Abstract
Both NLRP3 inflammasome and Th17 cells play important roles in the pathogenesis of systemic lupus erythematosus (SLE). Here we tried to investigate whether leptin promotes the differentiation of Th17 cells from lupus mice by activating the NLRP3 inflammasome. Th17 cells induced from MRL/Mp-Fas lpr mice splenocytes under Th17 polarizing condition were treated with leptin at scalar doses during the last 18 h of culture. The mRNA levels of IL-17A, IL-17F, RORγt, IL-1β, IL-18, NLRP3, ASC, and IL-1R1 were detected by quantitative PCR. IL-17A, IL-17F, IL-1β, and IL-18 were tested by ELISA, while the activity of caspase-1 and number of Th17 cells were counted by flow cytometry before/after inhibition of the NLRP3 inflammasome. We found that leptin pushed up the expression of IL-17A, IL-17F, NLRP3, and IL-1β and increased the number of Th17 cells in lupus mice, while the expression of IL-17A, RORγt, and IL-1β and the number of Th17 cells were decreased after inhibition of the NLRP3 inflammasome. Leptin promoted the differentiation of Th17 cells from lupus mice by activating the NLRP3 inflammasome.
Keywords: Leptin, NLRP3 inflammasome, systemic lupus erythematosus, Th17 cells, MRL/Mp-Fas lpr mice
Introduction
The pathogenesis of systemic lupus erythematosus (SLE), in which Th17 cells extensively participate, is still ill-defined. As a kind of major pathological effector T cells, Th17 cells, which play a key role in inducing tissue damage in autoimmune diseases, secrete IL-17A, the leading cytokine and crucial inflammatory mediator to activate the inflammatory cascade effect. Both IL-17A and Th17 cells are at the core position in the pathogenesis of SLE. IL-17A was much higher in serum from lupus mice, activating multiple immune signaling pathways, and played an important role in the pathogenesis of SLE.1 Furthermore, circulating Th17 cells were significantly raised in patients with active proliferative lupus nephritis, but significantly reduced in responder patients following therapy.2 The role of Th17 cells in the pathogenesis of SLE provides a potential and promising approach for treatment of SLE.
Leptin is a cytokine-like hormone, mainly secreted by adipocytes, controlling energy expenditure and metabolism. Leptin also plays a crucial part in the immune imbalance of SLE. Several studies showed that leptin was increased in serum from SLE patients and promoted SLE by increasing auto-Ab production and inhibiting immune regulation. Leptin was related with atherosclerosis and lupus nephritis.1,3,4 Leptin gene deficiency alleviated disease activity of MRL/Mp-Fas lpr (MRL/lpr) lupus mice,5 which are generated from spontaneous mutation of the Fas gene that encodes a membrane receptor to mediate apoptosis signal. MRL/lpr lupus mice exhibit remarkable lymphadenopathy and SLE-like manifestations, including auto-Ab production, glomerulonephritis, systemic vasculitis, and others. We found that the number of Th17 cells was decreased in leptin-deficient ob/ob mice but exogenous leptin supplementary reversed it to the normal level.6 Besides, fast induced hypoleptinemia could expand functional regulatory T cells in (NZB/NZW)F1 lupus mice.7
NLRP3, also named NLAP3, recruits apoptosis-associated speck-like protein containing a CARD region and ASC caspase-1, constituting the NLRP3 inflammasome. Activation of the NLRP3 inflammasome induces subsequent caspase-1 activation to promote the secretion of pro-inflammatory cytokines such as IL-1β and IL-18 to regulate immune and inflammatory responses. The NLRP3 inflammasome plays a key role in infectious, metabolic and autoimmune diseases. Especially its relationship with Th17/IL-17 attracts a wide-spread attention. The NLRP3 inflammasome also plays an inflammatory role in the pathogenesis of SLE. Shin et al. found that self-double-stranded (ds)DNA induced IL-1β production from human monocytes by activating the NLRP3 inflammasome in the presence of anti-dsDNA Abs.8 Anti-dsDNA Abs activated NLRP3 inflammasome in monocytes/macrophages from SLE patients by binding TLR4 to induce the production of mitochondrial reactive oxygen species (ROS).9 NLRP3/caspase-1/IL-1β promoted Th17 differentiation in other disease models.10,11 Recently, it was found that NLRP3 is expressed in Th1,12 Th2,13 and Th17 cells.14
Since Th17 cells, NLRP3 inflammasome and leptin all play roles in the pathogenesis of SLE, we asked the question whether they are connected. Here we report on our investigation whether leptin facilitates the differentiation of Th17 cells from lupus mice by activating the NLRP3 inflammasome.
