Abstract
Objective:
Spasmodic dysphonia (SD) is a debilitating voice/speech disorder without an effective cure. To obtain a better understanding of the underlying cortical neural mechanism of the disease we analyzed electroencephalographic (EEG) signals of people with SD during voice production.
Method:
Ten SD individuals and 10 healthy volunteers produced 50 vowel vocalization epochs of 2500ms duration. Two EEG features were derived: (1) event-related change in spectral power during vocalization relative to rest, (2) inter-regional spectral coherence.
Results:
During early vocalization (500–1000ms) the SD group showed significantly larger alpha band spectral power over the left motor cortex. During late vocalization (1000–2500ms) SD patients showed a significantly larger gamma band coherence between left somatosensory and premotor cortical areas.
Conclusions:
Two atypical patterns of cortical activity characterize the pathophysiology of spasmodic dysphonia during voice production: (1) a reduced movement-related desynchronization of motor cortical networks, (2) an excessively large synchronization between left somatosensory and premotor cortical areas.
Significance:
The pathophysiology of SD is characterized by an abnormally high synchronous activity within and across cortical neural networks involved in voice production that is mainly lateralized in the left hemisphere.
Keywords: Dystonia, Larynx, Sensorimotor Cortex, Speech, Voice Disorder, EEG Coherence
1. Introduction
Spasmodic dysphonia (SD) is the third most prevalent type of dystonia (Castelon Konkiewitz et al., 2002) manifesting itself through speech-related involuntary contractions of the laryngeal musculature (Ludlow, 2009). Clinical manifestations of SD include difficulty in pronouncing words beginning with or containing vowels (adductor SD) or voiceless consonants (abductor SD) (Ludlow, 2011). A subgroup of patients also experiences the co-occurrence of voice tremor. Treatment options for SD are symptomatic in effect; mainly, the cyclical injection of Botulinum Toxin (BOTOX) to the laryngeal musculature, and selective laryngeal denervation surgeries (Ludlow, 2009).
The underlying pathophysiological mechanism of SD is largely unknown. Abnormal long-latency responses to peripheral nerve stimulation have been observed in SD (Ludlow et al., 1995) and other forms of focal dystonia such as blepharospasm and oromandibular dystonia (Berardelli et al., 1985). A shortened cortical silent period, indicative of reduced cortical inhibition, is another consistent feature of focal dystonia that has recently been confirmed in SD (Samargia et al., 2014). In the last decade, postmortem histological studies identified a range of structural abnormalities in SD, such as white matter changes (loss of axonal density and myelin content) in the genu of the internal capsule where head and neck muscles are represented (Simonyan et al., 2008). This was followed by the observation of subtle brainstem abnormalities in the reticular formation, and mild degeneration and depigmentation of the substantia nigra and the locus coeruleus (Simonyan et al., 2010b). Furthermore, functional brain imaging assessments identified areas of abnormal activation that include the primary motor, premotor, and somatosensory cortex. However, the results are mixed; with one study showing reduced activation (Haslinger et al., 2005), while another reported increased activation of sensorimotor cortical areas in SD during voice production (Simonyan et al., 2010a), compared to healthy adults. The discrepancy in outcomes might be attributable to differences in the vocalization task, i.e., testing symptomatic versus asymptomatic voice/speech production.
With respect to other forms of focal dystonia, analysis of EEG signals has confirmed signs of reduced cortical inhibition during movement execution. A movement-related reduction of beta band desynchronization has been shown in cervical and segmental dystonia (Crowell et al., 2012; Miocinovic et al., 2015) and in writer’s cramp (Toro et al., 2000). For the SD, however, no equivalent movement-related EEG data exist, except for one report documenting a widespread abnormal bilateral resting-state activity in the delta and theta bands (Devous et al., 1990).
Previous work by our team had shown a generalized proprioceptive deficit in musician’s dystonia (Konczak et al., 2013) and in SD (Konczak et al., 2015). This raises the question if one can identify atypical patterns of somatosensory activation and/or abnormal interaction between somatosensory and motor cortical areas that underlie the observed proprioceptive and motor dysfunction in spasmodic dysphonia. Previous studies using transcranial magnetic stimulation and functional brain imaging techniques have already provided evidence for abnormal patterns of functional connectivity between and within sensorimotor cortical areas in SD. For example, Giovanelli et al. (2015) reported abnormal excitability of the motor cortical area of the dominant hand during specific linguistic tasks in SD, which was interpreted as an atypical functional connectivity between the primary motor cortex and the cortical speech network. Battistella et al. (2016) showed significant decreases in the resting-state functional connectivity of the sensorimotor cortex and frontoparietal network in SD in relation to healthy controls. Putzel et al. (2018) showed that the polygenic risk of spasmodic dystonia was significantly correlated with a decline of functional connectivity in the left premotor/primary sensorimotor and inferior parietal cortices.
While functional neuroimaging assessments have advanced our understanding of the underlying neural mechanisms of SD, the inherent slow dynamics of these technologies necessitates the implication of higher temporal-resolution electrophysiological recordings that allow for the investigation of the transient brain processes that may contribute to the pathomechanism of the disease. Thus, this study recorded EEG signals to unveil the cortical activation dynamics associated with voice production in spasmodic dysphonia. Our specific aims were to a) characterize the transient cortical processes underlying the voice/speech production, and b) to extend previous work that had documented abnormal cortical activity during rest in SD (Devous et al., 1990). Characterizing the cortical activation patterns associated with voice production in patients with spasmodic dysphonia helps to elucidate further the underlying mechanisms of the disease. It may yield a more objective approach to differentiate SD from other voice disorders with overlapping symptoms such as muscle tension dysphonia.
