Abstract
Background:
The variation in articular cartilage thickness (ACT) in healthy knees is difficult to quantify and therefore poorly documented. Our aims are to (1) define how machine learning (ML) algorithms can automate the segmentation and measurement of ACT on magnetic resonance imaging (MRI) (2) use ML to provide reference data on ACT in healthy knees, and (3) identify whether demographic variables impact these results.
Methods:
Patients recruited into the Osteoarthritis Initiative with a radiographic Kellgren-Lawrence grade of 0 or 1 with 3D double-echo steady-state MRIs were included and their gender, age, and body mass index were collected. Using a validated ML algorithm, 2 orthogonal points on each femoral condyle were identified (distal and posterior) and ACT was measured on each MRI. Site-specific ACT was compared using paired t-tests, and multivariate regression was used to investigate the risk-adjusted effect of each demographic variable on ACT.
Results:
A total of 3910 MRI were included. The average femoral ACT was 2.34 mm (standard deviation, 0.71; 95% confidence interval, 0.95-3.73). In multivariate analysis, distal-medial (−0.17 mm) and distal-lateral cartilage (−0.32 mm) were found to be thinner than posterior-lateral cartilage, while posterior-medial cartilage was found to be thicker (0.21 mm). In addition, female sex was found to negatively impact cartilage thickness (OR, −0.36; all values: P < .001).
Conclusion:
ML was effectively used to automate the segmentation and measurement of cartilage thickness on a large number of MRIs of healthy knees to provide normative data on the variation in ACT in this population. We further report patient variables that can influence ACT. Further validation will determine whether this technique represents a powerful new tool for tracking the impact of medical intervention on the progression of articular cartilage degeneration.
Keywords: machine learning, cartilage segmentation, cartilage thickness, arthritis, knee cartilage
Knee osteoarthritis (OA) is an increasingly common joint disease—almost 15% of US citizens will develop OA in their lifetime [1]. Establishing clinical measures associated with cartilage health and identifying changes in cartilage thickness may be useful when evaluating the effectiveness of protocols to reduce the risk of knee OA progression [2,3]. Although the very earliest OA stages may result in an increase in cartilage thickness [4], structural changes in the development and progression of clinical OA are commonly understood to be characterized by erosion and loss of articular cartilage. Individuals with symptomatic knee OA have been shown to have particularly significant changes in their posterior femoral condylar cartilage [5], which engages with the tibia in flexion, and in their distal femoral condylar cartilage [6,7], which engages with the tibia in extension. Current knowledge on the variation in normal and abnormal cartilage thickness, however, is outdated [8] and often studied in small patient samples [9] and with older imaging technologies [10].
In order to further understand the impact of the disease on articular cartilage thickness (ACT), it would be helpful to understand the variation in physiologic cartilage thickness across a large population of healthy patients and identify demographic factors that influence this variation. Furthermore, accurate measurement of cartilage thickness may be clinically useful in detecting and monitoring treatment effects for focal and nonfocal disease. In other areas, such as surgical technique for total knee arthroplasty, assumptions are made around ACT that impact implant positioning.
Magnetic resonance imaging (MRI) has become a gold standard for assessing knee cartilage thickness [11]. However, manual calculation of cartilage thickness from MRI is challenging and prone to error rendering the analysis of larger cohorts of patients or individualized therapy planning impractical [12,13]. Therefore, automated cartilage segmentation methods have been designed which are increasing in both popularity and accuracy [12,14]. Machine learning (ML), specifically neural networks, has been shown to successfully elucidate complex spatial relationships in images [15] and to segment cartilage successfully [12,16].
The objectives of this article were to determine (1) whether the automated segmentation and measurement of cartilage thickness using ML algorithms applied to a large number of MRIs can be used to (2) provide reference data on cartilage thickness in a large patient (control subjects in this case) population and (3) identify how demographic variables impact these results.
