Abstract
Protein/nucleic acid deglycase DJ-1 (DJ-1) is a 20-kDa conserved protein, which belongs to the DJ-1/ThiJ/Pfp I protein superfamily. Immunohistochemistry was performed to investigate the expression of DJ-1 in a colorectal cancer (CRC) tissue microarray containing tumor and corresponding adjacent normal tissues. In the present study, DJ-1 expression was significantly upregulated in CRC cells and tissues, compared with that in normal colon cells and adjacent normal tissues, respectively. In addition, patients with high DJ-1 expression levels had a worse overall survival (OS) compared with patients with low expression levels. Multivariate Cox regression analysis revealed that high DJ-1 expression levels was an independent prognostic factor for patients with CRC. Moreover, DJ-1 was able to regulate the PI3K/Akt/p27/cyclin E and PI3K/Akt/mTOR signaling pathways to promote CRC cell growth and metastasis in vitro and in vivo. In addition, DJ-1 regulated the NF-κB/Snail signaling pathway to induce CRC cell epithelial-mesenchymal transition to promote migration and invasion. Notably, patients receiving LFP treatment (oxaliplatin, 5-FU and tetrahydrofolate) had an increased OS compared with patients who underwent only surgery and low DJ-1 expression levels. The findings from the present study suggest that DJ-1 may serve as a promising prognostic marker and predicts chemotherapy efficacy in patients with CRC.
Keywords: DJ-1, colorectal cancer, prognosis, marker, chemotherapy
Introduction
Colorectal cancer (CRC) is the second most commonly diagnosed gastrointestinal malignant neoplasm, and one of the leading causes of cancer-associated mortality all over the world in 2015 (1). There were ~1.4 million new cases and 0.7 million deaths worldwide in 2012 (2). The recurrence rate is higher than 11 and 40–50% in postoperative patients with stage II and III CRC, respectively (3). The high recurrence rate of CRC is a major contributor to poor prognosis, and poor prognosis in patients with CRC is associated with tumor invasion and metastasis (4,5). The development of metastasis is complex, and includes proliferation, angiogenesis, invasion, detachment, migration, adhesion and extravasation into target organs (4). Research investigating the mechanism into the origin and development of CRC has identified a high number of biomarkers, as well as identifying signaling pathways that are vital for tumorigenesis and progression of CRC (5). However, only a few of the identified biomarkers have clinicopathological significance in CRC. Therefore, it is important to identify additional master genes that are associated with the progression and metastasis of CRC, which may provide more reliable molecular targets for therapy and improve the prognosis of CRC patients.
Protein/nucleic acid deglycase DJ-1 (DJ-1) is a 20-kDa conserved protein, which belongs to the DJ-1/ThiJ/Pfp I protein superfamily. DJ-1 is widely expressed in various tissues, and previous studies have shown that DJ-1 is associated with early-onset Parkinson's disease (6). Subsequent research has found that DJ-1 is associated with numerous types of cancer, such as lung and pancreatic cancer (7–9). DJ-1 promotes the active efflux of drugs and enhances the anti-apoptotic ability of multidrug resistant gastric cancer cells by upregulating P-gp and Bcl-2 (10). As an oncogene, DJ-1 was found to promote tumor cell migration and invasion through the PI3K/Akt/mTOR and SRC/ERK/uPA signaling pathways in pancreatic cancer (11,12). DJ-1 is suggested to promote the survival of human CRC cells through the PTEN-AKT, PLAGL2/Wnt/BMP4 or PI3K-AKT pathways (13–15). In addition, a number of studies have investigated treatment and diagnosis in clinical cancer. Overexpression of DJ-1 has been reported in numerous types of cancer, including breast cancer (9), melanoma (16), pancreatic cancer (17), astrocytic gliomas (18) and endometrial cancer (19). However, the precise role of DJ-1 in the occurrence and development, and clinical treatment of CRC remains unknown.
In the present study, DJ-1 was identified as a novel biomarker to predict the prognosis of patients with CRC. DJ-1 may predict the effect of chemotherapy based on clinical samples from CRC database, but further investigation is required to identify how DJ-1 regulates CRC cell proliferation, migration, invasion in vitro and in vivo.
Materials and methods
Patient specimens and tissue samples
The tissue microarray (TMA) cohort consisted of 470 CRC surgical samples from Yixing Hospital (Jiangsu, China) recruited between January 2000 to December 2006. These patients were followed up for at least 5 years. Overall survival (OS) was the primary endpoint of the analysis, and survival time was calculated from the date of surgery to the date of death or to the last follow-up. The median follow-up time was 59.3 months. In the CRC database, the mean age of all patients was 63 years. The age range was 30–88 years. There were 281 male and 189 female patients. The clinicopathological characteristics of the patients are summarized in Table SI.
A total of 8-paired fresh samples that were collected most recently were frozen in liquid nitrogen immediately for western blot analysis. The present study was granted ethical approval by the Institutional Review Board of Yixing Hospital Affiliated to Medical College of Yangzhou University (Yixing, Jiangsu). All patients provided written informed consent and all acquired data were assured of anonymity and confidentiality.
Construction of TMA
The CRC samples and matched non-cancerous colon tissues of patients with CRC were collected to construct the TMA. All tissue sections were fixed in formalin and embedded in paraffin. The CRC TMAs included 940 cores and were constructed by the Shanghai National Engineering Center for Biochip. Each sample was punched to a 1.0-mm diameter from the paraffin tumor block and the corresponding non-tumoral tissues. The thickness of each slide was 4-µm.
Immunohistochemistry (IHC)
The standard protocol used for the immunostaining was used as previously described (20). The TMA was heated at 55°C for 20 min and then washed with xylene 3 times to remove the paraffin. Following which, the chip was washed with absolute ethyl alcohol. Antigen retrieval step was then performed using 10 mmol/l sodium citrate (pH 6.0) and the samples were incubated at 95°C for 30 min. Serum blocking was subsequently performed for 30 min. The monoclonal mouse anti-DJ-1 (dilution 1:200; cat. no. sc-55572; Santa Cruz Biotechnology, Inc.), monoclonal rabbit anti-cyclin E (dilution 1:200; cat. no. 4136; Cell Signaling Technology, Inc.), anti-phosphorylated(p)-PI3K (dilution 1:200; cat. no. 17366; Cell Signaling Technology, Inc.), anti-p-Akt (dilution 1:200; cat. no. 4060; Cell Signaling Technology, Inc.), anti-p-mTOR (dilution 1:200; cat. no. 5536; Cell Signaling Technology, Inc.), anti-p27 (dilution 1:200; product code ab32034; Epitomics; Abcam), anti-cyclin E (dilution 1:200; product code ab32103; Epitomics; Abcam), anti-NF-κB (dilution 1:200; cat. no. 8242; Cell Signaling Technology, Inc.), anti-Snail (dilution 1:200; cat. no. 3879; Cell Signaling Technology, Inc.), anti-N-cadherin (dilution 1:200; cat. no. 13116; Cell Signaling Technology, Inc.), anti-E-cadherin (dilution 1:200; cat. no. 14472; Cell Signaling Technology, Inc.) and anti-vimentin (dilution 1:200; product code ab92547; Epitomics; Abcam) antibodies were used for primary antibody incubation overnight at 4°C. The samples were incubated with the respective rabbit or mouse secondary antibody (dilution 1:200; product codes 150077 or 150177; Epitomics; Abcam) for 30 min followed by hematoxylin staining using a 3,3′-diamido-plate. Dehydration was subsequently performed and sample sections were sealed by cover glasses. Once the tissue microarray was detected by immunohistochemistry, the dot array tissue would surely fall off. Thus, we could not acquire the data of 470 cases. We controlled the stripping rate to less than 5%.
Assessment of IHC
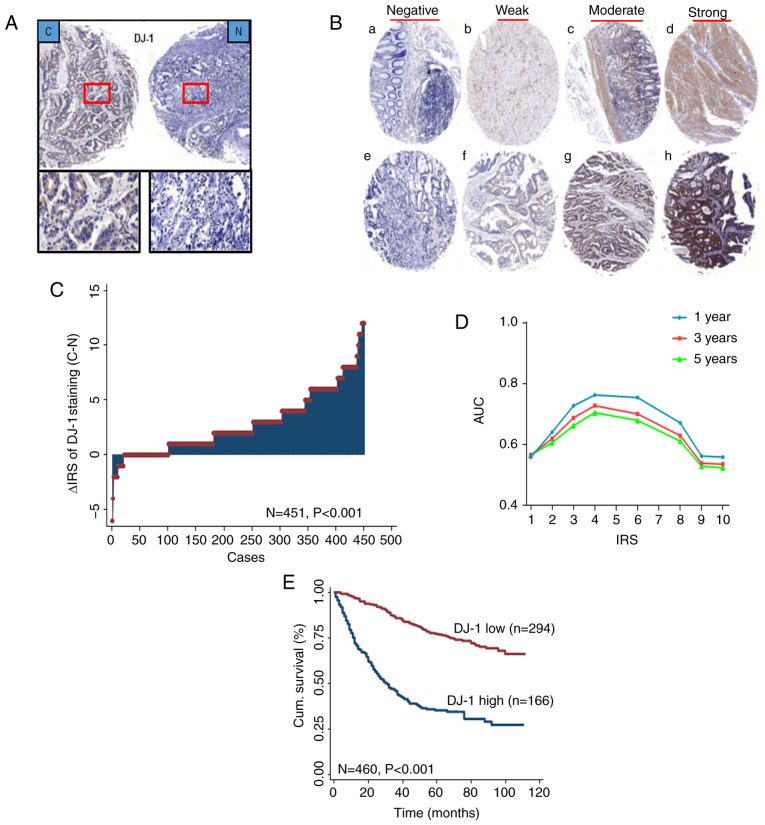
A semi-quantitative immunoreactivity score (IRS) was applied as previously described (21,22). A total of two independent pathologists, blinded to the study data, analyzed the staining of DJ-1 in the tissues, as shown in Fig. 2B. The receiver operator characteristic (ROC) analysis was used to determine the optimum cut-off value of IRS. The optimal cut-off value of DJ-1 IRS was 4, as it had the best predictive value for survival (Fig. 2D). The tissues with IRS 0–3 and 4–12 were classified as low or high expression of DJ-1 in tumors, respectively. In TMA, there were 465 plots in 470 tumor tissues. The few discrepancies were resolved by consensus using a multihead microscope.
