Abstract
To understand the contribution of intrinsic membrane properties to the different in vivo firing patterns of oxytocin (OT) and vasopressin (VP) neurones, in vitro studies are needed, where stable intracellular recordings can be made. Combining immunochemistry for OT and VP and intracellular dye injections allows characterisation of identified OT and VP neurones, and several differences between the two cell types have emerged. These include a greater transient K+ current that delays spiking to stimulus onset, and a higher Na+ current density leading to greater spike amplitude and a more stable spike threshold, in VP neurones. VP neurones also show a greater incidence of both fast and slow Ca2+-dependent depolarising afterpotentials, the latter of which summate to plateau potentials and contribute to phasic bursting. By contrast, OT neurones exhibit a sustained outwardly rectifying potential (SOR), as well as a consequent depolarising rebound potential, not found in VP neurones. The SOR makes OT neurones more susceptible to spontaneous inhibitory synaptic inputs and correlates with a longer period of spike frequency adaptation in these neurones. Although both types exhibit prominent Ca2+-dependent afterhyperpolarising potentials (AHPs) that limit firing rate and contribute to bursting patterns, Ca2+-dependent AHPs in OT neurones selectively show significant increases during pregnancy and lactation. In OT neurones, but not VP neurones, AHPs are highly dependent on the constitutive presence of the second messenger, phosphatidylinositol 4,5-bisphosphate, which permissively gates N-type channels that contribute the Ca2+ during spike trains that activates the AHP. By contrast to the intrinsic properties supporting phasic bursting in VP neurones, the synchronous bursting of OT neurones has only been demonstrated in vitro in cultured hypothalamic explants and is completely dependent on synaptic transmission. Additional differences in Ca2+ channel expression between the two neurosecretory terminal types suggests these channels are also critical players in the differential release of OT and VP during repetitive spiking, in addition to their importance to the potentials controlling firing patterns.
Keywords: afterhyperpolarisations, depolarising afterpotentials, ion channels, oxytocin, vasopressin
1 |. INTRODUCTION
Action potentials generated in the cell bodies of magnocellular, hypothalamic vasopressin (VP) and oxytocin (OT) neurones result in depolarisation of their nerve terminals in the neurohypophysis, initiating exocytotic hormone release leading to important physiological changes in peripheral organs. Our report in the inaugural issue of Journal of Neuroendocrinology1 was one of the first studies to temporally correlate electrical activity of supraoptic neurones with direct measurement of VP release from the neurohypophysis. Subsequent work from multiple laboratories over the past three decades has resulted in substantial insights into the ionic and cellular mechanisms underlying the activity both VP and OT neurones. This work is reviewed below.
In the 1970s, Wakerley and colleagues performed a series of incisive experiments on lactating female rats that demonstrated the relationship between the pattern of electrical discharge of putative OT and VP neurones and systemic neurohypophysial hormone release.2 Key to these studies was the ability to antidromically identify supraoptic (SON) or paraventricular (PVN) neurones with neural stalk stimulation and monitor action potential discharge at the same time whilst correlating this discharge with the release of OT, monitored in real time by intra-mammary pressure. Approximately half of the neurones in either the SON or PVN emit a high-frequency burst (50–100 Hz) for 2–4 seconds every 5–10 minutes, preceding the precipitous increase in intra-mammary pressure as sociated with milk let-down by 15–20 seconds. This stereotyped, synchronised bursting pattern characterised putative OT neurones in both the SON or PVN, and was repeatedly observed by other groups successfully investigating its mechanisms.3,4
The conclusion that OT neurones were associated with milk ejection led to the obvious hypothesis that non-milk ejection neurones were VP neurones, and concurrent immunochemical studies established that both VP and OT neurones were found in both the PVN and SON.5,6 When VP release was stimulated, these putative VP neurones adopted an asynchronous pattern of bursting, with bursts and interburst intervals in the order of 10–30 seconds.2 For both OT and VP release, a phasic bursting pattern of electrical activity maximises hormone release.7–10 Despite phasic bursting being the more efficient mode, both neurone types can also adopt a fast, continuous pattern of activity when stimulated (eg, with hyperosmolality) and both can exhibit a slow irregular pattern at rest. This latter pattern is common for OT neurones and, for VP neurones, the phasic vs continuous patterns may evolve depending on the intensity of stimulation.2
The electrical activity of OT and VP neurones in vivo depends on synaptic properties, as well as intrinsic membrane characteristics. In this review, we focus on the intrinsic properties of identified OT and VP neurones as assessed primarily from sharp electrode intracellular or whole-cell recordings from in vitro preparations. Although magnocellular OT and VP neurones also are found in the PVN, with smaller numbers in accessory neurosecretory nuclei, this review focuses on studies of the SON of rats, where the majority of this work has been conducted.
2 |. MEANS OF IDENTIF YING OT AND VP NEURONES COUPLED WITH ELECTRICAL RECORDING
2.1 |. Firing patterns
Identification of OT vs VP neurones based on the firing patterns found in vivo has obvious drawbacks, given that VP neurones exhibit both phasic and continuous patterns in vivo, and that the stereotyped, synchronous bursting of OT neurones is considered to be specific to lactating rats. A final caveat is that, even in vivo, a small percentage of milk-ejection neurones fired both synchronous milk ejection bursts, and exhibited asynchronous VP-like bursting inbetween bursts.11,12
Phasic bursting similar to that observed in putative VP neurones in vivo was observed in a variety of in vitro preparations in early research, such as acute13–17 and organotypic18 slices (Figure 1). Other types of activity, including slower, irregular firing, continuous firing, and even silent neurones, were often observed. A meta-analysis from Armstrong and Sladek19 revealed a similar distribution of these different patterns in the acutely prepared hypothalamic explant but, quantitatively, the phasic bursting patterns observed corresponded well with those reported in vivo for putative VP neurones regarding burst length and intraburst frequency with minimal activation,20 although they had had longer interburst intervals. Although there are many caveats to the assumption that phasic bursting in either the SON or PVN represents VP neurones exclusively, the probability is nevertheless high (see below), and the expression of bursting in vitro allowed investigations of its mechanisms, such as depolarising afterpotentials leading to plateau potentials,21 as discussed below (Figure 1).
FIGURE 1.
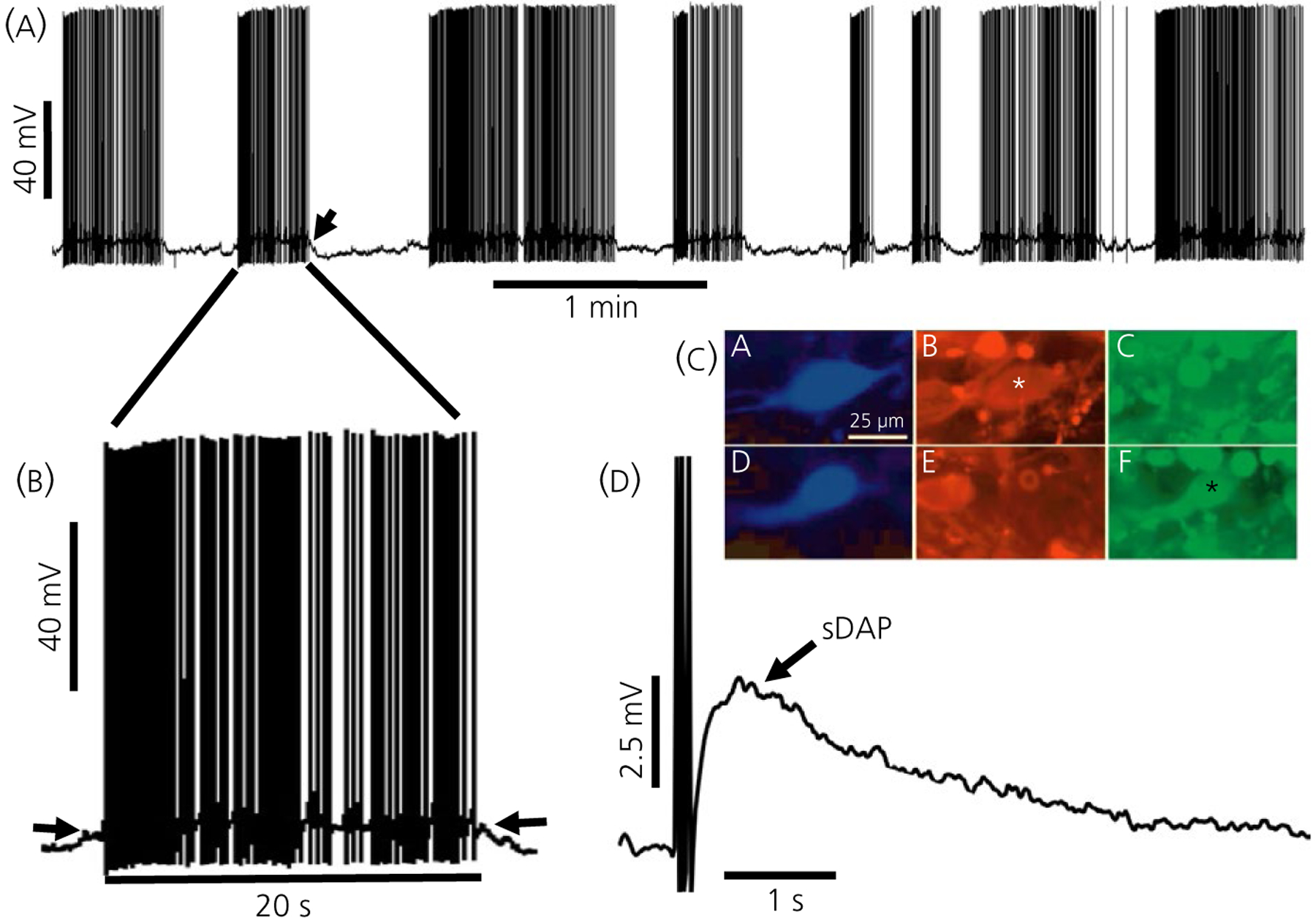
Characteristics of vasopressin (VP) neurones. A, Phasic bursting in an immunopositive VP neurone from a hypothalamic slice. The burst indicated by the arrow is expanded in (B), where the plateau potential underlying the burst is evident (arrows). C, Examples of immunochemically identified oxytocin (OT) and VP neurones. CA, Biocytin-filled neurone OT stained with avidin-aminomethylcoumarin. CB, The neurone is positive for OT-neurophysin (NP) and negative for VP-NP (CC). CD, Biocytin-filled neurone VP stained with avidin-AMCA. CE, The neurone was negative for OT-NP and positive for VP-NP (CF). D, Slow depolarising afterpotential (sDAP) in an identified VP neurone. Parts (A), (B) and (D) are modified from Armstrong.185 Part (C) is modified from Li et al44
2.2 |. Response characterisation
Additional tests can support cell identification in vivo. VP neurones can adopt, or enhance if already active, phasic bursting patterns in response to potent VP-releasing stimuli such as haemorrhage, water deprivation or carotid occlusion,11,20,22–24 whereas putative OT neurones accelerate their continuous firing rate monotonically when stimulated (eg, with hyperosmotic challenge that also release OT).2 A more reliable test for nonlactating female and male rats in vivo combines acute hypertension prompted by i.v. phenylephrine injection to selectively inhibit putative VP neurones, and systemic cholecystokinin (CCK) administration that activates gastric afferents and selectively excites OT neurones, similar to gastric expansion.25,26 Unfortunately, these stimuli are not useful in in vitro preparations such as the hypothalamic explant, where CCK directly activates the majority of SON neurones and releases VP.27
Despite the many other neuroactive substances localised in magnocellular nuclei, only the responses to VP and OT appear to be useful in distinguishing the two cell types because each type selectively carries an appropriate autoreceptor.28,29 VP30,31 and OT32,33 have been shown to specifically autoregulate their respective cell types via somatodendritically released peptide in vivo, where VP appears to be auto-inhibitory, and OT auto-excitatory. In vitro, direct application of OT or VP on dissociated SON neurones increases [Ca2+]i selectively in the immunochemically identified parent cell type.33–35 In slices, however, although OT was found to excite nonphasic neurones preferentially over phasic (putative VP neurones) by Yamashita et al,36 comparable differential effects of the two peptides on OT and VP neurones have not been reported.