Methods
Study Sample
Patients in our analysis were selected from the Osteoarthritis Initiative (OAI), which contains MRIs of 4796 patients. The OAI is a public-private partnership focused on understanding the development and progression of symptomatic knee OA. Only patients without OA were included in this analysis, defined as patients having a Kellgren-Lawrence grade of 0 or 1. The Kellgren-Lawrence grade was determined through the OAI where 2 expert readers independently assessed each X-ray and were blinded to both each other’s reading and the subject’s clinical data. Furthermore, only patients with double-echo steady-state (DESS) MRI series were included in our analysis (n = 3910). DESS MRI series are 3D DESS MRIs performed on a 3T MRI Machine from Siemens (repetition time/echo time, 16.2/4.7; field of view, 14 cm; matrix, 307 × 348; bandwidth, 62.5 kHz; and image resolutions, [0.3646 0.3646 0.7] mm).
Cartilage Segmentation
A validated ML segmentation model was used in order to identify which pixels of each MR image represented which tissue type for each series of MR images [17].
The model used in this study was a convolutional neural network which converts the greyscale value of each pixel of a radiograph to 1 of 6 numbers each representing 6 different tissue types. The identified tissue types included femoral cartilage, lateral tibial cartilage, medial tibial cartilage, patellar cartilage, lateral meniscus, and medial meniscus.
The neural network model chosen for this problem is based on the U-Net architecture which has previously shown promising results in the task of segmentation particularly for medical images [18-20]. The model was trained on 167 images which had been manually segmented by senior radiologists and technicians at our host institution. The automatic segmentation model used in this study has previously been found to have a correlation with manual segmentation for calculation of cartilage volume and thickness of 0.9349 and 0.9384, respectively, and the automatic segmentation was found to have comparable longitudinal precision to manual segmentation [12,14,21]. The server used to conduct this analysis had 64 processors, 251.6 GB of memory, and 4× Titan 12 GB GPUs.
Data Analysis
We investigated the cartilage thickness across 4 points in the knee: the distal most point of the medial and lateral femoral condyle (DM, DL), and the posterior most point of the medial and lateral femoral condyle (PM, PL). We chose to calculate thickness at these landmarks rather than averages across compartments because of their function in knee kinematics and how cartilage thickness changes in these locations affect clinical disease progression. Specifically, the distal most point of each femoral condyle engages with and demonstrates high levels of contact pressure with the tibia in extension [22,23] and has shown particularly sharp changes in medial knee OA [6,7]. In addition, the posterior most point on each femoral condyle engages with and shows high levels of contact pressure with the tibia in flexion [17,24,25] and has also demonstrated sharp changes, particularly in valgus knee OA [5].
Cartilage thickness was calculated based on previously published methodology to calculate thickness from MRIs. Several methods have been proposed which calculate the thickness of cartilage based on a vector perpendicular to the surface of the subchondral femur [26,27]. Certain methods use a 3D method in a grid created by the MRI to find a vector which is perpendicular to the point on the knee of interest [28]. Others align the MR image so that the y-axis of the image is aligned to the long axis of the femur and then use a 2D vector to calculate thickness from the point of interest on the femur [29]. Our study similarly aligned the images to the axis of the femoral condyle and used a 2D vector to calculate cartilage thickness.
The distal most points were identified by aligning the MRI so that the femur was parallel to the vertical axis of the image (the femoral y-axis was aligned with the vertical axis of the sagittal plane) and finding the most distal point that coded for femoral cartilage on each condyle. Thickness was measured by calculating how many pixels coded for femoral cartilage proximal to the most distal point within the same anterior-posterior slice and lateral-medial slice as the distal point. Each pixel on the vertical axis represented 0.346 mm. The pixel representation of distal cartilage thickness was converted into millimeters.
The posterior most point was found by finding the point most posterior in reference to the long axis of the femur that coded for femoral cartilage on each respective condyle. Thickness was calculated by calculating how many pixels coded for femoral cartilage anteriorly to the most posterior point within the same inferior-superior slice and lateral-medial slice as the posterior most point. Each pixel on the anterior-posterior axis represented 0.346 mm. The pixel representation of cartilage thickness was converted into millimeters.
We calculated the average and standard deviation for cartilage thickness in the 4 sites defined above. In univariate analysis, we investigated the association between age, sex, and body mass index (BMI) and cartilage thickness at each site with correlation coefficients. Pearson correlation coefficients were used for continuous variables (age, BMI), and Spearman correlation coefficients were used for dichotomous variables (sex). We then compared the differences in cartilage thickness at each of the 4 sites with paired t-tests. Finally, a multivariate linear regression was run to investigate the risk-adjusted effect of each demographic variable and cartilage location on cartilage thickness.