Figure 2.
DJ-1 is elevated in CRC and associated with poor prognosis of CRC patients. (A) Representative images of DJ-1 immunohistochemical staining in TMA are shown: Top panels, original magnification, ×40; bottom panels, ×200. (B) Representative images of DJ-1 immunohistochemical staining in CRC cancer and adjacent normal tissues. (a-d) Adjacent normal tissue and (e-h) cancer tissue. (a and e) Negative staining, (b and f) weak staining, (c and g) moderate staining and (d and h) strong staining. All panels, original magnification, ×40. (C) Distribution of the difference in DJ-1 staining in CRC compared with that in the paired normal tissues in the TMA. The expression of DJ-1 was higher in cancer tissues than normal tissues (P<0.001). (D) Area under the curve (AUC) at different cut-off values for DJ-1 immunoreactivity score (IRS) for 1-, 3- and 5-year OS. The optimal cut-off point of DJ-1 IRS was 4. (E) Kaplan-Meier curves of the patients with low/high DJ-1 expression. CRC patients with high DJ-1 expression had a worse OS than the patients with low expression (P<0.001). CRC, colorectal cancer; TMA, tumor microarray; OS, overall survival.
Animals and cell lines
Forty-eight female BALB/c nude mice were purchased from the Comparative Medicine Laboratory Animal Center (license no. scxk (SU) 2012-0004) of Yangzhou University. The mice, aged 6–8 weeks, 18–26 g, were maintained in specific pathogen-free conditions and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (https://www.ncbi.nlm.nih.gov/books/NBK54050/). The protocols were approved by the Institutional Animal Care and Use Committee of Yangzhou University. The human SW620, DLD-1, RKO, HCT116, SW480, HT29, HCT15 CRC and normal FHC cell lines were purchased from the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. Cells were cultured in RPMI-1640 medium containing 100 U/ml penicillin, 100 µg/ml streptomycin and 10% fetal bovine serum (FBS) and maintained at 37°C in a humidified incubator with 5% CO2.
Lentiviral infection and generation of stable cell lines
The HCT116 cells were infected with lentivirus (LV)-DJ-1, LV-DJ-1-control (ctrl), LV-DJ-1-RNA interference (RNAi) and LV-DJ-1-RNAi-ctrl with a multiplicity of infection (MOI) 20 and 10 µg/ml polybrene (Shanghai GeneChem Co., Ltd.). A total of 8 h after lentiviral infection, the HCT116 cells were maintained in RPMI-1640 medium. Subsequently after 24 h, the cells were selected using puromycin (Gibco; Thermo Fisher Scientific, Inc.) at a final concentration of 2 µg/ml. The transgenic efficiency was detected using fluorescence microscopy according to the GFP of the lentivirus. The knockdown and overexpression efficiency of DJ-1 was further analyzed using western blot analysis.
Cell Counting Kit (CCK)-8 assay
The LV-DJ-1 and LV-DJ-1-RNAi HCT116 cells and the corresponding controls were seeded at a density of 8×103 cells/100 µl culture medium per well in 96-well plates. The cell proliferation ability was analyzed 12, 24, 48, 60 and 72 h after cell culture using a CCK-8 solution (Dojindo Molecular Technologies, Inc.) according to the manufacturer's instructions. An automatic microplate reader measured the optical density of each well at 450 nm.
EdU immunofluorescence assay
The LV-DJ-1 and LV-DJ-1-RNAi HCT116 cells and the corresponding controls were seeded in 96-well plates at a density of 5×103 cells/100 µl culture medium. After 24 h of culture, EdU immunofluorescence analysis was performed using the EdU kit according to manufacturer's protocol (Guangzhou RiboBio Co., Ltd.).
Cell cycle analysis
The LV-DJ-1 and LV-DJ-1-RNAi HCT116 cells and the corresponding controls were fixed with 70% cold ethanol overnight at 4°C and stained with 20 µg/ml propidium iodide (PI) in 0.1% Triton X-100 for 15 min. Samples were subsequently analyzed using a flow cytometer (BD Biosciences).
Transwell and Matrigel assays
The LV-DJ-1 and LV-DJ-1-RNAi HCT116 cells and the corresponding controls (5×105 cells/100 µl serum-free RPMI-1640) were seeded onto the upper chamber of Transwell filters (8-µm pore size; EMD Millipore; Merck KGaA). The Transwell filter inserts were coated with or without Matrigel (EMD Millipore; Merck KGaA) for the cell invasion and migration assays, respectively. The bottom chamber was filled with 500 µl RPMI-1640 medium containing 10% FBS. After incubation for 24 h the cells on the upper surface of the filters were removed with a cotton swab. The cells that had traversed the membrane were fixed with 4% paraformaldehyde and stained with 0.4% crystal violet solution for 30 min at room temperature. The number of invaded and migrated tumor cells was counted under an inverted microscope (original magnification, ×40) and images were captured by the 1X73 inverted fluorescence microscope.
Wound healing assay
The LV-DJ-1 and LV-DJ-1-RNAi HCT116 cells and the corresponding controls (2×105 cells) were cultured in 6-well plates and grown to 80% confluence. The wound was scratched using a 10-µl pipette tip across the entire diameter of the well, and rinsed with PBS to remove all cellular debris. RPMI-1640 medium containing 2% FBS was then added to maintain cell growth during the assay. The process of tumor cell migration was observed and images were obtained at a low-power field (×50) under the 1X73 inverted fluorescence microscope at 0, 24 and 48 h after the wound was created. The closure rate reflected the migratory ability of the tumor cells. Three random measurements were made per photographed sample at every time point, which was used as baseline. AxioVision Rel. 4.8 software was used for the measurements.
Western blot analysis
Cells or tissues were lysed with cold lysis buffer supplemented with a protease inhibitor mixture on ice for 30 min. The total protein concentration was measured using the Bicinchoninic Acid Protein assay kit (Thermo Fisher Scientific, Inc.). Western blots were performed as previously described (23). The monoclonal mouse anti-DJ-1 (dilution 1:1,000; Santa Cruz Biotechnology, Inc.), monoclonal rabbit anti-cyclin E (dilution 1:1,000; Cell Signaling Technology, Inc.), anti-PI3K (dilution 1:1,000; Cell Signaling Technology, Inc.), anti-Akt (dilution 1:1,000, Cell Signaling Technology, Inc.), anti-p-PI3K (dilution 1:1,000; Cell Signaling Technology, Inc.), anti-p-Akt (dilution 1:1,000; Cell Signaling Technology, Inc.), anti-mTOR (dilution 1:1,000; Cell Signaling Technology, Inc.), anti-p-mTOR (dilution 1:1,000; Cell Signaling Technology, Inc.), anti-P27 (dilution 1:2,000; Epitomics; Abcam), anti-cyclin E (dilution 1:2,000; Epitomics; Abcam), anti-NF-κB (dilution 1:1,000; Cell Signaling Technology, Inc.), anti-Snail (dilution 1:1,000; Cell Signaling Technology, Inc.), anti-N-cadherin (dilution 1:1,000; Cell Signaling Technology, Inc.), anti-E-cadherin (dilution 1:1,000; Cell Signaling Technology, Inc.) and anti-vimentin (dilution 1:1,000, Epitomics; Abcam) antibodies were used for antibody incubation overnight at 4°C. The polyclonal mouse anti-actin (dilution 1:2,000; cat. no. CSB-PA007670HA01; Wuhan Boster Biological Technology, Ltd.) was used for the protein loading control. Each blot was repeated three times. The intensity of the protein bands was analyzed using densitometry by Image J software (National Institutes of Health, Bethesda, MD, USA) after normalization to the corresponding protein controls.