2. Methods
2.1. Participants
Ten spasmodic dysphonia patients (6 females, 4 males) from the Fairview Clinic at the University of Minnesota (mean age ± standard deviation: 58.4 ± 13.3 years), and ten age and gender-matched control participants attended the study. The experimental protocol was approved by the University of Minnesota Institutional Review Board. All participants gave their informed consent prior to the experiment. Patients receiving botulinum toxin treatment were tested towards the end of their injection cycle (see Table 1 and Table 2 for the clinical characteristics of SD participants).
Table 1.
Clinical characteristics of the study participants.
| Subject ID | Gender | Age | SD Type | Diagnosis Duration (mo.) | BOTOX Cycle (mo.) | Last BOTOX Injection (mo.) |
|---|---|---|---|---|---|---|
| SD 01 | Male | 60 | Adductor | 50 | >3 | 3 |
| SD 02 | Male | 73 | Adductor | 180 | NA | 36 |
| SD 03 | Female | 57 | Adductor | 36 | NA | NA |
| SD 04 | Female | 62 | Adductor | 411 | 5 | 2 |
| SD 05 | Male | 26 | Adductor | 93 | 2–5 | 2.5 |
| SD 06 | Female | 65 | Adductor | 204 | 2 | 2 |
| SD 07 | Female | 56 | Adductor | 324 | 6 | 6 |
| SD 08 | Female | 57 | Adductor | 15 | 3 | 3 |
| SD 09 | Male | 74 | Adductor | 396 | 4 | 4 |
| SD 10 | Female | 54 | Adductor | 288 | 3 | 3.5 |
Table 2.
Clinical characteristics of the recorded voice data.
| Subject ID | Number of Voice Breaks | Voice Tremor | HNR (dB) | Self-Rated Effort Scale (Abductor/Adductor Sentences) | Self-Rated Effort Scale (Vowel /a/) |
|---|---|---|---|---|---|
| SD 01 | 0 | No | 10.10 | 2 | 3 |
| SD 02 | 12 | No | 15.20 | 3 | 2 |
| SD 03 | 9 | Moderate | 8.80 | 5 | 7 |
| SD 04 | 0 | Mild to moderate | 7.01 | 4 | 5 |
| SD 05 | 2 | No | 13.02 | 7 | 6 |
| SD 06 | 4 | Mild to moderate | 7.67 | 9 | 8 |
| SD 07 | 2 | Mild | 17.09 | 2 | 2 |
| SD 08 | 0 | No | 13.67 | 2 | 3 |
| SD 09 | 0 | No | 17.75 | 2 | 2 |
| SD 10 | 0 | No | 19.71 | 3 | 3 |
A potential concern with regard to the recruitment of SD participants might be the issue of vocal fold immobility that is usually observed with higher dose, unilateral BOTOX injections. Patients in this study, however, had low dose injections given into both vocal folds (ranging between 0.2–2 units per vocal fold with the dose based on symptom response). With this technique, the vocal fold immobility is typically not present. Vocal fold bowing can occasionally be seen with laryngoscopy in the early (breathy) period after the first few days and can last for weeks following the injection. However, these laryngoscopic findings are absent during the period of improved vocal quality or the period of recurrence of vocal spasms (Brin et al., 1989). Since the timing of the subsequent BOTOX injection and, thus, inclusion in the study were symptom-based, vocal fold immobility was not expected to be present.
2.2. Description of the Behavioral Task
The experiment was held in an electrically and acoustically shielded chamber. Participants sat on a comfortable chair and were asked to restrict limb and body movements during recording. Upon receiving a 250ms-long auditory cue (1000Hz, 98dB) they pronounced the vowel /a/ for 2500ms. Cessation of vocalization was triggered by a second 250ms-long auditory cue. Vocalization was repeated 50 times with 3000ms resting intervals in between trials. Cortical EEG was recorded simultaneously. The task was performed in an eyes-open condition with participants focusing gaze at a fixation point on the front wall.
The following measures were used to examine the quality of voice in SD participants: (1) the number of voice breaks, (2) voice tremor, (3) the harmonics-to-noise ratio (HNR), and (4) a self-rated effort scale (range: 0 (easiest) to 10 (hardest)) for the vocalization of vowel /a/ and for reading a series of standard sentences devised for the assessment of voice quality in adductor/abductor spasmodic dysphonia (Woodson, 2010) (see the Appendix). The HNR, measured in dB, is an indication of the level of hoarseness of the voice, defined as the ratio of the periodic aspect of the voice to the random noise (Yumoto et al., 1982). It is reported that healthy adults have an HNR of around 10dB (de Felippe et al., 2006). The PRAAT software was used for the analysis of the voice data (Boersma & van Heuven, 2001).
2.3. EEG Recording
Electroencephalographic data were recorded using the ActiveTwo data acquisition system (Biosemi B.V. Ltd, Amsterdam, Netherlands) at the sampling rate of 512 Hz. Brain potentials were captured over 64 active electrodes embedded in the Biosemi EEG cap according to the Biosemi designed equiradial system. Participants were guided throughout the experiment by a series of auditory cues generated by the RPvdsEx software and the Tucker-Davis system (Tucker-Davis Technologies Ltd., Alachua, FL, USA). The event time-stamps were captured by the ActiveTwo system in a separate channel.