Results
A total of 3910 MRI series met the inclusion criteria used in our study from the OAI. Demographic information on the average patient is included in Table 1. The time required to process all the MRIs was 68 hours. The average femoral ACT was 2.34 mm (standard deviation, 0.71; 95% confidence interval [CI], 0.95-3.73).
Table 1.
The Distribution of Demographic Variables With Respect to Age, Sex, and Body Mass Index (BMI).
| Age | |
| <50 | 678 (17.3%) |
| 50-60 | 1505 (38.4%) |
| 60-70 | 1062 (27.1%) |
| >70 | 665 (17%) |
| Sex | |
| Male | 1692 (43.2%) |
| Female | 2218 (56.7%) |
| BMI | |
| <25 | 1239 (31.6%) |
| 25-30 | 1578 (40.3%) |
| 30-35 | 852 (21.7%) |
| >35 | 241 (6.1%) |
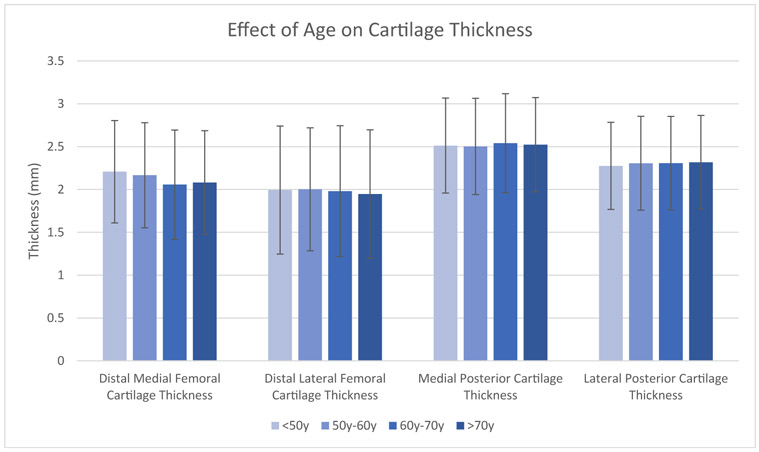
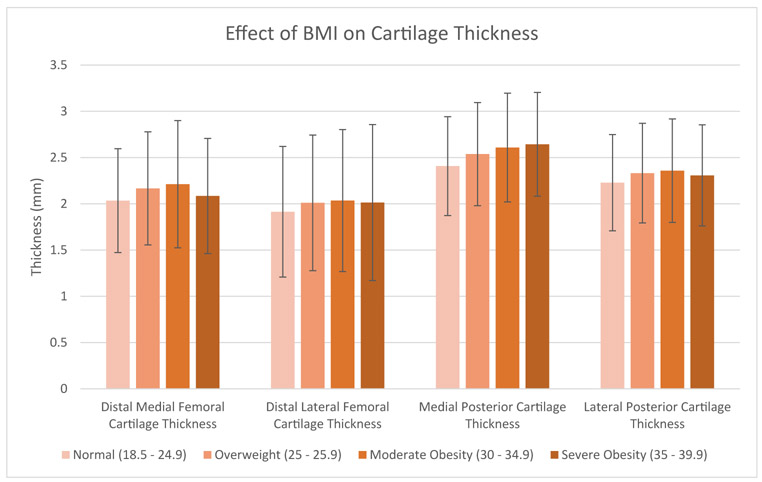
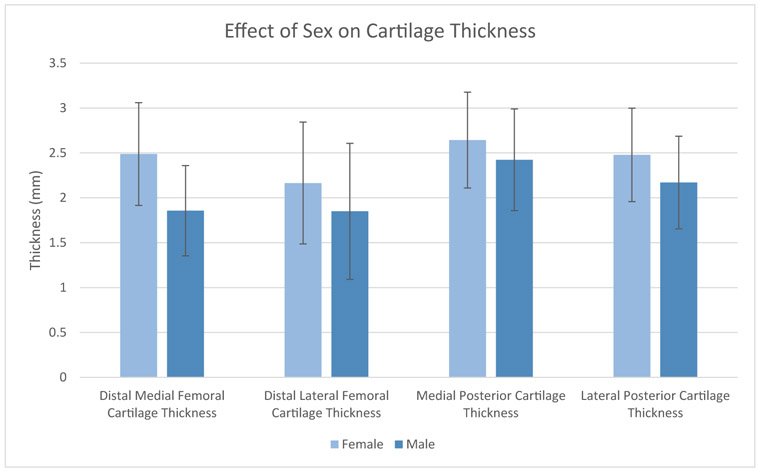
The average thickness of the distal medial, distal lateral, posterior medial, and posterior lateral cartilage was 2.13 mm, 1.99 mm, 2.52 mm, and 2.30 mm (Table 2). There was a wide range of distal cartilage thickness, as the 95% CI spanned almost 3 mm on both condyles. The effect of age, gender, and BMI on average cartilage thickness is shown in Figures 1-3 and Table 3. Male sex had a significant positive correlation with thickness at all 4 locations (ρ = 0.21-0.55, P < .0001), and this correlation was particularly marked for distal medial cartilage thickness. In addition, BMI had a significant positive correlation with thickness at all 4 locations (r = 0.067-0.14, P < .0001). The difference in cartilage thickness between locations in univariate analysis is displayed in Table 4. Distal medial cartilage was significantly thinner than posterior medial and lateral cartilage (−0.39 mm and −0.17 mm, respectively, P < .0001 for both). Distal lateral cartilage also was significantly thinner than posterior medial and lateral cartilage (−0.53 mm and −0.32 mm, respectively, P < .001 for both). Finally, distal medial cartilage was found to be thicker than distal lateral cartilage by 0.14 mm (P < .0001).
Table 2.
Average and Standard Deviation of Cartilage Thickness and Joint Space in Healthy Knees.
| Average (SD) | 95% Confidence Interval |
|
|---|---|---|
| Cartilage thickness (mm) Distal medial condyle |
2.13 (0.62) | 0.91-3.34 |