Reverse transcription-quantitative PCR (RT-qPCR)
The total RNAs from CRC and FHC cells were extracted using RNeasy Mini kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions using RNase-free conditions. The purified RNAs were reversely transcribed to first strand cDNA using a RevertAid RT reverse transcription kit (Thermo Fisher Scientific, Inc.). SYBR Green Real-Time qPCR analysis was performed using an Applied Biosystems 7500 Real-Time PCR System (Roche Applied Science). The thermocycling conditions are as follows: Pre-denaturation temperature: 95°C, 6 min; melting temperature: 95°C, 10 sec, 65°C, 60 sec, 97°C, 1 sec; amplification temperature: 95°C, 10 sec, 60°C, 10 sec, 72°C, 10 sec; then went through 40 cycles.
Human DJ-1 and GAPDH specific primers (DJ-1 forward, 5′-CCATATGATGTGGTGGTTCTAC-3′ and reverse, 5′-CGTCTGGGCTGTAGTCGGAT-3′; GAPDH forward, 5′-ACGGATTTGGTCGTATTGGG-3′ and reverse, 5′-CGCTCCTGGAAGATGGTGAT-3′) (Sangon Biotechnology Inc.) were used. The relative expression level of DJ-1 mRNA was normalized to GAPDH internal control and analyzed using the 2−ΔΔCq method (24). All reactions were performed in duplicate.
Tumor xenograft and abdominal metastasis model
In the tumor xenograft model, the LV-DJ-1 and LV-DJ-1-RNAi HCT116 cells and the corresponding controls (0.2 ml 1×107 cells/mouse; 5 mice/group) were injected subcutaneously into the flanks of BALB/c nude mice. The tumor size was measured using a caliper upon palpable every 3 days. The following equation was used to calculate the tumor volume: V = L × W2 × π/6 (V, volume; L, length; W, width). After 24 days, the mice were sacrificed by cervical dislocation. The tumors were removed and images were obtained. Each tumor was divided into 2 pieces and fixed in 10% buffered formalin.
In the peritoneal metastasis model, the same cells and groups were used as in the xenograft model, and inoculated into the peritoneal cavity of BALB/c nude mice. The weight of each mouse was recorded every 2 days. Mice were euthanized and examined macroscopically for the presence of peritoneal metastasis after 22 days. Intraperitoneal metastatic tumors were displayed, images were obtained and fixed in 10% formalin.
All experimental animal procedures were performed in compliance with the institutional ethics requirements and approved by the Institutional Animal Care and Use Committee of Yangzhou University.
Statistical analysis
The significance of associations between DJ-1 staining patterns and clinicopathological data was evaluated using Fisher's exact test. The paired Wilcoxon test (raw scores) was used to assess the significance of the difference of DJ-1 staining levels in tumor samples compared with that in the paired non-tumor samples. We used ANOVA (Tukey's) method to compare CRC cells with FHC cells, and the DJ-1 transfection groups with the control group. Kaplan-Meier survival analysis was performed to calculate OS and evaluate the prognostic value of patients with DJ-1 expression. Cox proportional hazards model was performed to analyze DJ-1 expression as a potential biomarker for predicting patient survival. All the statistical analyses were performed using STATA software (version 10.1; StataCorp LP). P<0.05 was considered to indicate a statistically significant difference.
Results
DJ-1 expression is increased in human CRC cells and tissues
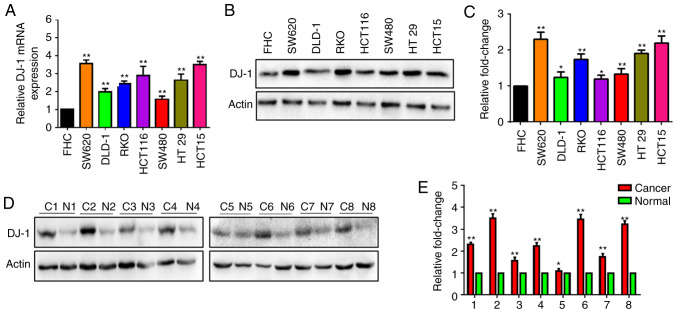
The DJ-1 mRNA level was determined in human CRC and FHC cells using RT-qPCR. The DJ-1 mRNA level was significantly higher in the SW620, DLD-1, RKO, HCT116, SW480, HT29 and HCT15 cells compared with that in normal FHC cells (Fig. 1A). Simultaneously, DJ-1 protein expression was determined in six CRC cell lines and FHC using western blot analysis. The results revealed that DJ-1 protein expression was increased in the CRC cells compared with that in FHC (Fig. 1B). The expression of DJ-1 protein was further detected in 8 CRC tissues (C1-C8) and corresponding normal tissues (N1-N8). The expression of DJ-1 protein was increased in the CRC tissues (Fig. 1D). A statistical analysis indicated that DJ-1 strip gray value was significantly overexpressed in the CRC cells or tumor tissues, respectively (Fig. 1C and E). These results indicated that DJ-1 expression was increased in CRC.
Figure 1.
DJ-1 is highly expressed in CRC tissues and cell lines. (A) DJ-1 mRNA in FHC and 7 CRC cell lines was analyzed using RT-qPCR. Data indicated that DJ-1 mRNA in FHC was lower than that in the CRC cell lines (**P<0.01). (B and C) DJ-1 protein was detected by western blot analysis. Actin served as an internal control of the protein level. The expression of DJ-1 was higher in the 7 CRC cells than that noted in the FHC cells. Densitometric analysis is presented as mean ± SD of 3 separate experiments (*P<0.05, **P<0.01). (D and E) Expression of DJ-1 was increased in cancer tissues (C1-C8) when compared with that noted in the paired normal colonic tissues (N1-N8) by western blot analysis. Densitometric analysis is presented as mean ± SD of 3 separate experiments (*P<0.05, **P<0.01). CRC, colorectal cancer.
Increased DJ-1 expression in CRC is associated with metastasis and poor OS in patients with CRC
To further reveal the role of DJ-1 in CRC, IHC was used to detect the expression of DJ-1 in a CRC TMA. Strong DJ-1 cytoplasm and nuclear staining was primarily found in CRC tissues and corresponding normal tissues (Fig. 2A). As shown in Fig. 2B, representative images of immunostaining revealed negative, weak, moderate, and strong expression levels in CRC tissues and corresponding normal tissues, and DJ-1 expression was upregulated in 351 of 451 (77.8%) tumors compared with that in the paired normal tissues (P<0.001; Fig. 2C).
In the CRC cohort, there was a significant association between high DJ-1 expression in cancerous tissues and depth of invasion (P<0.001), lymph node metastasis (P<0.001), and TNM stages (P<0.001; Table I). DJ-1 expression had a trend with age (P=0.050). There was no association between DJ-1 expression and sex, pathological classification, and tumor diameter (Table I). In addition, Kaplan-Meier survival curves were used to determine 5-year overall cumulative survival in patients with high and low DJ-1 expression levels. Patients with high DJ-1 expression levels had a worse OS compared with patients with low expression (P<0.001; Fig. 2E) and DJ-1 expression had the best predictive value for survival (Fig. 2D) upon assessment of IHC.
Table I.
Association between expression levels of DJ-1 and clinicopathological features in the CRC patients (N=460).
| DJ-1 expression | |||
|---|---|---|---|
| Variables | Low, n (%) | High, n (%) | P-valuea |
| All patients | 294 (63.9) | 166 (36.1) | |
| Age (years) | 0.050 | ||
| ≤65 | 177 (67.3) | 86 (32.7) | |
| >65 | 117 (59.4) | 80 (40.6) | |
| Sex | 0.229 | ||
| Male | 180 (65.5) | 95 (34.5) | |
| Female | 114 (61.6) | 71 (38.4) | |
| Pathological classificationb | 0.454 | ||
| I | 3 (60.0) | 2 (40.0) | |
| II | 267 (64.3) | 148 (35.7) | |
| III | 19 (54.3) | 16 (45.7) | |
| Depth of invasionb | <0.001 | ||
| T1/T2 | 78 (78.8) | 21 (21.2) | |
| T3/T4 | 211 (59.3) | 145 (40.7) | |
| Lymph node metastasisb | <0.001 | ||
| N0 | 204 (75.8) | 65 (24.2) | |
| N1/N2 | 86 (46.0) | 101 (54.0) | |
| TNM stageb | <0.001 | ||
| I | 68 (80.0) | 17 (20.0) | |
| II | 133 (76.0) | 42 (24.0) | |
| III | 82 (46.3) | 95 (53.7) | |
| IV | 6 (35.3) | 11 (64.7) | |
| Tumor diameterb | 0.529 | ||
| ≤5 cm | 237 (63.9) | 134 (36.1) | |
| >5 cm | 56 (63.6) | 32 (36.4) | |
| Distant metastasis | <0.001 | ||
| M0 | 288 (65.2) | 154 (34.8) | |
| M1 | 6 (33.3) | 12 (66.7) | |
| Adjuvant therapy | 0.071 | ||
| LFP regimen | 60 (71.4) | 24 (28.6) | |
| Surgery alone | 234 (66.7) | 142 (33.3) | |
Two-sided Fisher's exact tests.