2.4. EEG Signal Processing
We used the MATLAB EEGLab toolbox version 13.6.5 (Delorme et al., 2004) for the offline analysis of EEG data. EEG recordings were referenced to the average of two external electrodes attached to the left and right mastoid bones. Baseline drifts were removed by zero-phase 1Hz high-pass filtering, and the power line noise (50Hz) was excluded by zero-phase notch-filtering. Channels were then re-referenced to the common average of all electrodes to reduce the effect of non-cortical sources that may have been commonly captured. Segments of recording from 1000ms before vocalization to 2500ms afterward were derived as data epochs. Subsequently, we performed independent component analysis (ICA) on all 64 channels of data using the ‘runica’ algorithm and applied an automated multiple artifact rejection algorithm ‘SASICA’ (Chaumon et al., 2015) on the generated components to remove the contaminated ICs. The ‘SASICA’ algorithm uses spatiotemporal criteria to distinguish cortical components from artifactual ICs. This step was critical for removing muscle artifacts that may have interfered with the EEG data during vocalization. Finally, the remaining components were linearly added, and the resultant dataset was used for EEG feature extraction.
The following two measures were derived from the EEG data using the EEGLab functions: (1) event-related spectral perturbation (ERSP) at individual somatosensory and pre-motor/motor cortical electrodes (Makeig, 1993); and (2) event-related coherence (ERCOH) between somatosensory and premotor cortical electrodes (Pfurtscheller & Andrew, 1999).
ERSP reflects the logarithm of the event-related deviation in spectral power in relation to the baseline resting state (in dB). ERCOH (unit-less, ranging from 0–1) calculates the event-related coherence at different frequency bins between two electrodes and represents the level of synchronous activity between the two sites. EEG data epochs were pre-whitened prior to coherence computations to remove potential autocorrelations or trends that might have interfered with the results.
The conventional event-related averaging of epochs aims at capturing the synchronous neural activity that is both time- and phase-locked to a given stimulus. This, however, does not allow for probing the ongoing oscillatory activity that underlies individual components. Thus, it is possible that the latency jitter of single trials generated by background noise or delayed response latencies distort the overall averaged outcome. In order to control for jitter, we first applied the time-frequency analysis on individual trials, and then averaged the outcomes across all trials. Such induced activity is known to be dominantly generated by neuronal synchronizations which are reflective of coherent firing patterns that induce large fluctuations in the membrane potential of a cluster of neurons and contribute to the transfer of information between different brain regions (David et al., 2006; Koerner & Zhang, 2018).
Band-specific measures were computed for the physiologically-relevant frequency bands (i.e. <50Hz): theta (4–8Hz), alpha (8–13Hz), beta (13–30Hz), and low gamma (30–49Hz). Because the first 500ms of the recording could also reflect cortical auditory evoked potentials (Alvarenga et al., 2013) or be affected by a participant’s reaction time (Santee & Kohfeld, 1977), EEG assessments corresponding to vocalization focused on 500ms post-stimulus to the end of the trial (2500ms). In order not to miss early transient movement-related cortical phenomena (Riehle et al., 2013), this 2000ms-long vocalization period was further divided into two windows: (1) early vocalization (500–1000ms); and (2) late vocalization (1000–2500ms).
EEG features were extracted for 12 electrode sites: CP3, CP4, CP5, CP6, C3, C4, C5, C6, FC3, FC4, FC5, and FC6. Centro-parietal electrodes (CP3, CP4, CP5, and CP6) were nearby somatosensory cortical areas. Central electrodes (C3, C4, C5, and C6) were nearby motor cortical areas. Fronto-central electrodes (FC3, FC4, FC5, and FC6) were nearby premotor areas. Locations ‘3’ and ‘5’ refer to the cortical areas nearby the vocalization region on the left somatosensory and motor homunculi, while locations ‘4’ and ‘6’ refer to the corresponding positions in the right hemisphere (Caviness et al., 2006; Mor et al., 2017). ERSP measures were derived for each of the 12 electrodes at 4 distinct frequency bands (theta, alpha, beta, and gamma). ERCOH measures were derived for 4 electrode pairs: (CP3, FC3), (CP5, FC5), (CP4, FC4), and (CP6, FC6), for the same 4 frequency bands.
2.5. Statistical Analysis
Statistical comparisons were applied for all electrodes/electrode-pairs, at each distinct frequency band between the two groups. The Kolmogorov-Smirnov test was used to assess the normality of the EEG data. Because the data were not normally distributed, the non-parametric Mann-Whitney U-test was applied for group comparisons. For each distinct frequency band, and for the cluster of electrodes within the left/right hemisphere, p-values were adjusted for multiple comparisons via false discovery rate (FDR) correction based on the Benjamini-Hochberg method (Benjamini & Hochberg, 1995). The significance level was set at 0.05. The effect size was computed using Cohen’s d.
3. Results
3.1. Focal Somatosensory/Motor Cortical Synchronization Based on ERSP
To illustrate the differences in cortical processing between healthy controls and people with SD during vocalization, Figure 1 presents an exemplary time-frequency plot of the somatosensory (CP5) and premotor (FC5) cortical ERSP for one healthy and one SD participant. Expectedly, vocalization in the healthy participant was associated with the suppression of cortical oscillations below 30Hz and the excitation of gamma band. In comparison, the SD patient showed higher levels of spectral power across most frequency bands.
