Abstract
Objective
This study evaluated hepatoprotective effect of aescin (AES) and diosmin (DIO), individually or in low-dose combination in chemically induced liver injury in rats. Rats were divided into 6 groups; Group 1, control, Group 2, injected with a single dose of a mixture of corn oil and carbon tetrachloride (CCl4) to induce hepatic toxicity. Before CCl4 injection, Groups 3–6 were treated daily for 14 days with silymarin (SIL) (200 mg/kg), aescin (AES; 3.6 & 1.75 mg/kg), Diosmin (DIO; 100 & 50 mg/kg). Serum samples were analyzed for different liver function, oxidative stress and antioxidant markers. Moreover, inflammation and tissue damage were confirmed by histological staining of liver tissue sections.
Results
Results indicated that CCl4 elevated serum levels of all assessed liver function markers and decreased levels of key antioxidants. Administration of AES and/or DIO significantly reversed all those CCl4-induced effects. Histopathological study showed disruption of the hepatic architecture, necrosis and inflammatory cells and depositions of glycogen and protein in the tissues of CCl4-treated group. Pretreatment with DIO and/or AES significantly improved histopathological structure of liver tissue. In conclusion, low-dose combination of AES and DIO exhibited significant and preferential hepatoprotective activity compared to individual treatment with AES or DIO.
Keywords: Aescin, Diosmin, Carbon tetrachloride, Liver, Rats
Introduction
Liver damage is viewed as a worldwide medical and economic burden that is often inclined by viral infection, poisonous synthetic concoctions, and hepatotoxic drugs [1]. The liver assumes an irreplaceable job in the digestion of xenobiotics and therapeutic agents being detoxified and excreted [2]. Chemically‐induced hepatic damage is mainly evoked by the metabolic change of chemical into free radicals, which can truly influence cell macromolecules [2]. Carbon tetrachloride (CCl4)—induced liver damage has been for quite some time utilized as hepatic damage model to evaluate the therapeutic potential of drugs. This model has also been used in mechanical liver injuries that are comparable to human liver disease both in morphology and the biochemical characteristics of cellular lesions [2]. This hepatotoxic agent induces hepatic necrosis through the generation of trichloromethyl free radical under the effect of cytochrome p-450 2E1 [3].
Previous investigations have reported that a wide spectrum of natural products can prevent or even treat different types of liver diseases [4–9]. β-Aescin (AES) is a triterpene saponin derived from the horse chestnut tree Aesculus hippocastanum L. (Hippocastanaceae). Traditionally, leaves, bark and seeds were used to treat arthritis, brain trauma, stroke, venous congestion and thrombophlebitis [10]. There are different formulations of AES in clinical applications, including oral tablets, injections, and topical gel. AES is widely used in the clinical therapy because of its anti-apoptotic, anti-edematous, anti-inflammatory, and antioxidant effects [11–13]. Moreover, some studies reported anti-inflammatory, anti-oxidative stress and protective effects of AES against liver damage induced in animals by endotoxin [14], methyl parathion [15] and CCl4 [16].
Diosmin (DIO) (3′5,7-trihydroxy-4′-methoxyflavone 7-rutinoside) is an unsaturated flavonoid glycoside, present in citrus fruits [17]. DIO formulations are used to treat chronic venous insufficiency, hemorrhoids, venous ulcers (especially of the lower limbs) and to prevent postoperative thromboembolism [18]. Previous investigations reported anti-inflammatory, anti-oxidative stress and protective effects of DIO against liver damage induced in animals by ethanol [19], methotrexate [20], iron [21] and by aflatoxin [22]. Moreover, DIO successfully relieved mitochondrial oxidative stress effect against isopropanol-induced myocardial injury in rats [23]. In that context, AES and DIO have received considerable attention to their tremendous potential for management of various forms of hepatopathy [14, 15, 19–22]. Assessing AES/DIO and their combination against induced-liver damage is the objective of this study.
Main text
Methods
Chemicals
All chemicals were provided as detailed in (Additional file 1).
