Abstract
Long non-coding RNAs (lncRNAs) have been validated to mediate the development of atherosclerosis (AS). In the present study, the molecular mechanisms and functions of lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) in the advancement of human aortic endothelial cells (HAECs) were investigated. The levels of lncRNA-NEAT1 and miR-638 expression in clinical samples and cells were explored via quantitative reverse transcription polymerase chain reaction. Colony formation and CCK-8 assays were performed to determine the proliferative capacity of cells, and the apoptotic capacity of cells was analyzed on the basis of apoptotic cell proportion and caspase-3 activity. Then, the proportion of cells and correlations among phosphoglycerate kinase 1 (PGK1), NEAT1, and miR-638 were determined through RNA immunoprecipitation and luciferase assays and bioinformatics analysis. Moreover, the expression levels of Ki-67, proliferating cell nuclear antigen, PGK1, Bax, Bcl-2, (p)-mTOR, (p)-AKT, and β-catenin were analyzed via western blot analysis. In the serum of patients with AS and HAECs induced by oxidized low-density lipoprotein (ox-LDL), the expression level of miR-638 was decreased, whereas that of NEAT1 was increased. After ox-LDL therapy, NEAT1 knockdown suppressed HAEC proliferation and stimulated HAEC apoptosis, which could be reversed by the miR-638 inhibitor. NEAT1 inhibited miR-638 expression through direct mutual action. The following mechanical investigations revealed that PGK1 was a miR-638 target, whose expression was increased by NEAT1, a competing endogenous RNA of miR-638. Additionally, the miR-638 inhibitor contributed to proliferation and suppressed apoptosis through the activation of the AKT/mTOR signaling pathway in ox-LDL-induced HAECs. NEAT1 adjusted the AKT/mTOR signaling pathway via miR-638 in ox-LDL-induced HAECs to accelerate their proliferation and impede their apoptosis. This result revealed that NEAT1 may be valuable in the treatment of AS.
Keywords: long non-coding RNA, nuclear paraspeckle assembly transcript 1, atherosclerosis, miR-638, phosphoglycerate kinase 1
Introduction
Cardiovascular diseases (CVDs) are major killers that cause over 17.3 million deaths each year worldwide (1). Atherosclerosis (AS), which accounts for a large proportion of CVDs, may lay a foundation for strokes and heart attacks. AS, an inflammatory disease, is characterized by fibrous cap generation, lipid assembly, and necrotic core formation (2). Human aortic endothelial cells (HAECs) are implicated in the generation of AS lesions (3). In addition, previous research has indicated that the aberrant migration, proliferation, and apoptosis of HAECs is closely related to AS advancement (4). HAEC dysfunction can be influenced by oxidized low-density lipoprotein (ox-LDL), a pivotal risk factor in AS advancement (5). Therefore, in the present research, ox-LDL-induced HAECs were selected as AS cell models to investigate the modulating mechanism implicated in AS.
Long non-coding RNAs (lncRNAs), which are 200-nt long, are mainstay modulators in AS and other complex diseases (6). The significance of lncRNAs in AS has received considerable attention since they can modulate inflammatory responses, and lipid metabolism as well as cell adhesion, migration, proliferation, and apoptosis (7). The expression of lncRNA H19 has been revealed to be markedly increased in AS, and to upregulate cell proliferation and suppress the apoptosis of HAECs (8). LncRNA RNCR3 was revealed to accelerate the development of AS by impeding the migration and proliferation and triggering the apoptosis of vascular smooth muscle cells in vitro (9).
The lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) has been confirmed to be a pivotally blotted gene in cell differentiation and growth (10). NEAT1 modulates ox-LDL-triggered inflammation and lipid uptake in macrophages via paraspeckle formation (11). In addition, NEAT1 blockade was revealed to suppress inflammation response and lipid intake by adjusting miR-342-3p in human macrophages (THP-1 cells) (12). However, the exact molecular mechanism of NEAT1 in the growth of AS requires further research.
miRNAs are long small ncRNAs with lengths of 22 nt and post-transcriptionally modulate genes (13). miRNAs critically adjust AS pathological processes, including cholesterol efflux and lipoprotein metabolism, lipid and cholesterol biosynthesis, endothelial cell biology, immune responses, and vascular function (14). miR-638 was revealed to suppress tumors in breast (15), hepatocellular (16), and other cancers. However, the roles of miR-638 in AS remain unclear.
The present research aimed to identify the underlying treatment targets for AS by further investigating the possible roles and molecular bases of NEAT1 and miR-638 in the advancement of HAECs.
Materials and methods
Clinical specimens and ethics statement
The present research was conducted with the approval of the Ethics Committee of Tianjin Chest Hospital. A total of 10 ml of blood specimens was harvested from 24 healthy volunteers without malignant tumors, recent infection, AS disease, autoimmune diseases, or inflammatory diseases (<1 month) and from 24 patients with AS. The samples were placed in centrifuge tubes without anticoagulant. Subsequently, the specimens were maintained for approximately 1 h at room temperature, and serum was collected through 50 min of centrifugation at 1,006 × g. All the patients and healthy volunteers signed informed consent to participate in this study.
Cell culture
HAECs were obtained from the American Type Culture Collection and cultured in DMEM containing 10% fetal bovine serum, 100 U/ml penicillin, and streptomycin in an incubator with 5% CO2 at 37°C.
