, are depicted in yellow. GF = growth factor; MMPs = matrix metalloproteinases; HSPs = heat shock proteins; CLICs = chloride intracellular ion channels.
2.3.1. Hypoxia
Low oxygen tension, or hypoxia, frequently occurs during tumor progression and metastasis. Tumor cells, unlike normal cells, are able to survive adverse hypoxic conditions through a diversity of mechanisms (Rong and Vande Woude, 1994; Reynolds et al., 1996; Maulik and Das, 2002; Birchmeier et al., 2003; Semenza, 2003; Xie and Huang, 2003). The transitions between “oxic” states can be additionally challenging for cells to endure. Cells that are able to resist the negative effects of low oxygen conditions and then competitively flourish in hypoxic environments have been shown to be highly dependent on the expression of the transcription factor hypoxia-inducible-factor-1 (HIF-1) (Semenza and Wang, 1992). Indeed, several studies have shown an association between hypoxia, tumor progression, stem-ness and metastasis (Young et al., 1988; Brizel et al., 1996; Hockel et al., 1996; Jang and Hill, 1997; De Jaeger et al., 1998; Rofstad and Danielsen, 1999; Rofstad et al., 2000; De Jaeger et al., 2001). Cancer cell adaptation of the HIF-1 pathway may occur secondary to genetic mutations or epigenetic events (Kapitsinou and Haase, 2008). HIF-1 promotes the expression of several metastasis-associated genes by binding to hypoxia-responsive elements within their respective promoters. Such HIF-1 responsive genes include NOS (McQuillan et al., 1994; Melillo et al., 1995), IGF-II (Kim et al., 1998), CXCR4 (Semenza, 2003), and VEGF (Shweiki et al., 1992; Minchenko et al., 1994). Increased expression of some of these genes (VEGF and CXCR4, for example) can also help to counteract hypoxia by increasing oxygen levels creating a more suitable environment for tumor growth (Xie and Huang, 2003). HIF target genes are also important for stem cell growth and maintenance and have recently been shown to play important roles in cancer (Keith and Simon, 2007). Hypoxia may also be crucial for the creation and/or maintenance of the undifferentiated state of cancer stem cells (Keith and Simon, 2007). Recently, Gustafsson et al. (2005) demonstrated that hypoxia promoted an undifferentiated cell state through Notch signaling. Other genes including hTERT, the catalytic component of human telomerase (Nishi et al., 2004), the multi-drug resistance ABC transporter Bcrp/ABCG2 (Krishnamurthy et al., 2004), and the MET tyrosine kinase receptor (Boccaccio and Comoglio, 2006), are HIF regulated, are expressed in both cancer cells and stem cells, and contribute to the metastatic phenotype of cells.
2.3.2. Reactive species
The transient and vacillating hypoxia of the tumor microenvironment confounds the problem of hypoxia during re-oxygenation through the formation of reactive oxygen or nitrogen species (Xie and Huang, 2003). These reactive species may have contrasting effects on metastasis (Ambs et al., 1997; Xie and Fidler, 1998; Xie and Huang, 2003). In some cancers the formation of RS, i.e. nitric oxide (NO), contributes to the metastatic phenotype while in other cancers, reduced RS are linked with metastasis (Yamamoto et al., 1994; Pipili-Synetos et al., 1995; Iwasaki et al., 1997; Yamamoto et al., 1998). Mechanistically, NO expression is associated with altered expression of many mediators of metastasis, including VEGF-C (Radomski et al., 1991; Franchi et al., 2006; Nakamura et al., 2006; Brideau et al., 2007), MMP9 (Marcet-Palacios et al., 2003), p53 (Cook et al., 2004), and HIF-1 α (Kimura et al., 2000; Sandau et al., 2001). Intratumoral RS may also contribute to genomic instability associated with advanced cancers and may play a trophic role in tumor progression (Ambs et al., 1997; Xie and Fidler, 1998; Radisky et al., 2005; Halliwell, 2007). The observation of reduced RS in metastatic versus primary tumors may represent an adaptive advantage of some metastatic cancers to effectively manage the detoxification of RS (Xie and Huang, 2003).
2.3.3. Inflammation
The link between inflammation and cancer has been recognized for many years now. For example, in, 1986, Dvorak described cancer as “wounds that fail to heal” (Dvorak, 1986). Both inflammatory mediators and cells involved in the inflammatory response react against cancer cells and contribute to tumor progression. As such, macrophages represent an important mediator of the cancer inflammatory environment and have been show to increase the incidence of metastases in both in vitro and in vivo (Gorelik et al., 1982, 1985). Their influence is determined, in large part, by the local cytokine profile. As part of the innate immune system, macrophages can be potent anticancer cells in a GM-CSF rich environment (Gillessen et al., 2003). Conversely, successful cancers appear to create a cytokine environment that is dominated by CSF-1 rather than GM-CSF (Nowicki et al., 1996). In the CSF-1 dominated environment, macrophages contribute to the metastatic phenotype through many paths, including generation of RS, initiation of invasion and angiogenesis (Coussens et al., 2000; Huang et al., 2002; Pollard, 2004; Chen et al., 2005), and contribution to genomic instability (Condeelis and Pollard, 2006). In this way, successful metastatic cells appear to engage their microenvironment and recruit beneficial rather than deleterious populations of inflammatory cells to create a more “cancer hospitable environment.” Beyond the macrophage, other inflammatory cells contribute to the metastatic phenotype of cancers, including lymphocytes, neutrophils (Aeed et al., 1988; Welch et al., 1989; Caruso et al., 2002), mast cells (Brideau et al., 2007), T-regulatory cells (Khazaie and von Boehmer, 2006; Wahl et al., 2006; Teicher, 2007), and platelets (Karpatkin et al., 1988; Borsig, 2008). These cells can negatively regulate the host immune response against cancer. This may be accomplished through production of inflammation-based products such as chemokines, ROS/RNS, cytokines, and growth factors (Reichert et al., 2002; Pawelec, 2004; Yang and Carbone, 2004). TGF-beta, which can be released by macrophages, lymphocytes, and/or platelets inhibits T-cell proliferation through suppression of interleukin (IL)-2 production (Becknell and Caligiuri, 2005; Ma et al., 2006) and blunts natural killer (NK) cell activity by interfering with IFN-gamma production (Rook et al., 1986; Bellone et al., 1995). Matrix metalloproteinases (MMPs) are a family of proteins capable of degrading the extra-cellular matrix. Higher levels of MMPs are often associated with enhanced tumor invasion and metastasis and can often be secreted by several of the stromal cells present in the tumor microenvironment including macrophages and fibroblasts (Stetler-Stevenson et al., 1993; Sternlicht et al., 1999; Boire et al., 2005; Deryugina and Quigley, 2006). The ability of cancer cells to evade parts of the immune response and direct inflammatory cells towards benefit involves a complex set of relationships between the tumor cells and their environment at primary and secondary sites and appears crucial to the initiation and progression of cancer.
2.3.4. Nutritional and pH stress
Deprivation of cellular nutrition and alterations in pH balance are common stresses linked to cancer. The mammalian target of rapamycin (mTOR) is a critical cell signalling mediator involved with sensing the nutritional environment of cancer cells. The mechanisms of sensing and responding to these stresses occur through signaling intermediates such as AKT and mitogen-activated protein kinase. The current dogma suggests that mTOR coordinates signals from the nutritional and stress status of a cell resulting in upregulation and activation of specific proteins to maintain cell homeostasis. For example, tumor cells are highly dependent on the targets of mTOR-mediated translation such as c-myc, VEGFR, hypoxia-inducible factor, and transforming growth factor-beta.
Reduction in the pH is often seen in tumors, and often occurs in conjunction with regions of hypoxia. One of the ways a tumor cell can manage this stress is through increased glucose metabolism, which is frequently observed in cancers. In the 1920s, Warburg (1956) reported the observation of increased aerobic glycolysis in tumor cells. This shift in cellular metabolism has recently been shown to be dependent on the M2 splice isoform of pyruvate kinase (Christofk et al., 2008). Other groups have hypothesized that the Warburg effect is a mechanism for pre-malignant cells to adapt to intermittent hypoxia (Gatenby and Gillies, 2004). GLUT proteins, which function in transporting glucose across the plasma membrane, may also play an important role in the increased glucose metabolism phenotype of tumor cells. Studies have shown that hypoxia-responsive GLUT family members GLUT1 and GLUT3 are frequently overexpressed in tumors (Binder et al., 1997; Younes et al., 1997; Smith, 1999; Medina and Owen, 2002) while GLUT12 is found distinctively in prostate and breast cancer (Chandler et al., 2003; Rogers et al., 2003).