Animals
Male Wistar rats, weighing 100–130 g, were provided by the animal house colony of the National Research Centre, Dokki, Giza, Egypt. Animal-related procedures were all approved and operated in accordance with the Ethics Committee of the National Research Centre and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Experimental design
Rats were randomized and divided into six groups (n = 6). Each was subjected to different treatment as detailed in (Additional file 1). Briefly, Control, received water (5 ml/kg b.wt.) for 14 days then vehicle (1 ml corn oil/kg b.wt.). CCl4 group received water (5 ml/kg b.wt) then CCl4 (50% CCl4/corn oil; 1 ml/kg body weight). Silymarin (SIL) + CCL4 group received SIL (200 mg/kg b.wt.) [24] and CCl4 (50% CCl4/corn oil; 1 ml/kg b.wt. i.p.). The AES + CCl4 group received AES (3.6 mg/kg b.wt.) [16] and CCl4 (50% CCl4/corn oil; 1 ml/kg b.wt. ip). DIO + CCl4 group received DIO (100 mg/kg) [25] and CCL4 (50% CCl4/corn oil; 1 ml/kg b.wt. ip). The ASE + DIO + CCl4 group received AES (1.75 mg/kg) and DIO (50 mg/kg) and then CCL4 (50% CCl4/corn oil; 1 ml/kg b.wt. ip).
Sample collection and preparation
Rats were anesthetized using diethyl ether then samples of blood were withdrawn from the retro-orbital plexus. Post blood collection, animals were euthanized by cervical dislocation under 3% sodium pentobarbital anesthesia and livers were swiftly dissected out, washed with cold normal saline and weighted. Blood samples were centrifuged at 3000 rpm g for 10 min and serum was used for further biochemical analyses.
Assessment of serum biochemical parameters
Kits used were purchased from sources detailed in (Additional file 1).
Histological and histochemical assessment studies
After blood collection, rats of each group were euthanized by cervical dislocation under 3% sodium pentobarbital anesthesia, and their livers were collected, dissected immediately after the sacrifice and were used for the histopathological analysis as described in [26, 27] and detailed in (Additional file 1).
Assessments of morphometric area percentage and optical density
This assessment was carried out on periodic acid Schiff (PAS) & Mercuric bromophenol blue stained slides. Assessed areas were analyzed as detailed in (Additional file 1).