2.3 |. Immunochemical identification after intracellular dye injection
Although magnocellular neurosecretory subtypes were first labelled with specific antibodies following intracellular recording and Lucifer Yellow (LY) dye injection in goldfish by Reaves and Hayward,37,38 no differences in the electrical properties or firing rates of the three different neurosecretory cell types found in fish were reported; thus, any comparison with the mammalian homologs in the SON and PVN is difficult. Yamashita et al17 and Cobbett et al39 immunochemically identified LY-injected SON neurones in rat hypothalamic slices, and found that the majority of phasic bursting neurones recorded could be labelled for VP or its associated neurophysin (VP-NP). Although these data were not compared with a similar labelling for OT neurones, nor were the nonphasic, continuously active neurones analysed in the same manner, the results nevertheless confirmed that phasically bursting neurones contained VP. For simplicity, VP or VP-NP immunoreactive neurones are referred to here as VP neurones. Similar experiments studying OT neurones have used antibodies either to OT or to its specific neurophysin (OT-NP).
Erickson et al40–42 first successfully immunolabelled both cell types following recording and biocytin injection in slices from guinea pig SON. Phasic bursting was only found in VP neurones, and only those exhibiting depolarising afterpotential (DAPs).41,42 Although OT neurones did not show this behaviour, the phasic bursting in guinea pigs demonstrated much shorter bursts, and shorter interburst intervals than those reported in rats. Armstrong et al43 found that phasic bursting patterns in the SON from rat hypothalamic slices was similar to that recorded in vivo43 (Figure 1), and was more often associated with biocytin filled, VP neurones; however, phasic bursting was observed in a few OT neurones, just as in vivo. In organotypic cultures, biocytin-injected VP neurones were found to fire in phasic bursting patterns asynchronously and, remarkably, OT neurones exhibited synchronous discharges mimicking those associated with milk ejections in vivo.45
In dissociated SON neurones, differential OT and VP immunolabelling is also possible after recording, if more difficult, allowing characterisation of many electrophysiological properties after recording.33,46–49
In single neurohypophysial terminals, which contain large quantities of hormone, investigators have immunoblotted terminal contents for VP and OT after recording,50 or used an enzyme-linked immunoassay.51 These methods have been valuable in determining many properties of OT and VP terminals following recording.52
2.4 |. Reverse transcriptase-polymerase chain reaction (RT-PCR)
Single cell RT-PCR has been used to categorise membrane channels and other gene products of OT and VP neurones,53–57 and has been applied following patch clamp characterisation of SON neurones.58 However, the sensitivity of this technique coupled with the known co-localisation of both OT and VP mRNAs in many SON neurones54 demands either real-time single-cell RT-PCR58 or some other means of quantification to distinguish VP-dominant from and OT-dominant neurones).54 The much smaller numbers of co-localised VP-OT neurones observed with immunochemistry suggests a lesser degree of protein co-expression. This observation matches physiological studies showing selective release of OT (eg, during the milk ejection response),2 despite over 50% of SON neurones containing both mRNAs.54
2.5 |. Transgenic rodent lines with fluorescent markers
Post-recording identification can be avoided altogether by using transgenic rats in which the VP59 or OT60,61 promoters control the expression of different fluorescent transgenes. For example, these transgenic strains have been used to identify differences in the two cell types with respect to acid-induced currents62 and Ca2+ oscillations.63 Although co-localisation is still an issue, cross-breeding these strains allows the focus to be on neurones exhibiting only one of the fluorescent markers, in much the same way that immunochemical studies are more definitive when positive neurones are negative for the heterotypic peptide.
3 |. PROPERTIES OF IDENTIFIED OT AND VP NEURONES
3.1 |. Passive electrical properties
Neuronal input resistance and membrane time constant are passive properties that contribute to the efficacy of synaptic inputs and their eventual summation at the spike initiating zone. No significant differences were reported in either input resistance (200–300 MΩ) or membrane time constant (11–16 milliseconds) for sharp electrode recordings from the two cell types in either male43 or virgin and lactating female rats.64,65 As expected, with whole-cell patch recording, input resistance was three to four times higher, and membrane time constant approximately twice as long in virgin and lactating rats but, again, no differences were observed across state or cell type66 (but see also Li et al44). Thus, it is unlikely that the differences in the passive properties of OT and VP neurones contribute greatly to any differences in electrical activity.
3.2 |. Action potentials (APs) or spikes
The frequency and patterning of APs determine hormone release. Thus, AP properties may contribute to the different patterns of OT and VP neuronal firing. In male rats, no differences were found in AP amplitude (approximately 75 mV) or half width (approximately 1.5 milliseconds), although spike broadening during AP trains was greater in VP neurones.43 In female rats, spike threshold was more depolarised and spike heights were smaller in OT neurones.64,65 Action potential widths increase during lactation, primarily, but not exclusively, in OT neurones.55,64 The wider spikes reflected slower rise and decay times.64 Similarly, frequency-dependent spike broadening characterises both cell types in female rats, although it is greater in OT neurones during pregnancy and lactation. The underlying mechanism for this difference is unknown but may relate to the weaker expression of Na+ currents and repolarising, A-type K+ currents in OT neurones (see below).
In adult female virgin rats, VP neurones have twice the Na+ current density compared to OT neurones, although no differences were found in voltage dependence or the kinetics of various aspects of activation and inactivation with whole-cell recordings in dissociated neurones49 (Figure 2). VP neurone spikes were larger in slice recordings, with a faster-rising slope compared to OT neurones.49,64 The difference in Na+ channel density in the two cell types may underlie the observation that spike threshold in OT neurones was increased with steady-state depolarisation (−65 to −55 mV) but was unaffected in VP neurones.49 In situ hybridisation and immunochemical studies show that SON neurones have tetrodotoxin (TTX)-sensitive NaV1.2, NaV1.6, and NaV1.7 channels, as well as associated β1 and β2 accessory subunits and, although no differences were reported between cell types in male rats, mRNA and protein levels for these subunits were up-regulated by hypertonic saline.67,68 A TTX-insensitive Na+ channel, NaV1.969 is also found in SON neurones but was not increased by salt loading.69 The K+ and Ca2+ currents that contribute to action potential shape are dealt with below.
FIGURE 2.
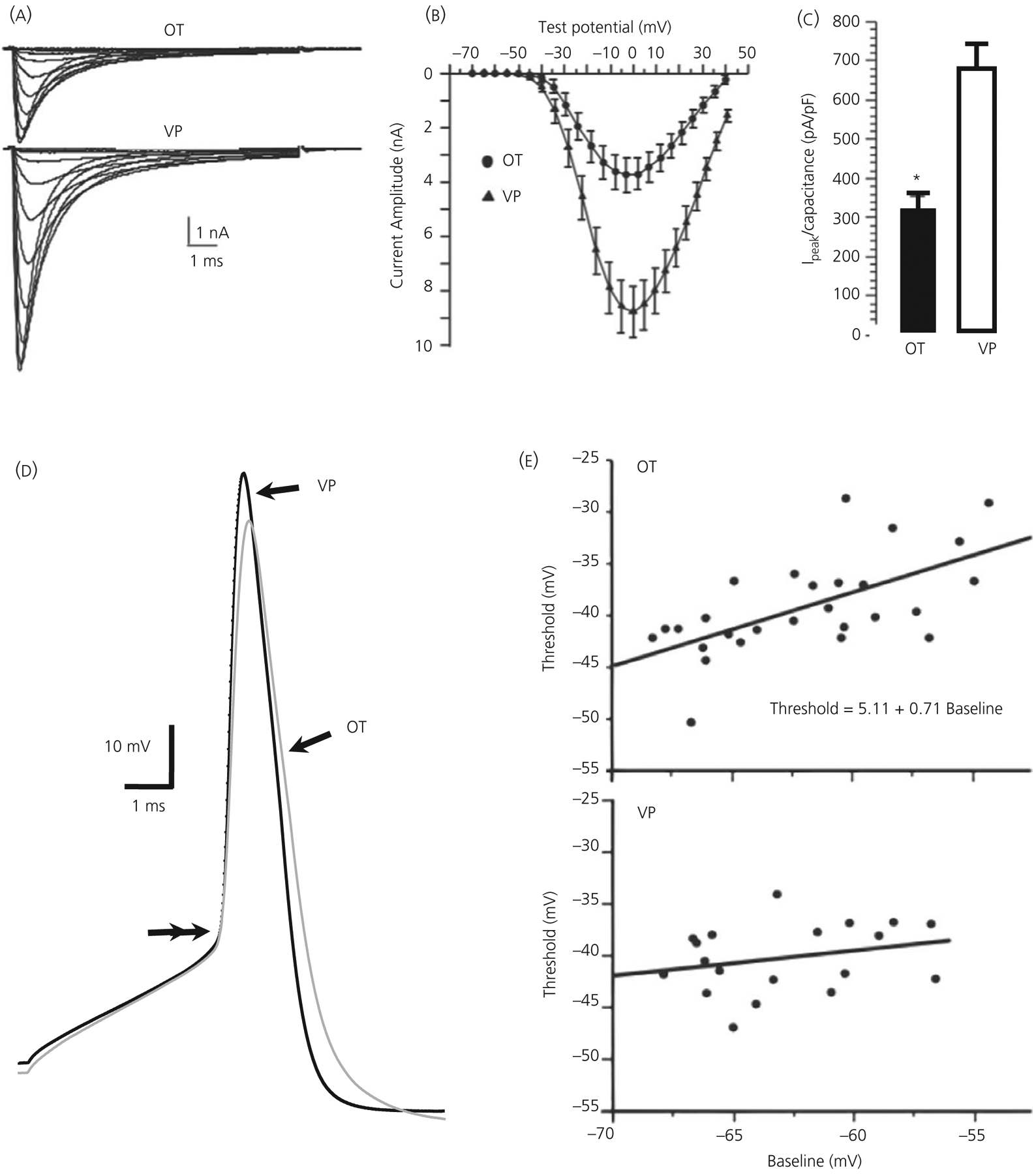
Transient voltage-dependent Na+ (INa+) currents in dissociated, immunopositive vasopressin (VP) and oxytocin (OT) neurones. A, Averaged INa+ records from 13 OT and 20 VP neurones showing voltage dependence of activation (steps to −55 through −5 mV from −90 mV). B, Plots of peak amplitude ( ± SEM) vs voltage from neurones shown in (A). C, Bar graph illustrating difference in peak current density ( ± SEM) for these OT and VP neurones, *P ≤ 0.05. D, Action potentials (APs) from OT (grey trace) and VP neurones (black trace) aligned at threshold (double arrows). Note the slower rise of the OT compared to the AP of the VP neurone, and its smaller amplitude. E, Spike threshold plotted against baseline membrane potential (Vm) in OT and VP neurones. Top: in OT neurones, threshold was positively correlated with Vm. Bottom: in VP neurones, no significant correlation was found. Modified from Scroggs et al49
3.3 |. Spike afterhyperpolarisations (AHPs)
3.3.1 |. Fast AHPs
Single APs in SON neurones are followed by an AHP lasting 10–20 milliseconds that gates firing rate by setting a minimum interspike interval. Multiple channels likely contribute to the fast AHP (fAHP), including a classical delayed rectifier K+ current and an A- type current, which, along with Na+ current inactivation, contribute to spike repolarisation. Large conducting, voltage- and Ca2+-dependent BK channels70,71 also contribute to the fAHP amplitude and duration but not to spike repolarisation per se.72,73 Although some differences between OT and VP neurones exist in the currents underlying fAHPs, the potentials themselves are similar in amplitude and duration in both cell types.43,64
3.3.2 |. Medium AHPs
The medium AHP (mAHP) in SON neurones lasts 200–500 milliseconds, and is carried by a Ca2+ -dependent K+ current.74 This mAHP accumulates with successive spikes in a train and is highly sensitive to the bee venom, apamin.41,75–77 Apamin binds with high affinity to small conductance (SK), Ca2+-dependent K+ channels that are found in the SON.78–82 Based on apamin sensitivity43,83,84 and immunocytochemistry,82 both cell types have SK channels (SK3 subtype), and apamin sensitivity accounts for approximately 80% of mAHP amplitude.43,84,85
The two cell types do not differ in the amplitude or decay of the mAHP in male43 or virgin female rats.64 By contrast, the mAHP in OT neurones increases and is associated with stronger spike frequency adaptation during pregnancy65 and lactation,64,65 suggesting that the enhanced bursting and excitation of OT neurones needed during reproduction is coupled with intrinsic restraints that limit the excitation to short periods of intense activity. The density of the underlying, apamin-sensitive current (ImAHP) is likewise increased in lactation, independent of any changes in Ca2+ current density or [Ca2+]i.83 This increase in ImAHP current density may rest in part as a result of changes in the calmodulin-SK channel complex, mediated by changes in the α subunit of the calmodulin-SK binding protein, casein kinase 2 (CK2). CK2 is down-regulated during pregnancy86 and, because CK2 typically phosphorylates calmodulin to reduce its Ca2+ sensitivity,87 this decrease would in turn allow increased activation of the coupled SK channels.