| Distal lateral condyle | 1.99 (0.74) | 0.53-3.44 |
| Posterior medial condyle | 2.52 (0.56) | 1.42-3.61 |
| Posterior lateral condyle | 2.30 (0.54) | 1.24-3.35 |
OA, osteoarthritis; SD, standard deviation.
Fig. 1.
The effect of age on average cartilage thickness on the distal medial, posterior medial, distal lateral, and posterior lateral femoral condyle. Error bars display 1 standard deviation.
Fig. 3.
The effect of BMI on average cartilage thickness on the distal medial, posterior medial, distal lateral, and posterior lateral femoral condyle. Error bars display standard deviation.
Table 3.
Correlation Between Demographic Variables and Cartilage Thickness at the Distal Medial, Distal Lateral, Posterior Medial, and Posterior Lateral Points of the Knee.
| Correlation With Cartilage Thickness Depending on Location (P Value) | ||||
|---|---|---|---|---|
| Distal Medial Cartilage Thickness |
Distal Lateral Cartilage Thickness |
Posterior Medial Cartilage Thickness |
Posterior Lateral Cartilage Thickness |
|
| Sex | 0.55 (P < .0001) | 0.24 (P < .0001) | 0.21 (P < .0001) | 0.30 (P < .0001) |
| Age | −0.086 (P < .0001) | −0.028 (P = .083) | 0.018 (P = .25) | 0.013 (P = .41) |
| BMI | 0.091 (P < .0001) | 0.067 (P < .0001) | 0.14 (P < .0001) | 0.090 (P < .0001) |
Spearman correlation used for dichotomous variables; Pearson correlation used for continuous variables. BMI, body mass index.
Table 4.
Univariate Analysis of the Differences in Cartilage Thickness at the Distal Medial, Distal Lateral, Posterior Medial, and Posterior Lateral Points of the Knee.
| Average Difference (mm) Standard Deviation of Difference (mm) P Value |
||||
|---|---|---|---|---|
| Distal Medial Cartilage Thickness |
Distal Lateral Cartilage Thickness |
Posterior Medial Cartilage Thickness |
Posterior Lateral Cartilage Thickness |
|
| Distal medial cartilage thickness | ||||
| Distal lateral cartilage thickness | 0.14 | |||
| 0.74 | ||||
| P < .0001 | ||||
| Posterior medial cartilage thickness | −0.39 | −0.53 | ||
| 0.75 | 0.86 | |||
| P < .0001 | P < .0001 | |||
| Posterior lateral cartilage thickness | −0.17 | −0.32 | 0.21 | |
| 0.69 | 0.79 | 0.65 | ||
| P < .0001 | P < .0001 | P < .0001 | ||
Paired t-tests used for statistical comparisons.