Statistical analyses
Using statistical package for social science (SPSS), collected data were statistically analyzed as detailed in (Additional file 1).
Results and discussion
AES, DIO and their combination attenuated liver lesions in CCl4-induced liver damage
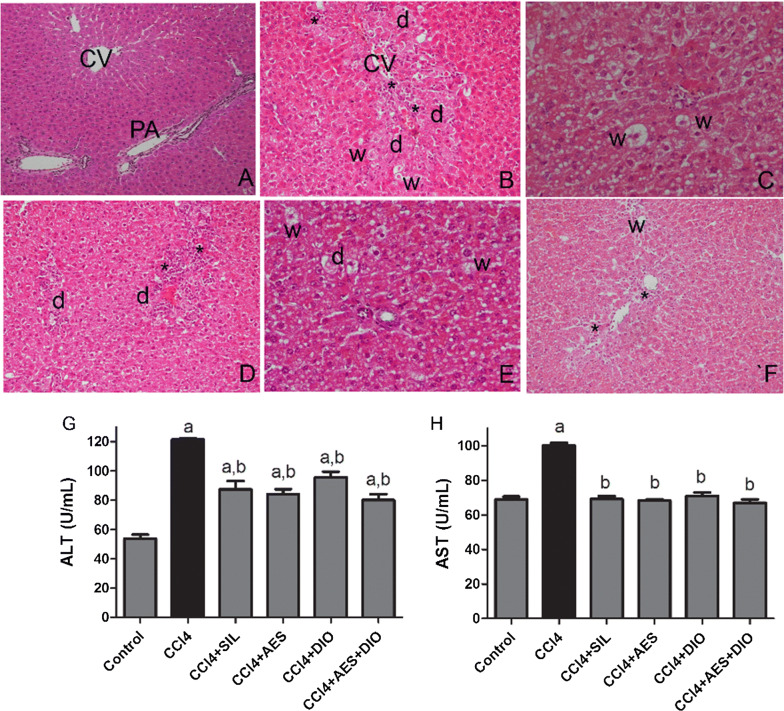
Normal liver sections “control group” showed typical hepatic architecture with the central vein centrally located and normally thickened hepatic cords radiating with well-formed hepatocytes with centrally located nuclei and intact cell membrane (Fig. 1A). CCl4-induced group showed hepatocytes degenerative changes including pyknotic nuclei. In addition, central veins were massively enlarged and clogged with ductular cells hyperplasia, scattered inflammatory cells which were concentrated around hepatic vessels (Fig. 1B). Compared to the SIL + CCl4 group (Fig. 3C) and to the protected groups; treated with ASE (Fig. 3D), DIO (Fig. 1E), animals intoxicated with CCl4 and treated with both AES and DIO at low dose combination showed marked degree of improvement of hepatic tissue as the hepatic tissue restored its normal structure and architecture with centrally located central vein and intact hepatocytes (Fig. 1F). Currently, CCl4-induced liver injury is a model that is widely employed to screen for anti-hepatotoxic/hepatoprotective drugs. It is also a useful tool to study different liver illnesses, such as fatty liver, fibrosis, and cirrhosis [2, 28]. The magnitude of CCl4 hepatic impairment is evaluated by the enhanced serum level of cytoplasmic enzymes and by histopathologic changes in liver [29, 30].
Fig. 1.
Effects of AES and DIO each alone or in low-dose combination on CCl4-induced liver damage in rats. (n = 6 per group). A–F Representative images of H&E-stained hepatic sections of the different groups (200 ×). C.V, (central vein), PA (portal area), Inflammatory cell infiltration (star), hepatic necrosis associated with degenerated nuclei (d) lipid droplets and vacuolization in hepatocytes (w). Serum markers of liver damage ALT (G), AST (H) activities. Data are presented as mean ± SEM. (n = 6/group). a P < 0.05 vs. control group. b P < 0.05 vs. CCL4 group
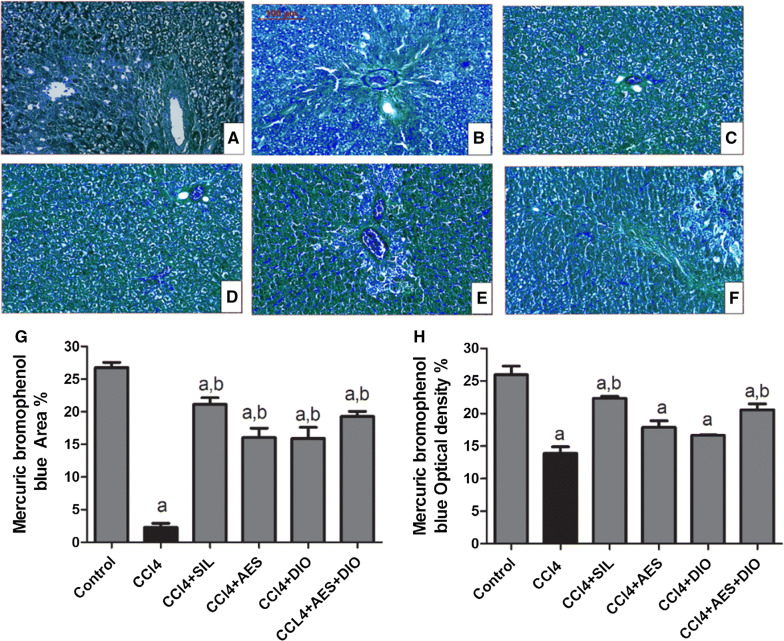
Fig. 3.