There is also a striking difference in the biochemical regulation of the mAHP between the two cell types. The mAHP in OT neurones, but not VP neurones, is dependent on the constitutive presence of the lipid, phosphatidylinositol 4,5-bisphosphate (PIP2),84 suggesting that the two cell types could differ in neuromodulation when the PIP2 pathway is targeted (Figure 3). The difference appears to relate not to the interaction of PIP2 with the SK3 channel but, more likely, to the modulation of high voltage-gated N-type Ca2+ channels, which supply the Ca2+ activating the mAHP.84,88 It is not known whether this difference plays a role in the mAHP plasticity of OT neurones.
FIGURE 3.
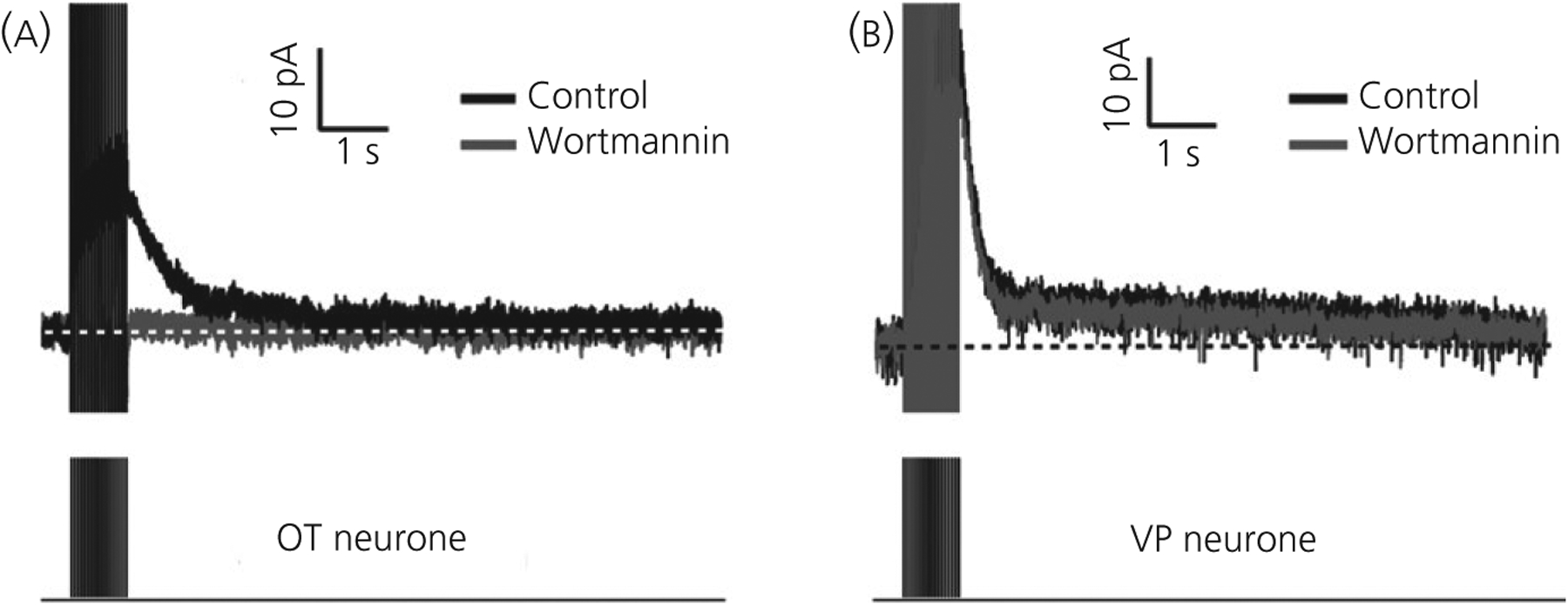
Afterhyperpolarisation current (IAHP) in immunopositive oxytocin (OT) and vasopressin (VP) neurones in slices. A, IAHP in an OT neurone produced by a train of 17, 5-ms voltage pulses (−60 mV to +10 mV) at 20 Hz. The IAHP is strongly inhibited by the PIP2 blocker, wortmannin (1 μmol L−1) (grey trace). B, In contrast, the IAHP to the same stimulus in a VP neurone is unaffected by wortmannin. In both (A) and (B), the amplitudes of voltages pulses have been truncated. Modified from Kirchner et al84
3.3.3 |. Slow AHPs (sAHPs)
A slower Ca2+-dependent AHP, the sAHP (1–2 seconds) also characterises SON neurones.83,89,90 The sAHP requires longer (or higher frequency) spike trains to reach its maximum compared to the fAHP or mAHP.90 As with the mAHP, the sAHP increases during pregnancy and/or lactation only in OT neurones.83,91 The extent of the sAHP is best appreciated when blocking the temporally overlapping slow depolarising afterpotentials (sDAP) with Cs+.90 The strong inhibition of the sAHP with muscarine,90 which operates on muscarinic receptors Gαq-coupled to the PIP2 pathway in the SON,92 suggests that cholinergic transmission could differentially target OT and VP neurones. As with the mAHP, Kirchner et al84 also found a strong dependence of the sAHP on constitutively expressed PIP2 in OT, but not VP neurones.
The channel underlying the sAHP is unknown. Greffrath et al89 were able to suppress the sAHP with charybdotoxin, which blocks some BK, intermediate conductance, and Ca2+-dependent K+ channels; however, the sAHP was not isolated from the time-overlapping sDAP. However, in another study when the sDAP was first blocked, both charybdotoxin and iberiotoxin failed to inhibit the sAHP,90 raising the possibility that Greffrath et al89 may have been modulating the sDAP. Like the mAHP, the sAHP in OT neurones depends on PIP2 expression and appears largely to be activated by Ca2+ from N-channels.84,88 In VP neurones, sAHPs are suppressed by R-type Ca2+ channel blockers.88
3.4 |. Depolarising afterpotentials (DAPs)
3.4.1 |. Slow depolarising afterpotentials (sDAPs)
sDAPs following spikes were the first specific membrane property ascribed to either cell type, and their occurrence increases the probability of APs occurring in close succession (Figure 1). Andrew and Dudek21,93 first reported sDAPs in the SON in association with the phasic bursting pattern typical of VP neurones in hypothalamic slices. The sDAP lasted 1–3 seconds (≤5 mV), could be observed after a single spike and summated to a plateau potential with repetitive spiking that supported long bursts of action potentials. sDAPs are activity- and Ca2+-dependent, independent of TTX-sensitive Na+ channels or synaptic transmission21,43,77,94 and, although more often associated with VP neurones, are present in a minority of OT neurones.43,45,64,65,95 Importantly, the sDAP can be masked by AHPs, and vice versa, making the study of specific spike afterpotentials difficult without pharmacological treatment.43,90,96 However, although a few OT neurones with sDAPs can adopt phasic bursting reminiscent of VP neurones, most do not.43,45 sDAPs do not underlie the synchronous, milk-ejection type bursting activity of OT neurones observed in organotypic cultures.45 Thus, VP neurones appear to have a different mechanism for the sDAP and the plateau potential compared to OT neurones.
sDAP currents show strong voltage dependence starting at approximately −80 mV and increasing near spike threshold. Different mechanisms have been proffered. The seminal study by Bourque94 suggested an inward cation current with a region of negative resistivity at approximately −65 mV consistent with a regenerative current. By contrast, Li and Hatton97 reported a decreased K+ conductance, consistent with the increased input resistance observed by many in current clamp.77,94,98 Modelling studies show that either mechanism faithfully reproduces a sDAP and its summation to a plateau potential, provided that there is a resting Na+ leak current,72,99 consistent with the Na+ dependence of the sDAP,97 and also with its sensitivity to nonselective cation channel blockers.100,101 sDAPs are inhibited by L- and N-type Ca2+ channel blockers and by reducing internal Ca2+ stores.102
sDAPs and the resulting plateau potentials underlying bursts are inhibited by κ-opiate receptor activation, an autocrine event driven by the activity-dependent, somatodendritic release of dynorphin from VP neurones.103–105 This inhibition may work by decoupling the sDAP/plateau mechanism from its Ca2+ dependence.99 Histamine, which promotes phasic bursting,106 also enhances the sDAP in VP neurones via H1-receptor activation.77
3.4.2 |. Fast DAPs (fDAPs)
fDAPs (approximately 200 milliseconds) are also activity-, voltage- and Ca2+-dependent, and are found in most (80%) VP neurones and less frequently (20%) in OT neurones.96 The underlying current is consistent with a nonselective cation current and may be critical in initiating bursts. Unlike the sDAP, however, the fDAP is insensitive to Cs+ blockade. An analysis of spike patterning of VP neurones in vivo suggests a prominent spike excitatory afterpotential with a time course similar to the fDAP, whereas the sDAP appears more dominant in vitro.107 VP neurones, but not OT neurones, are immunoreactive for the transient receptor potential melastatin channel 5 (TrpM5), whereas both cell types are immunoreactive for TrpM4.108 Elsewhere, these channels underlie transient, Ca2+-dependent cation ion currents which have characteristics similar to those of the IfDAP96
3.5 |. Voltage-dependent Ca2+ channels
3.5.1 |. High-threshold Ca2+ currents
Pharmacological investigations indicate the presence of high voltage-activated (HVA) L-, N-, and P/Q channels in most SON neurones in dissociated cell preparations109–111 and, more recently, in identified OT and VP neurones.88 Molecular studies have revealed expression of all HVA channel subunits in both cell types except α1E (Cav2.3, or R-type) channels.53 Although considered as HVA-type currents, R-type currents may have a lower voltage dependence and faster inactivation kinetics than L, N or P/Q channels.112
N-type followed by L-type channels account for most of the whole cell Ca2+ current,109,110 as well as the increases in [Ca2+]i measured with imaging following spike trains88; neither channel type appears to differ between cell types. Similarly, although P/Q channels contribute a relatively smaller percentage of either Ca2+ current109,110 or spike induced increases in [Ca2+]I, they do not appear different between OT and VP neurones. However, the R-type channel contribution may be more prominent in VP neurones,113 which may account for its stronger relationship with the sAHP in those neurones.88 This is in contrast to neurohypophysial terminals, where R-channel blockers inhibited a portion of OT, but not VP, release that was resistant to L-, N- and P/Q blockers.114
A splice variant for Cav2.1 (P/Q type) lacking the so-called synprint motif that interacts with exocytotic proteins is present in the SON.115 This variant appears to be exclusively related to OT neurones and terminals116 and may relate to the inability of P/Q channel blockers to influence OT release to the same degree as they do VP release, either from terminals117 or from the somatodendritic region.113 Tobin et al113 found N-channels to be more reliably coupled to local OT release than any other channel type. Both L- and N-type channels are important for terminal hormone release.114,117
3.5.2 |. Low-threshold Ca2+ currents
The presence of low-threshold, transient, T-type Ca2+ currents in magnocellular neurones has been inconsistently reported, although studies have suffered from a lack of specific blockers and the possibility of confusion with R-channels that activate at more negative potentials than L, N, or P/Q HVA channels. Based on voltage threshold, a transient current and sensitivity to Ni2+, T-like channels have been reported in SON neurones in some studies41,48,109,118,119 but not others.110,111,120 This inconsistency makes it likely that a type of low-threshold Ca2+ channel is at least minimally present, although detection may be dependent on particular recording conditions. Erickson et al41 reported no difference between OT and VP SON neurones in guinea pig slices in the presence of a low-threshold, transient Ca2+ current. To date, there is no molecular evidence for the presence of CaV3 (Cacna1g-i: T-type) or CaV2.3 (Cacna1e: R-type) channels specifically in magnocellular OT or VP neurones. In subclasses of parvocellular PVN neurones, however, well described low-threshold Ca2+ currents120–123 are likely to be a result of T-type (Cacna1 g, h) and possibly R-type (Cacna1e) Ca2+ channels.124
3.5.3 |. Plasticity of Ca2+ channels
Although whole-cell Ca2+ currents in OT neurones are larger during lactation because of cell size increases, current density does not change.83 It is nevertheless possible that specific Ca2+ currents could increase relative to a decrease in others. In dissociated SON neurones, spontaneous Ca2+ oscillations, although found in both cell types, are more prevalent in VP than OT neurones of virgin rats, whereas approximately twice as many OT neurones dissociated from lactating rats show these oscillation63 and this could be channel specific.