Figure 1.
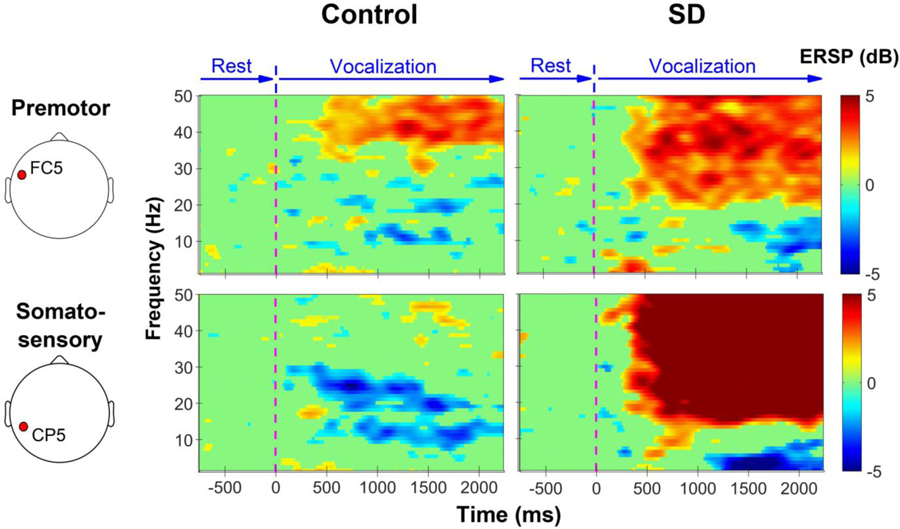
Example spectrograms of somatosensory (CP5) and premotor (FC5) cortical electrodes in one healthy and one SD participant during vocalization. Green indicates no significant change in relation to baseline (rest). Blue color depicts the suppression of oscillatory activities in relation to the baseline, and the red color shows the excitation of oscillations in relation to the baseline level. Vocalization in the healthy participant is associated with the suppression of lower frequency oscillations and excitation of gamma band. The SD patient shows smaller suppression (or excitation) in the majority of time-frequency bins.
The first step of the EEG analysis focused on markers of intra-regional cortical synchronization/desynchronization over those electrodes that encompass laryngeal somatosensory, premotor and motor cortical areas. We found that during the early stage of vocalization, the median of the alpha band ERSP in the SD group was significantly higher over the left motor cortex (C5: p = 0.03, Cohen’s d = 1.21, and C3: p = 0.03, Cohen’s d = 1.28). In contrast, assessment of the late vocalization period did not show any significant median ERSP differences between the two groups (see Figure 2).
Figure 2.
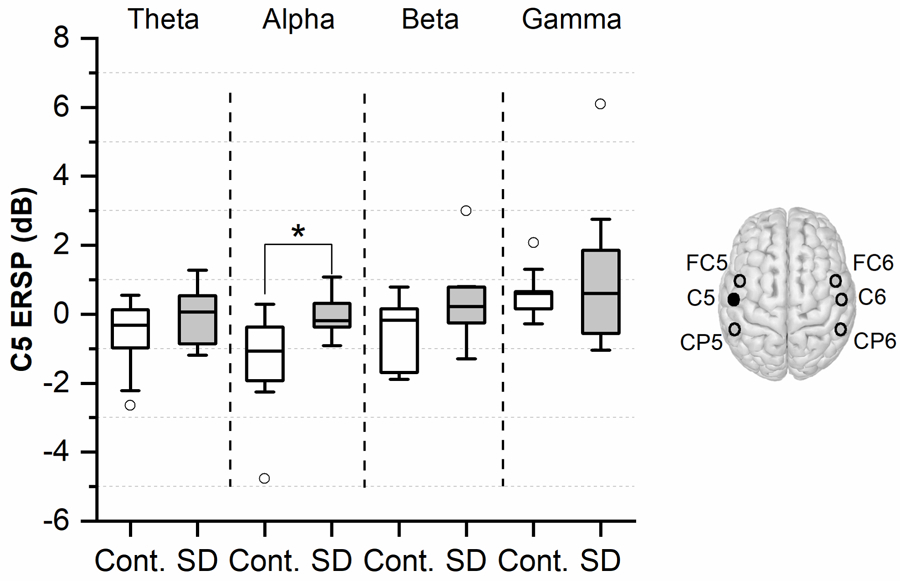
Comparison of motor cortical ERSP at electrode ‘C5’ between the SD and control groups during early vocalization (500–1000ms post-vocalization). The boxplots are generated based on individual ERSP values within each group and for four distinct frequency bands (theta, alpha, beta, and gamma). Lower and upper boundary of each box indicates the 25% and 75% quartiles, respectively. The horizontal line within the box depicts the median. The upper and lower whiskers extend to +1.5 and −1.5 inter-quartile range, respectively. Outliers are shown as white circles. * indicates a p-value < 0.05.