Effects of AES and DIO each alone or in low-dose combination on the distribution of protein contents in liver tissue stained by Mercuric bromophenol blue reagent in the CCl4-induced liver rat model. A–F Representative images of mercuric bromophenol blue staining (blue area), 200 × magnification, scale bar equals 500 µm. Mercuric bromophenol blue-positive area expression % (G) and optical density % (H) across10 different fields for each rat section using Leica Qwin 500 Image Analyzer. Data are presented as mean ± SEM (n = 6/group). a P < 0.05 vs. control group. b P < 0.05 vs. CCl4 group
Compared to control, CCl4-induced group had significantly higher levels of serum alanine aminotransferase (ALT) (130%), and aspartate aminotransferase (AST) (40%) (Fig. 1G, H). Levels of ALT and AST were significantly (P ≤ 0.05) improved upon treatment with SIL, AES and DIO. Pretreatment with SIL, AES, DIO and their combination reduced the elevated serum ALT by 28%, 34%, 31% and 21%, respectively, and AST levels by 31%, 33%, 29% and 32%, respectively, compared to animals in CCl4 group (Fig. 1G, H). Upregulation of ALT and AST have been ascribed to hepatotoxicity as they are released into blood serum upon hepatocyte degeneration [31]. The current study showed quite a restoration of almost normal levels especially when combined treatment was applied.
Glycogen and proteins distribution
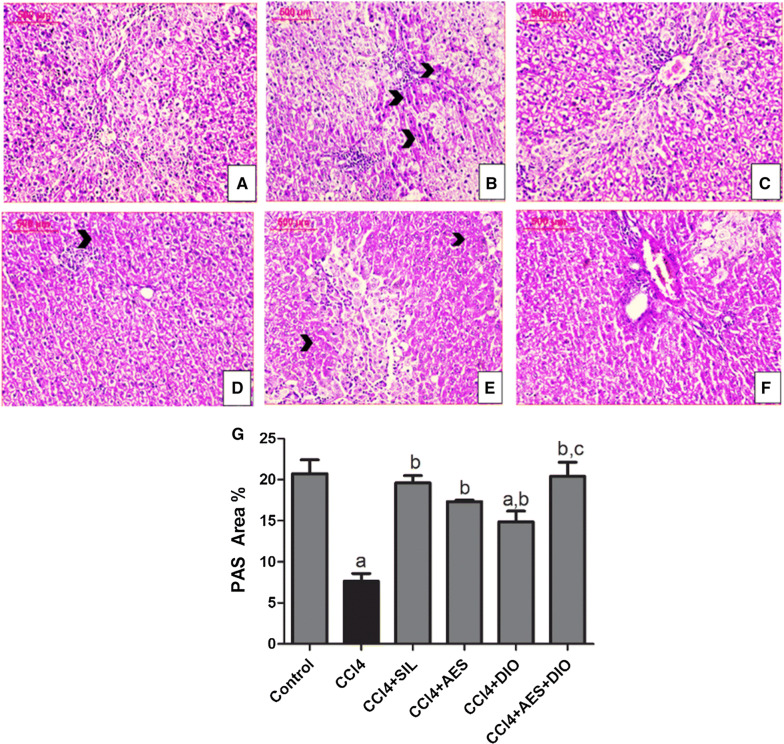
Figure 2 shows the distribution of glycogen contents in normal liver tissue as reflected by PAS positivity area (Fig. 3A; 20.74 ± 1.65). CCl4 group showed severe depletion of glycogen 6.63 ± 1.6 with a reduction percent of 82.45% from the control group.
Fig. 2.
