Abstract
The matrix metalloproteinase (MMP) family is widely involved in the destruction of the pulp and apical tissues in the inflammatory process. MMP9 is closely related to oral inflammation. Nevertheless, the specific function of MMP9 during oral inflammation, as well as its mechanism, is not well understood. Our previous studies found that in experimentally induced apical periodontitis, more severe inflammation occurred in MMP9 knockout mice compared with the wild type mice. Moreover, the pathology phenomenon of alveolar bone destruction was even more evident in MMP9 knockout mice compared with the wild type mice. We proposed that MMP9 has “anti-inflammatory” properties. We aimed to study the effects of MMP9 on inflammatory response as well as on bone formation and bone destruction. We found a specific relationship between MMP9 and inflammation. qRT-PCR and Western blot revealed that the production of IL-1β, TNF-α, RANK, RANKL, TLR2, and TLR4 was reduced by MMP9 in LPS-stimulated MC3T3-E1 cells. Meanwhile, the expressions of OPG and OCN were increased by MMP9 in LPS-stimulated cells. MMP9 plays a protective role in LPS-induced inflammation, thereby providing new clues to the prevention and treatment of apical periodontitis.
Keywords: Inflammation, LPS, matrix metalloproteinase 9, osteogenesis, osteolysis
Introduction
Chronic apical periodontal diseases lead to bone destruction in the apical region. This is a complex physiological change mediated by multiple inflammatory factors. Numerous data show that matrix metalloproteinases (MMPs) are widely involved and play an important role in the destruction of the pulp and apical tissues in the process of inflammation.1–3
MMPs are a family of zinc enzymes responsible for degradation and remodeling of the extracellular matrix proteins (ECMs) during normal developmental processes, such as organ morphogenesis and angiogenesis in pathological processes, such as inflammation and tumor invasion. They are synthesized and secreted in the cell surface or extracellular matrix when a clear signal arrives, such as physical agents (heat shock, UV irradiation) and cell cytokines (IL-1β, TNF-α). The synthesis stops or falls to a low level when the signal ceases or negative signal arrives, such as retinoic acid and TNF-β.4,5 MMPs start the osteoclast resorption by removing the collagen from the bone surface before the initiation of demineralization.6 They can degrade ECM and play subtle roles such as affecting cell activities by modifying the extracellular environment.7 Moreover, MMPs are reported to determine where and when bone resorption will be initiated. They are required for the recruitment of osteoclast to a future resorption site.8 Amongst MMPs, MMP9 appears to be a main regulator involved in the invasive activity of osteoclast.6,9
MMP9 (92 kDa type IV collagenase; gelatinase B) was discovered by Wilhelm in 1989 and is the largest MMP.10 It reportedly binds to its substrates, namely, type IV collagen, gelatin, and laminin.11 MMP9 is mainly secreted by neutrophils and macrophages,12,13 and it regulates inflammation in tissues and diseases.12,14–19 MMP9 is also closely related to periodontal inflammation. Significant elevation of MMP9 expression was observed in chronically infected areas in apical periodontitis.20–24 Overexpression of MMP9 attenuated osteoclast formation and inhibited pro-inflammatory cytokines secretion.25 MMP9 initiates osteoclasts by removing collagen from the demineralized bone, which is essential for resorption.26 Therefore, its role is to mediate and promote bone destruction. However, MMPs may have “anti-inflammatory properties.” When the expression of MMPs was inhibited by chemical substances, the periapical lesions were significantly aggravated, and the necrosis rate is increased.27 Our previous work showed that MMP9 knockout mice developed larger periapical lesions with greater inflammatory response compared with the wild type mice.28 This finding suggests that the role of MMP9 in bone destruction is complex and diverse. Therefore, the effects of MMP9 on inflammation require further investigation.
The aim of this investigation was to clarify the effects of MMP9 on inflammatory response and on bone formation and destruction in apical periodontitis. We found a specific relationship between MMP9 and inflammation. We analyzed the expression levels of receptor activator of NF-κB (RANK), receptor activator of NF-κB ligand (RANKL), osteoprotegerin (OPG), osteocalcin (OCN), TNF-α, and IL-1β by Western blot and quantitative real time PCR (qRT-PCR).
Materials and methods
Cell culture
The mouse osteoblastic cell line MC3T3-E1 cells were obtained from HYcell Biotechnology (Wuhan, China) and were cultured in α-MEM (Hyclone, USA) containing 10% FBS (Hyclone, USA) plus penicillin (100 U/ml) and streptomycin (100 mg/ml) (Hyclone, USA) at 37°C in a humidified atmosphere containing 5% CO2 and 5% air. The medium was refreshed every 2 d.
Culture of Porphyromonas endodontalis and preparation of LPS
P. endodontalis (ATCC35406) was obtained from BIOBW (Beijing, China) and was cultured anaerobically at 37°C. LPS was extracted by the hot phenol-water method as previously described.29 The bioactivity of purified P. endodontalis LPS was measured with the limulus amoebocyte lysate (LAL) endotoxin assay kit (GenScript, USA).