3.2. Inter-Regional Somatosensory-Motor Cortical Synchronization Based on ERCOH
A coherence analysis was performed to examine potential differences in the spectral characteristics of somatosensory-motor cortical interactions in each hemisphere. We found that during the early stage of vocalization median ERCOH in all frequency bands tended to be larger for SD patients for both hemispheres when compared to healthy volunteers. The relative median ERCOH surplus for SD patients in the left hemisphere (CP5, FC5) was computed as follows: theta band = +22%; alpha band = +28%; beta band = +27%; gamma band = +13%. For the right hemisphere (CP6, FC6) the same analysis yielded: theta band = +30%; alpha band = +37%; beta band = +6%; gamma band = +18%. However, no statistically significant difference was detected between the two groups.
Analysis of the gamma band ERCOH during late vocalization yielded a significantly higher median for the SD group in comparison to controls ((CP5, FC5): p = 0.03, Cohen’s d = 1.06). Figure 3B highlights that during late vocalization, the SD group tended to exhibit an enlarged somatosensory-premotor cortical coherence in both hemispheres and in the other three frequency bands as well. The relative median ERCOH change for SD patients in the left hemisphere (CP5, FC5) was: theta band = +34%; alpha band = +57%; beta band = +30%; gamma band = +28%. The respective relative median ERCOH change in the right hemisphere (CP6, FC6) was: theta band = +36%; alpha band = +11%; beta band = −4%; gamma band = +35%).
Figure 3.
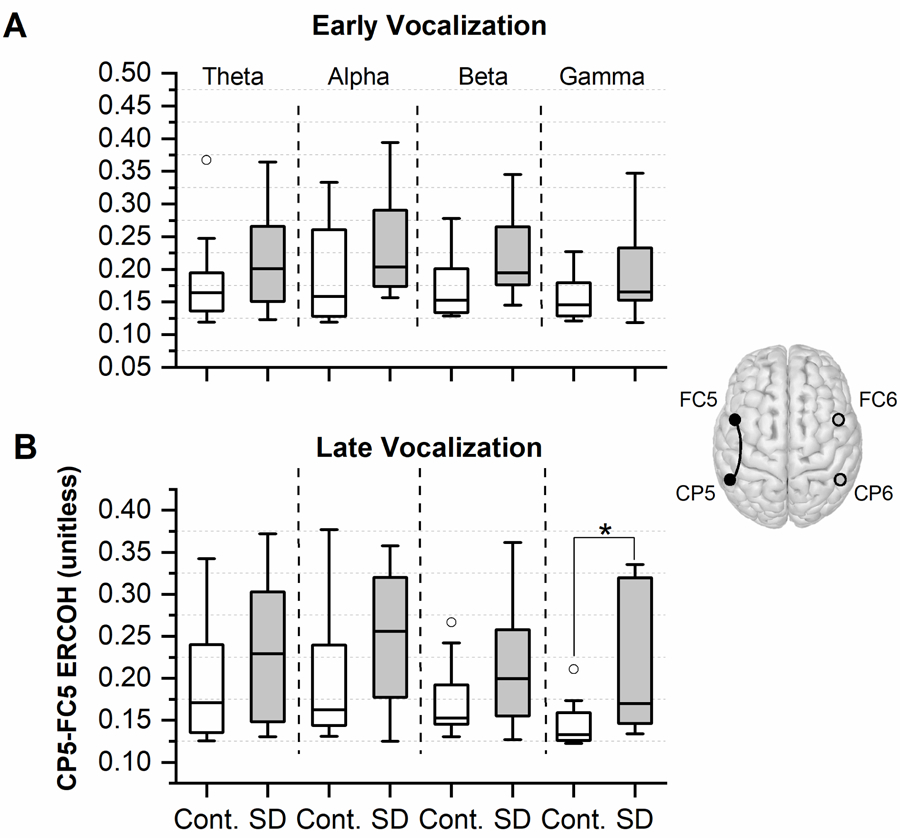
Comparison of (CP5, FC5) ERCOH for four distinct frequency bands (theta, alpha, beta, and gamma) between SD participants and the control group A) during early vocalization (500–1000ms post-vocalization), and B) during late vocalization (1000–2500ms post-vocalization). The boxplots are generated based on individual coherence values within each group. The lower and upper boundaries of each box depict the 25% and 75% quartiles, respectively. The median is marked by the horizontal line within the box. The upper and lower whiskers extend to +1.5 and −1.5 inter-quartile range, respectively. Outliers are shown as white circles. * indicates a p-value < 0.05.
Although the SD and control participants in this study were gender- and age-matched, we sought to account for the effect of age on the outcome measures. To that effect, statistical group comparisons were evaluated via linear mixed effect modeling (Koerner & Zhang, 2018). ERSP or ERCOH constituted the dependent variable. GROUP (SD or Control) was set as the independent variable, and AGE represented the random effect variable. Controlling for the effect of age, a significant group-difference was detected for the alpha band ERSP during early vocalization (p = 0.04), not for gamma band ERCOH during late vocalization (p = 0.06).
In addition, the relationship between disease duration and each of the significantly different neural measures (i.e., alpha band ERSP and gamma band ERCOH) was evaluated. The results revealed a significant positive relationship between the late-vocalization gamma band ERCOH and disease duration (p = 0.008). This indicates that patients with longer disease duration tended to exhibit higher levels of left hemispheric parieto-motor cortical interactions.
4. Discussion
The purpose of this study was to provide a systematic assessment of the spectral characteristics of somatosensory-motor cortical activities and interactions during vowel vocalization in spasmodic dysphonia. Our findings revealed that an excessive intra- and inter-regional somatosensory-motor cortical synchronization during vowel vocalization is a characteristic feature of spasmodic dysphonia.