Effects of AES and DIO each alone or in low-dose combination on the distribution of glycogen contents in liver tissue stained by PAS reagent in the CCl4-induced liver rat model. A–F Representative images of PAS staining (red area staining, arrow heads). 200 × magnification, scale bar equals 500 µm. G PAS -positive area expression as a percentage of the total area across10 different fields for each rat section using Leica Qwin 500 Image Analyzer. Data are presented as mean ± SEM (n = 6/group). a P < 0.05 vs. control group. b P < 0.05 vs. CCL4 group, c P < 0.05 vs SIL group
Groups treated with DIO, AES and both showed varying improvements in the glycogen content as reflected by the PAS area % of 15.84 ± 1.23, 16.54 ± 0.79 and 22.56 ± 1.36 respectively. Hepatotoxicity is always correlated with disruption in the synthesis and metabolism of glycogen and proteins. From the present findings, both protein and glycogen were reduced in CCl4 group. Poisonous effect on liver may mainly contribute to such reduction through the drug-induced necrosis of the plasma membrane, or via depleting energy sources needed for synthesizing protein and other metabolic events through obstructing the oxidative phosphorylation activity as documented elsewhere [32]. All tested preventive treatments successfully reversed such effect.
The control group showed the highest protein content (as reflected by Mercuric bromophenol blue positively stained area of the hepatocytes and its optical density of 26.75 ± 0.812 and 25.76 ± 1.66; Fig. 3). CCl4-intoxicated group showed the lowest comparable values of 2.29 ± 0.61 and 13.9 ± 0.98. Protected groups treated by AES and DIO showed varying degrees of protein content improvements; 16.037 ± 1.45 and 15.913 ± 1.7 and optical density of 17.88 ± 1.01 and 16.66 ± 0.08 respectively. The combined administration of AES and DIO at low dose, strengthened the stain reaction and showed marked improvement in the protein content of hepatocytes of 19.27 ± 0.8 and optical density of 20.59 ± 0.9. The present results of pre-administration of AES improved liver histology and function and significantly enhanced total hepatic glycogen and protein content. These results confirmed the hepatoprotective effect of AES as previously reported against liver damage induced by methyl parathion [15] and CCl4 [16, 22] in rats. Although treatment with DIO do positively contribute to liver histology and function, the protective effect of DIO was less evident than that of AES. These hepatoprotective effects of DIO coincide with those of [15, 19, 20]. Interestingly, livers of rats in CCl4 group treated with both AES and DIO showed minimal inflammation, eosinophilic staining and degenerations with significant enhancements in overall glycogen and protein contents in liver.
AES, DIO and their combination ameliorated oxidative stress and inflammation in CCl4-induced liver damage in rats
The administration of SIL, AES, DIO and AES/DIO combination significantly attenuated the increment in serum MDA and nitric oxide (NO) levels and partly attenuated the decrement in glutathione (GSH), Catalase and protein kinase C (PKC). Compared to SIL, AES, DIO and AES/DIO groups, the treatment with combined AES and DIO showed the best effects against all examined oxidative stress markers (Additional file 2). Abundant evidence suggests that reactive oxygen species (ROS) and other free radicals are induced following hepatic insults with drugs like CCl4. The enzymatic/non-enzymatic defense system is the natural protec-tor against free radicals accumulation [28, 30]. The accumulation of free radicals can cause decreased Catalase and GSH levels and a decline in capacity of scavenging free radicals. Consisting with previous report [30, 33], liver damage shown in CCl4-treated group was associated with enhanced MDA and NO as well as depletion of GSH and Catalase. Oxidative stress is known to cause activation of several stress kinases such as PKC and leads to exacerbation of cell toxicity [34]. The elevation of PKC in the present work was in line with the results of the previous studies which suggested that the hepatic oxidative stress and injury induced by bezafibrate [34] and acetaminophen [35] and CCl4 [36] was mediated by elevation of PKC. PKC induces upregulation of tumor necrosis factor- α (TNF-α), interleukin-6 (IL-6), and ROS, a major contributor to CCl4-induced liver injury [36]. Here, and in agreement with results of [16], AES ameliorated CCl4-induced hepatotoxicity by reducing MDA and NO and increasing GSH activity. Similarly, in this study, DIO mitigated CCl4-induced oxidative and nitrative stresses confirming previous studies that showed that DIO alleviates the oxidative stress caused by aflatoxin [22], methotrexate [20] by increasing GSH levels, lowering NO levels, and enhancing the activity of antioxidant enzymes. Most interestingly, much improvement in oxidative stress and antioxidants status was observed here when AES was combined with DIO.