Cell transfection
MC3T3-E1 cells were seeded onto 24-well plates and allowed to proliferate until 70–90% before DNA transfection. Cells were then transfected with pCMV3-SP-N-His (pCMV3, control group) (Sino Biological, Beijing, China) or pCMV3-MMP9 (MMP9 overexpression group) (Sino Biological, Beijing, China) at a final concentration of 0.8 μg/ml by Lipofectamine 2000 (Thermo, USA). After 48 h, cells were stimulated with different concentrations of P. endodontalis LPS for the indicated time. The total RNA and protein extracted from both groups were used for qRT-PCR and Western blot assay.
MC3T3-E1 cells were seeded onto 24-well plates and allowed to proliferate until 30–50% before siRNA transfection. Cells were transfected with si-MMP9-1 (Ribobio, Guangzhou, China), si-MMP9-2 (Ribobio, Guangzhou, China), si-MMP9-3 (Ribobio, Guangzhou, China), and a negative control siRNA (Ribobio, Guangzhou, China) at a final concentration of 50 nmmol/l using Lipofectamine 2000 (Thermo, USA) according to the manufacturer’s instructions. After 48 h, cells were stimulated with different concentrations of P. endodontalis LPS for the indicated time. Total RNA and protein extracted from both groups were used for qRT-PCR and Western blot assay.
qRT-PCR analysis
Total RNA was extracted using TRIpure total RNA extraction reagent (ELK Biotechnology, Wuhan, China). First-strand cDNA synthesis was performed using the reverse transcription system (ELK Biotechnology) according to the manufacturer’s instructions. qRT-PCR was performed with the StepOne™ real-time PCR system (Life Technologies, USA). The following genes were quantified: MMP9, IL-1β, TNF-α, RANK, RANKL, OPG, OCN, TLR2, and TLR4. GAPDH was used as the internal normalization control. Primer sequences are shown in Table 1. The expression of each gene was calculated using the 2−ΔΔCT methods. The gene expression ratio was shown as mean ± SD from three independent experiments.
Table 1.
Oligonucleotide primer sequences used in qRT-PCR.
| Gene | Sequence (5′-3′) | Size | |
|---|---|---|---|
| GAPDH | Forward | TGAAGGGTGGAGCCAAAAG | 227 |
| Reverse | AGTCTTCTGGGTGGCAGTGAT | ||
| MMP9 | Forward | AAGGGTACAGCCTGTTCCTGGT | 149 |
| Reverse | CTGGATGCCGTCTATGTCGTCT | ||
| IL-1β | Forward | TCATTGTGGCTGTGGAGAAGC | 164 |
| Reverse | AATGGGAACGTCACACACCAG | ||
| RANKL | Forward | CAGGACTCGACTCTGGAGAGTG | 152 |
| Reverse | AACCATGAGCCTTCCATCATAG | ||
| TNF-α | Forward | TCCCCAAAGGGATGAGAAGTT | 298 |
| Reverse | GAGGAGGTTGACTTTCTCCTGG | ||
| RANK | Forward | CTTGGACCAACTGCACCCTC | 201 |
| Reverse | CCTTCCTGTAGTAAACGCCGA | ||
| OPG | Forward | GGAGGAAGACATTGTGTGTCCC | 157 |
| Reverse | TCCTCACACTCACACACTCGGT | ||
| OCN | Forward | GCAGGAGGGCAATAAGGTAGTG | 165 |
| Reverse | CCATAGATGCGTTTGTAGGCG | ||
| TLR2 | Forward | ACGTTTGCTATGATGCCTTTGT | 109 |
| Reverse | AGACACAGCTTAAAGGGCGG | ||
| TLR4 | Forward | ACACTTTATTCAGAGCCGTTGGT | 297 |
| Reverse | CAGGTCCAAGTTGCCGTTTC | ||
Western blot analysis
Cells were harvested, lysed with lysis buffer (ASPEN, Wuhan, China), and centrifuged at 16,200 g for 10 min. Total proteins in the supernatant were measured using a BCA protein assay kit (ASPEN, Wuhan, China). Total proteins were extracted from MC3T3-E1 cells. The protein at 30 µg was resolved by 10% SDS-PAGE gels (ASPEN, Wuhan, China). After electrophoresis, the proteins were transferred onto nitrocellulose membrane (Millipore, USA). The membranes were blocked with 5% nonfat milk (ASPEN, Wuhan, China) at room temperature for 1 h. The samples were probed with anti-GAPDH (1:10,000; Abcam, UK), anti-IL-1β (1:1000; Abcam, UK), anti-TNF-α (1:500; Abcam, UK), anti-RANK (1:1000; Abcam, UK), anti-RANKL (1:500; Novusbio, Shanghai, China), anti-OPG (1:1000; Abcam, UK), anti-OCN (1:500; Abcam, UK), anti-TLR2 (1:1000; Abcam, UK), and anti-TLR4 (1:500; Abcam, UK) Abs. HRP-conjugated goat anti-rabbit IgG (1:10000; ASPEN, Wuhan, China) was used for detection.