Materials and methods
Mice
Eight-to-12 wk old female C57BL6/J (B6) and MRL/lpr mice were purchased from SLAC Laboratory Animal (Shanghai, China). The mice were maintained at the Department of Laboratory Animal Science of Fudan University with a 12 h light/dark cycle and age-matched when used for the experiments, under approved protocols, in accordance with regulations.
Th17 cells induction and culture
Mice spleens were teased into single-cell suspensions and filtered through a 70 mm cell strainer. After RBC lysis, splenocytes were either cultured in vitro or used for the purification of CD4+ T cells by positive selection using magnetic beads (Miltenyi Biotec, Auburn, CA), according to the manufacturer’s instructions. Splenocytes were cultured in complete medium or under Th17 polarizing conditions (2 mg/ml anti-CD3 Ab, 2 mg/ml anti-CD28 Ab, 40 U/ml IL-2, 20 ng/ml IL-6, 5 ng/ml TGF-β, 10 mg/ml anti-IL-4 Ab, 10 mg/ml anti-IFN-γ Ab) for 4 d in a 37°C/5% CO2 incubator. Leptin (250 ng/ml, 500 ng/ml) was added during the last 18 h in the presence of MCC950 (10 μmol/l) or Ac-YVAD-cmk (18.4 μmol/l) before cells underwent intracellular cytokine staining and flow cytometry analysis.
Flow cytometry analysis
For intracellular staining, cells were incubated for 4–5 h with 25 ng/ml PMA (Sigma-Aldrich), 2 mg/ml ionomycin (Sigma-Aldrich), and 10 mg/ml brefeldin A (eBioscience) at 37°C/5% CO2. After surface staining with fluorescent-labeled anti-CD4 (BD Bioscience), cells were re-suspended in fixation/permeabilization buffer (eBioscience) for intracellular staining with fluorescent-labeled anti-IL-17 (eBioscience). Caspase-1 activity was detected with FLICA kit (Immunochemistry, Shanghai, China), according to the manufacturer’s instructions. Flow cytometry was performed on a FACSCalibur instrument (BD Biosciences) and analysis was done using FlowJo software (Tree Star, Ashland, OR).
RNA isolation, cDNA synthesis, and real-time quantitative PCR (qPCR)
Total cellular RNA was isolated using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. cDNA was synthesized from 500 ng total RNA in 10 μl volume using a Superscript kit (Invitrogen, Thermo Fisher Scientific, Inc.). The housekeeping gene GAPDH was used as internal standard. Real time qPCR was performed on an ABI Prism 7500 real‑time PCR system (Thermo Fisher Scientific, Inc.). Primer sequences were as follows: NLRP3-F: 5′- ATTACCCGCCCGAGAAAGG-3′, NLRP3-R: 5′-CATGAGTGTGGCTAGATCCAAG-3′; IL-1β-F: 5′-GTACAAGGAGAACCAAGCAA-3′; IL-1β-R: 5′-CCGTCTTTCATTACACAGGA-3′; IL-18-F: 5′-AGGACACTTTCTTGCTTGCC-3′; IL-18-R: 5′-CACAAACCCTCCCCACCTAA-3′; IL-1R1-F: 5′-CCCGAGGTCCAGTGGTATAA-3′; IL-1R1-R: 5′-CTTCAGCCACATTCCTCACC-3′; ASC exon-F: 5′-CAAATGCGCGAAGGCTATGG-3′; ASC exon-R: 5′-CCAAGCCATACGCTCCA-GA-3′; IL-17A-F: 5′-GTCCAGGGAGAGCTTCATCTG-3′; IL-17A-R: 5′-CTTGGCCTCAGTGTTTGGAC-3′; IL-17F-F: 5′-GCATCTCGAGAAAGGTAATGGGAGTGGAAG-3′; IL-17F-R: 5′-GCATAAGCTTGGTTTCTCCAATGGCTGCTT C-3′; RORγt-F: 5′-AGTGTAATGTGGCCTACTCCT-3′; RORγt-R: 5′-GCTGCTGTTGCAGTTGTTTCT-3′; GAPDH-F: 5′-ATGGGGAAGGTGAAGGTCG-3′; GAPDH-R: 5′-GGGGTCATTGATGGCAACAATA-3′.