Limitations
This study was primarily focused on the assessment of tested extracts in induced liver fibrosis in animal model, more investigations are required for more understanding of the molecular mechanisms by which AES and DIO each alone or in low-dose combination might exert their beneficial hepatoprotective effect such as their effects on the inhibition of cytochrome CYP2E1 activity.
Supplementary information
Additional file 2. Effects of AES and DIO each alone or in low-dose combination on oxidative stress and inflammatory markers in rats. Data are presented as mean ± SEM (n = 6/group). a P < 0.05 vs. control group. b P < 0.05 vs. CCl4 group, c P < 0.05 vs SIL group, d P < 0.05 vs AES group. e P < 0.05 vs DIO group, f P < 0.05 vs AES +DIO.
Acknowledgements
Authors thank Al-Jalila Foundation No. 21S098 for partially funding this study for Amr Amin.
Abbreviations
- AES
Aescin
- DIO
Diosmin
- CCl4
Carbon tetrachloride
- SIL
Silymarin
- PAS
Periodic acid Schiff
- SPSS
Statistical package for social science
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- MDA
Malondialdehyde
- NO
Nitric oxide
- GSH
Glutathione
- PKC
Protein kinase C
- TNF-α
Tumor necrosis factor α
- IL-6
Interleukin-6
- ROS
Reactive oxygen species
Authors’ contributions
D, AS, NY, and SH conceived and designed research. SD, AS, NY, and SH conducted experiments. SD, AS, NY, and SH contributed materials, technical and analytical tools. SD, AS, NY, SH and AH analyzed data. AH, SH and AA wrote the manuscript. All authors read and approved the manuscript.
Funding
Partially support by Al-Jalila Foundation No. 21S098 was provided for Amr Amin.
Availability of data and materials
All data supporting the conclusions of this article are included within the article and all datasets supporting our findings are available.
Ethics approval and consent to participate
All procedures in the animal studies complied with and are approved in accordance with the Ethics Committee of the National Research Centre and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13104-020-05094-2.
References
- 1.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology (Baltimore, MD) 2014;60(6):2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingawale DK, Mandlik SK, Naik SR. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism (s): a critical discussion. Environ Toxicol Pharmacol. 2014;37(1):118–133. doi: 10.1016/j.etap.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Boll M, Lutz W, Becker E, Stampfl A. Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Zeitschrift für Naturforschung C. 2001;56(7–8):649–659. doi: 10.1515/znc-2001-7-826. [DOI] [PubMed] [Google Scholar]
- 4.Hamza AA, Heeba GH, Elwy HM, Murali C, El-Awady R, Amin A. Molecular characterization of the grape seeds extract’s effect against chemically induced liver cancer: in vivo and in vitro analyses. Sci Rep. 2018;8:1–16. doi: 10.1038/s41598-018-19492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Kharrag R, Amin A, Hisaindee S, Greish Y, Karam SM. Development of a therapeutic model of precancerous liver using crocin-coated magnetite nanoparticles. Int J Oncol. 2017;50:212–222. doi: 10.3892/ijo.2016.3769. [DOI] [PubMed] [Google Scholar]
- 6.Amin A, Hamza AA, Daoud S, Khazanehdari K, Al Hrout A, Baig B, Chaiboonchoe A, Adrian TE, Zaki N, Salehi-Ashtiani K. Saffron-based crocin prevents early lesions of liver cancer: in vivo, in vitro & network analyses. Recent Patents Anti Cancer Drug Discov. 2016;11:121–133. doi: 10.2174/1574892810666151102110248. [DOI] [PubMed] [Google Scholar]
- 7.Amin A, Mahmoud-Ghoneim D. Texture analysis of liver fibrosis microscopic images: a study on the effect of biomarkers. Acta Biochim Biophys Sin. 2011;43(3):193–203. doi: 10.1093/abbs/gmq129. [DOI] [PubMed] [Google Scholar]