Cell transfection and treatment
The full-length NEAT1 sequence was amplified through polymerase chain reaction (PCR), and pcDNA-NEAT1 overexpression plasmids were constructed following the subcloning of the sequence into pcDNA3.1 vectors (Invitrogen; Thermo Fisher Scientific, Inc.). Shanghai GenePharma Co., Ltd. synthesized miR-638 mimics, the miR-638 inhibitor, and their negative control (miR-con), as well as small interference RNA (siRNA) specific for NEAT1 (si-NEAT1#1 and si-NEAT1#2) and its negative control (si-con). Cells were transfected with Lipofectamine 2000 reagent acquired from Invitrogen in accordance with the manufacturer's instructions. HAECs were treated with different concentrations of ox-LDL (0, 25, 50 and 75 µg/ml) for 24 h to elucidate the effects of ox-LDL, which was provided by Biosynthesis Biotechnology, on NEAT1 and miR-638 expression levels.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assay
TRIzol® reagent obtained from Invitrogen; Thermo Fisher Scientific, Inc. was employed for total RNA extraction in accordance with the manufacturer's guidelines. Following the measurement of the concentration and purity of RNA specimens, RT was performed to synthesize cDNA specimens with U6 and GAPDH as the internal reference. A PCR solution was prepared with a premixed solution containing forward (F)/reverse (R) primers, DEPC from Beyotime Institute of Biotechnology, SYBR Green from Applied Biosystems; Thermo Fisher Scientific, Inc., and templates. Subsequently, the prepared solution was placed on an RT-PCR instrument for PCR amplification. miRNAs underwent RT into cDNAs with the use of a miRNA RT Kit from Tiangen Biotech Co., Ltd. and then subjected to PCR and quantitative analysis on the basis of the instructions of the miRNA qPCR kit from the same company. The primer sequences were as follows: lnc-NEAT1 F, TGTCCCTCGGCTATGTCAGA and R, GAGGGGACGTGTTTCCTGAG; GAPDH F, TGACCACAGTCCATGCCATCAC and R, GCCTGCTTCACCACCTTCTTGA; miR-638 F, AAGGGATCGCGGGCGGGT and R, CAGTGCAGGGTCCGAGGT; and U6 F, CTCGCTTCGGCAGCACATATACTA and R, ACGAATTTGCGTGTCATCCTTGC.
Western blot analysis
Total proteins were extracted with RIPA lysis buffer acquired from Beyotime Institute of Biotechnology and then quantified with a Pierce BCA Protein Assay Kit obtained from Thermo Fisher Scientific, Inc. SDS-PAGE (10%) was used for the isolation of 50 µg of proteins, which were then transferred to a PVDF membrane (EMD Millipore). The membrane was sealed with 5% skim milk at room temperature for 1 h and incubated with primary antibodies against proliferating cell nuclear antigen (PCNA product no. ab92552; dilution 1:1,000), β-actin (product no. ab8226; dilution 1:2,000), proliferation marker protein Ki-67 (Ki-67 product no. ab92742; dilution 1:2,000), PGK1 (product no. ab38007; dilution 1:1,000), AKT (product no. ab179463; dilution 1:1,000), p-AKT (product no. ab38449; dilution 1:1,500), mTOR (product no. ab2732; dilution 1:1,000), p-mTOR (product no. ab109268; dilution 1:1,500), Bax (product no. ab182733; dilution 1:1,000) and Bcl-2 (product no. ab196495; dilution 1:1,000) at 4°C overnight. Then, at room temperature, the membrane was further probed for 1 h using secondary antibodies (product nos. ab97040 and ab97080; dilution 1:3,000) coupled with HRP. All the antibodies were purchased from Abcam. Finally, Clarity Max™ Western ECL Substrate obtained from Bio-Rad Laboratories, Inc. was used to improve particular protein signals. The antibodies aforementioned were commercially obtained from Abcam.
CCK-8 assay
Approximately 1×104 HAECs were inoculated into each well of a 96-well plate overnight. A total of 10 µl of CCK-8 solution (Dojindo Molecular Technologies, Inc.) was added to each well following transfection for 0, 24, 48, or 72 h. Next, the cells were further cultured at 37°C for 3 h. Cell proliferation was evaluated after the measurement of the absorbance (A)450.
Colony formation assay
After seeding in a 12-well plate for 15 days of culture in complete medium, the transfected HAECs were immobilized with methanol and then dyed with 0.1% crystal violet solution (room temperature for 20 min) provided by Sigma-Aldrich; Merck KGaA. Finally, colonies containing at least 50 cells were quantified with the use of an DMi1 inverted microscope (Leica Microsystems).
Cell apoptosis assays
Cells undergoing apoptosis were examined using Annexin V FITC/PI apoptosis detection kit procured from Vazyme Biotech Co., Ltd. in accordance with the manufacturer's instructions. Briefly, harvested cells were resuspended in 1X binding buffer (100 µl), and a dyeing solution consisting of PI (5 µl) and Annexin V FITC (5 µl) was added. After mixing, the cells were incubated for 15 min at room temperature away from light. Then, prior to the addition of 1X binding buffer (240 µl), apoptotic cells were counted with the use of a flow cytometric apparatus supplied by BD Biosciences.
Examination of caspase-3 activity
Caspase-3 activity was assessed using Caspase-3 Assay Kit (Colorimetric) supplied by Abcam in accordance with the manufacturer's instructions. Briefly, 5×106 transfected cells were resuspended with 50 µl of cell lysate cooled in advance. Then, cell supernatant separation and protein concentration assessment were performed. Subsequently, 4 mM DEVD-p-NA substrate (5 µl) with a final concentration of 200 µM and 2X reaction buffer with 10 mM DTT (50 µl) were added to 50 µl of each sample.
Subcellular fractions
In reference to the manufacturer's instructions, RNA in HAEC cytoplasm or nucleus was isolated and purified with a cytoplasmic and nuclear RNA purification kit supplied by Norgen Biotek. Then, NEAT1, U6 and GAPDH expression levels in the cytoplasmic and nuclear fractions were independently assessed via qRT-qPCR.
Luciferase activity analysis
The 3′UTR fragments of PGK1 and NEAT1 comprising the binding sites of miR-638 were subjected to PCR amplification and established with psiCHECK-2 vector supplied by Promega Corporation to form PGK1-WT and NEAT1-WT reporters. Additionally, PGK1-MUT and NEAT1-MUT reporters comprising MUT miR-638 binding sites were produced via a Quickchange Multi Site-Directed Mutagenesis kit purchased from Stratagene; Agilent Technologies, Inc. Subsequently, HAECs were treated with the established luciferase reporters and plasmids or miRNAs. After 48 h, cell luciferase activity was assessed with the use of a dual luciferase reporter assay kit supplied by Promega Corporation in accordance with the manufacturer's instructions.