Statistical analysis
The data are presented as the mean ± SD. Statistical analysis was performed with SPSS15.0. Differences between individual groups were analyzed by Student’s t-test (two-tailed) with subsequent Bonferroni correction. The statistical significance was determined at P < 0.05 or P < 0.01. All experiments in this study were independently repeated at least thrice.
Results
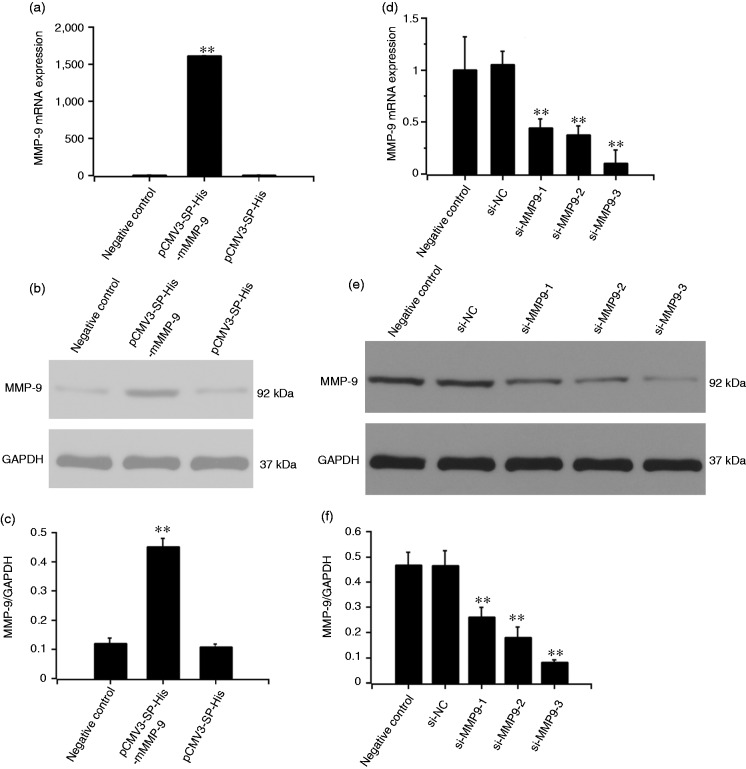
MMP9 expression was increased by MMP9 DNA transfection
Plasmid DNA pCMV3 or pCMV3-MMP9 was transfected into MC3T3-E1 cells. After 48 h, MMP9 expression was examined by Western blot and qRT-PCR. MMP9 production in the MMP9 overexpression group was significantly higher than in the control group at both mRNA and protein level (P < 0.01) (Figure 1a to c).
Figure 1.
Effect of MMP9 overexpression plasmid and MMP9 siRNAs on MMP9 expression. MC3T3-E1 cells were stimulated with pCMV3-SP-His-mMMP9 plasmid for 48 h. cDNA and protein were analyzed by RT-PCR (a) and Western blot (b), respectively. The cells were treated with the three different MMP9 siRNAs for 48 h.MMP9 expression was detected by qRT-PCR (d) and Western blot (e). Target sequences: si-m-Mmp9-1: GACTTGCCGCGAGACATGA, si-m-Mmp9-2: GCGCTCTGCATTTCTTCAA, si-m-Mmp9-3: GGAACTCACACGACATCTT. (c, f) Quantification of protein expression was normalized to GAPDH using a densitometer (imaging system). The data are representative of three independent experiments and expressed as the mean ± SD. **P < 0.01.
MMP9 expression was inhibited by MMP9 siRNAs
MC3T3-E1 cells were transfected with si-MMP9-1, si-MMP9-2, si-MMP9-3, or control siRNA for 48 h. MMP9 expression was detected by Western blot and qRT-PCR. MMP9 expression was inhibited by si-MMP9-1, si-MMP9-2, or si-MMP9-3 at mRNA and protein levels. MMP9 expression in the si-MMP9-3 group was the lowest both at mRNA and protein levels (P < 0.01) (Figure 1d to f).
LPS regulated IL-1β expression
MC3T3-E1 cells were treated without or with different concentrations of LPS (1, 5, 10, 20, and 50 µg/ml) for 24 h. qRT-PCR and Western blot were conducted to determine if LPS regulated IL-1β expression. qRT-PCR and Western blot results showed that IL-1β expression slightly decreased after the cells were treated with LPS at 1 and 5 µg/ml compared with the control group (P > 0.05). IL-1β expression increased significantly after treatment with LPS at 10 μg/ml (P < 0.01) and peaked at 20 μg/ml (P < 0.01), followed by a slight decrease at 50 μg/ml (P < 0.01) (Figure 2a to c).
Figure 2.
Effect of different concentrations and different time points of LPS stimulation on IL-1β expression. MC3T3-E1 cells were treated with or without LPS at different concentrations (1, 5, 10, 20, and 50 μg/ml) for 24 h. DNA samples were analyzed by qRT-PCR (a), and protein samples were analyzed by Western blot (b). The cells were then treated with 20 μg/ml of LPS at different time points (0, 6, 12, 24, and 48 h). The expression of IL-1β was detected by qRT-PCR (d) and Western blot (e). (c, f) Quantification of protein expression was normalized to GAPDH using a densitometer (imaging system). The data are representative of three independent experiments and expressed as the mean ± SD. *P < 0.05 vs. LPS; **P < 0.01 vs. LPS.