Another family of proteins that may play a role in helping cells cope with similar microenvironmental stresses are the chloride intracellular channels (CLICs). CLIC proteins are intimately involved in maintaining electrogenic gradients across both plasma and organelle membranes and thereby also serve in regulating organelle volume and pH (Jentsch et al., 2002). Beyond managing pH, cytoplasmic CLIC4 is also a direct response gene for c-myc and p53 with consensus binding sites for each in its promoter (Fernandez-Salas et al., 1999, 2002). CLIC4 is required for p53-induced apoptosis (Fernandez-Salas et al., 2002) and knockdown of CLIC4 in tumor cell lines results in inhibited tumor growth (Suh et al., 2005). In response to various forms of stress, CLIC4 will translocate to the nucleus resulting in cell cycle arrest and accelerated apoptosis (Suh et al., 2004). In addition, with advancing malignant progression, CLIC4 expression decreases in tumors while increasing in stromal cells (Suh et al., 2007) such as myofibroblasts (Seemayer et al., 1979; Ronnov-Jessen et al., 1995; Martin et al., 1996; Bhowmick et al., 2004). This linked expression of CLIC4 between the tumor and stromal cells is suggestive of a molecular cross-talk between the tumor and its microenvironment. The mechanisms regulating activation and intracellular transit of CLIC proteins are not well understood, but may be connected to the metastatic phenotype of cancers.
2.3.5. Heat shock proteins
An overarching mechanism by which cancer cells resist stress is through the stabilization and protection of many of the proteins described above. Heat shock proteins (Hsp) including Hsp70 and Hsp90 are molecular chaperones of many of these proteins (referred to as “client” proteins) that in many cases are linked to oncogenic and metastatic cancer phenotypes. For the most part, Hsp–client protein interactions protect these proteins from degradation, and physiologic protection of specific proteins has emerged as a physiologic means to overcome short-term cellular stressors. Several Hsp clients include many of the same proteins that are often mutated or have deregulated expression in cancer. The fusion product of the Bcr and Abl genes, p210Bcr-Abl, is intimately involved in both acute lymphocytic leukemia and chronic myelogenous leukemia and its stability is dependent on its association with Hsp90 (Blagosklonny et al., 2001). Hsp client HER-2 is commonly overexpressed in several cancers and it too is reliant on its association with Hsp90 for stability (Xu et al., 2001). Inhibition of HSP90 can also reduce hypoxia-induced HIF1 α transcription (Hur et al., 2002). Other oncogenic proteins known to be stabilized by Hsps include mutated p53 (Zhang and Burrows, 2004) and the stress-responsive kinase Akt, a key player in cell survival and metastatic spread (Toker and Yoeli-Lerner, 2006). Overexpression of other HSPs is associated with various malignancies and/or poor prognosis in patients. For example, Tomasovic et al. (1984) was able to show that metastatic clones had a higher levels of thermal resistance and also displayed enhanced rates of synthesis of fours HSPs when compared to clones from the primary tumor. Hsp27 is correlated with poor survival in liver cancer (King et al., 2000), gastric carcinoma (Cardones et al., 2003), osteosarcoma (Uozaki et al., 2000), and colorectal cancer (Zhao et al., 2007). Enhanced Hsp70 expression is correlated with outcome in lymph node metastases in squamous cell carcinoma (Kawanishi et al., 1999) and prognosis in bladder (Syrigos et al., 2003) and breast cancer (Thanner et al., 2003). The stress-mediated induction of the ER-chaperone HSP70 family member GPR78 by the unfolded protein response can lead to tumor proliferation (Luo et al., 2006; Dong et al., 2008), chemotherapeutic resistance (Pyrko et al., 2007), survival (Ranganathan et al., 2006; Fu et al., 2007), and metastasis (Fu and Lee, 2006; Zhang et al., 2006). These data additionally link the success of metastatic cells with the ability to effectively and selectively manage the stresses of protein accumulation.
2.4. Characteristics and emergence of the tumor microenvironment (the “soil”)
Upon arrival at a distant site, the new microenvironment (the “soil”) encountered by the metastatic cell is considered to be foreign and/or inhospitable. Survival of metastatic cells at the secondary site is likely a consequence of intrinsic features of the metastatic cell and an ability to effectively engage in a molecular cross-talk with its surroundings and modulate the environment of the secondary site. This engagement can occur by several mechanisms. One such mechanism may be through the use of the cytoskeletal protein ezrin. Ezrin is necessary and/or sufficient for metastasis in several cancers (Khanna et al., 2004; Yu et al., 2004) and links the plasma membrane as well as plasma membrane associated proteins with the actin cytoskeleton. Through this linker function, ezrin is thought to help cells resist stress (unpublished data) and improve metastatic efficiency by better enabling the cell to physically engage its microenvironment and transduce microenvironmental cues in order to respond to stress. Recent studies have suggested that ezrin contributes to metastatic efficiency through inhibition of apoptotic death experienced by most cancer cells upon their arrival at secondary sites (personal communication, C. Khanna).
Similar to ezrin’s role, cell–cell interactions are likely necessary for effective modulation of secondary sites. Integrins, key mediators of cell–cell interactions, often have deregulated expression in tumor cells (Juliano and Varner, 1993; Kurschat and Mauch, 2000). This aberrant expression is thought to provide enhanced proliferative and survival capabilities (Aplin et al., 1999; Hynes, 2002; Nikolopoulos et al., 2004; Naylor et al., 2005; Reddig and Juliano, 2003) in the tumor cell’s new microenvironment. Growth factor receptor–ligand interactions between tumor cells and host stromal cells can often result in an interactive signaling loop between tumor and host cells (Condeelis and Pollard, 2006; Yamaguchi et al., 2006) resulting in induction of angiogenesis (Pollard, 2004) or upregulation of pro-survival pathways (Derynck et al., 2001; Kalluri and Neilson, 2003; Bhowmick et al., 2004; Mueller and Fusenig, 2004; Kalluri and Zeisberg, 2006). Stromal cells such as macrophages and fibroblasts are often involved in such signaling loops (Forsberg et al., 1993; Condeelis and Pollard, 2006; Kalluri and Zeisberg, 2006; Yamaguchi et al., 2006) and can contribute to the invasive behavior of tumor cells through release of chemokines (Negus et al., 1997; Brigati et al., 2002; Coussens and Werb, 2002; Pollard, 2004) or production of matrix-degrading proteases (Stetler-Stevenson et al., 1993; Sternlicht et al., 1999; Giraudo et al., 2004; Pollard, 2004; Boire et al., 2005). Work by Kitadai et al. (2006a, b) suggests that secretion of PDGF by tumor cells may also contribute to metastasis by acting on tumor PDGF-R positive stromal cells, which in turn may secrete growth factors supportive for the tumor. Similar mechanisms are seen in prostate and breast cancer cells that are capable of secreting endothelin-1 (ET-1). ET-1 binds the endothelin A receptor expressed in osteoblasts, resulting in their activation. The activated osteoblast produces growth factors essential for metastatic tumor growth in bone, thereby leading to bone metastases (Yin et al., 2003).
In summary, successful metastatic cells that arrive at secondary sites engage cells in the microenvironment as a means to modulate the secondary site and produce an environment conducive to metastatic cell survival. Recent data now suggest that the development of such an environment may occur in advance of the arrival of metastatic cells themselves via priming by bone marrow derived cells (Kaplan et al., 2005).