RNA immunoprecipitation (RIP) assay
To probe the presence of NEAT1 in the RNA-induced silencing complex (RISC), an EZ-Magna RIP kit commercially provided by EMD Millipore was utilized for a RIP assay with the use of Ago2 antibody (product no. ab32381; dilution 1:150) from Abcam. Briefly, HAECs were lysed using RIP lysis buffer and incubated with anti-IgG antibody (cat. no. 12-370; dilution 1:150; Merck Millipore) supplied by EMD Millipore or anti-Ago2 antibody obtained from Abcam and protein A/G magnetic beads. RNAs in complexes linked with magnetic beads were subsequently purified. Ultimately, the expression levels of miR-638 and NEAT1 were examined via qRT-PCR using Ago2 or IgG antibody.
Statistical analysis
Each assay was implemented at least thrice, and the obtained data were presented as the mean ± SEM. Intergroup data differences were probed using Student's t-test or one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. P<0.05 indicated a statistically significant difference.
Results
NEAT1 is increased and miR-638 is decreased in the serum of patients with AS and ox-LDL-triggered HAECs
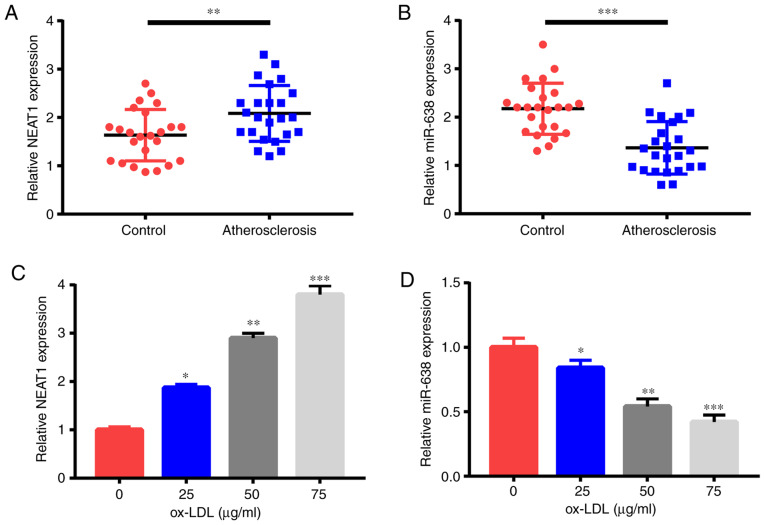
First, NEAT1 and miR-638 expression levels were detected through qRT-PCR. Compared with the 24 healthy controls, the 24 patients with AS had significantly increased NEAT1 expression (Fig. 1A) but a significantly reduced miR-638 expression (Fig. 1B) in their serum samples. Moreover, ox-LDL treatment triggered the increase of NEAT1 expression (Fig. 1C) and the decrease of miR-638 expression (Fig. 1D) in HAECs in a dose-dependent manner. Therefore, NEAT1 and miR-638 were revealed to be possible pivotal controllers in AS advancement.
Figure 1.
NEAT1 expression is increased and miR-638 is decreased in the serum of AS cases and in ox-LDL-triggered HAECs. Expression levels of (A) NEAT1 and (B) miR-638 in the serum of 24 AS cases and 24 healthy controls. HAECs were treated with ox-LDL (0, 25, 50, and 75 µg/ml) for 1 day, and (C) NEAT1 and (D) miR-638 expression levels were examined. *P<0.05, **P<0.01 and ***P<0.001. NEAT1, nuclear paraspeckle assembly transcript 1; AS, atherosclerosis; HAECs, human aortic endothelial cells; ox-LDL, oxidized low-density lipoprotein.
NEAT1 downregulation decreases the proliferation and triggers the apoptosis of ox-LDL-induced HAECs
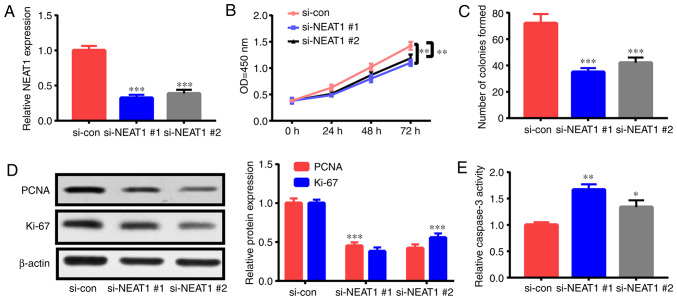
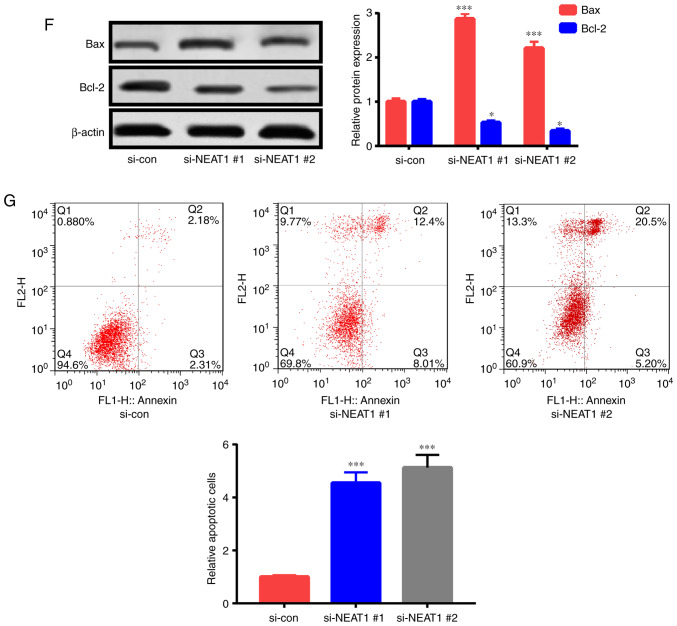
The roles of NEAT1 in the apoptosis and proliferation of ox-LDL-triggered HAECs were investigated by interfering with NEAT1 expression via siRNA (si-NEAT1#1 and si-NEAT1#2) treatment. After transfection with si-NEAT1#1 or si-NEAT1#2, HAECs were treated with 50 µg/ml ox-LDL for 24 h, and their apoptosis rate and proliferation capacity were assessed. NEAT1 expression was significantly suppressed by transfection with si-NEAT1#1 and si-NEAT1#2 (Fig. 2A). As revealed in the CCK-8 assay, NEAT1 downregulation suppressed HAEC proliferation (Fig. 2B). Furthermore, colony formation assay ascertained that NEAT1 downregulation impeded the colony formation ability of HAECs (Fig. 2C). Moreover, the expression levels of proliferation-related indexes [Ki-67 and proliferating cell nuclear antigen (PCNA)] were detected. Their expression levels in HAECs were significantly decreased subsequent to NEAT1 downregulation (Fig. 2D). In addition, the flow cytometric experiment revealed that apoptosis in HAECs treated with NEAT1 siRNAs was increased relative to that in the control (Fig. 2G). Fig. 2E and F revealed that NEAT1 downregulation significantly enhanced caspase-3 activity and Bax expression and decreased Bcl-2 expression. The aforementioned findings indicated that NEAT1 downregulation inhibited HAEC proliferation and stimulated apoptosis.