After 24 hours of dehydration, L-channel Ca2+ density accounts for an increase in Ca2+ current density in both OT and VP neurones.125 Water deprivation or salt loading not only activates both OT and VP neurones, but also leads to many morphological, electrophysiological and synaptic changes in magnocellular neurones and associated glia similar to those observed specifically in OT neurones during lactation.126–128
3.6 |. Voltage-dependent K+ channels
3.6.1 |. A-type currents
SON and magnocellular PVN neurones have a prominent A-type current (IA), comprising a K+ current that activates rapidly at low voltage and decays rapidly.47,73,120,129–134 The IA underlies the prominent transient outward rectification (TOR) that delays spike activation in magnocellular PVN and SON neurones when depolarised from hyperpolarised membrane potentials, contributes to spike repolarisation, and likely plays a significant role in somatic and terminal spike broadening (ie, which facilitates Ca2+ influx) via its activity-dependent inactivation47,129,130,135,136. The strong presence of TOR in the magnocellular neurones distinguishes them from many surrounding parvocellular neurones in the PVN122 and SON.137,138 Most SON neurones are immunopositive for an A-type channel subunit, KV4.2.139 In the PVN, single cell RT-PCR studies show that approximately 75% of magnocellular VP and OT neurones IA have mRNA for KV4.3 channels, although approximately 33% also have Kv4.2.140 Unfortunately, because of the extensive co-localisation of OT and VP mRNA, discerning a difference between two types was not possible in these studies.
In current clamp studies, the TOR is stronger in VP than in OT neurones and this difference results in a significant delay to spiking from hyperpolarised membrane potentials47,64 in VP neurones. This difference can be directly related to the greater density of IA under voltage clamp in VP neurones, reflected by both peak amplitude, and in a larger contribution from a slowly inactivating component of the current47 (Figure 4). Together, these studies suggest that OT neurones would be more excitable than VP to depolarising inputs in the range where IA is active.
FIGURE 4.
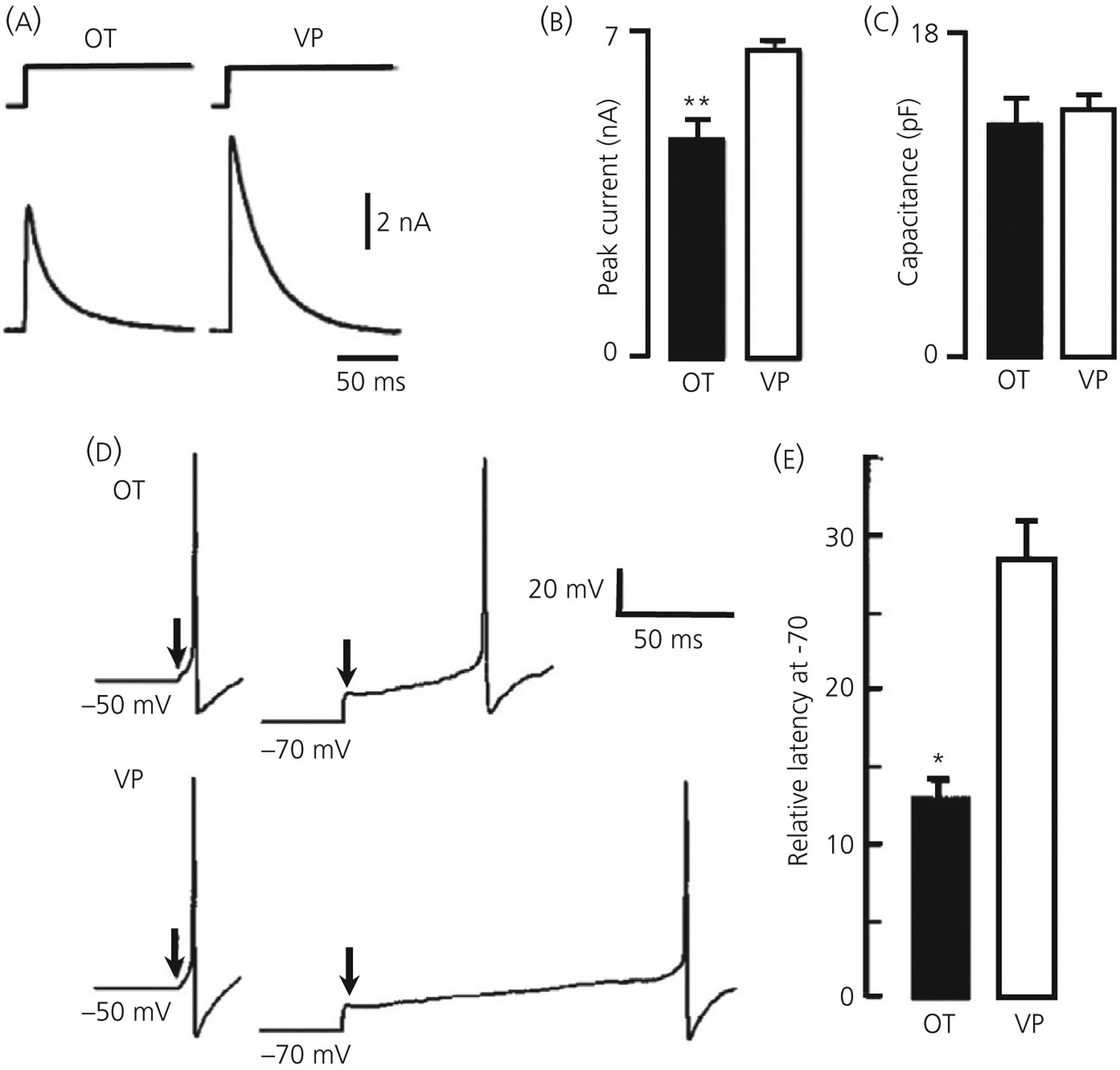
Transient K+ current (IA) is greater in vasopressin (VP) neurones. A, IA evoked with 500-ms pulse from −130 to −10 mV in an oxytocin (OT) and VP neurone under voltage clamp. B, Bar graph illustrating differences in peak current for 18 OT and 52 VP neurones ( ± SEM), ** P ≤ 0.001. C, The whole cell capacitance is not different in these cells, indicating a larger current density in VP neurones. D, Current clamp recording showing the latency to excitation with a depolarising pulse in OT and VP neurones from holding potentials of −50 mV vs −70 mV. The time between the arrows and the action potential is the latency. E, bar histograms showing the mean ± SEM latency to excitation following release from −70 mV expressed relative to that at 50 mV. Note that the relative latency in OT neurones is <50% of that in VP neurones (*P < 0.01). Modified from Fisher et al47
In another study of dissociated neurones, however, identified VP neurones were found to exhibit no IA whatsoever compared to its presence in every identified OT neurone.46 Fisher et al47 suggested that, because Widmer et al46 blocked Ca influx with Co2+ to unmask IA from voltage-dependent Ca2+ currents, this result could be attributed to a differential Ca2+-dependence between the two neurone types, a dependence shown in unidentified SON neurones.129 However, the apparent Ca2+- dependence has been at least partly attributed to charge screening effects of different divalent cations on voltage dependence because IA is prominently displayed in Ca2+-free media73,132,133 and its voltage dependence is shifted to more depolarised potentials as Ca2+ is increased.133 This is also consistent with the strong suppression of IA in PVN magnocellular neurones by Co2+, but not in Ca2+-free media.132 Regardless, the dramatic absence of IA in VP neurones reported by Widmer et al46 suggests that there may be some important difference in its regulation between the two cell types, aside from channel density.
3.6.2 |. Sustained outward rectifier (SOR)
An outwardly rectifying K+ current (SOR) characterised most OT but not VP neurones recorded from the SON using sharp electrodes.64,141,142 The SOR required depolarisation to approximately −60 mV, and was most easily seen making long hyperpolarising steps from approximately −50 mV, where a sag associated with its deactivation was observed at potentials nearest to −50 mV, and which was followed by a rebound depolarisation when returning to −50 mV (Figure 5). Althougth resembling the muscarine-activated K+ current (M-current) that has been identified in SON neurones,143 muscarine did not affect the SOR.142
FIGURE 5.
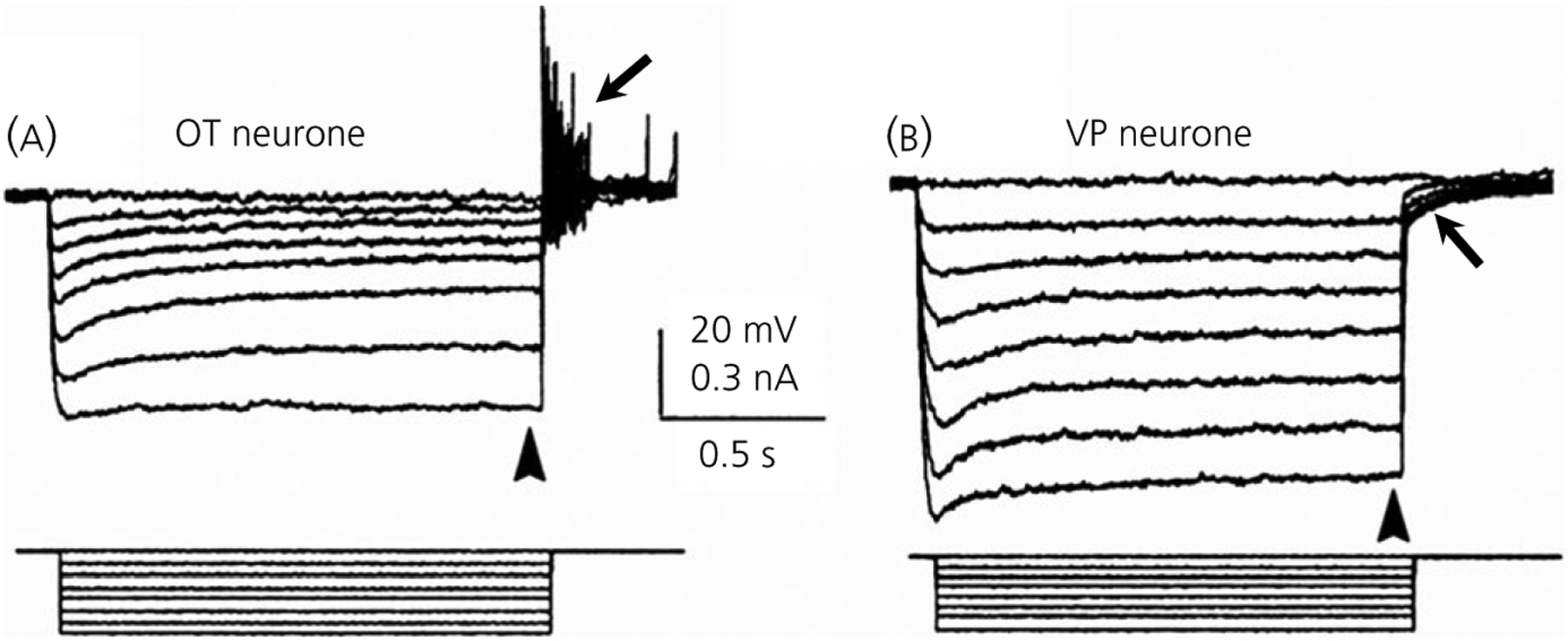
Oxytocin (OT) neurones exhibit a sustained outward rectification (SOR) with sharp electrode recordings in slices. A, When hyperpolarised from −50 mV, a biocytin-injected, immunopositive OT neurone shows a time and voltage-dependent SOR (arrowhead) followed by a rebound depolarisation at the current offset (arrow). Note that the sag representing the SOR decreases with hyperpolarisation. B, In contrast, the vasopressin (VP) neurone hyperpolarised from −50 mV shows a slow sag that increases with hyperpolarisation consistent with some IH, although with an otherwise almost linear I/V relationship (arrowhead). Rather than a rebound depolarisation, VP neurones show transient outward rectification at the current offset (arrow). In both (A) and (B), current steps are shown below voltage traces. Modified from Stern and Armstrong141
The SOR is sensitive to millimolar concentrations of tetraethylammonium (TEA), but not to Cs+, or 4-aminopyridine. Although showing some Ca2+-dependence, the SOR is unaffected by SK or BK channel blockers. OT neurones show a longer latency spike frequency adaptation not seen in VP neurones and this may be SOR-dependent.64 In addition, the strong presence of this persistent current at depolarised potentials might contribute to the elevated spike threshold sometimes observed in OT relative to VP neurones.49,64,65 Its role in the prominent rebound potential upon its deactivation and subsequent reactivation also suggests that inhibitory inputs occurring near rest could significantly modify ongoing firing patterns. Thus, the SOR may contribute to the irregular firing patterns produced by GABAergic synaptic potentials in OT neurones44 and to the facilitation of OT bursts induced by local GABA application in lactating rats.144
Unfortunately, the SOR has not been observed with the same prevalence with whole-cell recordings and thus is not useful for distinguishing the two cells with this method.44,58 However, the SOR, as well as an additional inward rectification, has been observed in OT neurones using the perforated patch method.145 This suggests that whole cell dialysis may dilute some intracellular constituent necessary for SOR expression.