For some patients data concerning these clinical pathological parameters were not available. CRC, colorectal cancer; TNM, Tumor-Node-Metastasis; LFP, (regimen including oxaliplatin, 5-FU and tetrahydrofolate).
From the univariate and multivariate Cox regression analysis, DJ-1 expression was found to be an independent risk factor for the prognosis of CRC. The univariate Cox regression analysis revealed that age, pathological classification, depth of invasion, lymph node metastasis, TNM stage, distant metastasis and DJ-1 expression were associated with OS in patients with CRC (Table II). Subsequently, multivariate Cox regression analysis was used to verify the effect of DJ-1 expression, and the clinical parameters (sex, pathological classification, TNM stage and tumor diameter). The results indicated that DJ-1 expression was an independent and unfavorable prognostic factor in patients with CRC (HR, 0.301; 95% CI, 0.224–0.405; P<0.001; Table II).
Table II.
Univariate and multivariate Cox regression analysis of DJ-1 expression and clinicopathological variables predicting survival in CRC patients.
| N=470 cases | ||
|---|---|---|
| Variables | HR (95% CI) | P-value |
| Univariate Cox regression analysis | ||
| Age (≤65 vs. >65 years) | 1.607 (1.215–2.126) | 0.001 |
| Sex (male vs. female) | 1.013 (0.762–1.347) | 0.927 |
| Pathological classification (I/II vs. III) | 2.475 (1.587–3.860) | <0.001 |
| Depth of invasion (T1/T2 vs. T3/T4) | 3.687 (2.270–5.990) | <0.001 |
| Lymph node metastasis (N0 vs. N1/N2) | 2.807 (2.112–3.731) | <0.001 |
| TNM stage (I/II vs. III/IV) | 3.214 (2.407–4.291) | <0.001 |
| Distant metastasis (M0 vs. M1) | 8.150 (4.849–13.699) | <0.001 |
| Tumor diameter (≤5 cm vs. >5 cm) | 1.196 (0.848–1.688) | 0.307 |
| DJ-1 expression (low vs. high) | 0.255 (0.191–0.340) | <0.001 |
| Multivariate Cox regression analysisa | ||
| Sex (male vs. female) | 0.914 (0.684–1.222) | 0.544 |
| Pathological classification (I/II vs. III) | 2.301 (1.420–3.731) | 0.001 |
| TNM stage (I/II vs. III/IV) | 2.452 (1.810–3.324) | <0.001 |
| Tumor diameter (≤5 cm vs. >5 cm) | 0.978 (0.675–1.415) | 0.904 |
| DJ-1 expression (low vs. high) | 0.301 (0.224–0.405) | <0.001 |
Multivariate Cox regression analysis including sex, pathological classification, TNM stage, tumor diameter and DJ-1 expression. CRC, colorectal cancer; HR, hazard ratio; CI, confidence interval.
Lentivirus-mediated DJ-1 overexpression and knockdown in CRC cells
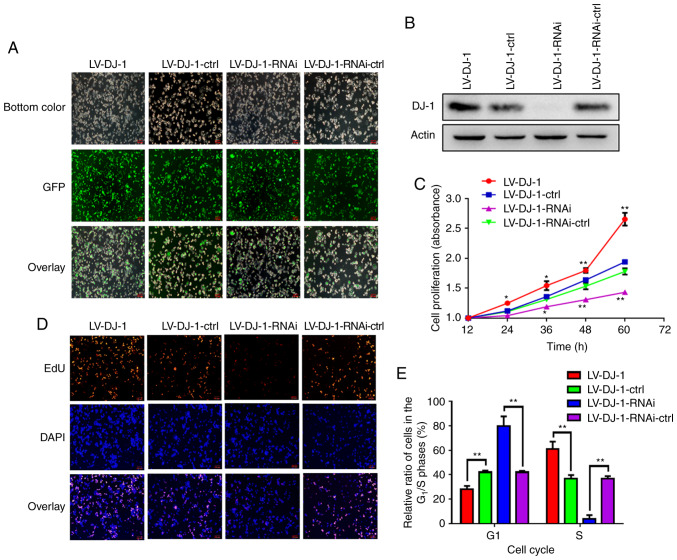
HCT116 cells transfected with either LV-DJ-1 or LV-DJ-1-RNAi exhibited increased or knocked down DJ-1 expression levels, respectively, compared with that in the respective control groups. As shown in Fig. 3A, the transfection efficiency with lentivirus and GFP was high. The lentivirus-mediated overexpression or knockdown of DJ-1 in HCT116 cells was subsequently analyzed using western blot analysis (Fig. 3B) and the results were consistent with fluorescence imaging.
Figure 3.
DJ-1 increases the cell proliferation in CRC cells in vitro. (A) Fluorescence microscopic analysis of green fluorescent protein (GFP) expression. The GFP expression in HCT116 cells that were infected by the lentivirus was examined under fluorescence microscopy. Representative images of GFP and overlay images are shown. (B) The lentivirus-mediated overexpression or knockout of DJ-1 in HCT116 cells was analyzed by western blot analysis. (C) The cell proliferation of HCT116 with different DJ-1 expression levels as detected by CCK-8 assay. The ability of cell proliferation was drastically and significantly increased after DJ-1 overexpression or decreased after DJ-1 knockout (*P<0.05, **P<0.01). (D) EdU immunofluorescence assay was utilized to detect the cell proliferation of HCT116 cells with differential DJ-1 expression levels. EdU-stained proliferating cells, DAPI-stained cell nuclei. Representative images of EdU, DAPI and overlay images are shown. (E) Alterations in the cell population in different phases of the cell cycle in regards to DJ-1 expression levels were evaluated by PI single staining method. Lower percentage in the G1 phase and higher percentage of cells in the S phase were noted in the LV-DJ-1 cells, while a higher percentage of cells in the G1 phase and lower percentage of cells in the S phase were noted in the LV-DJ-1-RNAi cells (**P<0.01). CRC, colorectal cancer. The HCT116 cells were infected with lentivirus (LV)-DJ-1, LV-DJ-1-control (ctrl), LV-DJ-1-RNA interference (RNAi) and LV-DJ-1-RNAi-ctrl.
Overexpression and knockdown of DJ-1 enhances and inhibits CRC cell proliferation
From the TMA data analysis, DJ-1 overexpression was associated with TNM stage in patients with CRC. It is unknown whether DJ-1 overexpression increases CRC cell growth. To investigate the biological role of DJ-1 in CRC cell proliferation, CCK-8 assay was used to observe the proliferation rate in DJ-1-overexpressing and -knockdown HCT116 cells and the corresponding control cells (Fig. 3C). Furthermore, the cell proliferation rate was investigated using EdU immunofluorescence assay (Fig. 3D). The results indicated that the cell proliferation ability was significantly increased after DJ-1 overexpression and significantly decreased when DJ-1 was knocked down in the HCT116 cells, when compared with that in the respective controls. To examine whether DJ-1 promoted CRC cell proliferation through directly acceleration of the progression of the cell cycle, cell cycle analysis was performed using PI and flow cytometry. As shown in Figs. 3E and S1, there was a significantly lower number of LV-DJ-1 cells in the G1 phase and a significantly higher number in the S phase, whereas significantly higher and lower numbers of LV-DJ-1-RNAi cells were observed in the G1 and S phase, respectively, compared with those of the corresponding control cells. The results indicate that DJ-1 promotes CRC cell proliferation and growth through regulation of the cell cycle.
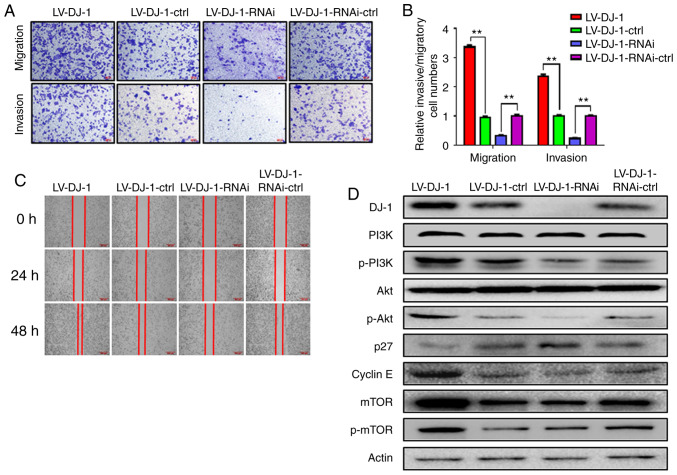
Overexpression and knockdown of DJ-1 promotes and inhibits CRC cell migration and invasion, respectively
By analyzing the CRC database, DJ-1 expression was associated with lymph node metastasis in patients with CRC. To further clarify the role of DJ-1 in the metastasis of CRC, the migration and invasion abilities of LV-DJ-1, LV-DJ-1-ctrl, LV-DJ-1-RNAi and LV-DJ-1-RNAi-ctrl cell lines were observed using Transwell and wound healing, and Matrigel assays respectively. As shown in Fig. 4A, the results revealed that the migration and invasion abilities of LV-DJ-1 cells were increased, whereas the migration and invasion abilities of the LV-DJ-1-RNAi cells were decreased when compared with that in the corresponding controls, respectively. The mean number of migrated and invaded cells in the LV-DJ-1 group was 3.36- and 2.37-fold, respectively, whereas the number of migrated and invaded cells decreased by 68.0 and 23.5%, respectively, in the LV-DJ-1-RNAi cells compared with that in the control group (Fig. 4B; P<0.01). In addition, the wound healing rate of LV-DJ-1 cells was higher compared with that in LV-DJ-1-ctrl cells, and the rate of LV-DJ-1-RNAi cells was lower compared with that in LV-DJ-1-RNAi-ctrl cells (Fig. 4C). These data indicated that DJ-1 promoted CRC cell migration and invasion.