ELISA
IL-17A, IL-17F, IL-1β, and IL-18 in the supernatant of the cell culture were measured with ELISA kits from R&D Systems. Measurements were done according to the manufacturer’s instruction.
Western blot
Equal number of cells was lysed in lysis buffer, and cell proteins were extracted. The protein levels in different groups were expressed as a ratio to the level of GAPDH. A rabbit polyclonal anti-mouse ASC Ab and rabbit polyclonal anti-mouse GAPDH Ab were used as primary Abs and an anti-rabbit IgG HRP-linked Ab was used as the secondary one. All Abs were from Cell Signaling Technology.
Statistical analysis
A paired t-test was employed for two group analyses and Kruskal–Wallis one-way ANOVA was used for analyses of > 3 groups with GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Results were expressed as the mean ± SD. P < 0.05 was considered to indicate a statistically significant difference and all experiments were repeated three times.
Results
High concentration of leptin promoted the differentiation of CD4+ T cells into Th17 cells in MRL/lpr lupus mice
CD4+ T cells from the spleen were separated, purified and co-cultured with leptin at scalar doses (250 ng/ml, 500 ng/ml). After 18 h co-culture, when compared to the low concentration, high concentration of leptin was found to significantly promote the transcription of IL-17A, IL-17F, and RORγt in these CD4+ T cells (Figure 1a to c). Since RORγt is a key functional transcription factor and IL-17A/IL-17F are key functional cytokines of Th17, these CD4+ T cells were further labeled with anti-IL-17 Ab to confirm their properties. The percentage of Th17 cells was increased in a dose-dependent manner under scalar doses of leptin (Figure 1f and g), consistent with our previously report,7 which showed that leptin promoted the differentiation of naive CD4+ T cells into Th17 cells. Furthermore, obvious expression of IL-17A and IL-17F were observed in the cell culture supernatant at the presence of a high dose of leptin (Figure 1d and e).
Figure 1.
Leptin promotes the differentiation of Th17 cells from MRL/lpr lupus mice. mRNA levels of (a) IL-17A, (b) IL-17F, and (c) RORγt by qPCR and (d) IL-17A and (e) IL-17F by ELISA in CD4+ T cells from MRL/lpr lupus mice stimulated under Th17 polarizing conditions and treated with leptin at scalar doses during the last 18 h of culture. Flow cytometry results of CD4+ IL-17+ from CD4+ T cells treated as above. (g) Representative and (F) cumulative data from two experiments (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001.
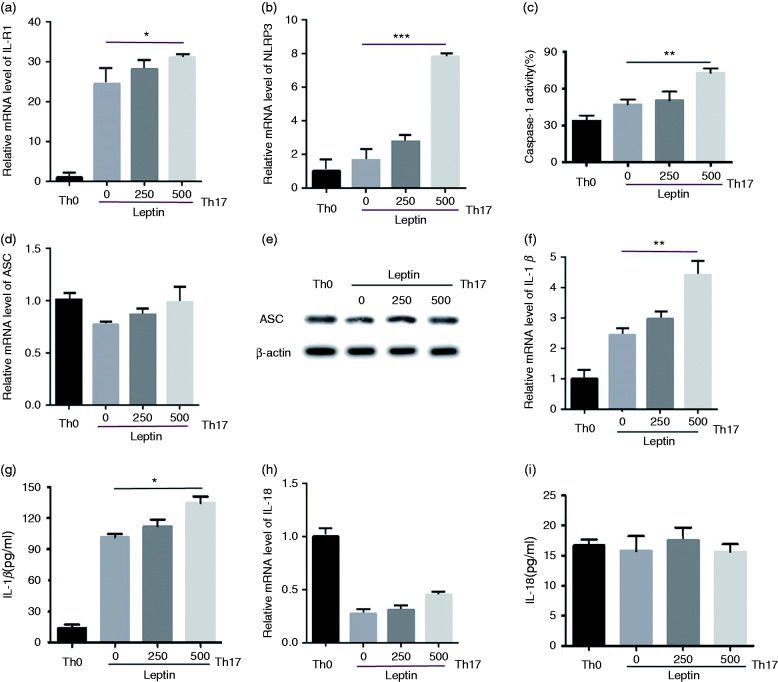
The NLRP3 inflammasome was activated in leptin-induced Th17 cells from MRL/lpr lupus mice