2.4.1. Cell–cell/microenvironment interactions
The ability of cells to effectively develop heterotypic interactions with other cells and to non-cellular elements in the microenvironment is pivotal in a metastatic tumor cell’s ability to successfully adapt to its new surroundings. Integrins are critical players in forming these interactions, are known to mediate anchorage-independent growth (Eble and Haier, 2006), angiogenesis (Eble and Haier, 2006), enhanced survival (Guo and Giancotti, 2004), and are thought to act as oncogenes and/or tumor-suppressor genes (Juliano and Varner, 1993; Hulleman and Boonstra, 2001). Briefly, integrins, which are frequently internalized and/or recycled (Caswell and Norman, 2006), consist of non-covalently associated α and β subunits with cytoplasmic, membrane, and extra-cellular domains (Hynes, 2002; Ramsay et al., 2007). Through extra-cellular matrix ligand binding, integrins can transduce signals to the cell and are also capable of responding to intracellular messages (Ramsay et al., 2007). Integrins may also have co-receptor functions that are adhesion-independent. Overexpression of β4 integrin, for example, can act as a signaling substrate for the HGF receptor C-met, resulting in enhanced anchorage-independent growth and tumorigenesis in nude mice (Bertotti et al., 2005, 2006).
Along with integrins, intercellular adhesion molecules (ICAMs), also play a significant role in mediating cellular interactions. ICAMs are members of the immunoglobulin superfamily and intimately involved in mediating cell–cell, cell–ECM, and immune interactions (Dustin et al., 1986; Roland et al., 2007). Because of their ability to influence cell–cell and cell–microenvironment interactions, it comes as no surprise that aberrant expression of these molecules is associated with both cancer development and metastatic progression. A summary of these associations with cancer is provided in Table 1.
Table 1.
Heterotypic interactions between a tumor cell and its microenvironment (both cellular and non-cellular elements) are critical to the cell’s ability to engage its surroundings in order to manage stress. Listed are some of the aberrantly expressed molecules that are thought to facilitate those interactions in specific cancers.
| Molecule | Associated cancer | Reference |
|---|---|---|
| ανβ3 | Melanoma | Nip et al. (1992) |
| α2β1, α3β1 | Gastric carcinoma liver metastases |
Ura et al. (1998) |
| ανβ3 | Bone-residing metastases | Liapis et al. (1996), Byzova et al. (2000) |
| ανβ5 | Colon carcinoma cells liver metastases |
Enns et al. (2005) |
| α6β6 | Colon carcinoma | Bates et al. (2005) |
| ανβ3 | Colon carcinoma | Max et al. (1997) |
| ICAM-1 | Melanoma, metastatic breast and liver carcinoma, gastric carcinoma | Natali et al. (1990, 1997), Sun et al. (1998), Rosette et al. (2005), Tachimori et al. (2005) |
| ICAM-2 | Metastatic gastric carcinoma | Tanaka et al. (2004) |
| ICAM-5 | Head and neck squamous carcinoma | Maruya et al. (2005) |
2.4.2. Tumor stroma
The activated stroma of the tumor microenvironment consists of several components including growth factors, other secreted molecules, and host cells that are all thought to highly influence the behavior of tumor cells (reviewed in Liotta and Kohn (2001)). Of the host cells present in the tumor microenvironment, endothelial cells, macrophages (discussed above) and fibroblasts are established players in the metastatic process (Kalluri and Zeisberg, 2006). The cellular and non-cellular features that make up the tumor stroma are not only necessary for the metastatic behavior of cells but in fact have been shown to be sufficient. An activated “metastatic tumor stroma” is sufficient to convert non-metastatic cells to metastatic. The determinants of the metastatic tumor stroma” is in part generated by macrophages and fibroblast secretion of matrix metalloproteinases, which liberate a cascade of events that contribute to stromal activation and to chemokines (chemotactic proteins) and their associated receptors, which direct and modulate the cellular component of the stroma (Deryugina and Quigley, 2006). Table 2 lists specific features of the “metastatic stroma” and associations made with specific metastatic cancers.
Table 2.
Tumor stroma consists of multiple components including growth factors, chemokines, various host cells, and other secreted molecules. “Activated” stroma is critical to the overall survival of the cancer and in many cases, is thought to enhance the tumor’s malignant capabilities. Listed below are some of the stromal components along with the cancer they have been associated with.
2.4.3. Pre-metastatic niche
A novel hypothesis that extends our understanding of role of the stroma in the formation of metastasis is the pre-metastatic niche hypothesis (Kaplan et al., 2005). Kaplan et al. recently showed that tumor cell secreted factors were able to direct bone marrow derived VEGFR-1 + hematopoietic progenitor cells (HPCs) to future metastatic sites prior to the arrival of metastatic cells. Blocking the formation of the pre-metastatic niche prior to tumor development through the use of antibodies against VEGFR1 and VEGFR2 significantly inhibited metastasis (Kaplan et al., 2005, 2006). The HPCs of the pre-metastatic niche are thought to prime the pre-metastatic site in order to make it more favorable for incoming tumor cells. The migration of HPCs within the bone marrow is influenced by the interaction between the integrin VLA-4 and its ligand fibronectin (Kaplan et al., 2006). Since upregulation of fibronectin occurs within the pre-metastatic niche and VEGFR-1+ HPCs express VLA-4, it is reasonable that the same interaction that influences migration of HPCs in bone marrow may also play a role in the adhesion of HPCs with the niche (Kaplan et al., 2005, 2006). The origins of the pre-metastatic niche in the bone marrow allows extension of a related hypothesis, that cancer cells may in fact transit to the bone marrow prior to their eventual spread to distant secondary sites. Similar to the pre-metastatic niche, the bone marrow microenvironment may provide a permissive environment for tumor cells (Kaplan et al., 2006). Indeed, several cancers metastasize to bone marrow in a CXCR4-dependent manner (Muller et al., 2001; Kaifi et al., 2005). Beyond protection, the bone marrow may in fact contribute to the metastatic phenotype of residing cells (Scadden, 2006). The reciprocal role of tumor cells on the bone marrow stroma is highlighted work from Nicola et al. (2003) who recently demonstrated that bone marrow stroma taken from breast cancer patients is significantly less adhesive towards tumor cells than normal bone marrow stroma. It is reasonable that this may make it easier for tumor cells to leave the niche after becoming ‘educated.’
2.5. Therapy
As discussed above, a better understanding of the molecular cross-talk between tumor cell and tumor microenvironment and how this affects the generally more agrgressive and chemotherapy-resistant biology of metastatic tumors is needed. However, despite new insights, metastasis still remains the overwhelming cause of death in cancer patients and new therapies are therefore needed that target the disease in a different way. An opportunity exists to improve outcomes for cancer patients by using our understanding the tumor cell (seed), the microenvironment (soil), and the molecular cross-talk between seed and soil.
Our understanding of the tumor seed and its stem-like features may predict the failure of treatments that target the tumor cell alone. Cancer cells with stem-like features (CSCs) may be best positioned to effectively resist the insult of many types of cancer therapy. While large fractions of tumor cells are sensitive, CSCs are thought to persist in a quiescent state only to recur at future times (Reya et al., 2001; Al-Hajj et al., 2004; Wicha et al., 2006). Targeting CSCs, or their protective niche may be the basis of more successful therapy (Yang and Wechsler-Reya, 2007). Exemplary of this, CSCs in brain tumors are thought to reside in “vascular niches” (regions rich in blood vessels) (Shen et al., 2004; Ramirez-Castillejo et al., 2006) that are often lined with endothelial cells that secrete stem cell survival and self-renewal factors. Calabrese et al. (2007) was able to show that by targeting these vascular niches with specific inhibitors in tumor-bearing mice, they were able to slow the growth rate of the tumor and significantly decrease the overall number of CSCs while having little effect on proliferation of most of the other tumor cells (Calabrese et al., 2007). Therefore, the addition of therapies that target CSCs in their niches to current treatment regimens may be valuable (Yang and Wechsler-Reya, 2007). An alternative means of targeting CSCs may include the use of differentiation agents. Compounds such as cyclopamine (hedgehog signaling) and imatinib (Wnt/β-catenin pathway) have been used to target pathways that are likely critical to a CSC’s ability to self-renew (Galmozzi et al., 2006; Li et al., 2007). It is tempting to speculate that the success of other differentiation inducers such as retinoic acid may be due to induction of differentiation in CSCs there by eliminating their ability to self-renew (Li et al., 2007). Such therapies may be useful as part of long-term combination therapy strategies that target quiescent cancers with stem-like features.