Average difference (mm), Standard deviation of difference (mm), and P value are represented. The reference value is the column header.
The results of our multivariate regression are displayed in Table 5. We found that female sex and lower BMI were associated with significantly thinner ACT, while male sex and higher BMI were associated with thicker ACT (all P < .001). In addition, we found that distal lateral and distal medial cartilage was thinner than posterior cartilage after risk adjustment (P < .001).
Table 5.
Multivariate Analysis of the Effect of Location and Patient Variables on Cartilage Thickness.
| Patient Characteristic | Odds Ratio (Confidence Interval) | P Value |
|---|---|---|
| Age | −0.001 (−0.011 to 0.007) | .7073 |
| BMI | 0.04 (0.03 to 0.051) | <.0001 |
| Female | −0.361 (−0.38 to −0.342) | <.0001 |
| Location of cartilage | ||
| Posterior lateral condyle | REF | |
| Posterior medial condyle | 0.214 (0.188 to 0.24) | <.0001 |
| Distal lateral condyle | −0.317 (−0.344 to −0.291) | <.0001 |
| Distal medial condyle | −0.173 (−0.199 to −0.147) | <.0001 |
BMI, body mass index.
Bold represents a P-value of less than 0.05 was considered statistically significant.
Discussion
A methodology that allows for an accurate and automated measurement of cartilage thickness may be clinically useful in detecting and monitoring treatment effects and natural progression of OA over time. Previous efforts to investigate the average cartilage thickness at weight-bearing points in the healthy knee are now outdated in terms of the technology used for the analysis and generally studied in a small number of patients. Furthermore, the methodologies described previously have been very resourceintensive. In this study, we document the use of a technique that was able to analyze cartilage thickness in the MRIs of the knees of a large population of patients by using ML methods. We found that healthy female patients and patient with lower BMIs have significantly thinner cartilage than male patients and those with higher BMIs. In addition, we found that distal femoral cartilage is significantly thinner than posterior cartilage in healthy and OA knees. Our data can serve as a baseline for referencing average ACT in future studies and provides a template on how to use ML to manage large imaging study datasets. Furthermore, we demonstrate that the use of ML-based algorithms presents a promising new methodology through which to study ACT and, by inference, OA progression, in large populations. Such capability is particularly relevant in the context of medical interventions designed to alter disease progression.
The trends in cartilage thickness presented here provide further insights in the context of past studies on the thickness of distal femoral articular cartilage. Several small wet lab-based studies have found distal cartilage thickness ranging from 1.65 mm to 2.65 mm [8,30] A study done on the same OAI dataset used in this analysis in 2017 similarly found that distal medial cartilage thickness in healthy knees was on average 1.820 mm [31]. However, this analysis was done on images segmented by a probably approximately correct learning method which is not validated. In addition, little information was provided on the site where thickness was calculated. Our study calculates thickness at specific clinical sites and used a validated method to segment cartilage. We found that distal medial cartilage was slightly thicker on average at 2.13 mm and that there is a wide range of thickness in healthy knees as the 95% CI spanned nearly 3 mm in both condyles.
Past research has investigated the effect of age on cartilage thickness. For example, a study done in 2017 on 10 knees under 30 years of age found femoral cartilage thickness of 3.6 mm to 4.3 mm [32], suggesting that age may negatively impact cartilage thickness, a finding which has also been shown in slightly larger studies [33,34]. In our study, older patients without radiographic signs of OA were found to have thinner cartilage than younger patients but this finding did not reach statistical significance. Furthermore, our study found that female patients had significantly thinner cartilage distally compared to males. This is a finding that has been published in small populations in the past [35-38], but our study is the first to demonstrate it in a large population and across several distinct areas of the knee. Additionally, our study found that increasing BMI is associated with thicker cartilage in healthy patients. This pattern of the effect of BMI on thickness has been reported before in small populations [39] but other studies have demonstrated the opposite effect [40]. Our study confirms the positive correlation of BMI on cartilage thickness with a much larger population than previously evaluated and across several areas in the knee. Finally, our study found that posterior condylar cartilage is thicker than distal cartilage in healthy patients, which has been supported elsewhere [6]. This information taken as a whole provides a comprehensive baseline on the normal physiologic variation in cartilage thickness of the distal femur in radiographically healthy knees across a broad population.
