The American College of Radiology (ACR)–endorsed Reporting and Data Systems (RADS) are guidelines for the evaluation and interpretation of disease-oriented imaging studies. Each system is devised by a group of experts in consensus and may be periodically updated to improve diagnostic parameters. Standardized reporting is preferred by referring physicians because it minimizes reporting variations and ambiguous terminology. Furthermore, the systematic nature of the ACR RADS allows consistent data collection. These qualities are critical for facilitating outcomes monitoring and for postadoption refinements and quality assurance.
The RADS are both modality and technique specific. All but one system, the Ovarian-Adnexal Reporting and Data System (O-RADS), use a stepwise numerical scoring system that corresponds to the degree of disease suspicion. Certain systems also allow modifiers to convey specific details, such as inadequate examination, negative examination, posttreatment findings, and non–disease-related findings. Certain systems also offer follow-up recommendations for each associated category. These recommendations add value to our reports and have the potential to improve practice standards across institutions.
The prototype system Breast Imaging Reporting and Data System (BI-RADS) was first published by the ACR in 1993 to address the lack of uniformity in mammography reporting. As of mid-2019, there are nine systems with full ACR endorsements and several new systems in various stages of development (Figure). BI-RADS (breast cancer; mammography, MRI, and US), C-RADS (colon cancer; CT colonography), LI-RADS (liver cancer; MRI, CT, US, and contrast-enhanced US), Lung-RADS (lung cancer; low-dose CT), NI-RADS (head and neck cancers; PET, CT, and MRI), O-RADS (adnexal masses; US), PI-RADS (prostate cancer; MRI), and TI-RADS (thyroid cancer; US) focus on malignancy, while CAD-RADS (CT angiography) focuses on coronary artery disease.
Figure.
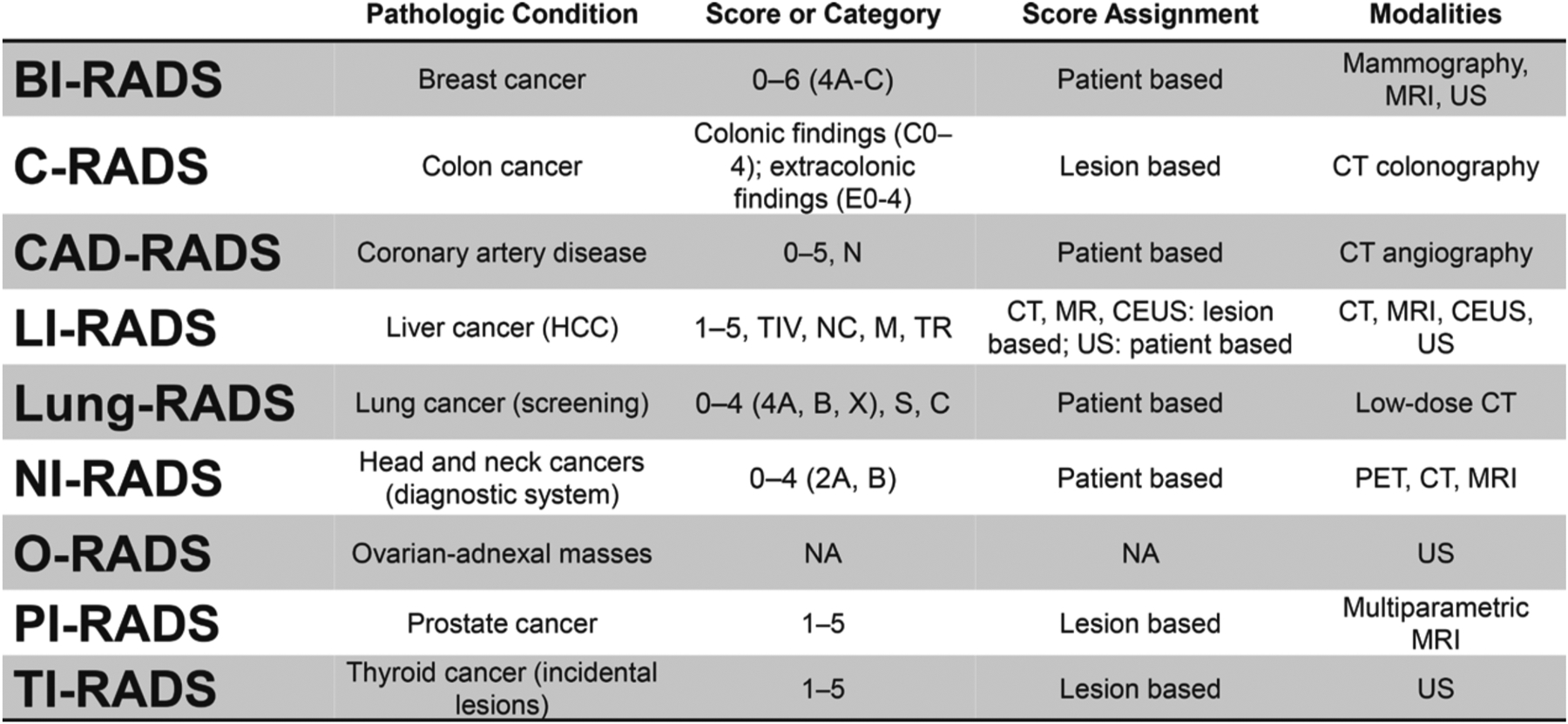
Overview of nine ACR RADS as of midyear 2019. C = prior lung cancer; C-RADS = CT Colonography Reporting and Data System; CAD-RADS = Coronary Artery Disease Reporting and Data System; CEUS = contrast-enhanced US; HCC = hepatocellular carcinoma; LI-RADS = Liver Imaging Reporting and Data System; M = malignant but not HCC; N, NC = nondiagnostic; NI-RADS = Neck Imaging Reporting and Data System; PI-RADS = Prostate Imaging Reporting and Data System; S = clinically significant or potentially clinically significant findings (nonlung cancer); TI-RADS = Thyroid Imaging Reporting and Data System; TIV = tumor in vein; TR = treated.