The role of the tumor microenvironment in promoting tumor development and progression has been highlighted above. Therapies that now seek to modulate the tumor–host microenvironment and its components are feasible and should be considered (Langley and Fidler, 2007). For example, targeting macrophage/tumor cell interactions may be an attractive therapeutic target due to the multifactorial role macrophages play in regulating inflammation, angiogenesis, invasion, and the ECM. In support of this idea, macrophage knock-out mice exhibit a reduced rate of tumor growth and a dramatic decrease in metastases compared to litter-mate controls (Lin et al., 2001), while overexpression of the macrophage growth factor colony-stimulating-factor-1 accelerates the rate of tumor growth and metastasis (Lin et al., 2001). Additionally, a high density of tumor-associated macrophages is associated with a poorer prognosis in a large proportion of published studies (Lin et al., 2002). Targeting non-cellular features of the tumor stroma should also be considered as a treatment for metastasis. For example, induction of NO in the tumor stroma via expression of the NOS II expression machinery may be part of the mechanism associated with IFN-β and IFN-γ therapy, which has been shown to suppress metastasis in pancreatic adenocarcinoma (Wang et al., 2001). The hypoxic and acidic environment that is characteristic of tumor microenvironments may also be exploited to kill tumor cells. Bioreductive drugs, compounds that are only toxic under low oxygen conditions or reduced pH, such as tirapazamine have shown promising results when used in combination with other chemotherapeutic agents in clinical trials (Kovacs et al., 1999; Craighead et al., 2000; Xie and Huang, 2003). Similar strategies to target the hypoxic conditions of the tumor microenvironment may be considered as part of gene therapy using hypoxia-responsive promoters for gene expression (Xie and Huang, 2003). Other physiological conditions unique to the tumor microenvironment may limit current therapeutic approaches and may also be targets of novel treatments. Specifically, P-glycoprotein is a major component of the blood–brain barrier and other pharmacologic sanctuaries that are responsible for the poor penetration of chemotherapeutic agents (Cordon-Cardo et al., 1989). Thus, therapies that seek to modulate this protein may prove useful in the treatment of patients with CNS cancer or CNS metastasis.
Finally, targeting the cross-talk that occurs between the tumor cell and the microenvironment may over-ride the influence of the microenvironment on the metastatic phenotype. Classical targets of this cross-talk include VEGF, FGF, and PDGF. It is likely that the benefit of strategies that target these growth factors extends beyond the process of antiangiogenesis. (Kabbinavar et al., 2005; Sandler et al., 2006).
While much progress has been made to understand the biology of metastasis and metastatic lesions, many questions remain unanswered. The biology is complex with the cell’s fate being heavily influenced by the concepts introduced over 100 years ago in Paget’s seed and soil hypothesis. The phenotype of a metastatic cancer results from the tumor cell, the tumor microenvironment, and the interaction between tumor cell and its microenvironment. The process of metastasis is taxing (stressful). Successful metastatic cells are uniquely able to manage the stresses of metastasis by virtue of intrinsic features of the cell, the tumor microenvironment that is in part activated by the tumor cell, and the ability of the tumor cell to successfully engage and interact with its microenvironment. As our understanding of this complex biology improves, new opportunities to target the tumor (seed), the tumor microenvironment (soil) and their interactions will emerge. It is hoped that these efforts will improve outcomes for patients with metastasis or at high risk for metastasis.
Acknowledgements
The authors would like to thank Drs. Joseph W. Briggs, Sung-Hyeok Hong, Lalage Wakefield, and Glenn Merlino for helpful comments and critical reading of the manuscript.
References
- Aeed PA, Nakajima M, Welch DR. The role of polymorphonuclear leukocytes (PMN) on the growth and metastatic potential of 13762NF mammary adenocarcinoma cells. Int J Cancer 1988;42:748–59. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Becker MW. Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev 2004; 14:43–7. [DOI] [PubMed] [Google Scholar]
- Ambs S, Hussain SP, Harris CC. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J 1997;11:443–8. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Howe AK, Juliano RL. Cell adhesion molecules, signal transduction and cell growth. Curr Opin Cell Biol 1999; 11:737–44. [DOI] [PubMed] [Google Scholar]
- Arteaga CL. EGF receptor mutations in lung cancer: from humans to mice and maybe back to humans. Cancer Cell 2006;9:421–3. [DOI] [PubMed] [Google Scholar]
- Barnhart BC, Simon MC. Metastasis and stem cell pathways. Cancer Metastasis Rev 2007;26:261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates RC, Bellovin DI, Brown C, Maynard E. Wu B. Kawakatsu H. et al. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest 2005;115:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol 2005;86:209–39. [DOI] [PubMed] [Google Scholar]
- Bellone G, Aste-Amezaga M, Trinchieri G, Rodeck U. Regulation of NK cell functions by TGF-beta 1. J Immunol 1995;155:1066–73. [PubMed] [Google Scholar]
- Belotti D, Calcagno C, Garofalo A, Caronia D, Riccardi E, Giavazzi R, et al. Vascular endothelial growth factor stimulates organ-specific host matrix metalloproteinase-9 expression and ovarian cancer invasion. Mol Cancer Res 2008;6:525–34. [DOI] [PubMed] [Google Scholar]
- Bertotti A, Comoglio PM, Trusolino L. Beta4 integrin is a transforming molecule that unleashes Met tyrosine kinase tumorigenesis. Cancer Res 2005;65:10674–9. [DOI] [PubMed] [Google Scholar]
- Bertotti A, Comoglio PM. Trusolino L. Beta4 integrin activates a Shp2-Src signaling pathway that sustains HGF-induced anchorage-independent growth.J Cell Biol 2006;175:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004;432:332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 2006;6:506–20. [DOI] [PubMed] [Google Scholar]
- Binder C, Binder L, Marx D, Schauer A, Hiddemann W. Deregulated simultaneous expression of multiple glucose transporter isoforms in malignant cells and tissues. Anticancer Res 1997;17:4299–304. [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E. Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003;4:915–25. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV, Fojo T, Bhalla KN, Kim JS, Trepel JB, Figg WD, et al. The Hsp90 inhibitor geldanamycin selectively sensitizes Bcr-Abl-expressing leukemia cells to cytotoxic chemotherapy. Leukemia 2001;15:1537–43. [DOI] [PubMed] [Google Scholar]
- Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer 2006;6:637–45. [DOI] [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, Jacques S, Sherifi S. Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 2005;120:303–13. [DOI] [PubMed] [Google Scholar]
- Borsig L The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther 2008;8:1247–55. [DOI] [PubMed] [Google Scholar]
- Brideau G, Makinen MJ, Elamaa H, Tu H, Nilsson G, Alitalo K. et al. Endostatin overexpression inhibits lymphangiogenesis and lymph node metastasis in mice. Cancer Res 2007;67:11528–35. [DOI] [PubMed] [Google Scholar]
- Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates:friends or foes? Clin Exp Metastasis 2002;19:247–58. [DOI] [PubMed] [Google Scholar]
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 1996;56:941–3. [PubMed] [Google Scholar]
- Brown LF, Guidi AJ, Schnitt SJ, Van De Water L, Iruela-Arispe ML, Yeo TK, et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res 1999;5:1041–56. [PubMed] [Google Scholar]
- Brown NS, Bicknell R. Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res 2001. ;3:323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzova TV, Kim W, Midura RJ, Plow EF. Activation of integrin alpha(V)beta(3) regulates cell adhesion and migration to bone sialoprotein. Exp Cell Res 2000;254:299–308. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007;11:69–82. [DOI] [PubMed] [Google Scholar]
- Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(l) integrin. Cancer Res 2003;63:6751–7. [PubMed] [Google Scholar]