MC3T3-E1 cells were then treated with 20 μg/ml LPS at different time points (0, 6, 12, 24, and 48 h). The IL-1β expression was detected by qRT-PCR and Western blot. IL-1β expression peaked at 12 h (P < 0.01), afterwards it decreased at 24 h and 48 h (Figure 2d to f). LPS regulated IL-1β expression in a time- and dose-dependent manner.
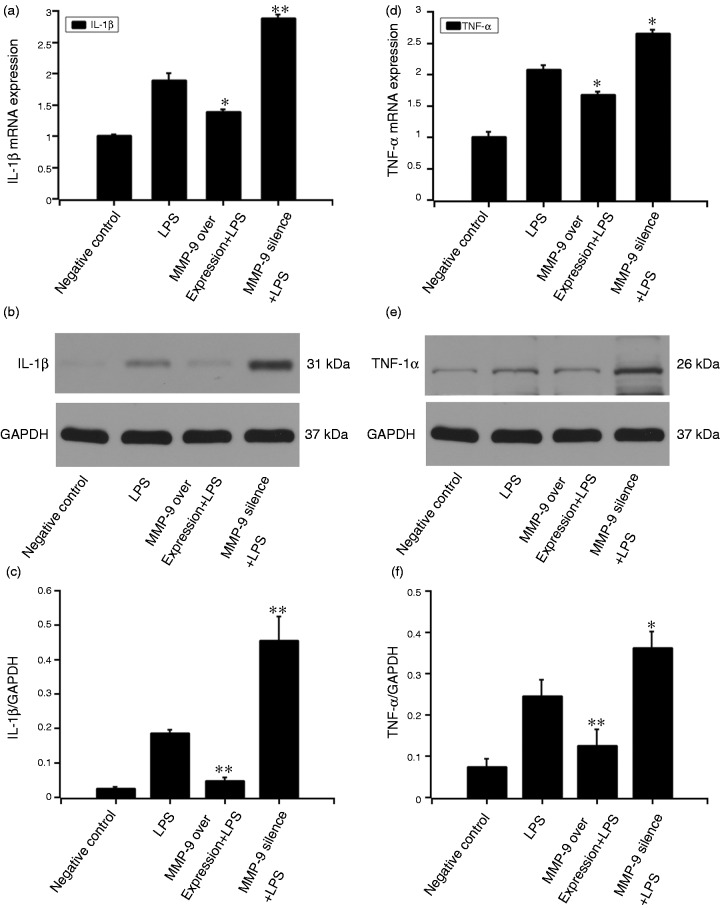
MMP9 inhibited LPS-induced IL-1β and TNF-α expression
After the MC3T3-E1 cells were pretreated with MMP9 DNA or si-MMP9-3 for 48 h, 20 μg/ml LPS was added to the culture medium for another 12 h. qRT-PCR and Western blot were performed to detect IL-1β and TNF-α expressions. At mRNA level, MMP9 suppressed LPS-induced IL-1β (P < 0.05) and TNF-α (P < 0.05) expressions. Moreover, pre-treatment with MMP9 siRNA-3 increased the LPS-induced IL-1β (P < 0.01) and TNF-α (P < 0.05) expressions. Similarly, at protein level, MMP9 suppressed LPS-induced IL-1β (P < 0.01) and TNF-α (P < 0.01) expressions. Pre-treatment with MMP9 siRNA-3 increased the LPS-induced IL-1β (P < 0.01) and TNF-α (P < 0.05) expressions (Figure 3).
Figure 3.
Effect of MMP9 on LPS-induced expression of IL-1β and TNF-α. MC3T3-E1 cells were pre-treated with MMP9 overexpression plasmid or si-MMP9-3 (target sequences: GGAACTCACACGACATCTT) for 24 h. The 20 μg/ml LPS was added to the culture medium for another 12 h. qRT-PCR and Western blot were performed to detect IL-1β (a–c) and TNF-α (d–f) expressions. Quantification of protein expression was normalized to GAPDH using a densitometer (imaging system). The data are representative of three independent experiments and expressed as the mean ± SD. *P < 0.05 vs. LPS; **P < 0.01 vs. LPS.
MMP9 inhibited LPS-stimulated RANKL and RANK expression while increased OPG and OCN expression
After pretreating MC3T3-E1 cells with MMP9 DNA or si-MMP9-3 for 48 h, 20 μg/ml LPS was added to the culture medium for another 12 h. qRT-PCR and Western blot were conducted to detect RANKL, RANK, OPG, and OCN expression. Treatment with LPS (20 μg/ml) increased RANKL expression. MMP9 suppressed the LPS-induced RANKL expression at both mRNA level (P < 0.05) and protein level (P < 0.05). Moreover, pre-treatment with MMP9 siRNA-3 increased the LPS-induced RANKL expression at both mRNA level (P < 0.05) and protein level (P < 0.01) (Figure 4a to c).