Figure 2.
NEAT1 downregulation decreases cell proliferation and triggers the apoptosis of ox-LDL-induced HAECs. HAECs were subjected to si-NEAT1#1, si-NEAT1#2, or si-con transfection and ox-LDL (50 µg/ml) treatment for 1 day. Then, (A) NEAT1 expression, (B) proliferation potential, (C) colony formation ability, (D) PCNA and Ki-67 expression levels, (E) caspase-3 activity, (F) Bcl-2 and Bax expression levels and (G) cells undergoing apoptosis were detected. *P<0.05, **P<0.01 and ***P<0.001. NEAT1, nuclear paraspeckle assembly transcript 1; ox-LDL, oxidized low-density lipoprotein; HAECs, human aortic endothelial cells; PCNA, proliferating cell nuclear antigen.
NEAT1 reduces miR-638 expression by direct mutual action
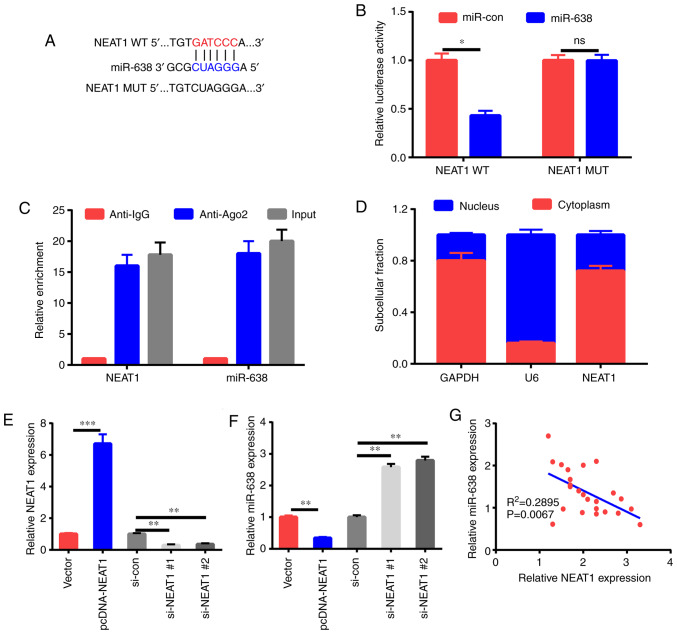
Bioinformatics analysis was applied to verify the possible miRNA sponged by NEAT1. Some potential binding sites between miR-638 and NEAT1 were reveealed (Fig. 3A). This speculation was further demonstrated by the transfection of HAECs with NEAT1-WT or NEAT1-MUT luciferase reporter and miR-638 or miR-con. The results of the following luciferase assay revealed that relative to miR-con treatment, miR-638 treatment decreased by half the luciferase activity of NEAT1-WT reporter but did not influence that of the NEAT1-MUT reporter (Fig. 3B). Then, the direct binding relationship between miR-638 and NEAT1 was elucidated via RIP assay. Fig. 3C revealed that miR-638 and NEAT1 were markedly upregulated by Ago2 antibody relative to the control IgG antibody, revealing the presence of miR-638 and NEAT1 in the RISC. The fractionation experiment in subcells revealed that NEAT1 was primarily present in the cytoplasm of HAECs, indicating that NEAT1 may sponge miR-638 (Fig. 3D). Furthermore, miR-638 expression significantly decreased upon overexpression of NEAT1 expression; these effects were abrogated by si-NEAT1 transfection (Fig. 3E and F). Lastly, an inverse relationship between miR-638 expression and NEAT1 expression was observed in the serum of patients (Fig. 3G).
Figure 3.
NEAT1 curbs miR-638 expression through direct mutual action. (A) Speculated binding sites between miR-638 and NEAT1 and MUT sites in NEAT1-MUT reporter are displayed. (B) Luciferase activity was assessed in HAECs co-transfected with NEAT1-WT or NEAT1-MUT reporter and miR-638 or miR-con. (C) Efficiency of binding between miR-638 and NEAT1 to Ago2 protein in HAECs was investigated via RIP experiment. (D) U6, GAPDH, and NEAT1 expression levels in HAEC cytoplasm and nucleus were assessed. (E and F) HAECs were treated with pcDNA-NEAT1 overexpression plasmid, pcDNA3.1 empty vector, si-con, si-NEAT1#1, or si-NEAT1#2, and miR-638 and NEAT1 levels were examined. (G) Correlation analysis of NEAT1 and miR-638 expression levels. *P<0.05, **P<0.01, and ***P<0.001. NEAT1, nuclear paraspeckle assembly transcript 1; HAECs, human aortic endothelial cells; RIP, RNA immunoprecipitation.