3.6.3 |. Kv3-like currents
The Kv3 family of K+ currents (Kv3.1–3.4; KCNC1–4) are high voltage, rapidly activating, TEA-sensitive (<1 mmol L−1) currents that are known to influence spike repolarisation, typically in fast-firing neurones in cortex and elsewhere.146 VP neurones more strongly express Kv3.1b subunits compared to OT neurones immunochemically, show a greater proportion of TEA-blockable (<1 mmol L−1), slowly in activating current, and show a greater degree of TEA-induced spike broadening (<1 mmol L−1).73 The sensitivity of both OT and VP neurones to the Kv3.4 toxin, BDS-I, although not as great as low doses of TEA, suggests that the high voltage, rapidly inactivating component of TEA-sensitive current may be a result of the Kv3.4 subunit. To date, no corroborative evidence for the existence of this family of currents in OT or VP neurones has been made available.
3.6.4 |. Na+-dependent K+ current
Both OT and VP neurones also exhibit a Na+-dependent, voltage-dependent persistent K+ current consistent with the properties of Slo2.1 (Slick) and Slo2.2 (Slack) K+ channels, which structurally belong to the Ca2+−dependent channel family, KCa4.1 (KCNT1) and KCa 4.2 (KCNT2).147 Given their Na+ activation and very depolarised half-activation values (approximately −4 mV), we can speculate that these channels could contribute to the small spike-activated sAHPs remaining following removal of the larger Ca2+-dependent AHPs in SON neurones,88,90 similar to that found in other neurones expressing these channels148.
3.7 |. Osmotically sensitive currents
In vivo, hyperosmotic stimuli activate both OT and VP neurones2 and release both hormones,149,150 although there may be differences in aspects of their release to hypertonic challenge.149–151 It is now understood that this activation results from: (i) the direct osmosensitivity of the OT and VP neurones as demonstrated in vitro; (ii) osmoresponsive elements projecting to these neurones from regions surrounding the rostral end of the third ventricle, such as the organum vasculosum of the lamina terminalis152,153; and (iii) the osmosensitive astrocytes surrounding the SON neurones releasing the inhibitory transmitter taurine, which acts on glycine ionotropic receptors that pass Cl−.154,155
3.7.1 |. The osmoreceptor current
Work carried out with synaptically uncoupled neurones in slices, as well as isolated, dissociated neurones, has shown that SON neurones are directly osmoreceptive.15,156–158 The basis for direct osmoreceptivity appears to be a mixed cation current carried by a stretch-inactivated type mechanoreceptor, identified as an N-terminal splice variant of the transient receptor potential vanilloid type-1 (Trpv1) channel; knockouts of Trpv1 render SON neurones insensitive to hyperosmotic activation.159 Almost all magnocellular SON neurones appear to be osmoreceptive160,161 and there are no data available suggesting that Trpv1 channels are differentially distributed between OT and VP neurones.
3.7.2 |. Osmotically sensitive K+ current
Liu et al162 identified a depolarisation-activated current in isolated SON neurones that was increased by hyperosmolality. This was subsequently found to be a voltage- and Ca2+-dependent K+ current that was inhibited by muscarine and by M-current blockers, and was increased by the M-current channel opener, retigibine. Although the current was present in all of the identified OT and VP neurones, its osmosensitivity was only evident in approximately 50% of each cell type.143 RT-PCR and immunocytochemistry data suggested that SON neurones expressed several members of the Kv7 (KCNQ) family of K+ channels, although it is unknown whether these channels mediate this particular current. Although similar in voltage dependence and pharmacology to Kv7 type currents, other properties of this osmosensitive current, such as its inactivation, suggest differences from a classic M-current.143 Interestingly, given its Ca2+ dependence, sensitivity to muscarine, and the ability of M-current drugs to modulate firing patterns, the current described by Zhang et al143 may be involved in the sAHP.90
3.8 |. Acid-sensing and related channels
SON neurones possess acid-sensing Na+ channels (ASIC1 and ASIC2) that are amiloride-sensitive, voltage-independent and which, when activated with low pH, produce a large, transient inward current.163 This current is much larger in the VP neurones studied in transgenic rats compared to OT neurones, and the expression of ASIC1 and ASIC2 channels has only been confirmed in VP neurones,62 suggesting a role for ASICs in stimulating VP release during localised hypoxia.163 However, OT release to this same stimulus was not examined.
ASIC channels are in the superfamily of epithelial Na+ channels (ENaCs) and ENaCs have also been found in SON and PVN magnocellular neurones.56 Similar to ACICs, ENaCs are sensitive to amiloride and are voltage-independent. Although α-ENaC was found in both OT and VP neurones, β- and γ-ENaCs were found only in VP neurones. Amiloride and the more specific ENaC blocker, benzamil, both reduced an inward leak current and more often hyperpolarised SON VP neurones, suggesting that ENaCs contribute to resting membrane potential to a greater degree in VP than in OT neurones. β- and γ-ENaCs neurones are up-regulated in VP neurones of rats given a high-salt diet,164 and γ-ENaCs are up-regulated in the SON of the salt-sensitive Dahl-strain of rats.165,166 Although changes in ENaCs in VP neurones could contribute to the development of salt-related hypertension via increases in VP release, their role in changes in VP neurone firing in these conditions is unknown. The aldosterone receptor, known to regulate ENaC expression, is also found in SON neurones, but appears to be equally distributed in VP and OT neurones.56,166
3.9 |. Differences in the Cl− equilibrium potential between OT and VP neurones
Haam et al167 found a striking difference in the Cl− equilibrium potential between OT and VP neurones that would markedly influence GABAergic neurotransmission, as well the osmotically regulated glial transmission mediated by activation by taurine of glycine ionotropic receptors. Using perforated patch recordings (gramicidin) that maintain [Cl−]i, GFP-VP neurones from transgenic rats had a depolarised ECl−(approximately −36 mV) compared to OT neurones (ECl− approximately −72 mV); this rendered GABAergic synaptic potentials as excitatory near the resting potential in VP neurones. This difference correlated with a differential expression of Cl− transporters, such that the inhibition of the K+-Cl− co-transporter 2 (KCC2) in VP neurones had little effect on ECl−, whereas inhibition of the Na K Cl− co-transporter 1 (NKCC1) produced a positive shift. On the other hand, OT neurones were unaffected by selective NKCC1 antagonism but exhibited a significant depolarising shift in ECl when KCC2 was antagonised. These results correlated with weaker immunochemical localisation of KCC2 in VP neurones compared to OT neurones. However, Choe et al,168 also using perforated patch recording, failed to find a difference in ECl- between RFP-OT and GFP-VP labelled neurones from two different strains of transgenic rats, and found abundant KCC2 in VP neurones. Kim and colleagues also found the percentage of identified VP neurones showing GABA-mediated excitation to be low.169
Although it is difficult to reconcile these disparate findings, it is evident that ECl– is labile and likely subject to different environmental conditions. Kim et al170 found a dramatic shift in ECl– in SON neurones after a chronic salt load, such that all neurones displayed a depolarised ECl, and found a similar depolarising shift in identified VP neurones made hypertensive with the deoxycortisone acetate-salt model.169 Similarly, Choe et al171 found that high-salt loading produced a depolarising shift in GABA-mediated Cl− currents in identified VP neurones. This shift correlated with a reduction of KCC2 immunolabelling, a decreased sensitivity of ECl– to its antagonism and an increased response to NKCC1 antagonism that resulted in GABAergic excitation of firing. Finally, a switch to GABAergic excitation also occurs with angiotensin II-induced hypertension,172 suggesting that more than one type of chronic challenge to the VP system can produce this change.
The differential expression, or sensitivity, of Cl− transporters in the two neurone types could have profound consequences for the efficacy of glial transmission through nonsynaptic glycine receptors,154,155 for nonsynaptic GABA receptors likely present in the SON,44,173 and for the synaptic plasticity observed in GABAergic synaptic density127 and in GABAergic neurotransmission174 in OT neurones during pregnancy and lactation. In addition, miniature inhibitory synaptic potentials are four to five times more prevalent in OT than VP neurones, at least in female rats, suggesting a basal, differential regulation of terminal GABA release that influences firing patterns.44
3.10 |. Neurohypophysial terminal currents
The magnocellular neurosecretory system is one of few central nervous system regions where axon terminals are accessible for a variety of electrophysiological analyses and imaging.135,175,176 Although differences between soma and terminals previously have been noted in Ca2+177 and BK71 channels, fewer studies have focused on differences between identified OT and VP terminals, primarily as a result of technical difficulties. Such differences would be important when considering the effect different types of AP activity arriving at the terminals during bursting. For example, although bursts of action potential are known to facilitate both VP and OT release, VP release fatigues much more rapidly than does OT release during continuous axonal stimulation.9,178 Furthermore, presynaptic opiate actions and those of other locally released transmitters, such as ATP, may depend not only on differentially distributed receptors on the terminals, but also on channel diversity.52
3.10.1 |. Terminal Ca2+ channels
Both terminal types appear to exhibit L- and N-type high voltage-gated channels but differ regarding putative P/Q or R- type channels. Using an isolated terminal preparation, VP but not OT terminals were found to be immunopositive for a P/Q antibody,179 and the K+-induced VP release that remained in this preparation after L- and N-type blockade was blocked by the P/Q toxin, ω-AgaIVA (P/Q toxin).117 Identified VP but not OT terminals also show a P/Q type Ca2+ current,178 as identified originally in approximately 50% of unidentified terminals.117 By contrast, the release of OT that is not dependent on L- or N- channel activity is inhibited by the R-type channel blocker, SNX-482.114 Unlike VP terminals, OT terminals exhibit an SNX-482 blockable current,178 a current that was previously noted in approximately 50% of unidentified terminals.114 However, immunochemical localisation with an R-type antibody indicated its presence both OT and VP terminals, suggesting that this antibody recognised a nonfunctional channel. This is likely because SNX-482 appears to block virtually all the remaining current after L- and N-type blockade.114,179
3.10.2 |. Terminal K+ currents
Spike broadening as a result of K+ current (primarily A-type) inactivation is critical to gaining the enhanced Ca2+ influx that underlies the facilitation of terminal hormone release.9,135 Although there are differences in the properties of frequency-dependent facilitation for OT and VP release, and their release in response to drugs blocking either A-type and delayed rectifying K+ currents,180 no evidence exists at present to suggest a difference in the currents themselves in the two terminal types, nor in Ca2+-dependent K+ currents.52
Although not covered in the current review, the neurohypophysis contains a variety of neuroactive substances either co-localised with OT or VP, or found in non-neurosecretory axons or pituicytes that can influence hormone release.52,181 Perhaps the best studied are the opiates dynorphin and enkephalin, ATP, adenosine, GABA and nitric oxide. The involvement of these neuromodulators in both positive- and negative-feedback during spike-dependent hormone release on hormone release could suggest a differential coupling to Ca2+ or K+ channels in these terminals.52 For example, it is clear that the elevation of nitric oxide from Ca2+ influx during spike trains facilitates oxytocin release via cGMP-mediated actions on BK channels,182 although it is unknown whether a similar mechanism exits for VP terminals. On the other hand, μ- or κ-opioid receptor activation may inhibit most Ca2+ channels to suppress oxytocin release, whereas μ-opioid receptor activation can actually facilitate N-channels VP neurones.183
4 |. CONCLUSIONS
Electrophysiological studies from identified OT and VP neurones and their terminals reveal intrinsic differences that influence their firing patterns, their ability to fire repetitively and the differential release of hormone. The original studies of phasic bursting activity in VP neurones in vivo have been corroborated in vitro, with underlying mechanisms such as sDAPs and plateau potentials. Caution is needed, however, because many OT neurones display sDAPs that only rarely summate and lead to phasic bursting, such that the great majority of OT neurones fire in an irregular-to-continuous mode, much as they do in vivo when not engaged in milk ejection. Surprisingly, VP neurones exhibited larger APs, a larger underlying Na+ current and a more stable AP threshold. When coupled with a stronger IA delaying spiking to depolarising inputs, this suggests that VP neurones are less excitable than OT neurones. The SOR and its related rebound potential in OT neurones further suggest an additional mode of excitability in response to pattern-shaping inhibitory inputs not present in VP neurones. However, the synchronous, bursting behaviour of OT neurones exhibited during lactation appears to have no responsible intrinsic property driving this behaviour, and is likely synaptically driven in combination with the somatodendritic release of OT.32,45,119 Thus, regardless of whether in organotypic cultures45,119 or in hypothalamic slices,184 the bursts of OT neurones observed in vitro that best mimic those in vivo are dependent upon both glutamatergic and oxytocinergic activity. Perhaps the most surprising difference between the two cell types is that the second messenger PIP2 is necessary for OT neurones to express Ca2+-dependent AHPs. Thus, although these AHPs are superficially similar in the two cell types, their potential for transmitter modulation is dramatically different. Considering that OT neurones show considerable up-regulation of the AHP during pregnancy and lactation part as a result of changes in the modulation SK3 channels by CK2, the obligatory role of PIP2 in AHPs in these cells suggests that it may play a significant role in their plasticity.