Figure 4.
Effect of MMP9 on LPS-induced expression of RANKL, RANK, OPG, and OCN. MC3T3-E1 cells were pre-treated with MMP9 overexpression plasmid or si-MMP9-3 (target sequences: GGAACTCACACGACATCTT) for 24 h. The 20 μg/ml of LPS was added to the culture medium for another 12 h. qRT-PCR and Western blot were performed to detect RANKL (a–c), RANK (d–f), OPG (g–i), and OCN (j–l) expressions. Quantification of protein expression was normalized to GAPDH using a densitometer (imaging system). The data are representative of three independent experiments and expressed as the mean ± SD. *P < 0.05 vs. LPS; **P < 0.01 vs. LPS.
Similarly, treatment with LPS (20 μg/ml) increased RANK expression. MMP9 suppressed the LPS-induced RANK expression at both mRNA level (P < 0.05) and protein level (P < 0.01). Pre-treatment with MMP9 siRNA-3 increased the LPS-induced RANK expression at both mRNA level (P < 0.05) and protein level (P < 0.05) (Figure 4d to f).
Conversely, treatment with LPS (20 μg/ml) decreased OPG expression. OPG expression increased after MMP9 overexpression compared with the LPS-stimulated group (P < 0.05). OPG expression decreased after MMP9 was inhibited by MMP9 siRNA-3 (P < 0.01). qRT-PCR and Western blot analysis showed the same results (Figure 4g to i).
Treatment with LPS (20 μg/ml) inhibited OCN expression. OCN expression increased after MMP9 over-expression compared with the LPS-stimulated group (P < 0.01). OCN expression decreased after MMP9 was inhibited by MMP9 siRNA-3 (P < 0.01). qRT-PCR and Western blot analysis showed the same results (Figure 4j to l).
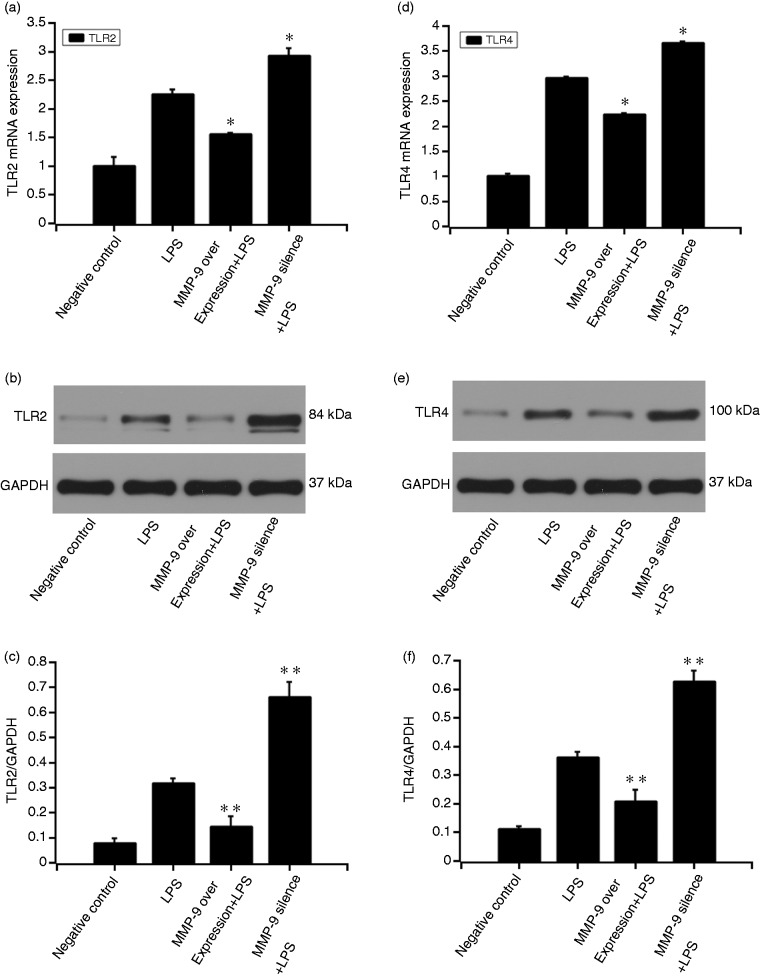
MMP9 inhibited LPS-induced TLR2 and TLR4 expression
After the MC3T3-E1 cells were pre-treated with MMP9 DNA or si-MMP9-3 for 48 h, 20 μg/ml LPS was added to the culture medium for another 12 h. qRT-PCR and Western blot were performed to detect TLR2 and TLR4 expressions. At mRNA level, MMP9 suppressed LPS-induced TLR2 (P < 0.05) and TLR4 (P < 0.05) expressions. Moreover, pre-treatment with MMP9 siRNA-3 increased the LPS-induced TLR2 (P < 0.05) and TLR4 (P < 0.05) expressions. At protein level, MMP9 suppressed LPS-induced TLR2 (P < 0.01) and TLR4 (P < 0.01) expressions. Pre-treatment with MMP9 siRNA-3 increased the LPS-induced TLR2 (P < 0.01) and TLR4 (P < 0.01) expressions (Figure 5).