Figure 4.
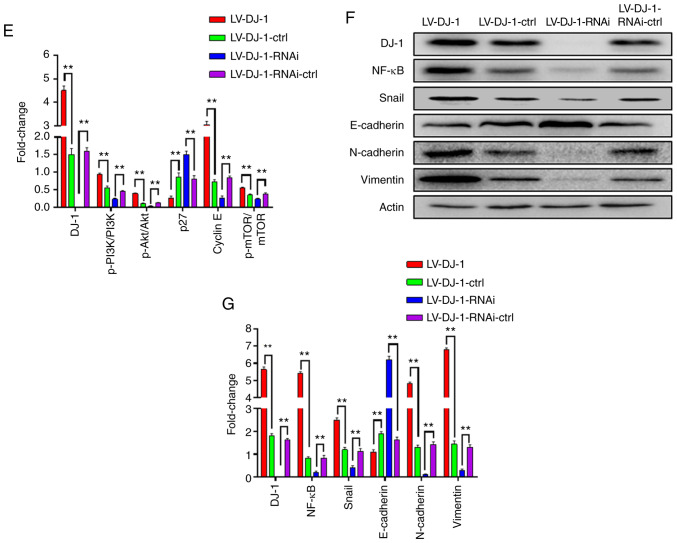
DJ-1 positively regulates CRC cell migration and invasion in vitro. (A and B) The cell migration and invasion results of HCT116 cells with differential DJ-1 expression levels. Numbers of cell migration and invasion per field were counted in five random fields for the DJ-1-overexpressing/knockout and control groups (n=3/group). The ability of cell migration and invasion was increased after DJ-1 over-expression, whereas the ability of cell migration and invasion was decreased after DJ-1 knockout (**P<0.01). (C) Wound healing assay was used to detect the migratory ability of HCT116 cells with differential DJ-1 expression levels. The wound healing rate of LV-DJ-1 cells was higher than that of the LV-DJ-1-ctrl cells, whereas the wound healing rate was lower in the LV-DJ-1-RNAi cells compared with the control groups. (D and E) The expression of PI3K/Akt downstream molecules such as p27, cyclin E, mTOR, p-mTOR was detected by western blot analysis. DJ-1 regulated PI3K/Akt/p27/cyclin E and PI3K/Akt/ mTOR signaling pathway to promote CRC cell growth and metastasis. Densitometric analysis is presented as mean ± SD of 3 separate experiments (**P<0.01). (F and G) Nuclear transcription factors (NF-κB, Snail), EMT markers (E-cadherin, N-cadherin, and vimentin) were evaluated by western blot analysis. Densitometric analysis is presented as mean ± SD of 3 separate experiments (**P<0.01). DJ-1 was able to regulate the NF-κB/Snail signaling pathway to induce EMT. CRC, colorectal cancer; EMT, epithelial-mesenchymal transition. The HCT116 cells were infected with lentivirus (LV)-DJ-1, LV-DJ-1-control (ctrl), LV-DJ-1-RNA interference (RNAi) and LV-DJ-1-RNAi-ctrl.
DJ-1 activates the PI3K/Akt signaling pathway to promote CRC cell proliferation, migration and invasion
To further explore the molecular mechanism of DJ-1 in promoting proliferation and metastasis in CRC, proliferation- and metastasis-related proteins were detected using western blot analysis. DJ-1 positively regulated p-PI3K and p-Akt expression however, there was no difference in total PI3K and Akt protein levels. The data indicate that DJ-1 is able to activate the PI3K/Akt signaling pathway. The expression of PI3K/Akt downstream molecules, such as p27, cyclin E, mTOR, p-mTOR were also analyzed and the results revealed that DJ-1 negatively regulated p27 and cyclin E expression and positively regulated mTOR and p-mTOR expression (Fig. 4D and E). These results from the present study suggest that DJ-1 regulates the PI3K/AKT/p27/cyclin E and PI3K/Akt/mTOR signaling pathways to promote CRC cell growth and metastasis.
DJ-1 induces CRC cell EMT to promote migration and invasion
Previous studies have demonstrated that DJ-1 is upregulated in renal fibrosis and DJ-1 mediates EMT by suppressing cytoplasmic PTEN expression and Akt activation (25). Epithelial marker (E-cadherin) and mesenchymal markers (N-cadherin and vimentin) are markers for the occurrence of EMT. We investigated whether DJ-1 stimulates CRC cells to induce EMT, which consequently promotes CRC cell invasion and metastasis. The results from western blot analysis revealed that protein expression level of E-cadherin was reduced following DJ-1 overexpression, whereas E-cadherin was upregulated following knockdown of DJ-1, when compared with the corresponding controls, respectively. The expression of N-cadherin and vimentin was inversely associated with DJ-1 expression. The data confirmed that DJ-1 was able to induce CRC cell EMT to promote migration and invasion. To investigate the related mechanism further, the effect of DJ-1 on the NF-κB/Snail signaling pathway was examined (Fig. 4F and G). From these results, we concluded that DJ-1 could regulate EMT signaling pathway through NF-κB/Snail.
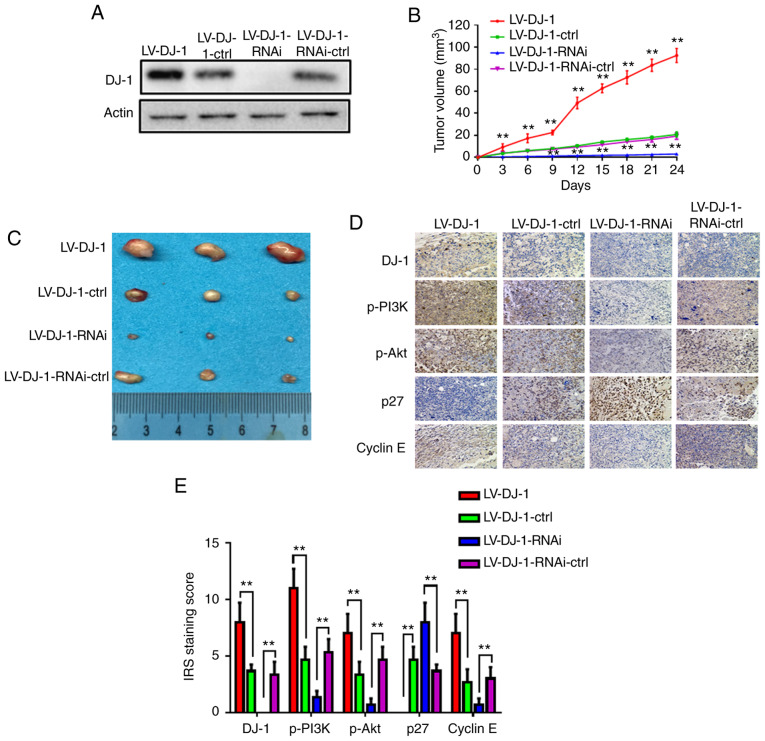
DJ-1 increases CRC cell growth and induces CRC cell metastasis in vivo
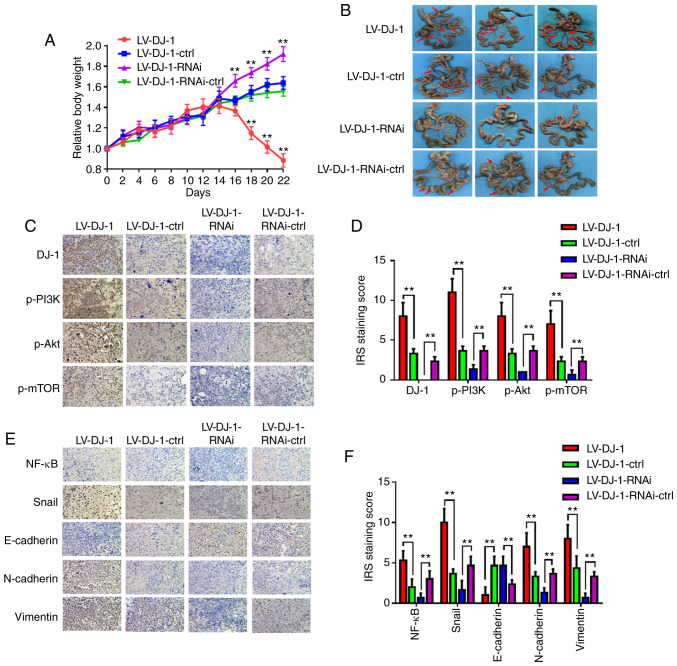
LV-DJ-1, LV-DJ-1-ctrl, LV-DJ-1-RNAi and LV-DJ-1-RNAi-ctrl cell lines exhibited differential levels of DJ-1 (Fig. 5A). These four groups cells were injected subcutaneously into nude mice, and tumor growth was monitored (Fig. 5B). Tumor volume was increased in the LV-DJ-1 group, whereas it was decreased in LV-DJ-1-RNAi group when compared with that in the respective control groups (Fig. 5B and C). Furthermore, the protein expression levels of DJ-1, p-PI3K, p-Akt, p27 and cyclin E in the xenograft tumors were determined using IHC. The results revealed that DJ-1 expression in tumors was higher in the LV-DJ-1 group and lower in the LV-DJ-1-RNAi group compared with that in the respective control groups. The DJ-1 expression was positively associated with the expression of p-PI3K, p-Akt and cyclin E, whereas it was negatively associated with p27 expression (Fig. 5D and E). In addition, the aforementioned transfected cells were inoculated into the peritoneal cavity of BALB/c nude mice. The weight of each mouse was monitored every 2 days. The relative weight of the mice in the LV-DJ-1 group was significantly reduced at days 18, 20 and 22 compared with that in the control group, whereas the weight of the mice in the LV-DJ-1-RNAi group increased at days 16, 18, 20 and 22 compared with that in the control group (Fig. 6A). These mice were sacrificed by cervical dislocation on day 22, and the number of metastatic nodules was higher and lower in the LV-DJ-1 and LV-DJ-1-RNAi group, respectively, compared with that in the corresponding control group (Fig. 6B). Subsequently, the protein expression of DJ-1, p-PI3K, p-Akt and p-mTOR in the metastatic nodules of the peritoneum was determined using IHC. The results indicated that DJ-1 expression was positively associated with the expression levels of p-PI3K, p-Akt and p-mTOR (Fig. 6C and D). Furthermore, the expression levels of EMT-related proteins (NF-κB, Snail, E-cadherin, N-cadherin and vimentin) were also determined in intraperitoneal metastasis. The expression levels of NF-κB, Snail, N-cadherin and vimentin were positively regulated by the expression of DJ-1, whereas E-cadherin was negatively associated with DJ-1 expression (Fig. 6E and F). The data from the present study revealed that DJ-1 was also capable of promoting CRC cell growth and metastasis in vivo, which is consistent with the results from CRC cell proliferation and invasion in vitro.