4.1. Abnormally Large Left Motor Cortical Synchronization in SD
To the best of our knowledge, our work is one of the first studies that attempted to characterize the dynamics of transient somatosensory/motor cortical processes associated with spasmodic dysphonia. Previous research had applied functional brain imaging methods for this purpose, which due to the slower dynamics of these techniques, did not allow for capturing the fast cortical neuronal phenomena that correspond to abnormal speech phonatory functioning. Moreover, the only study which used EEG to identify cortical abnormalities in SD only captured the resting-state patterns (Devous et al., 1990).
It is well established that rhythmic neuronal activities over the sensorimotor cortex within the alpha and beta frequency bands are modulated during goal-directed movements (Brinkman et al., 2014). The rise of alpha band amplitude has been interpreted to reflect either the inhibition of task-irrelevant or the integration of task-relevant activity (Doesburg et al., 2009). Moreover, the increase of alpha band oscillatory amplitude is believed to indicate synchronized firing patterns in the underlying neuronal populations (Palva & Palva, 2011). In our assessments, both the healthy controls and SD participants exhibited the expected pattern of low-frequency cortical desynchronization during vocalization in relation to the resting state. However, in the SD group, this cortical inhibition tended to be lower over bilateral laryngeal somatosensory-motor cortical regions and was significantly lower over the left motor cortex in the alpha band. Our finding is compatible with previous reports of reduced cortical inhibition in SD as indicated by a reduced cortical silent period (Samargia et al., 2015) and reduced movement-related cortical desynchronization in other forms of focal dystonia (Toro et al., 2000, Crowell et al., 2012, Miocinovic et al., 2015) and may be reflective of overlapping underlying neural mechanisms in these dystonia subtypes.
4.2. Abnormally Large Left Somatosensory-Premotor Cortical Synchronization in SD
Our second aim in this study was to evaluate interregional interactions between somatosensory and motor cortical areas during active voice production in SD. Our analysis revealed that the synchronized activity between somatosensory and premotor cortices was abnormal for SD individuals. Within the patient group, the event-related coherence, as a frequency-specific measure of functional connectivity, was significantly elevated in the left hemisphere in the gamma band during late vocalization. Synchronization of neuronal activities in the gamma band is known to correspond to task-specific functions such as somatosensory processing (Bauer et al., 2006) and motor preparation (Engel et al., 2001; Nowak et al., 2018). Cortical gamma oscillations have been detected during the speech (Palva et al., 2002) and are known to be related to the semantic retrieval of individual words and the integration between word pairs (Maguire & Abel, 2013). The gamma rhythm is also considered as a temporal code for performing complicated forms of information processing through neuronal coherence (Varela et al., 2001). Because the coordinated activity of neuronal networks across dispersed brain regions is implicated in neuronal communication (Arce-McShane et al., 2016), the excessive somatosensory-premotor coherence in SD suggests an abnormally large degree of interaction between these cortical regions in this disorder.
In addition, previous research documented defective sensorimotor integration mechanisms in other forms of dystonia (Breakefield et al., 2008; Butterworth et al., 2003; Quartarone et al., 2006). Accordingly, our observation of an excessive gamma band spectral coherence between somatosensory and motor cortical areas in SD might be a sign of atypical processes of sensorimotor integration in this disorder.
The observed pattern of excessive cortical synchronization can also be linked to the previously reported structural abnormalities in SD, e.g., the loss of axonal density and myelin content in the genu of the internal capsule (Simonyan et al., 2008) and the depigmentation of the substantia nigra (Simonyan et al., 2010b). These structural changes can affect the regulation of brain’s inhibitory processes, such as the reduction of the GABAergic outputs of the substantia nigra (Lee et al., 2011), which may consequently contribute to the disinhibition of the basal ganglia-thalamocortical circuitry and the atypical rise of task-specific cortical synchronizations in SD.
Moreover, abnormal patterns of cortical processing in SD were found to be largely lateralized over the left cortex, which likely reflects the functional lateralization of voice and speech production in the left hemisphere.
4.3. Limitations of the Study
A main limitation of this study lies in the general weakness of EEG to localize the exact source of the detected cortical activity. While the EEG feature extraction was performed using electrodes that were nearby the vocalization region of the somatosensory and motor cortical homunculi (Mor et al., 2017), the precise localization of the detected abnormalities cannot be obtained. Another limitation of the study is that our analysis cannot establish a causal relationship between the excessive cortical synchronization observed in SD patients and the overt symptoms. That is to say, we cannot differentiate from our data, if the observed cortical patterns reflect a primary deficit or have to be understood as a compensatory response of the sensorimotor networks involved in voice production.
In addition, we cannot claim that the observed cortical abnormalities are specific to SD. On the contrary, similar movement-related reductions of cortical desynchronization were observed in cervical and segmental dystonia (Crowell et al., 2012; Miocinovic et al., 2015) as well as in writer’s cramp (Toro et al., 2000). This elucidates a common underlying pathomechanism between SD and other focal dystonia subtypes.
Moreover, it needs to be considered that the recorded EEG signals reflect the activity of a somatosensory-motor cortical network. That implies that the dystonic muscle contractions can alter the proprioceptive input to the somatosensory cortex, which then affects motor cortical activity. That is, the observed EEG output may be affected by abnormal muscle activity as well.