Figure 5.
Effect of MMP9 on LPS-induced expression of TLR2 and TLR4. MC3T3-E1 cells were pre-treated with MMP9 overexpression plasmid or si-MMP9-3 (target sequences: GGAACTCACACGACATCTT) for 24 h. The 20 μg/ml LPS was added to the culture medium for another 12 h. qRT-PCR and Western blot were performed to detect TLR2 (a–c) and TLR4 (d–f) expressions. Quantification of protein expression was normalized to GAPDH using a densitometer (imaging system). The data are representative of three independent experiments and expressed as the mean ± SD. *P < 0.05 vs. LPS; **P < 0.01 vs. LPS.
Discussion
We previously found that the loss of MMP9 induced a great inflammation response in experimentally induced mouse apical periodontitis.28 This finding suggested the important role of MMP9 in the host’s immune and inflammatory response to pulp and periapical infection.
P. endodontalis is considered an important member of the Gram-negative anaerobic microorganisms involved in infected root canals and apical periodontitis.30,31 A primary virulence factor of P. endodontalis is LPS. Certain studies have reported that P. endodontalis LPS play a critical role in initiating inflammation, thereby resulting in the synthesis and release of cytokines and inflammatory mediators.32,33 IL-1β is an important pro-inflammatory cytokine which is mainly expressed in macrophages and neutrophils. It is a critical cytokine associated with the initiation and the persistence of inflammation.34,35 We first confirmed LPS’ regulatory function on pro-inflammatory factor IL-1β by stimulating MC3T3-E1 cells with different concentrations at different time points. LPS induced the IL-1β mRNA and protein expressions in a time- and dose-dependent manner. IL-1β expression peaked after stimulation with LPS at 20 μg/ml for 12 h. MMPs can mediate neutrophil response to inflammation.12,36 MMP9 is closely related to inflammation development and can mediate the recruitment of pro-inflammatory cells to the inflammatory zone.37,38 We found that MMP9 inhibited the LPS-induced IL-1β up-regulation in MC3T3-E1 cells in the subsequent detection. TNF-α is also a potent pro-inflammatory cytokine that plays an important role in immunity and inflammation.39,40 TNF-α expression decreased following MMP9 over-expression, thereby indicating that MMP9 can protect against LPS-induced inflammation.
LPS administration induces inflammation and osteoclastic bone resorption.41,42 We next examined the RANKL-OPG bi-molecular system. By activating the cognate RANK receptor on the surface of pre-osteoclasts, it triggers their differentiation into mature osteoclasts, thereby activating bone resorption.43 The action of RANKL can be blocked by OPG, which has structural homology to RANK. By binding to RANKL, OPG prevents further interaction with RANK and indirectly protects bone from resorption.44 In this study, RANKL and RANK expressions were decreased by MMP9. Conversely, OPG expression was increased after MMP9 over-expression. Thus, MMP9 might inhibit bone resorption by down-regulating RANKL and RANK and by up-regulating OPG.
We then examined a marker gene for osteogenesis, OCN, which can regulate bone mineralization and bone turnover.45,46 OCN is synthesized by osteoblastic cells and is the most abundant non-collagenous protein.47 LPS down-regulated osteogenic differentiation by inhibiting OCN expression in MC3T3-E1 cells.48 OCN expression increased in MMP9 over-expressed cells, which indicated that MMP9 might stimulate bone formation in LPS-induced inflammation.
Our study showed that under the stimulation of P. endodontalis LPS, MMP9 inhibited IL-1β, TNF-α, RANKL, and RANK expressions and increased OPG and OCN expressions. In order to explore the mechanism of MMP9’s regulation of the cells’ responses to LPS, we examined the expressions of TLR2 and TLR4. Previous studies reported that LPS triggered inflammation response through its binding with the cell membrane receptor, TLR4.49–51 There is also work indicating TLR2 being involved in mediating responses to LPS.52,53 We found that MMP9 inhibited the LPS-induced TLR2 and TLR4 expression.
In summary, the findings of this study showed that MMP9 is a potent inhibitor of LPS-induced IL-1β and TNF-α production. It regulates osteogenesis/osteolysis by inhibiting bone resorption and promoting bone formation. This “anti-inflammation” effect of MMP9 is consistent with the hypothesis we proposed in our previous study.28 Furthermore, the regulatory effects of MMP9 are found to be associated with TLR2 and TLR4. However, more researches are needed to explore the regulatory mechanism of MMP9.
Declaration of conflicting interests
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Nature Science Foundation of China (grant number 81700952) and the Natural Science Foundation of Shandong Province (grant number ZR2017BH075).