The strengths of this study are that it uses ML to automatically segment cartilage in knee MRIs using ML algorithms in a large population. Several past research projects have demonstrated how ML can automatically segment knee MRIs with equal performance to manual segmentation [12,14,41]. Our study builds on these frameworks to perform an automated analysis of nearly 4000 images using a validated ML methodology. Furthermore, the study sample was balanced with respect to age, gender, and BMI.
There are several limitations to this study. First, this study is based on a US population who participated in the OAI and therefore our findings need to be replicated in other populations before they are generalized beyond the United States. In addition, the analysis was done on 3.0 Tesla MRI studies which create a high-quality image to perform the analysis. However, our methodology uses a 2D landmarked-based method that can be easily translated to standard MRI sequences. We acknowledge that we relied on prior validation of this technique and did not attempt to replicate work performed elsewhere showing ML to be an accurate method to analyze cartilage thickness and to be faster, cheaper, less laborintensive, and less subjective than manual segmentation [13,15,17]. Additionally, we have not accounted for race in the analysis as that data point was not available. It is important to note that the goal of this investigation was to create a template for how to analyze a large number of MRIs in a large population in hopes that further research can build on our work. To validate this tool in the context of disease progression, a large data set of sequential images would need to be studied. However, we have shown that the methodology we report is sufficiently sensitive in differentiating ACT that it can be used in such a context.
In conclusion, this study provides insight into the normal physiologic variation in cartilage thickness in a large cohort of patients considered to have healthy knees based on their radiographic and clinical findings. It also confirms that ML can be used to analyze cartilage thickness in a large number of MRI studies in an automated and efficient manner. Further study is needed to evaluate the ability of these techniques to track disease progression over time and determine the feasibility of applying ML as a longitudinal clinical tool for the evaluation of treatment modalities for diseases of articular cartilage.
Supplementary Material
Fig. 2.
The effect of sex on average cartilage thickness on the distal medial, posterior medial, distal lateral, and posterior lateral femoral condyle. Error bars display standard deviation.
Acknowledgments
Funding Information: Partially funded by the following NIAMS/NIH grants—R61AR073552 (SM/VP) and R00AR070902 (VP).
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.arth.2019.07.022.
References
- [1].Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26: 355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Buck RJ, Wyman BT, Hellio Le Graverand MP, Hudelmaier M, Wirth W, Eckstein F. Osteoarthritis may not be a one-way-road of cartilage loss – comparison of spatial patterns of cartilage change between osteoarthritic and healthy knees. Osteoarthritis Cartilage 2010;18:329–35. [DOI] [PubMed] [Google Scholar]
- [3].Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage 2006;14:46–75. [DOI] [PubMed] [Google Scholar]
- [4].Frobell RB, Nevitt MC, Hudelmaier M, Wirth W, Wyman BT, Benichou O, et al. Femorotibial subchondral bone area and regional cartilage thickness: a cross-sectional description in healthy reference cases and various radiographic stages of osteoarthritis in 1,003 knees from the Osteoarthritis Initiative. Arthritis Care Res 2010;62:1612–23. [DOI] [PubMed] [Google Scholar]
- [5].Omoumi P, Michoux N, Roemer FW, Thienpont E, Vande Berg BC. Cartilage thickness at the posterior medial femoral condyle is increased in femorotibial knee osteoarthritis: a cross-sectional CT arthrography study (Part 2). Osteoarthritis Cartilage 2015;23:224–31. [DOI] [PubMed] [Google Scholar]