Finally, one needs to recognize that this study is exploratory in nature. To our knowledge, this is the first study that examined cortical activation patterns during vocalization in SD. We fully recognize that additional research is warranted to confirm our finding of excessive cortical activation during vocalization in SD. Although the statistical significance of our outcome measure provide support for the generalizability of our findings, follow up studies with a larger sample size could provide additional information about how consistent the observed abnormalities in cortical processing are across the SD population, and how these abnormalities relate to the severity of their voice symptoms.
5. Conclusions
In summary, this study revealed that two atypical patterns of cortical activity characterize the pathophysiology of spasmodic dysphonia. The first pattern is a reduced movement-related desynchronization of motor cortical neural networks during voice production. The second pattern is an excessively large synchronized activity between left somatosensory and premotor cortical areas during voice production. These neurophysiological findings could provide a basis for the development of more effective treatment opportunities for spasmodic dysphonia by examining ways to reduce this abnormal activity. For example, neuromodulation therapies such as the transcranial alternating current stimulation could use these insights in order to optimize stimulation waveform parameters with the goal to normalize the cortical oscillations in patients with SD.
Supplementary Material
Highlights.
Inhibition of slow motor cortical rhythms during vocalization is reduced in spasmodic dysphonia
Somatosensory-motor cortical synchrony during vocalization is excessive in spasmodic dysphonia
Abnormal cortical processing in spasmodic dysphonia is mainly lateralized in the left hemisphere
Acknowledgments
We appreciate all participants for devoting their time to this study. Our gratitude is extended to the Center for Applied and Translational Sensory Sciences (CATSS), University of Minnesota, for providing the recording space and facilities. This research was supported by NIH 1R21DC011841 to PW and JK and by 1 R01 DC016315–01A1 to JK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflicts of interest, financial, or otherwise, are declared by the authors. All authors have approved the final article.
References
- Arce-McShane FI, Ross CF, Takahashi K, Sessle BJ, Hatsopoulos NG. Primary motor and sensory cortical areas communicate via spatiotemporally coordinated networks at multiple frequencies. Proc Natl Acad Sci USA 2016;113:5083–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN. Oscillatory interactions between sensorimotor cortex and the periphery. Curr. Opin. Neurobiol 2007;17:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Fuertinger S, Fleysher L, Ozelius LJ, & Simonyan K. Cortical sensorimotor alterations classify clinical phenotype and putative genotype of spasmodic dysphonia. Eur J Neurol, 2016;23(10):1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas, J. Neurosci, 2006;26:490:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- Berardelli A, Rothwell JC, Day BL, Marsden CD. Pathophysiology of blepharospasm and oromandibular dystonia. Brain. 1985;108:593–608. [DOI] [PubMed] [Google Scholar]
- Boersma P, & van Heuven V. Speak and unspeak with PRAAT. Glot International, 2001;5(9/10):341–347. [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nature Rev Neurosci 2008;9:222–234. [DOI] [PubMed] [Google Scholar]
- Brin MF, Blitzer A, Fahn S, Lovelace RE, & Gould W. Adductor laryngeal dystonia (spastic dysphonia): treatment with local injections of botulinum toxin (Botox). Mov Disord, 1989;4(4): 287–296. [DOI] [PubMed] [Google Scholar]
- Brinkman L, Stolk A, Dijkerman HC, de Lange FP, Toni I. Distinct roles for alpha- and beta-band oscillations during mental simulation of goal-directed actions. J Neurosci 2014;34:14783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth S, Francis S, Kelly E, McGlone F, Bowtell R, Sawle GV. Abnormal cortical sensory activation in dystonia: An fMRI study. Mov Disord 2003;18:673–82. [DOI] [PubMed] [Google Scholar]
- Castelon Konkiewitz E, Trender-Gerhard I, Kamm C, Warner T, Ben-Shlomo Y, Gasser T, et al. Service-based survey of dystonia in munich. Neuroepidemiol 2002;21:202–206. [DOI] [PubMed] [Google Scholar]
- Caviness JN, Liss JM, Adler C, Evidente V. Analysis of high-frequency electroencephalographic-electromyographic coherence elicited by speech and oral nonspeech tasks in Parkinson’s disease. J Speech Lang Hear Res 2006;49(2):424–38. [DOI] [PubMed] [Google Scholar]
- Chaumon M, Bishop DVM, Busch NA. A practical guide to the selection of independent components of the electroencephalogram for artifact correction. J Neurosci Methods. 2015;250:47–63. [DOI] [PubMed] [Google Scholar]
- Crowell AL, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Shimamoto S, Lim DA, et al. Oscillations in sensorimotor cortex in movement disorders: an electrocorticography study. Brain. 2012;135:615–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O, Kilner JM, & Friston KJ. Mechanisms of evoked and induced responses in MEG/EEG. NeuroImage, 2006;31(4):1580–1591. [DOI] [PubMed] [Google Scholar]
- de Felippe ACN, Grillo MHMM, & Grechi TH. Standardization of acoustic measures for normal voice patterns. Braz J Otorhinolaryngol, 2006;72(5):659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods, 2004;134:9–21. [DOI] [PubMed] [Google Scholar]
- Devous MD Sr, Pool KD, Finitzo T, Freeman FJ, Schaefer SD, Watson BC, et al. Evidence for cortical dysfunction in spasmodic dysphonia: regional cerebral blood flow and quantitative electrophysiology. Brain Lang, 1990;39:331–44. [DOI] [PubMed] [Google Scholar]