Figure 5.
DJ-1 induces CRC cell growth in vivo. (A) Western blot analysis was used to validate the expression of DJ-1. LV-DJ-1, LV-DJ-1-ctrl, LV-DJ-1-RNAi and LV-DJ-1-RNAi-ctrl cell lines were generated. (B) The tumor volume was calculated in the 4 groups every 3 days. The tumor volume was significantly larger in the LV-DJ-1 group, and smaller in the LV-DJ-1-RNAi group compared with the control group, respectively (**P<0.01). (C) Images of the xenograft tumors in the stable DJ-1-over-expression or knockout and the corresponding vector control group. (D) The expression levels of DJ-1, p-PI3K, p-Akt, p27, cyclin E in the xenograft tumors were tested by IHC. (E) The IRS staining scores of DJ-1, p-PI3K, p-Akt, p27, cyclin E in the xenograft tumors were evaluated (n=3). Data are presented as mean ± SD (**P<0.01). CRC, colorectal cancer; IHC, immunohistochemistry; IRS, immunoreactivity score.
Figure 6.
DJ-1 promotes cell metastasis of CRC cells in vivo. (A) The body weight of mice in the four groups were monitored every 2 days. After 2 weeks, the mouse weights in the LV-DJ-1 group were lower than that in the LV-DJ-1-ctrl group; whereas in the LV-DJ-1-RNAi group, the opposite phenomenon was observed. (B) Representative images of the metastatic nodules of the peritoneal cavity in the four groups. Overexpression of DJ-1 was found to promote the metastasis of CRC cells. In the LV-DJ-1-RNAi group, metastasis of the CRC cells was reduced much more than in the control group. (C) Expression of DJ-1, p-PI3K, p-Akt and p-mTOR in the metastatic nodules was assessed by IHC. (D) IRS staining scores of DJ-1, p-PI3K, p-Akt, p-mTOR were evaluated (n=3). Data are presented as mean ± SD (**P<0.01). (E) Protein expression of NF-κB, Snail, E-cadherin, N-cadherin, and vimentin in the metastatic nodules was assessed by IHC. (F) IRS staining scores of NF-κB, Snail, E-cadherin, N-cadherin, and vimentin were evaluated (n=3). Data are presented as mean ± SD (**P<0.01). The HCT116 cells used in the mouse model were infected with lentivirus (LV)-DJ-1, LV-DJ-1-control (ctrl), LV-DJ-1-RNA interference (RNAi) and LV-DJ-1-RNAi-ctrl. CRC, colorectal cancer; IHC, immunohistochemistry.
Patients with low DJ-1 expression levels are more sensitive to adjuvant chemotherapy
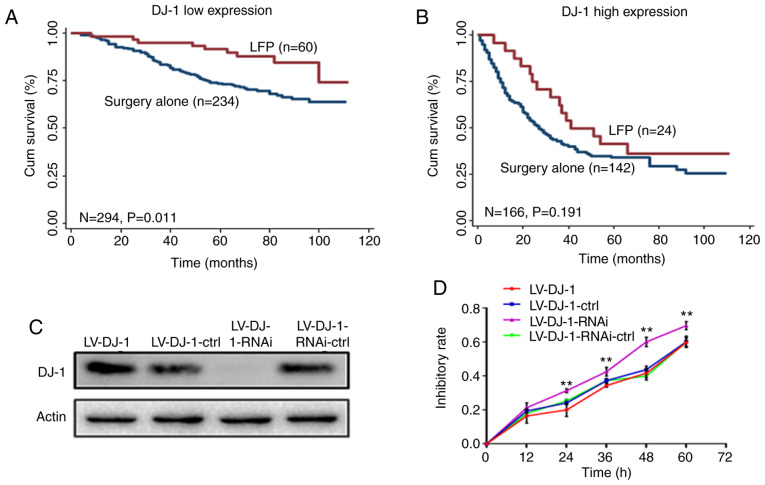
In the CRC database, the postoperative chemotherapy of each patient with CRC was recorded in detail. According to the National Comprehensive Cancer Network guidelines at that time, the recommended first-line postoperative adjuvant chemotherapy for patients with CRC is the LFP regimen, which includes oxaliplatin, 5-FU and tetrahydrofolate (26). Subsequently, the correlation between DJ-1 expression and the therapeutic effect of chemotherapy was investigated. Kaplan-Meier curve analysis revealed that patients who received LFP treatment and had low DJ-l expression levels in tumor tissues had a significantly longer survival time compared with that in patients who received surgery alone (Fig. 7A; P=0.011). However, patients with high DJ-1 expression level did not benefit from LFP treatment (Fig. 7B; P=0.191). Multivariate Cox proportional hazard regression analysis, including 6 variables (age, sex, TNM stage, pathological classification, tumor diameter and adjuvant chemotherapy) was used to evaluate the benefit of chemotherapy on OS. Notably, LFP treatment increased OS compared with that with surgery alone in patients with low DJ-1 expression levels (HR, 0.410; 95% CI, 0.200–0.837; P=0.014; Table III). However, this effect was not observed in patients with high DJ-1 expression levels (HR, 0.634; 95% CI, 0.355–1.32; P=0.124; Table III).
Figure 7.
Kaplan-Meier curves depicting OS according to DJ-1 expression and adjuvant chemotherapy. (A) Patients with low DJ-1 expression and who received LFP-based chemotherapy (oxaliplatin, 5-FU and tetrahydrofolate) had a prolonged survival time (P=0.011). (B) Patients with high DJ-1 expression did not benefit from LFP-based chemotherapy (P>0.05). (C) LV-DJ-1, LV-DJ-1-ctrl, LV-DJ-1-RNAi and LV-DJ-1-RNAi-ctrl cell lines were validated by western blot analysis. (D) In addition, the association between DJ-1 expression and chemosensitivity was investigated in vitro. A drug which contains 5-FU (25 µg/ml) and L-OHP (20 µg/ml) was used to act on LV-DJ-1, LV-DJ-1-ctrl, LV-DJ-1-RNAi and LV-DJ-1-RNAi-ctrl cell lines. The inhibition rate was determined using CCK-8 assay. The results indicate that the inhibition rate in the LV-DJ-1-RNAi group was significantly higher when compared with that in LV-DJ-1-RNAi-ctrl group (**P<0.01); however, the inhibition rate in the LV-DJ-1 group was not statistically significant compared with that in the control group (P>0.05). OS, overall survival.
Table III.