- Caruso RA, Bellocco R, Pagano M, Bertoli G, Rigoli L, Inferrera C. Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy, Mod Pathol 2002;15:831–7. [DOI] [PubMed] [Google Scholar]
- Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic 2006;7:14–21. [DOI] [PubMed] [Google Scholar]
- Chandler JD, Williams ED, Slavin JL, Best JD, Rogers S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer 2003;97:2035–42. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, et al. Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol 2005;23:953–64. [DOI] [PubMed] [Google Scholar]
- Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, et al. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells 2008;26:364–71. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008;452:230–3. [DOI] [PubMed] [Google Scholar]
- Ciampolillo A, De Tullio C, Perlino E, Maiorano E. The IGF-I axis in thyroid carcinoma. Curr Pharm Des 2007;13:729–35. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263–6. [DOI] [PubMed] [Google Scholar]
- Cook T, Wang Z, Alber S, Liu K, Watkins SC, Vodovotz Y, et al. Nitric oxide and ionizing radiation synergistically promote apoptosis and growth inhibition of cancer by activating p53. Cancer Res 2004;64:8015–21. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A 1989;86:695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000;103:481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead PS, Pearcey R, Stuart G. A phase I/II evaluation of tirapazamine administered intravenously concurrent with cisplatin and radiotherapy in women with locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2000;48: 791–5. [DOI] [PubMed] [Google Scholar]
- Croker AK, Allan AL. Cancer stem cells: implications for the progression and treatment of metastatic disease. J Cell Mol Med 2008;12:374–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer 2003;107:1–10. [DOI] [PubMed] [Google Scholar]
- De Jaeger K, Kavanagh MC, Hill RP. Relationship of hypoxia to metastatic ability in rodent tumours. Br J Cancer 2001;84:1280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jaeger K, Merlo FM, Kavanagh MC, Fyles AW, Hedley D, Hill RP. Heterogeneity of tumor oxygenation: relationship to tumor necrosis, tumor size, and metastasis. Int J Radiat Oncol Biol Phys 1998;42:717–21. [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 2001;29:117–29. [DOI] [PubMed] [Google Scholar]
- El Deryugina, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 2006;25:9–34. [DOI] [PubMed] [Google Scholar]
- Dickson RB, Deb TB. EGF receptor in breast cancer chemoresistance. Adv Exp Med Biol 2007;608:113–8. [DOI] [PubMed] [Google Scholar]
- Ding Y, Shimada Y, Maeda M, Kawabe A, Kaganoi J, Komoto I, et al. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res 2003;9:3406–12. [PubMed] [Google Scholar]
- Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res 2008;68:498–505. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol 1986;137:245–54. [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315:1650–9. [DOI] [PubMed] [Google Scholar]
- Eble JA, Haier J, Integrins in cancer treatment. Curr Cancer Drug Targets 2006;6:89–105. [DOI] [PubMed] [Google Scholar]
- Enns A, Korb T, Schluter K, Gassmann P, Spiegel HU, Senninger N, et al. Alphavbeta5-integrins mediate early steps of metastasis formation. Eur J Cancer 2005;41:1065–72. [DOI] [PubMed] [Google Scholar]
- Fang S, Jin X, Wang R, Li Y, Guo W, Wang N, et al. Polymorphisms in the MMP1 and MMP3 promoter and non-small cell lung carcinoma in North China. Carcinogenesis 2005;26:481–6. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salas E, Sagar M, Cheng C, Yuspa SH, Weinberg WC. p53 and tumor necrosis factor alpha regulate the expression of a mitochondrial chloride channel protein. J Biol Chem 1999;274:36488–97. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salas E, Suh KS, Speransky W, Bowers WL, Levy JM, Adams T, et al. mtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and participates in the apoptotic response to p53. Mol Cell Biol 2002;22:3610–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor, Endocr Rev 1997;18:4–25. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science 1977;197:893–5. [DOI] [PubMed] [Google Scholar]
- Forsberg K, Valyi-Nagy I, Heldin CH, Herlyn M, Westermark B. Platelet-derived growth factor (PDGF) in oncogenesis: development of a vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF-BB. Proc Natl Acad Sci U S A 1993;90:393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi A, Massi D, Santucci M, Masini E, Degl’lnnocenti DR, Magnelli L, et al. Inducible nitric oxide synthase activity correlates with lymphangiogenesis and vascular endothelial growth factor-C expression in head and neck squamous cell carcinoma. J Pathol 2006;208:439–45. [DOI] [PubMed] [Google Scholar]
- Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer BiolTher 2006;5:741–4. [DOI] [PubMed] [Google Scholar]
- Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res 2007;67:3734–40. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell 1998;94:715–25. [DOI] [PubMed] [Google Scholar]
- Galmozzi E, Facchetti F, La Porta CA. Cancer stem cells and therapeutic perspectives. Curr Med Chem 2006;13:603–7. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4:891–9. [DOI] [PubMed] [Google Scholar]
- Gillessen S, Naumov YN, Nieuwenhuis EE, Exley MA, Lee FS, Mach N, et al. CD1d-restricted T cells regulate dendritic cell function and antitumor immunity in a granulocyte-macrophage colony-stimulating factor-dependent fashion. Proc Natl Acad Sci U S A 2003;100:8874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 2004;114:623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik E, Wiltrout RH, Brunda MJ, Holden HT, Herberman RB. Augmentation of metastasis formation by thioglycollate-elicited macrophages. Int J Cancer 1982;29:575–81. [DOI] [PubMed] [Google Scholar]
- Gorelik E, Wiltrout RH, Copeland D, Herberman RB. Modulation of formation of tumor metastases by peritoneal macrophages elicited by various agents. Cancer Immunol Immunother 1985;19:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CB, Wang S, Deng C, Zhang DL, Wang FL, Jin XQ. Relationship between matrix metalloproteinase 2 and lung cancer progression. Mol Diagn Ther 2007;11:183–92. [DOI] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 2004;5:816–26. [DOI] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 2005;9:617–28. [DOI] [PubMed] [Google Scholar]
- Halliwell B Oxidative stress and cancer: have we moved forward? Biochem J 2007;401:1–11. [DOI] [PubMed] [Google Scholar]
- Hedley BD, Welch DR, Allan AL, Al-Katib W, Dales DW, Postenka CO, et al. Downregulation of osteopontin contributes to metastasis suppression by breast cancer metastasis suppressor 1. Int J Cancer 2008;123:526–34. [DOI] [PubMed] [Google Scholar]
- Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 1996;56:4509–15. [PubMed] [Google Scholar]
- Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice.J Natl Cancer Inst 2002;94:1134–42. [DOI] [PubMed] [Google Scholar]
- Hulleman E, Boonstra J. Regulation of G1 phase progression by growth factors and the extracellular matrix. Cell Mol Life Sci 2001. ;58:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K, Welch DR, Liu ET. Genetic background is an important determinant of metastatic potential. Nat Genet 2003;34:23–4 [author reply 25]. [DOI] [PubMed] [Google Scholar]
- Hur E, Kim HH, Choi SM, Kim JH, Yim S, Kwon HJ, et al. Reduction of hypoxia-induced transcription through the repression of hypoxia-inducible factor-1alpha/aryl hydrocarbon receptor nuclear translocator DNA binding by the 90-kDa heat-shock protein inhibitor radicicol. Mol Pharmacol 2002;62:975–82. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110: 673–87. [DOI] [PubMed] [Google Scholar]
- Ingram JL, Bonner JC. EGF and PDGF receptor tyrosine kinases as therapeutic targets for chronic lung diseases. Curr Mol Med 2006;6:409–21. [DOI] [PubMed] [Google Scholar]
- Ishida T, Ishii T, Inagaki A, Yano H, Kusumoto S, Ri M, et al. The CCR4 as a novel-specific molecular target for immunotherapy in Hodgkin lymphoma. Leukemia 2006;20:2162–8. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Higashiyama M, Kuriyama K, Sasaki A, Mukai M, Shinkai K, et al. NG-nitro-l-arginine methyl ester inhibits bone metastasis after modified intracardiac injection of human breast cancer cells in a nude mouse model. Jpn J Cancer Res 1997;88:861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang A, Hill RP. An examination of the effects of hypoxia, acidosis, and glucose starvation on the expression of metastasis-associated genes in murine tumor cells. Clin Exp Metastasis 1997;15:469–83. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev 2002;82:503–68. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Varner JA. Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol 1993;5:812–8. [DOI] [PubMed] [Google Scholar]
- Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 2005;23:3697–705. [DOI] [PubMed] [Google Scholar]
- Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, et al. Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J Natl Cancer Inst 2005;97:1840–7. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003;112:1776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006;6:392–401. [DOI] [PubMed] [Google Scholar]
- Kapitsinou PP, Haase VH. The VHL tumor suppressor and HIF: insights from genetic studies in mice. Cell Death Differ 2008;15:650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev 2006;25:521–9. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005;438:820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest 1988;81:1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi K, Shiozaki H, Doki Y, Sakita I, Inoue M, Yano M, et al. Prognostic significance of heat shock proteins 27 and 70 in patients with squamous cell carcinoma of the esophagus. Cancer 1999;85:1649–57. [DOI] [PubMed] [Google Scholar]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007;129:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin.J Clin Invest 2008;118:1367–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna C, Wan X, Bose S, Cassaday R, Olomu 0, Mendoza A, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med 2004;10:182–6. [DOI] [PubMed] [Google Scholar]
- Khazaie K, von Boehmer H. The impact of CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol 2006; 16:124–36. [DOI] [PubMed] [Google Scholar]
- Kim KW, Bae SK, Lee OH, Bae MH, Lee MJ, Park BC. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res 1998;58:348–51. [PubMed] [Google Scholar]
- Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D’Acquisto F, et al. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood 2000;95:189–97. [PubMed] [Google Scholar]
- King KL, Li AF, Chau GY, Chi CW, Wu CW, Huang CL, et al. Prognostic significance of heat shock protein-27 expression in hepatocellular carcinoma and its relation to histologic grading and survival. Cancer 2000;88:2464–70. [DOI] [PubMed] [Google Scholar]
- Kitadai Y, Sasaki T, Kuwai T, Nakamura T, Bucana CD, Fidler IJ. Targeting the expression of platelet-derived growth factor receptor by reactive stroma inhibits growth and metastasis of human colon carcinoma. Am J Pathol 2006a;169:2054–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitadai Y, Sasaki T, Kuwai T, Nakamura T, Bucana CD, Hamilton SR, et al. Expression of activated platelet-derived growth factor receptor in stromal cells of human colon carcinomas is associated with metastatic potential. Int J Cancer 2006b;119:2567–74. [DOI] [PubMed] [Google Scholar]
- Knowles HJ, Harris AL. Hypoxia and oxidative stress in breast cancer. Hypoxia and tumourigenesis. Breast Cancer Res 2001. ;3:318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs MS, Hocking DJ, Evans JW, Siim BG, Wouters BG, Brown JM. Cisplatin antitumour potentiation by tirapazamine results from a hypoxia-dependent cellular sensitization to cisplatin. Br J Cancer 1999;80:1245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S. Mercer KE, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem 2004;279:24218–25. [DOI] [PubMed] [Google Scholar]
- Kurschat P, Mauch C. Mechanisms of metastasis. Clin Exp Dermatol 2000;25:482–9. [DOI] [PubMed] [Google Scholar]
- Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta 2008;1785:232–65. [DOI] [PubMed] [Google Scholar]
- Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev 2007;28:297–321. [DOI] [PubMed] [Google Scholar]
- LeMaistre CF, Meneghetti C, Howes L, Osborne CK. Targeting the EGF receptor in breast cancer treatment. Breast Cancer Res Treat 1994;32:97–103. [DOI] [PubMed] [Google Scholar]
- Letsch A. Keilholz U. Schadendorf D, Assfalg G. Asemissen AM, Thiel E, et al. Functional CCR9 expression is associated with small intestinal metastasis. J Invest Dermatol 2004;122:685–90. [DOI] [PubMed] [Google Scholar]
- Levy L, Hill CS. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 2006;17:41–58. [DOI] [PubMed] [Google Scholar]
- Li F, Tiede B, Massague J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res 2007;17:3–14. [DOI] [PubMed] [Google Scholar]
- Liapis H, Flath A, Kitazawa S. Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol 1996;5:127–35. [DOI] [PubMed] [Google Scholar]
- Lievre A, Milet J, Carayol J, Le Corre D, Milan C, Pariente A, et al. Genetic polymorphisms of MMP1, MMP3 and MMP7 gene promoter and risk of colorectal adenoma. BMC Cancer 2006;6:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia 2002;7:147–62. [DOI] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001; 193: 727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature 2001;411:375–9. [DOI] [PubMed] [Google Scholar]
- Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol 2007;23:675–99. [DOI] [PubMed] [Google Scholar]
- Luker KE, Luker GD. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett 2006;238:30–41. [DOI] [PubMed] [Google Scholar]
- Luo S Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol 2006;26:5688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A, Koka R, Burkett P. Diverse functions of IL-2, 1L-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol 2006;24:657–79. [DOI] [PubMed] [Google Scholar]
- Maihle NJ, Baron AT, Barrette BA, Boardman CH, Christensen TA, Cora EM, et al. EGF/ErbB receptor family in ovarian cancer. Cancer Treat Res 2002;107:247–58. [DOI] [PubMed] [Google Scholar]
- Marcet-Palacios M, Graham K, Cass C, Befus AD, Mayers I, Radomski MW. Nitric oxide and cyclic GMP increase the expression of matrix metalloproteinase-9 in vascular smooth muscle. J Pharmacol Exp Ther 2003;307:429–36. [DOI] [PubMed] [Google Scholar]
- Martin M, Pujuguet P, Martin F. Role of stromal myofibroblasts infiltrating colon cancer in tumor invasion. Pathol Res Pract 1996;192:712–7. [DOI] [PubMed] [Google Scholar]
- Martin MD, Carter KJ, Jean-Philippe SR, Chang M, Mobashery S, Thiolloy S, et al. Effect of ablation or inhibition of stromal matrix metalloproteinase-9 on lung metastasis in a breast cancer model is dependent on genetic background. Cancer Res 2008;68:6251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruya SI, Myers JN, Weber RS, Rosenthal DI, Lotan R, El-Naggar AK. ICAM-5 (telencephalin) gene expression in head and neck squamous carcinoma tumorigenesis and perineural invasion! Oral Oncol 2005;41:580–8. [DOI] [PubMed] [Google Scholar]
- Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res 2002;62:2937–41. [PubMed] [Google Scholar]
- Maulik N, Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med 2002;33:1047–60. [DOI] [PubMed] [Google Scholar]
- Max R, Gerritsen RR, Nooijen PT, Goodman SL, Sutter A, Keilholz U, et al. Immunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomas. Int J Cancer 1997;71:320–4. [DOI] [PubMed] [Google Scholar]
- McQuillan LP, Leung GK, Marsden PA, Kostyk SK, Kourembanas S. Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am J Physiol 1994;267:H1921–7. [DOI] [PubMed] [Google Scholar]
- Medina RA, Owen Gl. Glucose transporters: expression, regulation and cancer. Biol Res 2002;35:9–26. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer 2006;6:449–58. [DOI] [PubMed] [Google Scholar]
- Melillo G. Musso T. Sica A, Taylor LS, Cox GW, Varesio L. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med 1995;182:1683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes O Kim HT. Stoica G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis 2005;22:237–46. [DOI] [PubMed] [Google Scholar]
- Minchenko A, Salceda S, Bauer T, Caro J. Hypoxia regulatory elements of the human vascular endothelial growth factor gene. Cell Mol Biol Res 1994;40:35–9. [PubMed] [Google Scholar]
- Monti S, Proietti-Pannunzi L, Sciarra A, Lolli F, Falasca P, Poggi M, et al. The IGF axis in prostate cancer. Curr Pharm Des 2007;13:719–27. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes – bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2004;4:839–49. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410:50–6. [DOI] [PubMed] [Google Scholar]