- [6].Favre J, Scanlan SF, Erhart-Hledik JC, Blazek K, Andriacchi TP. Patterns of femoral cartilage thickness are different in asymptomatic and osteoarthritic knees and can be used to detect disease-related differences between samples. J Biomech Eng 2013;135:101002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hada S, Kaneko H, Sadatsuki R, Liu L, Futami I, Kinoshita M, et al. The degeneration and destruction of femoral articular cartilage shows a greater degree of deterioration than that of the tibial and patellar articular cartilage in early stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 2014;22:1583–9. [DOI] [PubMed] [Google Scholar]
- [8].Shepherd DET, Seedhom BB. Thickness of human articular cartilage in joints of the lower limb. Ann Rheum Dis 1999;58:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Adam C, Eckstein F, Milz S, Putz R. The distribution of cartilage thickness within the joints of the lower limb of elderly individuals. J Anat 1998;193 (Pt 2):203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Faisal A, Ng SC, Goh SL, Lai KW. Knee cartilage segmentation and thickness computation from ultrasound images. Med Biol Eng Comput 2018;56:657–69. [DOI] [PubMed] [Google Scholar]
- [11].Stammberger T, Eckstein F, Englmeier KH, Reiser M. Determination of 3D cartilage thickness data from MR imaging: computational method and reproducibility in the living. Magn Reson Med 1999;41:529–36. [DOI] [PubMed] [Google Scholar]
- [12].Ambellan F, Tack A, Ehlke M, Zachow S. Automated segmentation of knee bone and cartilage combining statistical shape knowledge and convolutional neural networks: data from the Osteoarthritis Initiative. Med Image Anal 2019;52:109–18. [DOI] [PubMed] [Google Scholar]
- [13].McWalter EJ, Wirth W, Siebert M, von Eisenhart-Rothe RM, Hudelmaier M, Wilson DR, et al. Use of novel interactive input devices for segmentation of articular cartilage from magnetic resonance images. Osteoarthritis Cartilage 2005;13:48–53. [DOI] [PubMed] [Google Scholar]
- [14].Ahn C, Bui TD, Lee YW, Shin J, Park H. Fully automated, level set-based segmentation for knee MRIs using an adaptive force function and template: data from the Osteoarthritis Initiative. Biomed Eng Online 2016;15:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chung SW, Han SS, Lee JW, Oh KS, Kim NR, Yoon JP, et al. Automated detection and classification of the proximal humerus fracture by using deep learning algorithm. Acta Orthop 2018;89:468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Deniz CM, Xiang S, Hallyburton RS, Welbeck A, Babb J, Honig S, et al. Segmentation of the proximal femur from MR images using deep convolutional neural networks. Sci Rep 2018;8:16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Einhorn T, Buckwalter J, O’Keefe R. Orthopaedic basic science: foundations of clinical practice. Rosemont, IL: Amer Academy of Orthopaedic; 2007. [Google Scholar]
- [18].Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology 2017;284:574–82. [DOI] [PubMed] [Google Scholar]
- [19].Ronneberger O, Fischer P, Brox T. U-Net: convolutional networks for biomedical image segmentation. Cham: Springer International Publishing; 2015. [Google Scholar]
- [20].Liu F, Zhou Z, Jang H, Samsonov A, Zhao G, Kijowski R. Deep convolutional neural network and 3D deformable approach for tissue segmentation in musculoskeletal magnetic resonance imaging. Magn Reson Med 2018;79: 2379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Norman B, Pedoia V, Majumdar S. Use of 2D U-Net convolutional neural networks for automated cartilage and meniscus segmentation of knee MR imaging data to determine relaxometry and morphometry. Radiology 2018;288:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Makinejad MD, Abu Osman NA, Wan Abas WAB, Bayat M. Preliminary analysis of knee stress in full extension landing. Clinics 2013;68:1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kubicek M Stress strain analysis of knee joint. Eng Mech 2009;16:315–22. [Google Scholar]
- [24].Escamilla RF. Knee biomechanics of the dynamic squat exercise. Med Sci Sports Exerc 2001;33:127–41. [DOI] [PubMed] [Google Scholar]