References
- 1.Accorsi-Mendonca T, Silva EJ, Marcaccini AM, et al. Evaluation of gelatinases, tissue inhibitor of matrix metalloproteinase-2, and myeloperoxidase protein in healthy and inflamed human dental pulp tissue. J Endod 2013; 39: 879–882. [DOI] [PubMed] [Google Scholar]
- 2.Romualdo PC, Lucisano MP, Paula-Silva FWG, et al. Ovariectomy exacerbates apical periodontitis in rats with an increase in expression of proinflammatory cytokines and matrix metalloproteinases. J Endod 2018; 44: 780–785. [DOI] [PubMed] [Google Scholar]
- 3.Jain A, Bahuguna R. Role of matrix metalloproteinases in dental caries, pulp and periapical inflammation: An overview. J Oral Biol Craniofac Res 2015; 5: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkedal-Hansen H, Moore WG, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993; 4: 197–250. [DOI] [PubMed] [Google Scholar]
- 5.Woessner JF., Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991; 5: 2145–2154. [PubMed] [Google Scholar]
- 6.Delaisse JM, Engsig MT, Everts V, et al. Proteinases in bone resorption: obvious and less obvious roles. Clin Chim Acta 2000; 291: 223–234. [DOI] [PubMed] [Google Scholar]
- 7.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell 1997; 91: 439–442. [DOI] [PubMed] [Google Scholar]
- 8.Blavier L, Delaisse JM. Matrix metalloproteinases are obligatory for the migration of preosteoclasts to the developing marrow cavity of primitive long bones. J Cell Sci 1995; 108: 3649–3659. [DOI] [PubMed] [Google Scholar]
- 9.Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998; 93: 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilhelm SM, Collier IE, Marmer BL, et al. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem 1989; 264: 17213–17221. [PubMed] [Google Scholar]
- 11.Allan JA, Docherty AJ, Barker PJ, et al. Binding of gelatinases A and B to type-I collagen and other matrix components. Biochem J 1995; 309: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley LM, Douglass MF, Chatterjee D, et al. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathog 2012; 8: e1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi K. Microbiological, pathological, inflammatory, immunological and molecular biological aspects of periradicular disease. Int Endod J 1998; 31: 311–325. [DOI] [PubMed] [Google Scholar]
- 14.Reif S, Somech R, Brazovski E, et al. Matrix metalloproteinases 2 and 9 are markers of inflammation but not of the degree of fibrosis in chronic hepatitis C. Digestion 2005; 71: 124–130. [DOI] [PubMed] [Google Scholar]
- 15.Esparza J, Kruse M, Lee J, et al. MMP-2 null mice exhibit an early onset and severe experimental autoimmune encephalomyelitis due to an increase in MMP-9 expression and activity. FASEB J 2004; 18: 1682–1691. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Zhang X, Guo Y, et al. MMP-9 inhibition suppresses wear debris-induced inflammatory osteolysis through downregulation of RANK/RANKL in a murine osteolysis model. Int J Mol Med 2012; 30: 1417–1423. [DOI] [PubMed] [Google Scholar]
- 17.Greenlee KJ, Corry DB, Engler DA, et al. Proteomic identification of in vivo substrates for matrix metalloproteinases 2 and 9 reveals a mechanism for resolution of inflammation. J Immunol 2006; 177: 7312–7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cataldo DD, Tournoy KG, Vermaelen K, et al. Matrix metalloproteinase-9 deficiency impairs cellular infiltration and bronchial hyperresponsiveness during allergen-induced airway inflammation. Am J Pathol 2002; 161: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMillan SJ, Kearley J, Campbell JD, et al. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J Immunol 2004; 172: 2586–2594. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed GM, El-Baz AA, Hashem AA, et al. Expression levels of matrix metalloproteinase-9 and gram-negative bacteria in symptomatic and asymptomatic periapical lesions. J Endod 2013; 39: 444–448. [DOI] [PubMed] [Google Scholar]
- 21.Corotti MV, Zambuzzi WF, Paiva KB, et al. Immunolocalization of matrix metalloproteinases-2 and -9 during apical periodontitis development. Arch Oral Biol 2009; 54: 764–771. [DOI] [PubMed] [Google Scholar]
- 22.Buzoglu HD, Unal H, Ulger C, et al. The zymographic evaluation of gelatinase (MMP-2 and -9) levels in acute and chronic periapical abscesses. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108: e121–e-126. [DOI] [PubMed] [Google Scholar]
- 23.Carneiro E, Menezes R, Garlet GP, et al. Expression analysis of matrix metalloproteinase-9 in epithelialized and nonepithelialized apical periodontitis lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campos K, Gomes CC, Farias LC, et al. DNA methylation of MMP9 is associated with high levels of MMP-9 messenger RNA in periapical inflammatory lesions. J Endod 2016; 42: 127–130. [DOI] [PubMed] [Google Scholar]
- 25.Guo J, Zeng X, Miao J, et al. MiRNA-218 regulates osteoclast differentiation and inflammation response in periodontitis rats through Mmp9. Cell Microbiol 2019; 21: e12979. [DOI] [PubMed] [Google Scholar]