- Murakami T, Cardones AR, Finkelstein SE, Restifo NP, Klaunberg BA, Nestle FO, et al. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. J Exp Med 2003;198:1337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res 2002;62:7328–34. [PubMed] [Google Scholar]
- Nakamura Y, Yasuoka H. Tsujimoto M, Yoshidome K, Nakahara M, Nakao K, et al. Nitric oxide in breast cancer: induction of vascular endothelial growth factor-C and correlation with metastasis and poor prognosis. Clin Cancer Res 2006; 12:1201–7. [DOI] [PubMed] [Google Scholar]
- Natali P, Nicotra MR, Cavaliere R, Bigotti A, Romano G, Temponi M, et al. Differential expression of intercellular adhesion molecule 1 in primary and metastatic melanoma lesions. Cancer Res 1990;50:1271–8. [PubMed] [Google Scholar]
- Natali PG, Hamby CV, Felding-Habermann B, Liang B, Nicotra MR, Di Filippo F, et al. Clinical significance of alpha(v)beta3 integrin and intercellular adhesion molecule-1 expression in cutaneous malignant melanoma lesions. Cancer Res 1997;57:1554–60. [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of betal integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol 2005;171:717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus RP, Stamp GW, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol 1997;150:1723–34. [PMC free article] [PubMed] [Google Scholar]
- Nicola MH, Bizon R, Machado JJ, Sollero T, Rodarte RS, Nobre JS, et al. Breast cancer micrometastases: different interactions of carcinoma cells with normal and cancer patients’ bone marrow stromata. Clin Exp Metastasis 2003;20:471–9. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell 2004;6:471–83. [DOI] [PubMed] [Google Scholar]
- Nip J, Shibata H, Loskutoff DJ, Cheresh DA, Brodt P. Human melanoma cells derived from lymphatic metastases use integrin alpha v beta 3 to adhere to lymph node vitronectin. J Clin Invest 1992;90:1406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi H, Nakada T, Kyo S, Inoue M, Shay JW, Isaka K. Hypoxia-inducible factor 1 mediates upregulation of telomerase (hTERT). Mol Cell Biol 2004;24:6076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notohamiprodjo M, Segerer S, Huss R, Hildebrandt B, Soler D, Djafarzadeh R, et al. CCR10 is expressed in cutaneous T-cell lymphoma. Int J Cancer 2005;115: 641–7. [DOI] [PubMed] [Google Scholar]
- Nowicki A Szenajch J, Ostrowska G, Wojtowicz A, Wojtowicz K, Kruszewski AA, et al. Impaired tumor growth in colony-stimulating factor 1 (CSF-1)-deficient, macrophage-deficient op/op mouse: evidence for a role of CSF-1-dependent macrophages in formation of tumor stroma. Int J Cancer 1996;65:112–9. [DOI] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 1999;59:5002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk MA, Guven GS, Haznedaroglu IC. How hematopoietic stem cells know and act in cardiac microenvironment for stem cell plasticity? Impact of local renin-angiotensin systems. Med Hypotheses 2004;63:866–74. [DOI] [PubMed] [Google Scholar]
- Paule B Potential therapeutic implications of EGF R/PDGF R signalling pathways in bone metastases of prostate cancer. Prog Urol 2005;15:616–20. [PubMed] [Google Scholar]
- Pawelec G Tumour escape: antitumour effectors too much of a good thing? Cancer Immunol Immunother 2004;53:262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipili-Synetos E, Papageorgiou A, Sakkoula E, Sotiropoulou G, Fotsis T, Karakiulakis G, et al. Inhibition of angiogenesis, tumour growth and metastasis by the NO-releasing vasodilators, isosorbide mononitrate and dinitrate. Br J Pharmacol 1995;116:1829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K, Du YC, Jechlinger M, Beverly LJ, Hambardzumyan D, Varmus H. Seeding and propagation of untransformed mouse mammary cells in the lung. Science 2008;321:1841–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004;4:71–8. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A 2007;104:973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res 2007;67:9809–16. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE. et al. Rac1 b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 2005;436:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski MW, Jenkins DC, Holmes L, Moncada S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res 1991. ;51:6073–8. [PubMed] [Google Scholar]
- Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci 2006;9:331–9. [DOI] [PubMed] [Google Scholar]
- Ramsay AG, Marshall JF, Hart IR. Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev 2007;26:567–78. [DOI] [PubMed] [Google Scholar]
- Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res 2006;66:1702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev 2005;24:425–39. [DOI] [PubMed] [Google Scholar]
- Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res 2002;8:3137–45. [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–11. [DOI] [PubMed] [Google Scholar]
- Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res 1996;56:5754–7. [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445:111–5. [DOI] [PubMed] [Google Scholar]
- Rofstad EK, Danielsen T. Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer 1999;80:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofstad EK, Sundfor K, Lyng H, Trope CG. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastasis. Br J Cancer 2000;83:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S, Docherty SE, Slavin JL, Henderson MA, Best JD. Differential expression of GLUT12 in breast cancer and normal breast tissue. Cancer Lett 2003;193:225–33. [DOI] [PubMed] [Google Scholar]
- Roland CL, Harken AH, Sarr MG, Barnett CC Jr. ICAM-1 expression determines malignant potential of cancer. Surgery 2007;141:705–7. [DOI] [PubMed] [Google Scholar]
- Rong S, Vande Woude GF. Autocrine mechanism for met proto-oncogene tumorigenicity. Cold Spring Harb Symp Quant Biol 1994;59:629–36. [DOI] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest 1995;95:859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook AH, Kehrl JH, Wakefield LM, Roberts AB, Spom MB, Burlington DB, et al. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol 1986;136:3916–20. [PubMed] [Google Scholar]
- Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J, et al. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis 2005;26:943–50. [DOI] [PubMed] [Google Scholar]
- Roussidis AE, Theocharis AD, Tzanakakis GN, Karamanos NK. The importance of c-Kit and PDGF receptors as potential targets for molecular therapy in breast cancer. Curr Med Chem 2007;14:735–43. [DOI] [PubMed] [Google Scholar]
- Saldivar JS, Chen Z, Sommer S. EGF receptor testing for non-small cell lung carcinomas Curr Protoc Hum Genet 2006; Chapter 10,Unit 10 9. [DOI] [PubMed] [Google Scholar]
- Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 2007;28:20–47. [DOI] [PubMed] [Google Scholar]
- Sandau KB, Zhou J, Kietzmann T, Brune B. Regulation of the hypoxia-inducible factor 1alpha by the inflammatory mediators nitric oxide and tumor necrosis factor-alpha in contrast to desferroxamine and phenylarsine oxide. J Biol Chem 2001;276:39805–11. [DOI] [PubMed] [Google Scholar]
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542–50. [DOI] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature 2006;441:1075–9. [DOI] [PubMed] [Google Scholar]
- Scheel C, Onder T, Karnoub A, Weinberg RA. Adaptation versus selection: the origins of metastatic behavior. Cancer Res 2007;67:11476–9 [discussion 11479–80]. [DOI] [PubMed] [Google Scholar]
- Seemayer TA, Lagace R, Schurch W, Tremblay G. Myofibroblasts in the stroma of invasive and metastatic carcinoma: a possible host response to neoplasia. Am J Surg Pathol 1979;3:525–33. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721–32. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 1992;12:5447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004;304:1338–40. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992;359:843–5. [DOI] [PubMed] [Google Scholar]
- Singh S, Singh UP, Stiles JK, Grizzle WE, Lillard JW Jr. Expression and functional role of CCR9 in prostate cancer cell migration and invasion. Clin Cancer Res 2004a. ;10:8743–50. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature 2004b;432:396–401. [DOI] [PubMed] [Google Scholar]
- Sisci D, Surmacz E. Crosstalk between IGF signaling and steroid hormone receptors in breast cancer. Curr Pharm Des 2007;13:705–17. [DOI] [PubMed] [Google Scholar]
- Smith TA. Facilitative glucose transporter expression in human cancer tissue. Br J Biomed Sci 1999;56:285–92. [PubMed] [Google Scholar]