- Doesburg SM, Green JJ, McDonald JJ, Ward LM. From local inhibition to long-range integration: a functional dissociation of alpha-band synchronization across cortical scales in visuospatial attention. Brain Res, 2009;151(303):97–110. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top–down processing. Nat Rev Neurosci, 2001;2:704–716. [DOI] [PubMed] [Google Scholar]
- Giovannelli F, Marsili L, Suppa A, Di Stasio F, Rocchi L, Upadhyay N, et al. Functional connectivity between cortical speech network and primary motor cortex is abnormal in spasmodic dysphonia. Clin Neurophysiol, 2015;126(1), e27. [Google Scholar]
- Haslinger B, Erhard P, Dresel C, Castrop F, Roettinger M, Ceballos-Baumann AO. “Silent event-related” fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology. 2005;65:1562–1569. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 2010;4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner TK, & Zhang Y Differential effects of hearing impairment and age on electrophysiological and behavioral measures of speech in noise. Hear Res, 2018;370:130–142. [DOI] [PubMed] [Google Scholar]
- Konczak J, Abbruzzese G. Focal dystonia in musicians: linking motor symptoms to somatosensory dysfunction. Front Hum Neurosci 2013;7:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konczak J, Aman JE, Chen Y-W, Li K-y, Watson PJ. Impaired limb proprioception in adults with spasmodic dysphonia. J Voice. 2015;29:777.e17-.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Witkovsky P, & Rice ME. Regulation of substantia nigra pars reticulata GABAergic neuron activity by H₂O₂ via flufenamic acid-sensitive channels and K(ATP) channels. Front Syst Neurosci, 2011;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL. Treatment for spasmodic dysphonia: limitations of current approaches. Curr Opin Otolaryngol Head Neck Surg 2009;17:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL. Spasmodic dysphonia: a laryngeal control disorder specific to speech. J Neurosci 2011;31:793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL, Yamashita T, Schulz GM, Deleyiannis FWB. Abnormalities in long latency responses to superior laryngeal nerve stimulation in adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol 1995;104:928–935. [DOI] [PubMed] [Google Scholar]
- Maguire MJ, Abel AD. What changes in neural oscillations can reveal about developmental cognitive neuroscience: language development as a case in point. Dev Cogn Neurosci, 2013;6:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol, 1993;86(4):283–293. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, de Hemptinne C, Qasim S, Ostrem JL, Starr PA. Patterns of cortical synchronization in isolated dystonia compared with Parkinson disease. JAMA neurol 2015;72:1244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor N, Simonyan K, & Blitzer A. Central voice production and pathophysiology of spasmodic dysphonia. Laryngoscope, 2017;128(1):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M, Zich C, Stagg CJ, Motor cortical gamma oscillations: what have we learnt and where are we headed? Curr Behav Neurosc Rep, 2018;5:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, & Palva JM. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front Psychol, 2011; 2: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Palva JM, Shtyrov Y, Kujala T, Ilmoniemi RJ, Kaila K, Näätänen R. Distinct gamma-band evoked responses to speech and non-speech sounds in humans. J Neurosci 2002; 15:22(4):RC211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, & Andrew C. Event-related changes of band power and coherence: methodology and interpretation. J Clin Neurophysiol, 1999;16(6):512–519. [DOI] [PubMed] [Google Scholar]
- Putzel GG, Battistella G, Rumbach AF, Ozelius LJ, Sabuncu MR, & Simonyan K. Polygenic risk of spasmodic dysphonia is associated with vulnerable sensorimotor connectivity. Cereb Cortex, 2018;28(1):158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, Siebner HR, Rothwell JC. Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci 2006;29:192–9. [DOI] [PubMed] [Google Scholar]
- Riehle A, Wirtssohn S, Gruen S, & Brochier T. Mapping the spatio-temporal structure of motor cortical LFP and spiking activities during reach-to-grasp movements. Front Neural Circuits, 2013; 7,48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samargia S, Schmidt R, Kimberley TJ. Shortened cortical silent period in adductor spasmodic dysphonia: Evidence for widespread cortical excitability. Neurosci Lett 2014;560:12–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santee JL, & Kohfeld DL. Auditory reaction time as a function of stimulus intensity, frequency, and rise time. Bull Psychon Soc, 1977;10(5):393–396. [Google Scholar]
- Simonyan K, & Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex 2010a;20:2749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Ludlow CL, Vortmeyer AO. Brainstem pathology in spasmodic dysphonia. Laryngoscope. 2010b;120:121–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, et al. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131:447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro C, Deuschl G, Hallet M. Movement-related electroencephalographic desynchronization in patients with hand cramps: Evidence for motor cortical involvement in focal dystonia. Ann Neurol 2000;47:456–61. [PubMed] [Google Scholar]
- van Wijk BCM, Willemse RB, Peter Vandertop W, Daffertshofer A. Slowing of M1 oscillations in brain tumor patients in resting state and during movement. Clin. Neurophysiol 2012;123:2212–9. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J, The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci, 2001;2:229–239. [DOI] [PubMed] [Google Scholar]
- Woodson G (2010) Spasmodic dysphonia and muscle tension dysphonia In: Laryngeal Evaluation. Thieme. [Google Scholar]
- Yumoto E, Gould W, & Baer T. Harmonics-to-noise ratio as an index of the degree of hoarseness. J Acous Soc of Am, 1982;71(6):1544–1549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