Recently it was reported that Th17 cells express the NLRP3 inflammasome.14 Our research revealed that leptin promoted Th17 response in SLE.6 Since leptin plays an important role in the pathogenesis of SLE, we wondered whether it impacts the expression of NLRP3 in Th17 cells. CD4+ T cells in lupus mice were co-cultured with leptin at scalar doses under Th17 polarizing condition. The NLRP3 inflammasome-related assembly components (NLRP3 and ASC) and related cytokines (IL-1β and IL-18) were evaluated on transcription and protein levels. It turned out that leptin facilitated the transcription of IL-1R1 (IL-1β receptor) (Figure 2a) and NLRP3 (Figure 2b), rather than ASC (Figure 2d). The protein expression of ASC was also not affected by leptin (Figure 2e). Furthermore, a high dose of leptin also enhanced the activity of caspase-1 (Figure 2c). Interestingly, when evaluating IL-1β and IL-18 which are the activation indicators of NLRP3 inflammation, the results showed that leptin promoted the transcription and release of IL-1β (Figure 2f and g), instead of IL-18 (Figure 2h and i).
Figure 2.
Leptin promotes the expression of NLRP3, IL-1β, and IL-1R1 in Th17 cells from MRL/lpr lupus mice. mRNA levels of (a) Il-1R1, (b) NLRP3, (d) ASC, (f) IL-1β, and (h) IL-18 determined by qPCR, (c) caspase-1 activity determined by intracellular FLICA staining, levels of (h) IL-1β and (i) IL-18 by ELISA, and (e) ASC by Western blot in CD4+ T cells from MRL/lpr lupus mice stimulated under Th17 polarizing conditions and leptin-treated with scalar doses during the last 18 h of culture. *P < 0.05, **P < 0.01, ***P < 0.001.
Inhibition of the NLRP3 inflammasome down-regulated the increased percentage of Th17 cells induced by leptin in MRL/lpr mice
Last, to test whether leptin promotes the differentiation of Th17 cells via activating the NLRP3 inflammasome, CD4+ T cells in MRL/lpr lupus mice were co-cultured with leptin and NLRP3 inhibitor MCC950 (10 μmol/l) or caspase-1 inhibitor Ac-YVAD-cmk (18.4 μmol/l) under Th17-polarizing condition. The results showed that the expression/activity of NLRP3 (Figure 3a), caspase-1 (Figure 3b), IL-1β (Figure 3c), and RORγt (Figure 3h) were decreased after inhibition of the NLRP3 inflammasome, but the level of IL-18 almost remained the same (Figure 3d). Both NLRP3 inhibitor and caspase-1 inhibitor were effective in decreasing leptin-induced Th17 differentiation (Figure 3e and f). However, caspase-1 inhibitor seemed to be more powerful than NLRP3 inhibitor in blocking the effect of leptin. Inhibition of the NLRP3 inflammasome activation also suppressed the IL-17A release in leptin-induced Th17 cells (Figure 3g).
Figure 3.