- 8.Mahmoud-Ghoneim D, Amin A, Corr P. MRI-based texture analysis, a potential technique to assess protectors against induced-liver fibrosis in rats. Radiol Oncol. 2009;43(1):30–40. [Google Scholar]
- 9.Amin A. Chemopreventive effect of chlorella on the antioxidant system in DMBA-induced oxidative stress in liver. Int J Pharmacol. 2008;4(3):169–176. [Google Scholar]
- 10.Celep AGS, Yilmaz S, Coruh N. Antioxidant capacity and cytotoxicity of Aesculus hippocastanum on breast cancer MCF-7 cells. J Food Drug Anal. 2002;20:692–698. [Google Scholar]
- 11.Xiao G-m, Wei J. Effects of β-Aescin on the expression of nuclear factor-κB and tumor necrosis factor-α after traumatic brain injury in rats. J Zhejiang Univ Sci B. 2005;6(1):28. doi: 10.1631/jzus.2005.B0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji DB, Xu B, Liu JT, Ran FX, Cui JR. beta-Escin sodium inhibits inducible nitric oxide synthase expression via downregulation of the JAK/STAT pathway in A549 cells. Mol Carcinog. 2011;50(12):945–960. doi: 10.1002/mc.20762. [DOI] [PubMed] [Google Scholar]
- 13.Xin W, Zhang L, Sun F, Jiang N, Fan H, Wang T, et al. Escin exerts synergistic anti-inflammatory effects with low doses of glucocorticoids in vivo and in vitro. Phytomedicine. 2011;18(4):272–277. doi: 10.1016/j.phymed.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Jiang N, Xin W, Wang T, Zhang L, Fan H, Du Y, et al. Protective effect of aescin from the seeds of Aesculus hippocastanum on liver injury induced by endotoxin in mice. Phytomedicine. 2011;18(14):1276–1284. doi: 10.1016/j.phymed.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Du Y, Wang T, Jiang N, Ren R-T, Li C, Li C-K, et al. Sodium aescinate ameliorates liver injury induced by methyl parathion in rats. Exp Ther Med. 2012;3(5):818–822. doi: 10.3892/etm.2012.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh H, Sidhu S, Chopra K, Khan M. The novel role of β-aescin in attenuating CCL4-induced hepatotoxicity in rats. Pharm Biol. 2017;55(1):749–757. doi: 10.1080/13880209.2016.1275023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szymański M, Młynarek D, Szymański A, Matławska I. Simultaneous determination of diosmin and hesperidin in pharmaceuticals by RPLC using ionic liquids as mobile phase modifiers. Iran J Pharm Res. 2016;15(1):141–148. [PMC free article] [PubMed] [Google Scholar]
- 18.Katsenis K. Micronized purified flavonoid fraction (MPFF): a review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr Vasc Pharmacol. 2005;3(1):1–9. doi: 10.2174/1570161052773870. [DOI] [PubMed] [Google Scholar]
- 19.Tahir M, Rehman MU, Lateef A, Khan R, Khan AQ, Qamar W, et al. Diosmin protects against ethanol-induced hepatic injury via alleviation of inflammation and regulation of TNF-alpha and NF-kappaB activation. Alcohol. 2013;47(2):131–139. doi: 10.1016/j.alcohol.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Daim MM, Khalifa HA, Abushouk AI, Dkhil MA, Al-Quraishy SA. Diosmin attenuates methotrexate-induced hepatic, renal, and cardiac injury: a biochemical and histopathological study in mice. Oxidative Med Cell Longevity. 2017;2017:10. doi: 10.1155/2017/3281670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Reheim AM, Messiha BS, Abo-Saif A. Hepatoprotective effect of diosmin on iron-induced liver damage. Int J Pharmacol. 2017;13:529–540. [Google Scholar]
- 22.Eraslan G, Sarica ZS, Bayram LC, Tekeli MY, Kanbur M, Karabacak M. The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ Sci Pollut Res Int. 2017;24(36):27931–27941. doi: 10.1007/s11356-017-0232-7. [DOI] [PubMed] [Google Scholar]