miR-638 inhibitor reverses the effects of NEAT1 downregulation on the apoptosis and proliferation of ox-LDL-induced HAECs
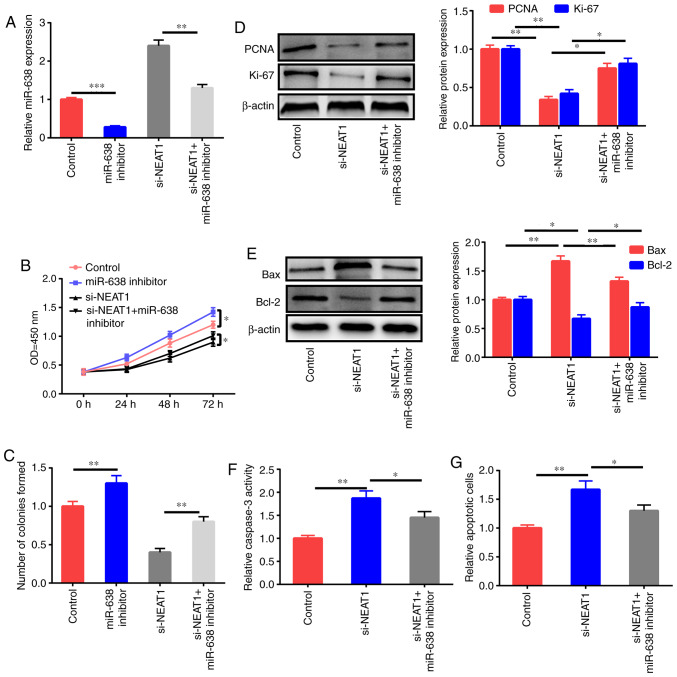
Whether the antiproliferation and proapoptosis effects of NEAT1 downregulation were mediated by miR-638 was further studied through the treatment of HAECs with the control, miR-638 inhibitor, si-NEAT1, and si-NEAT1+miR-638 inhibitor after ox-LDL stimulation, and miR-638 expression was assessed (Fig. 4A). miR-638 inhibition significantly enhanced the proliferation ability and colony formation ability of HAECs, and NEAT1 downregulation decreased cell growth; this effect was reversed by the miR-638 inhibitor (Fig. 4B and C). The downregulation of PCNA and Ki-67 expression after treatment with si-NEAT1 was partly reversed by inhibition of miR-638 (Fig. 4D). Impeding miR-638 expression partly reversed the increase in Bax expression, the decrease of Bcl-2, and the upregulation of caspase-3 activity and cell apoptosis induced by si-NEAT1 (Fig. 4E-G).
Figure 4.
miR-638 inhibitor reverses the effects of NEAT1 downregulation on the apoptosis and proliferation of ox-LDL-induced HAECs. (A) HAECs were treated with the control, miR-638 inhibitor, si-NEAT1, and si-NEAT1+miR-638 inhibitor and induced by ox-LDL (50 µg/ml) for 1 day, and the expression level of miR-638 was assessed. (B) Cell proliferation potential. (C) Colony formation ability. (D) Ki-67 and PCNA expression levels. (E) Bax and Bcl-2 expression levels. (F) Caspase-3 activity. (G) Cells subjected to apoptosis. Data are presented as the mean ± SEM (n=3) of three independent experiments. *P<0.05, **P<0.01 and ***P<0.001. NEAT1, nuclear paraspeckle assembly transcript 1; HAECs, human aortic endothelial cells; ox-LDL, oxidized low-density lipoprotein; PCNA, proliferating cell nuclear antigen.
PGK1 is a target of miR-638
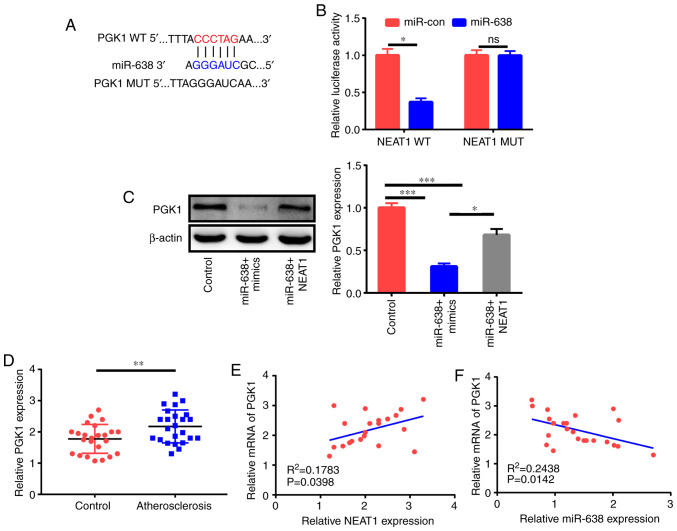
LncRNAs function as competing endogenous RNAs (ceRNAs) of miRNAs to adjust target mRNA expression (17). Thus, bioinformatics analysis was applied to elucidate the possible targets of miR-638. As revealed in Fig. 5A, the PGK1 3′UTR comprised the potential binding sequences of miR-638. The subsequent luciferase assay demonstrated that miR-638 decreased the luciferase activity of the PGK1-WT reporter but not that of the PGK1-MUT reporter (Fig. 5B). The western blot assay further revealed that miR-638 significantly inhibited PGK1 expression, and NEAT1 could partly reverse this effect (Fig. 5C). Clinical data indicated that PGK1 expression in the serum of patients with AS was higher than that in the serum of healthy controls (Fig. 5D). In addition, PGK1 expression had a positive relationship with NEAT1 expression and an inverse association with miR-638 expression (Fig. 5E and F) in patients with AS.
Figure 5.
PGK1 is a miR-638 target. (A) Forecasted binding sequences between miR-638 and PGK1 3′UTR. (B) Examination of luciferase activity at 48 h following the co-transfection of HAECs with PGK1-WT or PGK1-MUT reporter and miR-638 and miR-con. (C) Assessment of the effects of miR-638 mimics and miR-638+NEAT1 on PGK1 protein expression using western blot assay at 48 h after HAEC transfection. (D) PGK1 expression in the serum of 24 healthy controls and 24 AS cases. (E and F) Assessment of the relationship between PGK1 and miR-638 or NEAT1 in the serum of 24 AS cases. *P<0.05, **P<0.01 and ***P<0.001. PGK1, phosphoglycerate kinase 1; HAECs, human aortic endothelial cells; NEAT1, nuclear paraspeckle assembly transcript 1; AS, atherosclerosis.