Funding information
NIH, Grant/Award Number: R01HD-072056 and R01NS-044163
REFERENCES
- 1.Armstrong WE, Wilson CJ, Gallagher MJ, Sladek CD. Quantitative comparisons between the electrical activity of supraoptic neurons and vasopressin release in vitro. J Neuroendocrinol. 1989;1:215–226. [DOI] [PubMed] [Google Scholar]
- 2.Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. [DOI] [PubMed] [Google Scholar]
- 3.Richard P, Moos F, Freund-Mercier MJ. Central effects of oxytocin. Physiol Rev. 1991;71:331–370. [DOI] [PubMed] [Google Scholar]
- 4.Leng G, Sabatier N. Electrophysiology of magnocellular neurons in vivo In: Armstrong WE, Tasker JG, eds. Neurophysiology of Neuroendocrine Neurons. Chichester, UK: John Wiley and Sons; 2015:1–28. [Google Scholar]
- 5.Swaab D, Pool CW, Nijveldt F. Immunofluorescence of vasopressin and oxytocin in the rat hypothalamo-neurohypophyseal system. J Neural Transm. 1975;36:195–215. [DOI] [PubMed] [Google Scholar]
- 6.Vandesande F, Dierickx K. Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretory system of the rat. Cell Tissue Res. 1975;164:153–162. [DOI] [PubMed] [Google Scholar]
- 7.Bicknell REJ, Leng G. Relative efficiency of neural firing patterns for vasopressin release from the rat neurohypophysis. Neuroendocrinology. 1981;33:295–299. [DOI] [PubMed] [Google Scholar]
- 8.Bicknell R, Flint APF, Leng G, Sheldrick EL. Phasic pattern of electrical stimulation enhances oxytocin secretion from the isolated neurohypophysis. Neurosci Lett. 1982;30:47–50. [Google Scholar]
- 9.Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979;290:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulain D, Wakerley JB, Dyball REJ. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B. 1977;196:367–384. [DOI] [PubMed] [Google Scholar]
- 12.Moos FC, Ingram CD. Electrical recordings of magnocellular supraoptic and paraventricular neurons displaying both oxytocin- and vasopressin-related activity. Brain Res. 1995;669:309–314. [DOI] [PubMed] [Google Scholar]
- 13.Hatton GI, Armstrong WE, Gregory WA. Spontaneous and osmotically stimulated activity in slices of rat hypothalamus. Brain Res Bull. 1978;3:497–508. [DOI] [PubMed] [Google Scholar]
- 14.Haller EW, Brimble MJ, Wakerley JB. Phasic discharge in supraoptic neurones recorded from hypothalamic slices. Exp Brain Res. 1978;331:31–34. [DOI] [PubMed] [Google Scholar]
- 15.Mason WT. Supraoptic neurones of rat hypothalamus are osmosensitive. Nature. 1980;287:154–157. [DOI] [PubMed] [Google Scholar]
- 16.Mason WT. Electrical properties of neurons recorded from the rat supraoptic nucleus in vitro. Proc R Soc Lond B. 1983;217:141–161. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita H, Inenaga K, Kawata M, Sano Y. Phasically firing neurons in the supraoptic nucleus of the rat hypothalamus: immunocytochemical and electrophysiological studies. Neurosci Lett. 1983;37:87–92. [DOI] [PubMed] [Google Scholar]
- 18.Gahwiler BH, Sandoz P, Dreifuss JJ. Neurones with synchronous bursting discharges in organ cultures of the hypothalamic supraoptic nucleus area. Brain Res. 1978;151:245–253. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong WE, Sladek CR. Spontaneous ‘phasic firing’ in supraoptic neurons recorded from hypothalamo-neurohypophysial explants in vitro. Neuroendocrinology. 1982;34:405–409. [DOI] [PubMed] [Google Scholar]
- 20.Wakerley JB, Poulain DA, Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res. 1978;148:425–440. [DOI] [PubMed] [Google Scholar]
- 21.Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. [DOI] [PubMed] [Google Scholar]
- 22.Dreifuss J-J, Harris MC, Tribollet E. Excitation of phasically firing hypothalamic supraoptic neurones by carotid occlusion in rats. J Physiol. 1976;257:337–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris MC, Dreifuss J-J, Legros J-J. Excitation of phasically firing supraoptic neurones during vasopressin release. Nature. 1975;258:80–82. [DOI] [PubMed] [Google Scholar]
- 24.Wakerley JB, Poulain DA, Dyball REJ, Cross BA. Activity of phasic neurosecretory cells during haemorrhage. Nature. 1975;25:882–884. [DOI] [PubMed] [Google Scholar]
- 25.Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am J Physiol. 1987;253:R661–R665. [DOI] [PubMed] [Google Scholar]
- 26.Leng G, Way S, Dyball RE. Identification of oxytocin cells in the rat supraoptic nucleus by their response to cholecystokinin injection. Neurosci Lett. 1991;122:159–162. [DOI] [PubMed] [Google Scholar]
- 27.Jarvis CR, Bourque CW, Renaud LP. Depolarizing action of cholecystokinin on rat supraoptic neurones in vitro. J Physiol. 1992;458:621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freund Mercier MJ, Stoeckel ME, Klein MJ. Oxytocin receptors on oxytocin neurones: histoautoradiographic detection in the lactating rat. J Physiol. 1994;480:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurbin A, Orcel H, Alonso G, Moos F, Rabie A. The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology. 2002;143:456–466. [DOI] [PubMed] [Google Scholar]
- 30.Brown CH, Ludwig M, Leng G. Temporal dissociation of the feedback effects of dendritically co-released peptides on rhythmogenesis in vasopressin cells. Neuroscience. 2004;124:105–111. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. [DOI] [PubMed] [Google Scholar]
- 32.Lambert RC, Moos FC, Richard P. Action of endogenous oxytocin within the paraventricular or supraoptic nuclei: a powerful link in the regulation of the bursting pattern of oxytocin neurons during the milk-ejection reflex in rats. Neuroscience. 1993;57:1027–1038. [DOI] [PubMed] [Google Scholar]
- 33.Lambert RC, Dayanithi G, Moos FC, Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994;478:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dayanithi G, Sabatier N, Widmer H. Intracellular calcium signalling in magnocellular neurones of the rat supraoptic nucleus: understanding the autoregulatory mechanisms. Exp Physiol. 2000;85:75S–84S. [DOI] [PubMed] [Google Scholar]
- 35.Gouzenes L, Sabatier N, Richard P, Moos FC, Dayanithi G. V1a- and V2-type vasopressin receptors mediate vasopressin-induced Ca2+ responses in isolated rat supraoptic neurones. J Physiol. 1999;517:771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamashita H, Okuya S, Inenaga K, et al. Oxytocin predominantly excites putative oxytocin neurons in the rat supraoptic nucleus in vitro. Brain Res. 1987;416:364–368. [DOI] [PubMed] [Google Scholar]
- 37.Reaves TA Jr, Hayward JN. Intracellular dye-marked enkephalin neurons in the magnocellular preoptic nucleus of the goldfish hypothalamus. Proc Natl Acad Sci USA. 1979;76:6009–6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reaves TA Jr, Hayward JN. Functional and morphological studies of peptide-containing neuroendocrine cells in goldfish hypothalamus. J Comp Neurol. 1980;193:777–788. [DOI] [PubMed] [Google Scholar]
- 39.Cobbett P, Smithson KG, Hatton GI. Immunoreactivity to vasopressin- but not oxytocin-associated neurophysin antiserum in phasic neurons of rat hypothalamic paraventricular nucleus. Brain Res. 1986;362:7–16. [DOI] [PubMed] [Google Scholar]
- 40.Erickson KR, Ronnekliev OK, Kelly MJ. Inward rectification (Ih) in immunocytochemically-identified vasopressin and oxytocin neurons of the guinea-pig supraoptic nucleus. J Neuroendocrinol. 1990;2:261–265. [DOI] [PubMed] [Google Scholar]
- 41.Erickson KR, Ronnekleiv OK, Kelly MJ. Role of a T-type calcium current in supporting a depolarizing potential, damped oscillations, and phasic firing in vasopressinergic guinea pig supraoptic neurons. Neuroendocrinology. 1993;57:789–800. [DOI] [PubMed] [Google Scholar]
- 42.Erickson KR, Ronnekleiv OK, Kelly MJ. Electrophysiology of guinea-pig supraoptic neurones: role of a hyperpolarization-activated cation current in phasic firing. J Physiol. 1993;460:407–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armstrong WE, Smith BN, Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurones in vitro. J Physiol. 1994;475:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C, Tripathi PK, Armstrong WE. Differences in spike train variability in rat vasopressin and oxytocin neurons and their relationship to synaptic activity. J Physiol. 2007;581:221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Israel J-M, Poulain D. Oxytocin neurons during suckling: lessons from organotypic cultures In: Armstrong W, Tasker J, eds. Neurophysiology of Neuroendocrine Neurons. Chichester, UK: John Wiley and Sons; 2015:29–62. [Google Scholar]
- 46.Widmer H, Boissin-Agasse L, Richard P, Desamenien MG. Differential distribution of a potassium current in immunocytochemically identified supraoptic magnocellular neurones of the rat. Neuroendocrinology. 1997;652:29–37. [DOI] [PubMed] [Google Scholar]
- 47.Fisher TE, Voisin DL, Bourque CW. Density of transient K+ current influences excitability in acutely isolated vasopressin and oxytocin neurones of rat hypothalamus. J Physiol. 1998;511:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabatier N, Richard P, Dayanithi G. L-, N- and T- but neither P- nor Q-type Ca2+ channels control vasopressin-induced Ca2 + influx in magnocellular vasopressin neurones isolated from the rat supraoptic nucleus. J Physiol. 1997;503:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scroggs R, Wang L, Teruyama R, Armstrong WE. Variation in sodium current amplitude between vasopressin and oxytocin hypothalamic supraoptic neurons. J Neurophysiol. 2013;109:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang XM, Treistman SN, Lemos JR. Direct identification of individual vasopressin-containing nerve terminals of the rat neurohypophysis after ‘whole-cell’ patch-clamp recordings. Neurosci Lett. 1991;124:125–128. [DOI] [PubMed] [Google Scholar]
- 51.Custer EE, Ortiz-Miranda S, Knott TK, et al. Identification of the neuropeptide content of individual rat neurohypophysial terminals. J Neurosci Methods. 2007;163:226–234. [DOI] [PubMed] [Google Scholar]
- 52.Lemos J, Wang G, Marrero H, Ortiz-Miranda S . Neurophysiology of neurohypophysial terminals In: Armstrong W, Tasker J, eds. Neurophysiology of Neuroendocrine Neurons. Chichester: John Wiley and Sons; 2015:163–188. [Google Scholar]
- 53.Glasgow E, Kusano K, Chin H, Mezey E, Young WS 3rd, Gainer H. Single cell reverse transcription-polymerase chain reaction analysis of rat supraoptic magnocellular neurons: neuropeptide phenotypes and high voltage-gated calcium channel subtypes. Endocrinology. 1999;140:5391–5401. [DOI] [PubMed] [Google Scholar]
- 54.Xi D, Kusano K, Gainer H. Quantitative analysis of oxytocin and vasopressin messenger ribonucleic acids in single magnocellular neurons isolated from supraoptic nucleus of rat hypothalamus. Endocrinology. 1999;140:4677–4682. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita M, Glasgow E, Zhang BJ, Kusano K, Gainer H. Identification of cell-specific messenger ribonucleic acids in oxytocinergic and vasopressinergic magnocellular neurons in rat supraoptic nucleus by single-cell differential hybridization. Endocrinology. 2002;143:4464–4476. [DOI] [PubMed] [Google Scholar]
- 56.Teruyama R, Sakuraba M, Wilson LL, Wandrey NE, Armstrong WE. Epithelial Na(+) sodium channels in magnocellular cells of the rat supraoptic and paraventricular nuclei. Am J Physiol Endocrinol Metab. 2012;302:E273–E285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haque M, Wilson R, Sharma K, Mills NJ, Teruyama R. Localisation of 11beta-hydroxysteroid dehydrogenase type 2 in mineralocorticoid receptor expressing magnocellular neurosecretory neurones of the rat supraoptic and paraventricular nuclei. J Neuroendocrinol. 2015;27:835–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.da Silva MP, Merino RM, Mecawi AS, Moraes DJ, Varanda WA. In vitro differentiation between oxytocin- and vasopressin-secreting magnocellular neurons requires more than one experimental criterion. Mol Cell Endocrinol. 2015;400:102–111. [DOI] [PubMed] [Google Scholar]
- 59.Ueta Y, Fujihara H, Serino R, et al. Transgenic expression of enhanced green fluorescent protein enables direct visualization for physiological studies of vasopressin neurons and isolated nerve terminals of the rat. Endocrinology. 2005;146:406–413. [DOI] [PubMed] [Google Scholar]
- 60.Katoh A, Fujihara H, Ohbuchi T, et al. Specific expression of an oxytocin-enhanced cyan fluorescent protein fusion transgene in the rat hypothalamus and posterior pituitary. J Endocrinol. 2010;204:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katoh A, Fujihara H, Ohbuchi T, et al. Highly visible expression of an oxytocin-monomeric red fluorescent protein 1 fusion gene in the hypothalamus and posterior pituitary of transgenic rats. Endocrinology. 2011;152:2768–2774. [DOI] [PubMed] [Google Scholar]