Multivariate Cox model analysis of the effect of LFP therapy on OS of CRC patients with low/high DJ-1 expression.
| DJ-1 low expression | DJ-1 high expression | |||||
|---|---|---|---|---|---|---|
| HR (95% CI) | β | P-valuea | HR (95% CI) | β | P-valuea | |
| Age (≤65 vs. >65 years) | 1.670 (1.072–2.604) | 2.27 | 0.023 | 1.552 (1.040–2.317) | 2.15 | 0.031 |
| Sex (male vs. female) | 0.734 (0.464–1.161) | −1.32 | 0.186 | 1.056 (0.723–1.542) | 0.28 | 0.779 |
| Pathological classification (I/II vs. III) | 1.037 (0.440–2.447) | 0.08 | 0.934 | 3.645 (1.948–6.819) | 4.05 | 0.001 |
| TNM stage (I/II vs. III/IV) | 2.390 (1.536–3.718) | 3.86 | 0.001 | 3.260 (2.061–5.157) | 5.05 | 0.001 |
| Tumor diameter (≤5 cm vs. >5 cm) | 1.306 (0.763–2.236) | 0.97 | 0.331 | 0.853 (0.502–1.451) | −0.59 | 0.558 |
| Adjuvant therapy (LFP vs. surgery alone) | 0.410 (0.200–0.837) | −2.45 | 0.014 | 0.634 (0.355–1.132) | −1.54 | 0.124 |
Multivariate Cox regression analysis including age, sex, pathological histological type, TNM stage, tumor diameter and adjuvant therapy (LFP vs. surgery alone). OS, overall survival; HR, hazard ratio; CI, confidence interval; CRC, colorectal cancer; TNM, Tumor-Node-Metastasis; LFP, (regimen including oxaliplatin, 5-FU and tetrahydrofolate).
In addition, the association between DJ-1 expression and chemosensitivity was investigated in vitro. A drug which contains 5-FU (25 µg/ml) and L-OHP (20 µg/ml) was used to act on LV-DJ-1, LV-DJ-1-ctrl, LV-DJ-1-RNAi and LV-DJ-1-RNAi-ctrl cell lines. As in Fig. 7C, DJ-1 expression in these four groups was again verified by Western blot analysis. The inhibition rate was determined using CCK-8 assay. The results indicate that the inhibition rate in the LV-DJ-1-RNAi group was significantly higher when compared with that in LV-DJ-1-RNAi-ctrl group (P<0.01; Fig. 7D); however, the inhibition rate in the LV-DJ-1 group was not statistically significant compared with that in the control group (P>0.05; Fig. 7D). These results suggest that DJ-1 expression may predict the effect of LFP chemotherapy in patients with CRC.
Discussion
The pathogenesis of colorectal cancer (CRC) is a complex process, which is associated with the abnormal expression of oncogenes and tumor-suppressor genes (27). In the progression of CRC, novel molecular markers may be valuable as early diagnostic markers or indicators of treatment efficacy (28). Our previous research revealed that molecular markers can predict metastasis and prognosis in patients with CRC (29,30). In the present study, protein/nucleic acid deglycase DJ-1 (DJ-1) expression was found to be significantly different between CRC and FHC cell lines, and between CRC tissues and normal adjacent tissues. Furthermore, subsequent experiments were performed to fully elucidate the underlying mechanisms and clinical significance of DJ-1 in CRC.
DJ-1 is associated with the development of cancer. Previous studies have revealed that DJ-1 promoted the invasion and metastasis in numerous types of tumors, including liver cancer (31), laryngeal carcinoma (32), lung cancer (33), and breast cancer (33). The mechanism involved may be associated with PI3K/Akt, SRC/ERK/uPA and other signaling pathways (12,25,34). Additionally, DJ-1 could induce apoptosis and promote cell EMT (25,35). In the present study, patients with CRC and high DJ-1 expression levels had a poorer disease-free survival, and multivariate Cox proportional hazards regression analysis revealed that DJ-1 expression was an independent negative prognostic factor following adjustment by sex, pathological classification, TNM stage and tumor diameter in CRC database analysis.
To validate these results, LV-DJ-1, LV-DJ-1-ctrl, LV-DJ-1-RNAi and LV-DJ-1-RNAi-ctrl cell lines were constructed to investigate the biological function of DJ-1 in CRC. In vitro, cell proliferation was significantly increased in LV-DJ-1 and decreased in LV-DJ-1-RNAi cells when compared with that in the respective controls. Moreover, the migration and invasion abilities of the LV-DJ-1 cells were increased, whereas these abilities were decreased in the LV-DJ-1-RNAi cells when compared with those in the corresponding controls. Previous research mechanisms have been clarified that DJ-1 could promote CRC cell growth or metastasis through the PTEN-AKT, PLAGL2/Wnt/BMP4 pathways (13,14). Subsequently, the related mechanisms involved were investigated in the present study. The results indicated that increased DJ-1 expression induced cell proliferation by regulating PI3K/Akt/p27/cyclin E signaling, and promoted cell invasion and metastasis by regulating PI3K/Akt/mTOR signaling or induced EMT in vitro. In addition, two models of subcutaneous implantation and peritoneal metastases were constructed to investigate the role of DJ-1 in tumorigenesis. The growth of tumors was observed and the regulation between DJ-1 and p-PI3K, p-Akt, p27 and cyclin E protein expression in xenograft tumor tissues was validated using IHC. In addition, the expression levels of DJ-1, p-PI3K, p-Akt, p-mTOR, NF-κB, Snail, E-cadherin, N-cadherin and vimentin were also analyzed in peritoneal metastasis tumor tissues. The analysis of these results indicates that DJ-1 promotes CRC cell growth and metastasis in vivo, which is consistent with the results from CRC cell proliferation and invasion in vitro.
Notably, the CRC database, which contains samples from 470 patients, was used to investigate the association between DJ expression and postoperative adjuvant chemotherapy. In the CRC database, 86 patients with CRC were treated with LFP chemotherapy. Using the Kaplan-Meier curve method and multivariate Cox proportional hazard regression, LFP treatment increased the overall survival (OS) compared with that in patients who underwent surgery alone and with low DJ-1 expression levels, whereas a lower OS was found in patients with high DJ-1 expression levels. To confirm this conclusion, the drugs 5-FU and L-OHP were used to act on LV-DJ-1, LV-DJ-1-ctrl, LV-DJ-1-RNAi and LV-DJ-1-RNAi-ctrl cell lines. The results indicate that the inhibition rate of the LV-DJ-1-RNAi group was higher compared with that in the LV-DJ-1-RNAi-ctrl group, but not in the LV-DJ-1 group.
In conclusion, DJ-1 was the most unfavorable prognostic factor for patients with CRC. High DJ-1 expression levels are positively associated with poorer survival in patients with CRC. The investigations into the molecular mechanisms revealed that DJ-1 increased cell proliferation by regulating the PI3K/Akt/p27/cyclin E signaling pathway and induced CRC metastasis via regulating the PI3K/Akt/mTOR signaling pathway or by inducing EMT in vitro and in vivo. DJ-1 may play a role as an oncogene in CRC tumorigenesis and may be involved in the progression of CRC. Therefore, DJ-1 appears to be a significant prognostic indicator for patients with CRC and an effective marker for predicting the efficacy of chemotherapy. However, the results from the present study require validation in larger retrospective and prospective CRC cohorts.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81773944), the Young Medicine Focus Talent Foundation of Jiangsu Province (grant no. QNRC2016206), the Postgraduate Research by Practice Innovation Program of Jiangsu Province (grant nos. KYCX18_2382 and KYCX17_1892), the Wuxi City Health Planning Commission project (grant no. MS201815), and the Natural Science Foundation of Jiangsu Province of China (grant no. BK20191149).