Because the various types of RADS are disease specific, there are inherent structural differences among the systems. Major differences include whether the study indication is for screening or diagnostic purposes, if scores are given per lesion or per patient, and the range of numbers included in the scoring categories. A summary of the nine systems and their key features is shown in the Figure. The primary literature is listed in the suggested readings.
In the online presentation, each system is individually reviewed. The scores or categories for each system are described, and imaging findings, management recommendations, and image examples for each are provided.
Clinical adoption of the ACR RADS varies greatly among radiologists, institutions, and practices. BI-RADS followed by PI-RADS and LI-RADS have gained the greatest acceptance and interest, as demonstrated by each of their sizeable and growing publication records. Further integration of RADS into clinical society guidelines, as demonstrated by BI-RADS with the American Society of Breast Surgeons and LI-RADS with the American Association for the Study of Liver Diseases, has potential to increase the use and impact radiology has on clinical care. Expert lectures and interactive cases are provided for more in-depth system-specific training.
TEACHING POINTS.
The ACR-endorsed RADS are expert-devised guidelines for the evaluation and interpretation of disease-oriented imaging studies.
The majority of systems are related to cancer imaging, with BI-RADS, PI-RADS, LI-RADS, and TI-RADS being the most widely accepted by radiologists and speciality physicians.
Because the various types of RADS are disease specific, there are inherent structural and scoring differences to be aware of when first applying these systems in practice.
Acknowledgments.—
The authors would like to thank Philip Araoz, MD, Wan Koo, MD, and Baris Turkbey, MD, for their guidance and review of this presentation.
Abbreviations:
- ACR
American College of Radiology
- RADS
Reporting and Data Systems
Footnotes
Disclosures of Conflicts of Interest.—
J.C.W. Activities related to the present article: member of the ACR LI-RADS and PI-RADS committees; received reimbursement for travel expenses for committee meetings from the ACR. Activities not related to the present article: disclosed no relevant relationships. Other activities: disclosed no relevant relationships.
The author J.C.W has provided disclosures (see end of article); all other authors have disclosed no relevant relationships.
Suggested Readings
- American College of Radiology. Liver Reporting and Data Systems (LI-RADS). https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS. Accessed June 4, 2019.
- Andreotti RF, Timmerman D, Benacerraf BR., et al. Ovarian-Adnexal Reporting Lexicon for Ultrasound: A White Paper of the ACR Ovarian-Adnexal Reporting and Data System Committee. Journal of the American College of Radiology 2018;15(10):1415–1429. [DOI] [PubMed] [Google Scholar]
- Cavazuti B, Hudgins P, Rath T, et al. Neck Imaging Reporting and Data System: An Atlas of NI-RADS Categories for Head and Neck Cancer. American College of Radiology. Accessed June 4, 2019. [Google Scholar]
- Cury RC, Abbara S, Achenbach S, et al. CAD-RADS Coronary Artery Disease: Reporting and Data System—An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI): Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2016;10(4):269–281. [DOI] [PubMed] [Google Scholar]
- D’Orsi CJ, Sickles EA, Mendelson EB et al. ACR BI-RADS atlas, Breast Imaging Reporting and Data System. Reston, Va: American College of Radiology, 2013. [Google Scholar]
- Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. Journal of the American College of Radiology 2017; 14(5):587–595. [DOI] [PubMed] [Google Scholar]
- Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, Margolis D, Schnall MD, Shtern F, Tempany CM, et al. PI-RADS Prostate Imaging: Reporting and Data System: 2015, Version 2. Eur Urol 2016;69(1):16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalis ME, Barish MA, Choi JR et-al. CT colonography reporting and data system: a consensus proposal. Radiology 2005; 236(1):3–9. [DOI] [PubMed] [Google Scholar]