- 62.Ohkubo J, Ohbuchi T, Yoshimura M, et al. Differences in acid-induced currents between oxytocin-mRFP1 and vasopressin-eGFP neurons isolated from the supraoptic and paraventricular nuclei of transgenic rats. Neurosci Lett. 2014;583:1–5. [DOI] [PubMed] [Google Scholar]
- 63.Kortus S, Srinivasan C, Forostyak O, et al. Physiology of spontaneous [Ca(2+)]i oscillations in the isolated vasopressin and oxytocin neurones of the rat supraoptic nucleus. Cell Calcium. 2016;59:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. J Neurosci. 1996;16:4861–4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teruyama R, Armstrong WE. Changes in the active membrane properties of rat supraoptic neurones during pregnancy and lactation. J Neuroendocrinol. 2002;14:933–944. [DOI] [PubMed] [Google Scholar]
- 66.Stern JE, Galarreta M, Foehring RC, Hestrin S, Armstrong WE. Differences in the properties of ionotropic glutamate synaptic currents in oxytocin and vasopressin neuroendocrine neurons. J Neurosci. 1999;19:3367–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanaka M, Cummins TR, Ishikawa K, Black JA, Ibata Y, Waxman SG. Molecular and functional remodeling of electrogenic membrane of hypothalamic neurons in response to changes in their input. Proc Natl Acad Sci USA. 1999;96:1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Black JA, Hoeijmakers JG, Faber CG, Merkies IS, Waxman SG. NaV1.7: stress-induced changes in immunoreactivity within magnocellular neurosecretory neurons of the supraoptic nucleus. Mol Pain. 2013;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Black JA, Vasylyev D, Dib-Hajj SD, Waxman SG. Nav1.9 expression in magnocellular neurosecretory cells of supraoptic nucleus. Exp Neurol. 2014;253:174–179. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Treistman SN, Lemos JR. Two types of high-threshold calcium currents inhibited by omega-conotoxin in nerve terminals of rat neurohypophysis. J Physiol. 1992;445:181–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dopico AM, Widmer H, Wang G, Lemos JR, Treistman SN. Rat supraoptic magnocellular neurones show distinct large conductance, Ca2+-activated K+ channel subtypes in cell bodies versus nerve endings. J Physiol. 1999;519:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roper P, Callaway J, Shevchenko T, Teruyama R, Armstrong W. AHP’s, HAP’s and DAP’s: how potassium currents regulate the excitability of rat supraoptic neurones. J Comput Neurosci. 2003;15:367–389. [DOI] [PubMed] [Google Scholar]
- 73.Shevchenko T, Teruyama R, Armstrong WE. High-threshold, Kv3-like potassium currents in magnocellular neurosecretory neurons and their role in spike repolarization. J Neurophysiol. 2004;92:3043–3055. [DOI] [PubMed] [Google Scholar]
- 74.Bourque CW, Randle JCR, Renaud LP. Calcium-dependent potassium conductance in rat supraoptic nucleus neurosecretory neurons. J Neurophysiol. 1985;54:1375–1382. [DOI] [PubMed] [Google Scholar]
- 75.Bourque C, Brown D. Apamin and d-tubocurarine block the afterhyperpolarization of rat supraoptic neurosecretory neurons. Neurosci Lett. 1987;82:185–190. [DOI] [PubMed] [Google Scholar]
- 76.Fagan M, Andrew RD. Intracellular study of calcium-related events in cat magnocellular neuroendocrine cells. J Physiol. 1991;434:337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith BN, Armstrong WE. Histamine enhances the depolarizing afterpotential of immunohistochemically identified vasopressin neurons in the rat supraoptic nucleus via H1-receptor activation. Neuroscience. 1993;53:855–864. [DOI] [PubMed] [Google Scholar]
- 78.Mourre C, Hugues M, Lazdunski M. Quantitative autoradiographic mapping in rat brain of the receptor of apamin, a polypeptide toxin specific for one class of Ca2+-dependent K+ channels. Brain Res. 1986;382:239–249. [DOI] [PubMed] [Google Scholar]
- 79.Köhler M, Hirschberg B, Bond CT, et al. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. [DOI] [PubMed] [Google Scholar]
- 80.Stocker M, Pedarzani P. Differential distribution of three Ca(2+)- activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. [DOI] [PubMed] [Google Scholar]
- 81.Greffrath W, Magerl W, Disque-Kaiser U, Martin E, Reuss S, Boehmer G. Contribution of Ca2+-activated K+ channels to hyperpolarizing after-potentials and discharge pattern in rat supraoptic neurones. J Neuroendocrinol. 2004;16:577–588. [DOI] [PubMed] [Google Scholar]
- 82.Armstrong WE, Rubrum A, Teruyama R, Bond CT, Adelman JP. Immunocytochemical localization of small-conductance, calcium-dependent potassium channels in astrocytes of the rat supraoptic nucleus. J Comp Neurol. 2005;491:175–185. [DOI] [PubMed] [Google Scholar]
- 83.Teruyama R, Armstrong WE. Enhancement of calcium-dependent afterpotentials in oxytocin neurons of the rat supraoptic nucleus during lactation. J Physiol. 2005;566:505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirchner MK, Foehring RC, Wang L, Chandaka GK, Callaway JC, Armstrong WE. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulates afterhyperpolarizations in oxytocin neurons of the supraoptic nucleus. J Physiol. 2017;595:4927–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirkpatrick K, Bourque CW. Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. J Physiol. 1996;494:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang L, Chandaka GK, Foehring RC, Callaway JC, Armstrong WE. Changes in potassium channel modulation may underlie afterhyperpolarization plasticity in oxytocin neurons during late pregnancy. J Neurophysiol. 2018;119:1745–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. 2012;74:245–269. [DOI] [PubMed] [Google Scholar]
- 88.Kirchner M, Foehring R, Callaway J, Armstrong W. Specificity in the interaction of high-voltage-activated Ca2+ channel types with Ca2+-dependent afterhyperpolarizations in magnocellular supraoptic neurons. J Neurophysiol. 2018;120:1728–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greffrath W, Martin E, Reuss S, Boehmer G. Components of afterhyperpolarization in magnocellular neurones of the rat supraoptic nucleus in vitro. J Physiol. 1998;513:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghamari-Langroudi M, Bourque CW. Muscarinic receptor modulation of slow afterhyperpolarization and phasic firing in rat supraoptic nucleus neurons. J Neurosci. 2004;24:7718–7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Armstrong WE, Wang L, Li C, Teruyama R. Performance, properties and plasticity of identified oxytocin and vasopressin neurones in vitro. J Neuroendocrinol. 2010;22:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shah L, Bansal V, Rye PL, Mumtaz N, Taherian A, Fisher TE. Osmotic activation of phospholipase C triggers structural adaptation in osmosensitive rat supraoptic neurons. J Physiol. 2014;592:4165–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andrew RD, Dudek FE. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol. 1984;51:552–566. [DOI] [PubMed] [Google Scholar]
- 94.Bourque C Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory cells. Neurosci Lett. 1986;70:204–209. [DOI] [PubMed] [Google Scholar]
- 95.Jourdain P, Poulain DA, Theodosis DT, Israel JM. Electrical properties of oxytocin neurons in organotypic cultures from postnatal rat hypothalamus. J Neurophysiol. 1996;76:2772–2785. [DOI] [PubMed] [Google Scholar]
- 96.Teruyama R, Armstrong WE. Calcium-dependent fast depolarizing afterpotentials in vasopressin neurons in the rat supraoptic nucleus. J Neurophysiol. 2007;98:2612–2621. [DOI] [PubMed] [Google Scholar]
- 97.Li Z, Hatton GI. Reduced outward K+ conductances generate depolarizing after-potentials in rat supraoptic nucleus neurones. J Physiol. 1997;505:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andrew RD. Endogenous bursting by rat supraoptic neuroendocrine cells is calcium dependent. J Physiol. 1987;384:451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roper P, Callaway J, Armstrong W. Burst initiation and termination in phasic vasopressin cells of the rat supraoptic nucleus: a combined mathematical, electrical, and calcium fluorescence study. J Neurosci. 2004;24:4818–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghamari-Langroudi M, Bourque CW. Caesium blocks depolarizing after-potentials and phasic firing in rat supraoptic neurones. J Physiol. 1998;510:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghamari-Langroudi M, Bourque CW. Flufenamic acid blocks depolarizing afterpotentials and phasic firing in rat supraoptic neurones. J Physiol. 2002;545:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Z, Hatton GI. Ca2+ release from internal stores: role in generating depolarizing after-potentials in rat supraoptic neurones. J Physiol. 1997;498:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown CH, Ghamari-Langroudi M, Leng G, Bourque CW. Kappa-opioid receptor activation inhibits post-spike depolarizing after-potentials in rat supraoptic nucleus neurones in vitro. J Neuroendocrinol. 1999;11:825–828. [DOI] [PubMed] [Google Scholar]
- 104.Brown CH, Bourque CW. Mechanisms of rhythmogenesis: insights from hypothalamic vasopressin neurons. Trends Neurosci. 2006;29:108–115. [DOI] [PubMed] [Google Scholar]
- 105.Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Armstrong WE, Sladek CD. Evidence for excitatory actions of histamine on supraoptic neurons in vitro: mediation by an H1-type receptor. Neuroscience. 1985;16:307–322. [DOI] [PubMed] [Google Scholar]
- 107.Sabatier N, Brown CH, Ludwig M, Leng G. Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. J Physiol. 2004;558:161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teruyama R, Sakuraba M, Kurotaki H, Armstrong WE. Transient receptor potential channel m4 and m5 in magnocellular cells in rat supraoptic and paraventricular nuclei. J Neuroendocrinol. 2011;23:1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fisher TE, Bourque CW. Voltage-gated calcium currents in the magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1995;486:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foehring RC, Armstrong WE. Pharmacological dissection of highvoltage-activated Ca2+ current types in acutely dissociated rat supraoptic magnocellular neurons. J Neurophysiol. 1996;76:977–983. [DOI] [PubMed] [Google Scholar]
- 111.Joux N, Chevaleyre V, Alonso G, et al. High voltage-activated Ca2+ currents in rat supraoptic neurones: biophysical properties and expression of the various channel alpha1 subunits. J Neuroendocrinol. 2001;13:638–649. [DOI] [PubMed] [Google Scholar]
- 112.Foehring RC, Mermelstein PG, Song WJ, Ulrich S, Surmeier DJ. Unique properties of R-type calcium currents in neocortical and neostriatal neurons. J Neurophysiol. 2000;84:2225–2236. [DOI] [PubMed] [Google Scholar]
- 113.Tobin VA, Douglas AJ, Leng G, Ludwig M. The involvement of voltage-operated calcium channels in somato-dendritic oxytocin release. PLoS ONE. 2011;6:e25366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang G, Dayanithi G, Newcomb R, Lemos JR. An R-type Ca(2+) current in neurohypophysial terminals preferentially regulates oxytocin secretion. J Neurosci. 1999;19:9235–9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rajapaksha WR, Wang D, Davies JN, Chen L, Zamponi GW, Fisher TE. Novel splice variants of rat CaV2.1 that lack much of the synaptic protein interaction site are expressed in neuroendocrine cells. J Biol Chem. 2008;283:15997–16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang D, Fisher TE. Expression of CaV 2.2 and splice variants of CaV 2.1 in oxytocin- and vasopressin-releasing supraoptic neurones. J Neuroendocrinol. 2014;26:100–110. [DOI] [PubMed] [Google Scholar]
- 117.Wang G, Dayanithi G, Kim S, et al. Role of Q-type Ca2+ channels in vasopressin secretion from neurohypophysial terminals of the rat. J Physiol. 1997;502:351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rose RA, Anand-Srivastava MB, Giles WR, Bains JS. C-type natriuretic peptide inhibits L-type Ca2+ current in rat magnocellular neurosecretory cells by activating the NPR-C receptor. J Neurophysiol. 2005;94:612–621. [DOI] [PubMed] [Google Scholar]
- 119.Israel JM, Poulain DA, Oliet SH. Oxytocin-induced postinhibitory rebound firing facilitates bursting activity in oxytocin neurons. J Neurosci. 2008;28:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luther JA, Tasker JG. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2000;523:193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poulain P, Carette B. Low-Threshold calcium spikes in hypothalamic neurons recorded near the paraventricular nucleus in vitro. Brain Res Bull. 1987;19:453–460. [DOI] [PubMed] [Google Scholar]