miR-638 blocks the proliferation of ox-LDL-induced HAECs and facilitates their apoptosis by modulating AKT/mTOR signaling
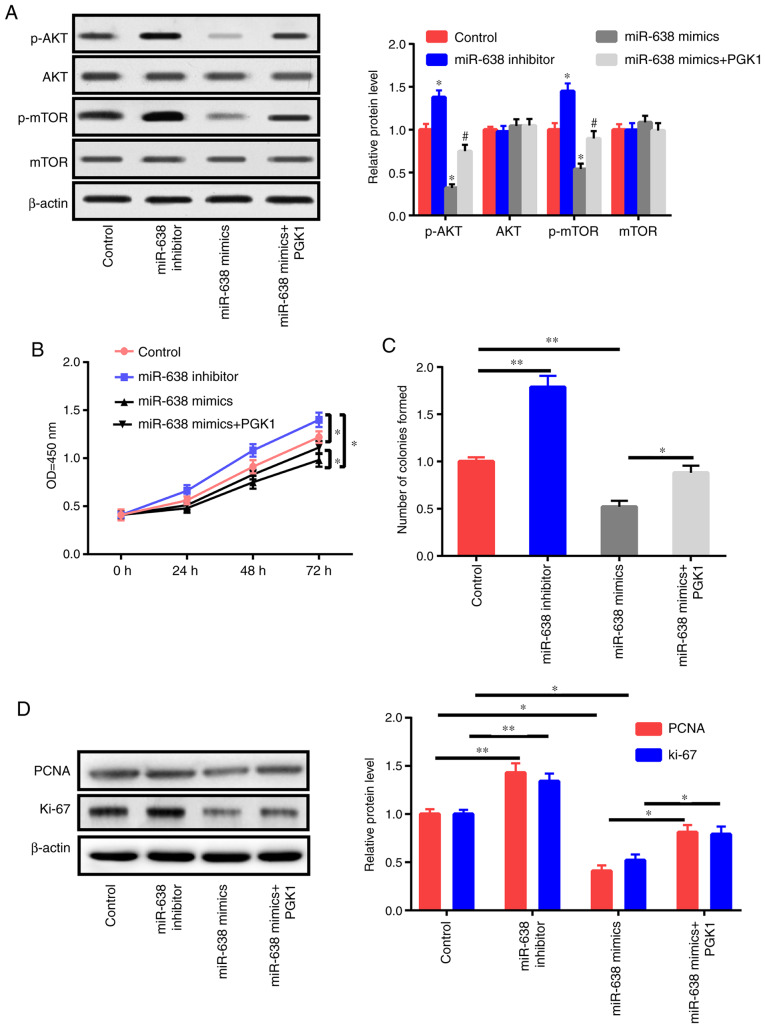
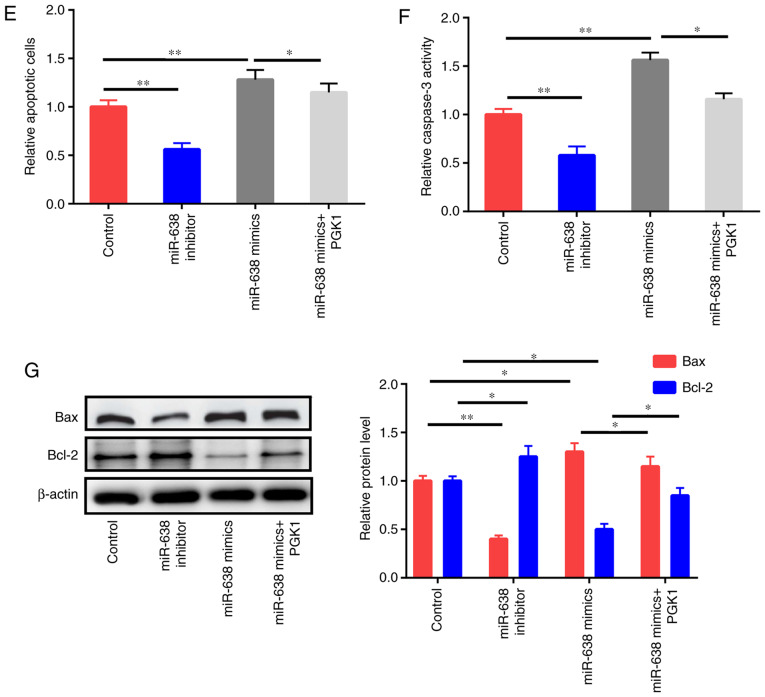
PGK1 activates the AKT/mTOR pathway (18). In the present study, the effects of PGK1 and miR-638 on the AKT/mTOR signaling pathway were also evaluated. Western blot results revealed that the inhibition of miR-638 increased p-AKT and p-mTOR instead of total AKT and mTOR (Fig. 6A). miR-638 overexpression decreased p-AKT and p-mTOR expression but did not affect total AKT and mTOR expression. In addition, the upregulation of PGK1 could partly reverse the effects of miR-638 on p-AKT and p-mTOR (Fig. 6A). Then, HAECs were treated with the control, miR-638 inhibitor, miR-638 mimics, and miR-638 mimics+PGK1 for 24 h. PGK1 upregulation partly reversed the suppressive effects of miR-638 on proliferation capacity and colony formation ability (Fig. 6B and C). The expression levels of PCNA and Ki-67 were also detected (Fig. 6D). PGK1 partly reversed the apoptosis of HAECs caused by the upregulation of miR-638 (Fig. 6E-G). To sum up, these findings indicated that miR-638 exerted its roles via modulation of the AKT/mTOR signaling by targeting PGK1.
Figure 6.
miR-638 adjusts HAEC proliferation and apoptosis through Akt/mTOR signaling. (A) HAECs were treated with the control, miR-638 inhibitor, miR-638 mimics, si-NEAT1, and si-NEAT1+miR-638 inhibitor mimics+PGK1 after 1 day of ox-LDL (50 µg/ml) stimulation, and mTOR, p-mTOR, AKT, and p-AKT expression levels were assessed. (B) Cell proliferation abilities were determined. (C) Colony formation ability. (D) Expression levels of PCNA and Ki-67. miR-638 adjusts HAEC proliferation and apoptosis through Akt/mTOR signaling. (A) HAECs were treated with the control, miR-638 inhibitor, miR-638 mimics, si-NEAT1, and si-NEAT1+miR-638 inhibitor mimics+PGK1 after 1 day of ox-LDL (50 µg/ml) stimulation, and mTOR, p-mTOR, AKT, and p-AKT expression levels were assessed. (B) Cell proliferation abilities were determined. (C) Colony formation ability. (D) Expression levels of PCNA and Ki-67. (E) Cells undergoing apoptosis. (F) Caspase-3 activity. (G) Bcl-2 and Bax expression levels. *P<0.05, **P<0.01 vs. control group, #P<0.05 vs. miR-638 mimics group. HAEC, human aortic endothelial cell; NEAT1, nuclear paraspeckle assembly transcript 1; ox-LDL, oxidized low-density lipoprotein; PCNA, proliferating cell nuclear antigen.