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
YuZ and YL conceived and coordinated the project. WW, HW, LX, TN and FJ performed experiments and collected the data. WW and LX performed overall data interpretation. JD, YaZ, and IS interpreted the data and critically reviewed the manuscript. WW wrote the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The present study was granted ethics approval by the Institutional Review Board of Yixing Hospital Affiliated to Medical College of Yangzhou University (Yixing, Jiangsu). All patients provided written informed consent and all acquired data were assured of anonymity and confidentiality. All experimental animal procedures were performed in compliance with the institutional ethics requirements and approved by the Institutional Animal Care and Use Committee of Yangzhou University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kang D, Park JM, Jung CK, Lee BI, Oh ST, Choi MG. Prognostic impact of membranous ATP-binding cassette Sub-family G member 2 expression in patients with colorectal carcinoma after surgical resection. Cancer Biol Ther. 2015;16:1438–1444. doi: 10.1080/15384047.2015.1071736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Tsikitis VL, Larson DW, Huebner M, Lohse CM, Thompson PA. Predictors of recurrence free survival for patients with stage II and III colon cancer. BMC Cancer. 2014;14:336. doi: 10.1186/1471-2407-14-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuo J, Ishikawa T, Boutros S, Xiao Z, Humtsoe JO, Kramer RH. Bcl-2 overexpression induces a partial epithelial to mesenchymal transition and promotes squamous carcinoma cell invasion and metastasis. Mol Cancer Res. 2010;8:170–182. doi: 10.1158/1541-7786.MCR-09-0354. [DOI] [PubMed] [Google Scholar]
- 5.Erstad DJ, Tumusiime G, Cusack JC., Jr Prognostic and predictive biomarkers in colorectal cancer: Implications for the clinical surgeon. Ann Surg Oncol. 2015;22:3433–3450. doi: 10.1245/s10434-015-4706-x. [DOI] [PubMed] [Google Scholar]
- 6.Bonifati V, Rizzu P, Squitieri F, Krieger E, Vanacore N, van Swieten JC, Brice A, van Duijn CM, Oostra B, Meco G, Heutink P. DJ-1( PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol Sci. 2003;24:159–160. doi: 10.1007/s10072-003-0108-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HJ, Siu MK, Jiang LL, Mak VC, Ngan HY, Cheung AN. Overexpression of the Parkinson disease protein DJ-1 and its regulator PTEN in gestational trophoblastic disease. Int J Gynecol Pathol. 2010;29:468–475. doi: 10.1097/PGP.0b013e3181de3068. [DOI] [PubMed] [Google Scholar]
- 8.Bai J, Guo C, Sun W, Li M, Meng X, Yu Y, Jin Y, Tong D, Geng J, Huang Q, et al. DJ-1 may contribute to metastasis of non-small cell lung cancer. Mol Biol Rep. 2012;39:2697–2703. doi: 10.1007/s11033-011-1024-7. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Kang M, Lu W, Guo Q, Zhang B, Xie Q, Wu Y. DJ-1, a novel biomarker and a selected target gene for overcoming chemoresistance in pancreatic cancer. J Cancer Res Clin Oncol. 2012;138:1463–1474. doi: 10.1007/s00432-012-1205-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu HY, Duan GL, Xu RY, Li XR, Xiao L, Zhao L, Ma ZX, Xu XW, Qiu LJ, Zhu ZM, Chen HP. DJ-1 overexpression confers the multidrug resistance phenotype to SGC7901 cells by upregulating P-gp and Bcl-2. Biochem Biophys Res Commun. 2019;519:73–80. doi: 10.1016/j.bbrc.2019.08.131. [DOI] [PubMed] [Google Scholar]
- 11.Aleyasin H, Rousseaux MW, Marcogliese PC, Hewitt SJ, Irrcher I, Joselin AP, Parsanejad M, Kim RH, Rizzu P, Callaghan SM, et al. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci USA. 2010;107:3186–3191. doi: 10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He X, Zheng Z, Li J, Ben Q, Liu J, Zhang J, Ji J, Yu B, Chen X, Su L, et al. DJ-1 promotes invasion and metastasis of pancreatic cancer cells by activating SRC/ERK/uPA. Carcinogenesis. 2012;33:555–562. doi: 10.1093/carcin/bgs002. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y, Chen Q, Liu QX, Zhou D, Lu X, Deng XF, Yang H, Zheng H, Qiu Y. High expression of DJ-1 promotes growth and invasion via the PTEN-AKT pathway and predicts a poor prognosis in colorectal cancer. Cancer Med. 2018;7:809–819. doi: 10.1002/cam4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Liu H, Zhang L, Liu X, Zhang C, Wang Y, He Q, Zhang Y, Li Y, Chen Q, et al. DJ-1 promotes colorectal cancer progression through activating PLAGL2/Wnt/BMP4 axis. Cell Death Dis. 2018;9:865. doi: 10.1038/s41419-018-0883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng H, Zhou C, Lu X, Liu Q, Liu M, Chen G, Chen W, Wang S, Qiu Y. DJ-1 promotes survival of human colon cancer cells under hypoxia by modulating HIF-1α expression through the PI3K-AKT pathway. Cancer Manag Res. 2018;10:4615–4629. doi: 10.2147/CMAR.S172008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardo M, García A, Thomas B, Piñeiro A, Akoulitchev A, Dwek RA, Zitzmann N. The characterization of the invasion phenotype of uveal melanoma tumour cells shows the presence of MUC18 and HMG-1 metastasis markers and leads to the identification of DJ-1 as a potential serum biomarker. Int J Cancer. 2006;119:1014–1022. doi: 10.1002/ijc.21942. [DOI] [PubMed] [Google Scholar]
- 17.Kawate T, Iwaya K, Koshikawa K, Moriya T, Yamasaki T, Hasegawa S, Kaise H, Fujita T, Matsuo H, Nakamura T, et al. High levels of DJ-1 protein and isoelectric point 6.3 isoform in sera of breast cancer patients. Cancer Sci. 2015;106:938–943. doi: 10.1111/cas.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haapasalo J, Nordfors K, Granberg KJ, Kivioja T, Nykter M, Haapasalo H, Soini Y. NRF2, DJ1 and SNRX1 and their prognostic impact in astrocytic gliomas. Histol Histopathol. 2018;33:791–801. doi: 10.14670/HH-11-973. [DOI] [PubMed] [Google Scholar]
- 19.Benati M, Montagnana M, Danese E, Paviati E, Giudici S, Ruzzenente O, Franchi M, Lippi G. The clinical significance of DJ-1 and HE4 in patients with endometrial cancer. J Clin Lab Anal. 2018;32:e22223. doi: 10.1002/jcla.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Wu X, Zhang J, Chen Y, Xu J, Xia X, He S, Qiang F, Li A, Shu Y, et al. CHIP functions as a novel suppressor of tumour angiogenesis with prognostic significance in human gastric cancer. Gut. 2013;62:496–508. doi: 10.1136/gutjnl-2011-301522. [DOI] [PubMed] [Google Scholar]
- 21.Bai J, Zhou Y, Chen G, Zeng J, Ding J, Tan Y, Zhou J, Li G. Overexpression of Cullin1 is associated with poor prognosis of patients with gastric cancer. Hum Pathol. 2011;42:375–383. doi: 10.1016/j.humpath.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Chen Y, Deng J, Zhou J, Gu X, Tang Y, Zhang G, Tan Y, Ge Z, Huang Y, et al. Cullin1 is a novel prognostic marker and regulates the cell proliferation and metastasis in colorectal cancer. J Cancer Res Clin Oncol. 2015;141:1603–1612. doi: 10.1007/s00432-015-1931-4. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Wu X, Chen Y, Zhang J, Ding J, Zhou Y, He S, Tan Y, Qiang F, Bai J, et al. Prognostic and predictive role of JWA and XRCC1 expressions in gastric cancer. Clin Cancer Res. 2012;18:2987–2996. doi: 10.1158/1078-0432.CCR-11-2863. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Yao Y, Wei H, Liu L, Liu L, Bai S, Li C, Luo Y, Zeng R, Han M, Ge S, Xu G. Upregulated DJ-1 promotes renal tubular EMT by suppressing cytoplasmic PTEN expression and Akt activation. J Huazhong Univ Sci Technolog Med Sci. 2011;31:469. doi: 10.1007/s11596-011-0475-3. [DOI] [PubMed] [Google Scholar]
- 26.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16:359–369. doi: 10.6004/jnccn.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papachristou DJ, Korpetinou A, Giannopoulou E, Antonacopoulou AG, Papadaki H, Grivas P, Scopa CD, Kalofonos HP. Expression of the ribonucleases Drosha, Dicer, and Ago2 in colorectal carcinomas. Virchows Arch. 2011;459:431–440. doi: 10.1007/s00428-011-1119-5. [DOI] [PubMed] [Google Scholar]
- 28.Oldenhuis CN, Oosting SF, Gietema JA, de Vries EG. Prognostic versus predictive value of biomarkers in oncology. Eur J Cancer. 2008;44:946–953. doi: 10.1016/j.ejca.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Deng J, Chen W, Du Y, Wang W, Zhang G, Tang Y, Qian Z, Xu P, Cao Z, Zhou Y. Synergistic efficacy of Cullin1 and MMP-2 expressions in diagnosis and prognosis of colorectal cancer. Cancer Biomark. 2017;19:57–64. doi: 10.3233/CBM-160341. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Deng J, Wang Q, Yao Q, Chen W, Tan Y, Ge Z, Zhou J, Zhou Y. Synergistic role of Cul1 and c-Myc: Prognostic and predictive biomarkers in colorectal cancer. Oncol Rep. 2017;38:245–252. doi: 10.3892/or.2017.5671. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Yang Z, Wei H, Shen W, Liu J, Yin Q, Li X, Yi J. Increased DJ-1 and its prognostic significance in hepatocellular carcinoma. Hepatogastroenterology. 2010;57:1247–1256. [PubMed] [Google Scholar]
- 32.Shen Z, Ren Y, Ye D, Guo J, Kang C, Ding H. Significance and relationship between DJ-1 gene and surviving gene expression in laryngeal carcinoma. Eur J Histochem. 2011;55:e9. doi: 10.4081/ejh.2011.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Vasseur S, Afzal S, Tardivel-Lacombe J, Park DS, Iovanna JL, Mak TW. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci USA. 2009;106:1111–1116. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasseur S, Afzal S, Tomasini R, Guillaumond F, Tardivel-Lacombe J, Mak TW, Iovanna JL. Consequences of DJ-1 upregulation following p53 loss and cell transformation. Oncogene. 2012;31:664–670. doi: 10.1038/onc.2011.268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.