Inhibition of the NLRP3 inflammasome down-regulates the percentage of Th17 cells induced by leptin from MRL/lpr lupus mice. mRNA levels of (a) NLRP3 determined by qPCR, (b) caspase-1 activity by intracellular FLICA staining, (c) IL-1β and (d) IL-18 of culture supernatant by ELISA from CD4+ T cells stimulated under Th17-polarizing conditions and treated with leptin at scalar doses and/or MCC950 or Ac-YVAD-cmk. (e) Representative and (f) cumulative data of flow cytometry results treated as above. (g) IL-17A of culture supernatant determined by ELISA and (h) mRNA level of RORγt by qPCR from CD4+ T cells treated as above (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In this study we found that a high concentration of leptin promoted the differentiation of Th17 cells from MRL/lpr lupus mice by activating the NLRP3 inflammasome. The effect of the NLRP3 inflammasome in autoimmune diseases, especially its relationship with Th17/IL-17, attracts extensive attention. Zhao et al. found that the NLRP3 inflammasome regulated Th17 differentiation in rheumatoid arthritis.15 Eljaafari et al. reported that mesenchymal stem cells from bone marrow and synoviocytes promoted proliferation and differentiation of Th17 via activating caspase-1 in rheumatoid arthritis.16 The NLRP3 inflammasome promoted the response of Th1 and Th17 via caspase-1 dependent cytokines and played a key role in the induction of experimental autoimmune encephalomyelitis.17 IL-1β and IL-18 induced by caspase-1 activation facilitated IL-17 production by γδT cells and CD4+ T cells in experimental autoimmune encephalomyelitis.18 Pro-inflammatory cytokines such as IL-1β secreted after NLRP3 inflammasome activation played positive feedback to further promote the expression and differentiation of Th17. Here, we also found that expression of RORγt, IL-17A, and Th17 were correspondingly decreased after inhibition of the NLRP3 inflammasome, which indicated that the interaction of Th17 and NLRP3 inflammasome as well as pro-inflammatory cytokines such as IL-1β, played positive feedback to the differentiation of leptin-induced Th17 cells.
Interestingly, here it turned out that leptin promoted the secretion of IL-1β instead of IL-18 from CD4+ T cells under Th17 polarizing condition. IL-18 and IL-1β, which are cytokines of the IL-1 family, are synthesized as precursor proteins and activated by the inflammasome via proteolytic processing. However recently it was found that IL-18 and IL-1β are differentially regulated. Type I IFN signaling is essential for induction of IL-18 and macrophages lacking type I IFN signaling were impaired in their ability to promote IL-18 induction.19 Dendritic cells (DCs) from caspase‑11‑deficient mice failed to secrete IL-1β in response to p63, while they were fully responsive for IL-18. Therefore, other disparate licensing factors may control IL-18 vs. IL-1β within DCs.20 Thus, there is a fundamental difference in IL-18 and IL-1β regulation, which indicates that leptin-mediated responses within immune cells may differ between species, lineage and differentiation state.
Surprisingly, compared with NLRP3, it seemed that caspase-1 played a more powerful role in the promotion of leptin to the expression Th17 in MRL/lpr lupus mice. Caspase-1 is an essential component in the development of lupus and associated with lupus vascular dysfunction. Mice lacking caspase-1 were significantly protected against the development of vascular dysfunction and immune complex glomerulonephritis.21 Previous studies identified a caspase-1 tetramer composed of two p20 and two p10 subunits (p20/p10) as an active species. Recently it was reported that in the cell, the dominant species of active caspase-1 dimers elicited by inflammasomes were in fact full-length p46 and a transient species, p33/p10. Further p33/p10 auto-processing occurred with kinetics specified by the inflammasome size and cell type, and this released p20/p10 from the inflammasome, whereupon the tetramer became unstable in cells and protease activity was terminated. The inflammasome-caspase-1 complex thus functioned as a holoenzyme that directed the location of caspase-1 activity but also incorporated an intrinsic self-limiting mechanism that ensured timely caspase-1 deactivation.22 Regrettably, these aspects could not be addressed in the current study. Next we are going to focus on the issue concerning which are the dominant species of active caspase-1, either the tetramer of two p20 and two p10 subunits (p20/p10) or a full-length p46 and a transient p33/p10 in leptin induced Th17 differentiation.
Conclusion
Leptin promoted the differentiation of Th17 cells from MRL/lpr lupus mice, via activating the NLRP3 inflammasome. This finding could provide a new idea of clinical prevention and therapy for SLE in future.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science and Technology Innovation Special Fund for Medical Health Project of BaoShan District (grant number 18-E-25), the National Natural Science Foundation of China (grant numbers 81401345, 81601396, and 31670885, and the Young Physician Training Plan of North Huashan Hospital, Fudan University (grant number 0000077).