Discussion
Despite the advanced treatment and clarified pathogenesis of AS, AS continues to severely endanger human health and exhibit high morbidity and mortality rates worldwide (19). A growing body of evidence has revealed that ncRNAs, including lncRNAs and miRNAs, exert mainstay effects on AS advancement (20). To further investigate the molecular mechanism of NEAT1, bioinformatics analysis was implemented to determine the possible targets of NEAT1. miR-638 was likely to mutually act with NEAT1. miR-638 has been revealed to be a tumor suppressor (21). The downregulation of miR-638 could promote cell proliferation and inhibit cell apoptosis (15,22). Ox-LDL could trigger AS by boosting EC dysfunction and activation, foaming cell formation, adjusting VSMC behavior, as well as several other mechanisms (23).
Endothelial cells also play a pivotal part in an early step in AS growth and contribute to the formation and progression of AS plaques as well as the occurrence of complications (24). Notably, endothelial dysfunction is associated with smoking, dyslipidemia, obesity, diabetes, mental stress, hypertension, and other cardiovascular risk factors (25). Endothelial cell apoptosis and death can lead to local lipid deposition and eventually AS and even thrombosis (26,27). Thus, the present study aimed to investigate the involvement of NEAT1 in the modulation of AS advancement by adjusting miR-638 in ox-LDL-triggered HAECs.
First, the expression of the level of NEAT1 was confirmed to be increased and the expression level of miR-638 to be decreased in serum samples from AS cases. Second, miR-638 expression was decreased but NEAT1 expression was increased in HAECs after ox-LDL treatment. As revealed by loss-of-function tests, NEAT1 downregulation significantly suppressed the proliferation and facilitated the apoptosis of ox-LDL-triggered HAECs.
Next, qRT-qPCR, fraction assay in subcells, bioinformatics analysis, luciferase assay, and RIP test further confirmed that NEAT1 interfered in miR-638 expression by binding to miR-638. Moreover, an inverse relationship was revealed between miR-638 expression and NEAT1 expression in the serum of AS cases. Thus, the mediation of miR-638 in the effects of NEAT1 on the apoptosis and proliferation of ox-LDL-triggered HAECs was further probed. Findings revealed that the proapoptosis and antiproliferation functions mediated by NEAT1 downregulation were markedly undermined subsequent to miR-638 reduction in ox-LDL-triggered HAECs. According to emerging evidence, miRNAs function by adjusting the stability or translation of target mRNAs. Thus, bioinformatics was utilized to identify the possible target mRNAs of miR-638. The findings revealed that PGK1 was an underlying target of miR-638 as demonstrated by the subsequent luciferase assay. The present study ascertained that NEAT1 increased the expression of the target gene PGK1 in HAECs by functioning as a ceRNA of miR-638. In addition, the mRNA expression of PGK1 was positively associated with NEAT1 expression and inversely correlated with miR-638 expression in the serum of AS cases.
PGK1 plays a key role in glycolytic ATP production. Several lines of evidence suggest that mitochondrial ATP production can activate the PI3K/AKT pathway via P2 receptors in multiple cell types (28,29). Moreover, an increased level of cytosolic free calcium, the vital second messenger of the ATP/P2 receptor-signaling pathway, can activate PI3K through direct linking with its p85 regulatory subunit, resulting in AKT/mTOR pathway activation (30). PGK1 was revealed to activate the AKT and ERK pathways in prostate cancer cells, resulting in the promotion of cell invasion (31). Thus, the effects of PGK1 and miR-638 on the expression levels of genes associated with AKT/mTOR signaling (p-AKT, p-mTOR, total AKT, and total mTOR) were examined in ox-LDL-induced HAECs. miR-638 downregulation activated the AKT/mTOR pathway in ox-LDL-triggered HAECs. In addition, the function of miR-638 in AKT/mTOR signaling was undermined subsequent to PGK1 upregulation. These findings demonstrated that miR-638 suppression increased AKT/mTOR signaling by adjusting PGK1. Then, whether miR-638 influences the apoptosis and proliferation of ox-LDL-triggered HAECs was investigated. The results revealed that PGK1 could partly reverse the effects of miR-638.
To sum up, the present study confirmed that NEAT1 downregulation restrained ox-LDL-triggered HAECs from proliferating and induced their apoptosis by inactivating AKT/mTOR signaling via miR-638. However, the present study has several limitations. First, the number of samples was small, and the results would be more convincing if we had collected additional samples. Second, whether other signaling pathways were affected was not verified in this study, and other key molecules should be detected. Third, in vivo experiments were not performed. In vivo experiments are planned in future studies to strengthen our conclusion.
The present study determined that the NEAT1/miR-638/ PGK1/AKT/mTOR regulatory axis is involved in HAEC proliferation, indicating that NEAT1 has a possible function in AS prevention. However, the mechanism and roles of NEAT1 in AS should be determined with the use of animal models in the future.