- 26.Martinho FC, Teixeira FF, Cardoso FG, et al. Clinical investigation of matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases, and matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase complexes and their networks in apical periodontitis. J Endod 2016; 42: 1082–1088. [DOI] [PubMed] [Google Scholar]
- 27.Tjaderhane L, Hotakainen T, Kinnunen S, et al. The effect of chemical inhibition of matrix metalloproteinases on the size of experimentally induced apical periodontitis. Int Endod J 2007; 40: 282–289. [DOI] [PubMed] [Google Scholar]
- 28.Wan C, Yuan G, Yang J, et al. MMP9 deficiency increased the size of experimentally induced apical periodontitis. J Endod 2014; 40: 658–664. [DOI] [PubMed] [Google Scholar]
- 29.Koga T, Nishihara T, Fujiwara T, et al. Biochemical and immunobiological properties of lipopolysaccharide (LPS) from Bacteroides gingivalis and comparison with LPS from Escherichia coli. Infect Immun 1985; 47: 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montagner F, Jacinto RC, Signoretti FG, et al. Clustering behavior in microbial communities from acute endodontic infections. J Endod 2012; 38: 158–162. [DOI] [PubMed] [Google Scholar]
- 31.Martinho FC, Leite FR, Chiesa WM, et al. Signaling pathways activation by primary endodontic infectious contents and production of inflammatory mediators. J Endod 2014; 40: 484–489. [DOI] [PubMed] [Google Scholar]
- 32.Yang D, Li R, Qiu LH, et al. Effects of lipopolysaccharides extracted from Porphyromonas endodontalis on the expression of IL-1beta mRNA and IL-6 mRNA in osteoblasts. Shanghai Kou Qiang Yi Xue 2009; 18: 194–197. [PubMed] [Google Scholar]
- 33.Guo J, Yang D, Okamura H, et al. Calcium hydroxide suppresses Porphyromonas endodontalis lipopolysaccharide-induced bone destruction. J Dent Res 2014; 93: 508–513. [DOI] [PubMed] [Google Scholar]
- 34.Kotecha S, Wilson L, Wangoo A, et al. Increase in interleukin (IL)-1 beta and IL-6 in bronchoalveolar lavage fluid obtained from infants with chronic lung disease of prematurity. Pediatr Res 1996; 40: 250–256. [DOI] [PubMed] [Google Scholar]
- 35.Nold MF, Mangan NE, Rudloff I, et al. Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proc Natl Acad Sci USA 2013; 110: 14384–14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Yu YY, Lieu S, et al. MMP9 regulates the cellular response to inflammation after skeletal injury. Bone 2013; 52: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin J, Cai L, Liu Z-M, et al. miRNA-218 inhibits osteosarcoma cell migration and invasion by down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev 2013; 14: 3681–3684. [DOI] [PubMed] [Google Scholar]
- 38.Kluger MA, Zahner G, Paust HJ, et al. Leukocyte-derived MMP9 is crucial for the recruitment of proinflammatory macrophages in experimental glomerulonephritis. Kidney Int 2013; 83: 865–877. [DOI] [PubMed] [Google Scholar]
- 39.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 2001; 11: 372–377. [DOI] [PubMed] [Google Scholar]
- 40.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 2003; 10: 45–65. [DOI] [PubMed] [Google Scholar]
- 41.Abu-Amer Y, Ross FP, Edwards J, et al. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J Clin Invest 1997; 100: 1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyaura C, Inada M, Matsumoto C, et al. An essential role of cytosolic phospholipase A2alpha in prostaglandin E2-mediated bone resorption associated with inflammation. J Exp Med 2003; 197: 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 2008; 473: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997; 89: 309–319. [DOI] [PubMed] [Google Scholar]
- 45.Young MF. Bone matrix proteins: their function, regulation, and relationship to osteoporosis. Osteoporos Int 2003. 14(Suppl 3): S35–S42 [DOI] [PubMed] [Google Scholar]
- 46.Gorski JP. Biomineralization of bone: a fresh view of the roles of non-collagenous proteins. Front Biosci 2011; 16: 2598–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ndiaye B, Prudhon C, Guillozo H, et al. Rat serum osteocalcin concentration is determined by food intake and not by inflammation. J Nutr 1992; 122: 1870–1874. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Hao W, Wang X, et al. miR-23b targets Smad 3 and ameliorates the LPS-inhibited osteogenic differentiation in preosteoblast MC3T3-E1 cells. J Toxicol Sci 2016; 41: 185–193. [DOI] [PubMed] [Google Scholar]
- 49.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 2008; 42: 145–151. [DOI] [PubMed] [Google Scholar]
- 50.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol 2005; 3: 36–46. [DOI] [PubMed] [Google Scholar]
- 51.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem 2002; 71: 635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Good DW, George T, Watts BA., 3rd. Toll-like receptor 2 is required for LPS-induced Toll-like receptor 4 signaling and inhibition of ion transport in renal thick ascending limb. J Biol Chem 2012; 287: 20208–20220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang Y, Sun F, Li X, et al. Porphyromonas endodontalis lipopolysaccharides induce RANKL by mouse osteoblast in a way different from that of Escherichia coli lipopolysaccharide. J Endod 2011; 37: 1653–1658. [DOI] [PubMed] [Google Scholar]