ORCID iD
References
- 1.Lourenço EV, Liu A, Matarese G, et al. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc Natl Acad Sci USA 2016; 113: 10637–10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenoy S, Chaurasia S, Edavalath S,et al. Effect of induction therapy on circulating T-helper 17 and T-regulatory cells in active proliferative lupus nephritis. Int J Rheum Dis 2018; 21: 1040–1048. [DOI] [PubMed] [Google Scholar]
- 3.McMahon M, Skaggs BJ, Sahakian L, et al. High plasma leptin levels confer increased risk of atherosclerosis in women with systemic lupus erythematosus, and are associated with inflammatory oxidised lipids. Ann Rheum Dis 2011; 70: 1619–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz-Rizo V, Bonilla-Lara D, Gonzalez-Lopez L, et al. Serum levels of adiponectin and leptin as biomarkers of proteinuria in lupus nephritis. PLoS One. 2017; 12: e0184056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita Y, Fujii T, Mimori T, et al. Deficient leptin signaling ameliorates systemic lupus erythematosus lesions in MRL/Mp-Fas lpr Mice. J Immunol 2014; 192: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y, Liu Y, Shi FD, et al. Cutting edge: leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J Immunol 2013; 190: 3054–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Yu Y, Matarese G, et al. Cutting edge: fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J Immunol 2012; 188: 2070–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin MS, Kang Y, Lee N, et al. Self double-stranded (ds)DNA induces IL-1β production from human monocytes by activating NLRP3 inflammasome in the presence of anti-dsDNA antibodies. J Immunol 2013; 190: 1407–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Fu R, Guo C, et al. Anti-dsDNA antibodies bind to TLR4 and activate NLRP3 inflammasome in lupus monocytes/macrophages. J Transl Med 2016; 14: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin RA, Ather JL, Lundblad LK, et al. Interleukin-1 receptor and caspase-1 are required for the Th17 response in nitrogen dioxide-promoted allergic airway disease. Am J Respir Cell Mol Biol 2013; 48: 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conforti-Andreoni C, Spreafico R, Qian HL, et al. Uric acid-driven Th17 differentiation requires inflammasome-derived IL-1 and IL-18. J Immunol 2011; 187: 5842–5850. [DOI] [PubMed] [Google Scholar]
- 12.Arbore G, West EE, Spolski R, et al. T helper 1 immunity requires complement driven NLRP3 inflammasome activity in CD4+ T cells. Science 2016; 352: aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruchard M, Rebé C, Derangère V, et al. The receptor NLRP3 is a transcriptional regulator of Th2 differentiation. Nat Immunol 2015; 16: 859–870. [DOI] [PubMed] [Google Scholar]
- 14.Martin BN, Wang C, Zhang CJ, et al. T cell-intrinsic ASC critically promotes T(H)17 mediated experimental autoimmune encephalomyelitis. Nat Immunol 2016; 17: 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao C, Gu Y, Zeng X, et al. NLRP3 inflammasome regulates Th17 differentiation in rheumatoid arthritis. Clin Immunol 2018; 197: 154–160. [DOI] [PubMed] [Google Scholar]
- 16.Eljaafari A, Tartelin ML, Aissaoui H, et al. Bone marrow-derived and synovium-derived mesenchymal cells promote Th17 cell expansion and activation through caspase 1 activation: contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum 2012; 64: 2147–2157. [DOI] [PubMed] [Google Scholar]
- 17.Gris D, Ye Z, Iocca HA, et al. Interleukin-1 receptor and caspase-1 are required for the Th17 response in nitrogen dioxide-promoted allergic airway disease. Am J Respir Cell Mol Biol 2013; 48: 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalor SJ, Dungan LS, Sutton CE, et al. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol 2011; 186: 5738–5748. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Q and, Kanneganti TD. Cutting edge: distinct regulatory mechanisms control proinflammatory cytokines IL-18 and IL-1β. J Immunol 2017; 198: 4210–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt RL, Lenz LL. Distinct licensing of IL-18 and IL-1 β secretion in response to NLRP3 inflammasome activation. PLoS One 2012; 7: e45186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahlenberg JM, Yalavarthi S, Zhao W, et al. An essential role for caspase-1 in the induction of murine lupus and its associated vascular damage. Arthritis Rheum 2014; 66: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucher D, Monteleone M, Coll RC, et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med 2018; 215: 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]