Supplementary Material
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
All authors participated in the study and manuscript preparation. XZ, MXG, ZGW and XHQ performed all experiments, QHJ, SL and HYZ performed the statistical analysis and drafted the manuscript. HLC conceived and designed the study and revised the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Tianjin Chest Hospital. All the patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Laslett LJ, Alagona P, Jr, Clark BA, III, Drozda JP, Jr, Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(25 Suppl):S1–S49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Dharmakidari S, Bhattacharya P, Chaturvedi S. Carotid artery stenosis: Medical therapy, surgery, and stenting. Curr Neurol Neurosci Rep. 2017;17:77. doi: 10.1007/s11910-017-0786-2. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: Successes, surprises, and future challenges. Circ Res. 2016;118:531–534. doi: 10.1161/CIRCRESAHA.116.308334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Yang C, Zhang L, Yang P. MicroRNA-210 induces endothelial cell apoptosis by directly targeting PDK1 in the setting of atherosclerosis. Cell Mol Biol Lett. 2017;22:3. doi: 10.1186/s11658-017-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Ren L, Meng Y, Shi L, Chen L, Yu B, Wu Q, Qi G. The protease inhibitor E64d improves ox-LDL-induced endothelial dysfunction in human aortic endothelial cells. Can J Physiol Pharmacol. 2018;96:120–127. doi: 10.1139/cjpp-2017-0016. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Yan CC, Zhang X, You ZH. Long non-coding RNAs and complex diseases: From experimental results to computational models. Brief Bioinform. 2017;18:558–576. doi: 10.1093/bib/bbw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Zheng L, Wang Q, Hu YW. Emerging roles and mechanisms of long noncoding RNAs in atherosclerosis. Int J Cardiol. 2017;228:570–582. doi: 10.1016/j.ijcard.2016.11.182. [DOI] [PubMed] [Google Scholar]
- 8.Pan JX. LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:322–328. [PubMed] [Google Scholar]
- 9.Shan K, Jiang Q, Wang XQ, Wang YN, Yang H, Yao MD, Liu C, Li XM, Yao J, Liu B, et al. Role of long non-coding RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell Death Dis. 2016;7:e2248. doi: 10.1038/cddis.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X, Li Z, Zheng H, Chan MT, Wu WK. NEAT1: A novel cancer-related long non-coding RNA. Cell Prolif. 2017;50 doi: 10.1111/cpr.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang-Fu N, Cheng JS, Wang Y, Li ZW, Wang SH. Neat1 regulates oxidized low-density lipoprotein-induced inflammation and lipid uptake in macrophages via paraspeckle formation. Mol Med Rep. 2018;17:3092–3098. doi: 10.3892/mmr.2017.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Xia JW, Ke ZP, Zhang BH. Blockade of NEAT1 represses inflammation response and lipid uptake via modulating miR-342-3p in human macrophages THP-1 cells. J Cell Physiol. 2019;234:5319–5326. doi: 10.1002/jcp.27340. [DOI] [PubMed] [Google Scholar]
- 13.Rupaimoole R, Slack FJ. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 14.Aryal B, Singh AK, Rotllan N, Price N, Fernández-Hernando C. MicroRNAs and lipid metabolism. Curr Opin Lipidol. 2017;28:273–280. doi: 10.1097/MOL.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Wang J, Liu H. Downregulation of miR-638 promotes progression of breast cancer and is associated with prognosis of breast cancer patients. Onco Targets Ther. 2018;11:6871–6877. doi: 10.2147/OTT.S182034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Zhang D, Jiang J, Dong L. Loss of miR-638 promotes invasion and epithelial-mesenchymal transition by targeting SOX2 in hepatocellular carcinoma. Oncol Rep. 2017;37:323–332. doi: 10.3892/or.2016.5273. [DOI] [PubMed] [Google Scholar]
- 17.Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA Interactions. Methods Mol Biol. 2016;1402:271–286. doi: 10.1007/978-1-4939-3378-5_21. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Ying G, Wang J, Jung Y, Lu J, Zhu J, Pienta KJ, Taichman RS. Characterization of phosphoglycerate kinase-1 expression of stromal cells derived from tumor microenvironment in prostate cancer progression. Cancer Res. 2010;70:471–480. doi: 10.1158/0008-5472.CAN-09-2863. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 20.Zhou T, Ding JW, Wang XA, Zheng XX. Long noncoding RNAs and atherosclerosis. Atherosclerosis. 2016;248:51–61. doi: 10.1016/j.atherosclerosis.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Zheng DH, Wang X, Lu LN, Chen DL, Chen JM, Lin FM, Xu XB. MiR-638 serves as a tumor suppressor by targeting HOXA9 in glioma. Eur Rev Med Pharmacol Sci. 2018;22:7798–7806. doi: 10.26355/eurrev_201811_16404. [DOI] [PubMed] [Google Scholar]
- 22.Zhao P, Zhang BL, Liu K, Qin B, Li ZH. Overexpression of miR-638 attenuated the effects of hypoxia/reoxygenation treatment on cell viability, cell apoptosis and autophagy by targeting ATG5 in the human cardiomyocytes. Eur Rev Med Pharmacol Sci. 2018;22:8462–8471. doi: 10.26355/eurrev_201812_16546. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima K, Nakano T, Tanaka A. The oxidative modification hypothesis of atherosclerosis: The comparison of atherogenic effects on oxidized LDL and remnant lipoproteins in plasma. Clin Chim Acta. 2006;367:36–47. doi: 10.1016/j.cca.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Dong Y, Fernandes C, Liu Y, Wu Y, Wu H, Brophy ML, Deng L, Song K, Wen A, Wong S, et al. Role of endoplasmic reticulum stress signalling in diabetic endothelial dysfunction and atherosclerosis. Diab Vasc Dis Res. 2017;14:14–23. doi: 10.1177/1479164116666762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gimbrone MA, Jr, Garcia-cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hainsworth AH, Oommen AT, Bridges LR. Bridges, Endothelial cells and human cerebral small vessel disease. Brain Pathol. 2015;25:44–50. doi: 10.1111/bpa.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goveia J, Stapor P, Carmeliet P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol Med. 2014;6:1105–1120. doi: 10.15252/emmm.201404156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osorio-Fuentealba C, Contreras-Ferrat AE, Altamirano F, Espinosa A, Li Q, Niu W, Lavandero S, Klip A, Jaimovich E. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kγ-Akt-AS160 in skeletal muscle cells. Diabetes. 2013;62:1519–1126. doi: 10.2337/db12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y. Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol. 2011;193:1257–1274. doi: 10.1083/jcb.201101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng A, Wang S, Yang D, Xiao R, Mattson MP. Calmodulin mediates brain-derived neurotrophic factor cell survival signaling upstream of Akt kinase in embryonic neocortical neurons. J Biol Chem. 2003;278:7591–7599. doi: 10.1074/jbc.M207232200. [DOI] [PubMed] [Google Scholar]
- 31.Lay AJ, Jiang XM, Kisker O, Flynn E, Underwood A, Condron R, Hogg PJ. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature. 2000;408:869–873. doi: 10.1038/35048596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.