- 122.Tasker JG, Dudek FE. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1991;434:271–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2001;537:161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zaman T, Zhou X, Pandey NR, et al. LMO4 is essential for paraventricular hypothalamic neuronal activity and calcium channel expression to prevent hyperphagia. J Neurosci. 2014;34:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang W, Star B, Rajapaksha WR, Fisher TE. Dehydration increases L-type Ca(2+) current in rat supraoptic neurons. J Physiol. 2007;580:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hatton GI. Glial-neuronal interactions in the mammalian brain. Adv Physiol Educ. 2002;26:225–237. [DOI] [PubMed] [Google Scholar]
- 127.Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. [DOI] [PubMed] [Google Scholar]
- 128.Armstrong WE. Central nervous system control of oxytocin secretion during lactation In: Plant T, Zelezkni A, eds. Knobil and Neill’s Physiology of Reproduction, 4th edn. New York, NY: Elsevier/Acadmic Press; 2015:527–563. [Google Scholar]
- 129.Bourque CW. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1988;397:331–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cobbett P, Legendre P, Mason WT. Characterization of three types of potassium current in cultured neurones of rat supraoptic nucleus area. J Physiol. 1989;410:443–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nagatomo T, Inenaga K, Yamashita H. Transient outward current in adult rat supraoptic neurones with slice patch-clamp technique: inhibition by angiotensin II. J Physiol. 1995;485:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Z, Ferguson AV. Electrophysiological properties of paraventricular magnocellular neurons in rat brain slices: modulation of IA by angiotensin II. Neuroscience. 1996;71:133–145. [DOI] [PubMed] [Google Scholar]
- 133.Hlubek MD, Cobbett P. Outward potassium currents of supraoptic magnocellular neurosecretory cells isolated from the adult guineapig. J Physiol. 1997;502:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schrader LA, Tasker JG. Modulation of multiple potassium currents by metabotropic glutamate receptors in neurons of the hypothalamic supraoptic nucleus. J Neurophysiol. 1997;78:3428–3437. [DOI] [PubMed] [Google Scholar]
- 135.Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci USA. 1991;88:380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hlubek MD, Cobbett P. Differential effects of K(+) channel blockers on frequency-dependent action potential broadening in supraoptic neurons. Brain Res Bull. 2000;53:203–209. [DOI] [PubMed] [Google Scholar]
- 137.Armstrong WE, Stern JE. Electrophysiological and morphological characteristics of neurons in perinuclear zone of supraoptic nucleus. J Neurophysiol. 1997;78:2427–2437. [DOI] [PubMed] [Google Scholar]
- 138.Wang L, Ennis M, Szabo G, Armstrong WE. Characteristics of GABAergic and cholinergic neurons in perinuclear zone of mouse supraoptic nucleus. J Neurophysiol. 2015;113:754–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alonso G, Widmer H. Clustering of KV4.2 potassium channels in postsynaptic membrane of rat supraoptic neurons: an ultrastructural study. Neuroscience. 1997;77:617–621. [DOI] [PubMed] [Google Scholar]
- 140.Lee SK, Lee S, Shin SY, Ryu PD, Lee SY. Single cell analysis of voltage-gated potassium channels that determines neuronal types of rat hypothalamic paraventricular nucleus neurons. Neuroscience. 2012;205:49–62. [DOI] [PubMed] [Google Scholar]
- 141.Stern JE, Armstrong WE. Electrophysiological differences between oxytocin and vasopressin neurones recorded from female rats in vitro. J Physiol. 1995;488:701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stern JE, Armstrong WE. Sustained outward rectification of oxytocinergic neurones in the rat supraoptic nucleus: ionic dependence and pharmacology. J Physiol. 1997;500:497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang W, Wang D, Liu XH, Kosala WR, Rajapaksha JS, Fisher TE. An osmosensitive voltage-gated K+ current in rat supraoptic neurons. Eur J Neurosci. 2009;29:2335–2346. [DOI] [PubMed] [Google Scholar]
- 144.Moos FC. GABA-induced facilitation of the periodic bursting activity of oxytocin neurones in suckled rats. J Physiol. 1995;488:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hirasawa M, Mouginot D, Kozoriz MG, Kombian SB, Pittman QJ. Vasopressin differentially modulates non-NMDA receptors in vasopressin and oxytocin neurons in the supraoptic nucleus. J Neurosci. 2003;23:4270–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. [DOI] [PubMed] [Google Scholar]
- 147.Bansal V, Fisher TE. Na(+)-Activated K(+) channels in rat supraoptic neurones. J Neuroendocrinol. 2016;28:. 10.1111/jne.12394 [DOI] [PubMed] [Google Scholar]
- 148.Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+. Trends Neurosci. 2005;28:422–428. [DOI] [PubMed] [Google Scholar]
- 149.Dunn FL, Brennan TJ, Nelson AE, Robertson GL. The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest. 1973;52:3212–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kasting NW. Simultaneous and independent release of vasopressin and oxytocin in the rat. Can J Physiol Pharmacol. 1988;66:22–26. [DOI] [PubMed] [Google Scholar]
- 151.Stricker EM, Verbalis JG. Interaction of osmotic and volume stimuli in regulation of neurohypophyseal secretion in rats. Am J Physiol. 1986;250:R267–R275. [DOI] [PubMed] [Google Scholar]
- 152.Richard D, Bourque CW. Synaptic control of rat supraoptic neurones during osmotic stimulation of the organum vasculosum lamina terminalis in vitro. J Physiol. 1995;489:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ciura S, Liedtke W, Bourque CW. Hypertonicity sensing in organum vasculosum lamina terminalis neurons: a mechanical process involving TRPV1 but not TRPV4. J Neurosci. 2011;31:14669–14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hussy N, Deleuze C, Bres V, Moos FC. New role of taurine as an osmomediator between glial cells and neurons in the rat supraoptic nucleus. Adv Exp Med Biol. 2000;483:227–237. [DOI] [PubMed] [Google Scholar]
- 155.Choe KY, Olson JE, Bourque CW. Taurine release by astrocytes modulates osmosensitive glycine receptor tone and excitability in the adult supraoptic nucleus. J Neurosci. 2012;32:12518–12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abe H, Ogata N. Ionic mechanism for the osmotically-induced depolarization in neurones of the guinea-pig supraoptic nucleus in vitro. J Physiol. 1982;327:157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Oliet SH, Bourque CW. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993;364:341–343. [DOI] [PubMed] [Google Scholar]
- 158.Bourque CW, Oliet SHR, Richard D. Osmoreceptors, osmoreception, and osmoregulation. Front Neuroendocrinol. 1994;15:231–274. [DOI] [PubMed] [Google Scholar]
- 159.Sharif Naeini R, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci. 2006;9:93–98. [DOI] [PubMed] [Google Scholar]
- 160.Bourque CW, Oliet SH. Osmoreceptors in the central nervous system. Annu Rev Physiol. 1997;59:601–619. [DOI] [PubMed] [Google Scholar]
- 161.Prager-Khoutorsky M, Bourque CW. Mechanical basis of osmosensory transduction in magnocellular neurosecretory neurones of the rat supraoptic nucleus. J Neuroendocrinol. 2015;27:507–515. [DOI] [PubMed] [Google Scholar]
- 162.Liu XH, Zhang W, Fisher TE. A novel osmosensitive voltage gated cation current in rat supraoptic neurones. J Physiol. 2005;568:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ohbuchi T, Sato K, Suzuki H, et al. Acid-sensing ion channels in rat hypothalamic vasopressin neurons of the supraoptic nucleus. J Physiol. 2010;588:2147–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sharma K, Haque M, Guidry R, Ueta Y, Teruyama R. Effect of dietary salt intake on epithelial Na(+) channels (ENaC) in vasopressin magnocellular neurosecretory neurons in the rat supraoptic nucleus. J Physiol. 2017;595:5857–5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mills NJ, Sharma K, Huang K, Teruyama R. Effect of dietary salt intake on epithelial Na(+) channels (ENaCs) in the hypothalamus of Dahl salt-sensitive rats. Physiol Rep. 2018;6:e13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mills NJ, Sharma K, Haque M, Moore M, Teruyama R. Aldosterone mediated regulation of epithelial sodium channel (ENaC) subunits in the rat hypothalamus. Neuroscience. 2018;390:278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haam J, Popescu IR, Morton LA, et al. GABA is excitatory in adult vasopressinergic neuroendocrine cells. J Neurosci. 2012;32:572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Choe KY, Trudel E, Bourque CW. Effects of salt loading on the regulation of rat hypothalamic magnocellular neurosecretory cells by ionotropic GABA and glycine receptors. J Neuroendocrinol. 2016;28:. 10.1111/jne.12372 [DOI] [PubMed] [Google Scholar]
- 169.Kim YB, Kim YS, Kim WB, et al. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circ Res. 2013;113:1296–1307. [DOI] [PubMed] [Google Scholar]
- 170.Kim JS, Kim WB, Kim YB, et al. Chronic hyperosmotic stress converts GABAergic inhibition into excitation in vasopressin and oxytocin neurons in the rat. J Neurosci. 2011;31:13312–13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Choe KY, Han SY, Gaub P, et al. High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron. 2015;85:549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Korpal AK, Han SY, Schwenke DO, Brown CH. A switch from GABA inhibition to excitation of vasopressin neurons exacerbates the development angiotensin II-dependent hypertension. J Neuroendocrinol. 2018;30:e12564. [DOI] [PubMed] [Google Scholar]
- 173.Park JB, Skalska S, Stern JE. Characterization of a novel tonic gamma-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology. 2006;147:3746–3760. [DOI] [PubMed] [Google Scholar]
- 174.Brussaard AB, Devay P, Leyting-Vermeulen JL, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. J Physiol. 1999;516:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bourque CW. Intraterminal recordings from the rat neurohypophysis in vitro. J Physiol. 1990;421:247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lemos J, Nowycky M. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989;2:1419–1426. [DOI] [PubMed] [Google Scholar]
- 177.Fisher TE, Bourque CW. Distinct ω-agatoxin-sensitive calcium currents in somata and axon terminals of rat supraoptic neurons. J Physiol. 1995;489:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bicknell RJ, Brown D, Chapman C, Hancock PD, Leng G. Reversible fatigue of stimulus-secretion coupling in the rat neurohypophysis. J Physiol. 1984;348:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lemos JR, Ortiz-Miranda SI, Cuadra AE, et al. Modulation/physiology of calcium channel sub-types in neurosecretory terminals. Cell Calcium. 2012;51:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bondy CA, Gainer H, Russell JT. Effects of stimulus frequency and potassium channel blockade on the secretion of vasopressin and oxytocin from the neurohypophysis. Neuroendocrinology. 1987;46:258–267. [DOI] [PubMed] [Google Scholar]
- 181.Falke N Modulation of oxytocin and vasopressin release at the level of the neurohypophysis. Prog Neurobiol. 1991;36:465–484. [DOI] [PubMed] [Google Scholar]
- 182.Klyachko VA, Ahern GP, Jackson MB. cGMP-mediated facilitation in nerve terminals by enhancement of the spike afterhyperpolarization. Neuron. 2001;31:1015–1025. [DOI] [PubMed] [Google Scholar]
- 183.Ortiz-Miranda S, Dayanithi G, Custer E, Treistman SN, Lemos JR. Micro-opioid receptor preferentially inhibits oxytocin release from neurohypophysial terminals by blocking R-type Ca2 + channels. J Neuroendocrinol. 2005;17:583–590. [DOI] [PubMed] [Google Scholar]
- 184.Hatton GI, Wang YF. Neural mechanism underlying the milk ejection burst and reflex. Prog Brain Res. 2008;170:155–166. [DOI] [PubMed] [Google Scholar]
- 185.Armstrong WE. Vasopressin (AVP) In: Coen C, ed. Reference Module in Neuroscience and Biobehavioral Psychology. Amsterdam: Elsevier; 2018:1–9. [Google Scholar]
- 186.Dyball RE. Oxytocin and ADH secretion in relation to electrical activity in antidromically identified supraoptic and paraventricular units. J Physiol. 1971;214:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]