- 23.Sharmila Queenthy S, Stanely Mainzen Prince P, John B. Diosmin prevents isoproterenol-induced heart mitochondrial oxidative stress in rats. Cardiovasc Toxicol. 2018;18(2):120–130. doi: 10.1007/s12012-017-9422-2. [DOI] [PubMed] [Google Scholar]
- 24.Li CC, Hsiang CY, Wu SL, Ho TY. Identification of novel mechanisms of silymarin on the carbon tetrachloride-induced liver fibrosis in mice by nuclear factor-kappaB bioluminescent imaging-guided transcriptomic analysis. Food Chem Toxicol. 2012;50(5):1568–1575. doi: 10.1016/j.fct.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Germoush MO. Diosmin protects against cyclophosphamide-induced liver injury through attenuation of oxidative stress, inflammation and apoptosis. Int J Pharmacol. 2016;12:644–654. [Google Scholar]
- 26.Hotchkiss RD. A microchemical reaction resulting in the staining of polysaccharide structures in fixed tissue preparations. Arch Biochem. 1948;16(1):131–141. [PubMed] [Google Scholar]
- 27.Mazia D, Brewer PA, Alfert M. The Cytochemical staining and measurement of protein with mercuric bromphenol blue. Biol Bull. 1953;104(1):57–67. [Google Scholar]
- 28.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 29.Awaad AS, Soliman GA, El-Sayed DF, El-Gindi OD, Alqasoumi SI. Hepatoprotective activity of Cyperus alternifolius on carbon tetrachloride-induced hepatotoxicity in rats. Pharm Biol. 2012;50(2):155–161. doi: 10.3109/13880209.2011.580351. [DOI] [PubMed] [Google Scholar]
- 30.Jayesh K, Helen LR, Vysakh A, Binil E, Latha Protective role of Terminalia bellirica (Gaertn.) Roxb fruits against CCL4 induced oxidative stress and liver injury in rodent model. Indian J Clin Biochem. 2019;34(2):155–163. doi: 10.1007/s12291-017-0732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdelhafez OH, Fawzy MA, Fahim JR, Desoukey SY, Krischke M, Mueller MJ, et al. Hepatoprotective potential of Malvaviscus arboreus against carbon tetrachloride-induced liver injury in rats. PLoS ONE. 2018;13(8):e0202362. doi: 10.1371/journal.pone.0202362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdel Salam OM, Baiuomy AR, El-Shenawy SM, Hassan NS. Effect of pentoxifylline on hepatic injury caused in the rat by the administration of carbon tetrachloride or acetaminophen. Pharmacol Rep. 2005;57(5):596–603. [PubMed] [Google Scholar]
- 33.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima T, Tanaka N, Li G, Hu R, Kamijo Y, Hara A, et al. Effect of bezafibrate on hepatic oxidative stress: comparison between conventional experimental doses and clinically-relevant doses in mice. Redox Rep. 2010;15(3):123–130. doi: 10.1179/174329210X12650506623807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saberi B, Ybanez MD, Johnson HS, Gaarde WA, Han D, Kaplowitz N. Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and-independent signaling pathways. Hepatology. 2014;59(4):1543–1554. doi: 10.1002/hep.26625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, Kim SJ, Lee H-S, Kwon O-S. PKCδ mediates NF-κB inflammatory response and downregulates SIRT1 expression in liver fibrosis. Int J Mol Sci. 2019;20(18):4607. doi: 10.3390/ijms20184607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Effects of AES and DIO each alone or in low-dose combination on oxidative stress and inflammatory markers in rats. Data are presented as mean ± SEM (n = 6/group). a P < 0.05 vs. control group. b P < 0.05 vs. CCl4 group, c P < 0.05 vs SIL group, d P < 0.05 vs AES group. e P < 0.05 vs DIO group, f P < 0.05 vs AES +DIO.
Data Availability Statement
All data supporting the conclusions of this article are included within the article and all datasets supporting our findings are available.