- [25].Iwaki H, Pinskerova V, Freeman MA. Tibiofemoral movement 1: the shapes and relative movements of the femur and tibia in the unloaded cadaver knee. J Bone Joint Surg Br 2000;82:1189–95. [DOI] [PubMed] [Google Scholar]
- [26].Pradsgaard D, Fiirgaard B, Spannow AH, Heuck C, Herlin T. Cartilage thickness of the knee joint in juvenile idiopathic arthritis: comparative assessment by ultrasonography and magnetic resonance imaging. J Rheumatol 2015;42: 534–40. [DOI] [PubMed] [Google Scholar]
- [27].Wirth W, Hunter DJ, Nevitt MC, Sharma L, Kwoh CK, Ladel C, et al. Predictive and concurrent validity of cartilage thickness change as a marker of knee osteoarthritis progression: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2017;25:2063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Argentieri EC, Sturnick DR, DeSarno MJ, Gardner-Morse MG, Slauterbeck JR, Johnson RJ, et al. Changes to the articular cartilage thickness profile of the tibia following anterior cruciate ligament injury. Osteoarthritis Cartilage 2014;22:1453–60. [DOI] [PubMed] [Google Scholar]
- [29].Freedman BR, Sheehan FT, Lerner AL. MRI-based analysis of patellofemoral cartilage contact, thickness, and alignment in extension, and during moderate and deep flexion. Knee 2015;22:405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kornaat PR, Reeder SB, Koo S, Brittain JH, Yu H, Andriacchi TP, et al. MR imaging of articular cartilage at 1.5T and 3.0T: comparison of SPGR and SSFP sequences. Osteoarthritis Cartilage 2005;13:338–44. [DOI] [PubMed] [Google Scholar]
- [31].Guillard G, Vincent GR, Brett A, Conaghan PG, Bowes M. Cartilage thickness, denudation and Kl grade: a study of medial femorotibial joints in 8,890 knees from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2017;25:S223–4. [Google Scholar]
- [32].Schmitz RJ, Wang HM, Polprasert DR, Kraft RA, Pietrosimone BG. Evaluation of knee cartilage thickness: a comparison between ultrasound and magnetic resonance imaging methods. Knee 2017;24:217–23. [DOI] [PubMed] [Google Scholar]
- [33].Ding C, Cicuttini F, Scott F, Cooley H, Jones G. Association between age and knee structural change: a cross sectional MRI based study. Ann Rheum Dis 2005;64:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hudelmaier M, Glaser C, Hohe J, Englmeier KH, Reiser M, Putz R, et al. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum 2001;44:2556–61. [DOI] [PubMed] [Google Scholar]
- [35].Faber SC, Eckstein F, Lukasz S, Muhlbauer R, Hohe J, Englmeier KH, et al. Gender differences in knee joint cartilage thickness, volume and articular surface areas: assessment with quantitative three-dimensional MR imaging. Skeletal Radiol 2001;30:144–50. [DOI] [PubMed] [Google Scholar]
- [36].Lukasz S, Muhlbauer R, Faber S, Englmeier KH, Reiser M, Eckstein F. [Sex-specific analysis of cartilage volume in the knee joint–a quantitative MRI-based study]. Ann Anat 1998;180:487–93. [PubMed] [Google Scholar]
- [37].Eckstein F, Winzheimer M, Westhoff J, Schnier M, Haubner M, Englmeier KH, et al. Quantitative relationships of normal cartilage volumes of the human knee joint–assessment by magnetic resonance imaging. Anat Embryol (Berl) 1998;197:383–90. [DOI] [PubMed] [Google Scholar]
- [38].Ding C, Cicuttini F, Scott F, Glisson M, Jones G. Sex differences in knee cartilage volume in adults: role of body and bone size, age and physical activity. Rheumatology (Oxford) 2003;42:1317–23. [DOI] [PubMed] [Google Scholar]
- [39].Blazek K, Favre J, Asay J, Erhart-Hledik J, Andriacchi T. Age and obesity alter the relationship between femoral articular cartilage thickness and ambulatory loads in individuals without osteoarthritis. J Orthop Res 2014;32:394–402. [DOI] [PubMed] [Google Scholar]
- [40].Keng A, Sayre EC, Guermazi A, Nicolaou S, Esdaile JM, Thorne A, et al. Association of body mass index with knee cartilage damage in an asymptomatic population-based study. BMC Musculoskelet Disord 2017;18:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhou Z, Zhao G, Kijowski R, Liu F. Deep convolutional neural network for segmentation of knee joint anatomy. Magn Reson Med 2018;80:2759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.