- Smits A, Funa K. Platelet-derived growth factor (PDGF) in primary brain tumours of neuroglial origin. Histol Histopathol 1998;13:511–20. [DOI] [PubMed] [Google Scholar]
- Sokolowska-Wojdylo M, Wenzel J, Gaffal E, Lenz J, Speuser P, Erdmann S, et al. Circulating clonal CLA(+) and CD4(+) T cells in Sezary syndrome express the skin-homing chemokine receptors CCR4 and CCR10 as well as the lymph node-homing chemokine receptor CCR7. Br J Dermatol 2005;152:258–64. [DOI] [PubMed] [Google Scholar]
- Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol 2008;5:206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999;98:137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 1993;9:541–73. [DOI] [PubMed] [Google Scholar]
- Suh KS, Crutchley JM, Koochek A, Ryscavage A, Bhat K, Tanaka T, et al. Reciprocal modifications of CLIC4 in tumor epithelium and stroma mark malignant progression of multiple human cancers. Clin Cancer Res 2007;13:121–31. [DOI] [PubMed] [Google Scholar]
- Suh KS, Mutoh M, Gerdes M, Crutchley JM, Mutoh T, Edwards LE, et al. Antisense suppression of the chloride intracellular channel family induces apoptosis, enhances tumor necrosis factor {alpha}-induced apoptosis, and inhibits tumor growth. Cancer Res 2005;65:562–71. [PubMed] [Google Scholar]
- Suh KS, Mutoh M, Nagashima K, Fernandez-Salas E, Edwards LE, Hayes DD, et al. The organellular chloride channel protein CLIC4/mtCLIC translocates to the nucleus in response to cellular stress and accelerates apoptosis. J Biol Chem 2004;279:4632–41. [DOI] [PubMed] [Google Scholar]
- Sun JJ, Zhou XD, Zhou G, Liu YK. Expression of intercellular adhesive molecule-1 in liver cancer tissues andliver cancer metastasis. World J Gastroenterol 1998;4:202–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrigos KN, Harrington KJ, Karayiannakis AJ, Sekara E, Chatziyianni E, Syrigou EI, et al. Clinical significance of heat shock protein-70 expression in bladder cancer. Urology 2003;61:677–80. [DOI] [PubMed] [Google Scholar]
- Tachimori A, Yamada N, Sakate Y, Yashiro M, Maeda K, Ohira M, et al. Up regulation of ICAM-1 gene expression inhibits tumour growth and liver metastasis in colorectal carcinoma. Eur J Cancer 2005;41:1802–10. [DOI] [PubMed] [Google Scholar]
- Takanami I Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis. Int J Cancer 2003; 105:186–9. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Fujimoto A, Tanaka M, Yamano T, Hsueh E, Hoon DS. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin Cancer Res 2004;10:2351–8. [DOI] [PubMed] [Google Scholar]
- Talmadge JE. Clonal selection of metastasis within the life history of a tumor. Cancer Res 2007;67:11471–5. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yashiro M, Sunami T, Sakate Y, Kosaka K, Hirakawa K. ICAM-2 gene therapy for peritoneal dissemination of scirrhous gastric carcinoma. Clin Cancer Res 2004;10:4885–92. [DOI] [PubMed] [Google Scholar]
- Teicher BA. Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res 2007;13:6247–51. [DOI] [PubMed] [Google Scholar]
- Thanner F, Sutterlin MW, Kapp M, Rieger L, Kristen P, Dietl J, et al. Heat-shock protein 70 as a prognostic marker in node-negative breast cancer. Anticancer Res 2003;23:1057–62. [PubMed] [Google Scholar]
- Till KJ, Lin K, Zuzel M, Cawley JC. The chemokine receptor CCR7 and alpha4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood 2002;99:2977–84. [DOI] [PubMed] [Google Scholar]
- Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol 2001;11:97–104. [DOI] [PubMed] [Google Scholar]
- Toker A, Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res 2006;66:3963–6. [DOI] [PubMed] [Google Scholar]
- Tomasovic SP, Rosenblatt PL, Johnston DA, Tang K, Lee PS. Heterogeneity in induced heat resistance and its relation to synthesis of stress proteins in rat tumor cell clones. Cancer Res 1984;44:5850–6. [PubMed] [Google Scholar]
- Uozaki H, Ishida T, Kakiuchi C, Horiuchi H, Gotoh T, Iijima T, et al. Expression of heat shock proteins in osteosarcoma and its relationship to prognosis, Pathol Res Pract 2000;196:665–73. [DOI] [PubMed] [Google Scholar]
- Ura H, Denno R, Hirata K, Yamaguchi K, Yasoshima T. Separate functions of alpha2beta1 and alpha3beta1 integrins in the metastatic process of human gastric carcinoma. Surg Today 1998;28:1001–6. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells—old concepts, new insights. Cell Death Differ 2008;15:947–58. [DOI] [PubMed] [Google Scholar]
- Wahl SM, Wen J, Moutsopoulos N. TGF-beta: a mobile purveyor of immune privilege. Immunol Rev 2006;213:213–27. [DOI] [PubMed] [Google Scholar]
- Wang B, Xiong Q, Shi Q, Le X, Abbruzzese JL, Xie K. Intact nitric oxide synthase II gene is required for interferon-beta-mediated suppression of growth and metastasis of pancreatic adenocarcinoma. Cancer Res 2001;61:71–5. [PubMed] [Google Scholar]
- Warburg O Origin of cancer cells. Oncologia 1956;9:75–83. [PubMed] [Google Scholar]
- Weiss L, Orr FW, Honn KV. Interactions of cancer cells with the microvasculature during metastasis, FASEB J 1988;2:12–21. [DOI] [PubMed] [Google Scholar]
- Welch DR, Schissel DJ, Howrey RP, Aeed PA. Tumor-elicited polymorphonuclear cells, in contrast to “normal” circulating polymorphonuclear cells, stimulate invasive and metastatic potentials of rat mammary adenocarcinoma cells. Proc Natl Acad Sci U S A 1989;86:5859–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res 2006;66:1883–90 [discussion 1895–6]. [DOI] [PubMed] [Google Scholar]
- Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst 2001;93:1638–43. [DOI] [PubMed] [Google Scholar]
- Witz IP, Levy-Nissenbaum O. The tumor microenvironment in the post-PAGET era. Cancer Lett 2006;242:1–10. [DOI] [PubMed] [Google Scholar]
- Wu LM, Zhang F, Xie HY, Xu X, Chen QX, Yin SY. et al. MMP2 promoter polymorphism (C-1306T)and risk of recurrence in patients with hepatocellular carcinoma after transplantation. Clin Genet 2008;73:273–8. [DOI] [PubMed] [Google Scholar]
- Xie K, Fidler IJ. Therapy of cancer metastasis by activation of the inducible nitric oxide synthase. Cancer Metastasis Rev 1998;17:55–75. [DOI] [PubMed] [Google Scholar]
- Xie K, Huang S, Regulation of cancer metastasis by stress pathways. Clin Exp Metastasis 2003;20:31–43. [DOI] [PubMed] [Google Scholar]
- Xu W, Mimnaugh E, Rosser MF, Nicchitta C, Marcu M, Yarden Y, et al. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem 2001;276:3702–8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Pixley F, Condeelis J. Invadopodia and podosomes in tumor invasion. Eur J Cell Biol 2006;85:213–8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Terada N, Nishizawa Y, Tanaka H, Akedo H, Seiyama A, et al. Effects of NG-nitro-l-arginine and/or l-arginine on experimental pulmonary metastasis in mice. Cancer Lett 1994;87:115–20. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Terada N, Seiyama A, Nishizawa Y, Akedo H, Kosaka H. Increase in experimental pulmonary metastasis in mice by l-arginine under inhibition of nitric oxide production by NG-nitro-l-arginine methyl ester. Int J Cancer 1998;75:140–4. [DOI] [PubMed] [Google Scholar]
- Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res 2004;92:13–27. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Wechsler-Reya RJ. Hit’em where they live: targeting the cancer stem cell niche. Cancer Cell 2007;11:3–5. [DOI] [PubMed] [Google Scholar]
- Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL. et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A 2003;100:10954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Immunohistochemical detection of Glut3 in human tumors and normal tissues. Anticancer Res 1997;17:2747–50. [PubMed] [Google Scholar]
- Young SD, Marshall RS, Hill RP. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A 1988;85:9533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med 2004; 10:175–81. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Strutz F, Muller GA. Role of fibroblast activation in inducing interstitial fibrosis. J Nephrol 2000;13(Suppl. 3):S111–120. [PubMed] [Google Scholar]
- Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med 2004;82:488–99. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jiang Y, Jia Z, Li Q, Gong W, Wang L, et al. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis 2006;23:401–10. [DOI] [PubMed] [Google Scholar]
- Zhao L, Liu L, Wang S, Zhang YF, Yu L, Ding YQ. Differential proteomic analysis of human colorectal carcinoma cell lines metastasis-associated proteins. J Cancer Res Clin Oncol 2007;133:771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]