Summary
The phytopathogen Erwinia carotovora carotovora (Ecc) has been used successfully to decipher some of the mechanisms that regulate the interactions between Drosophila melanogaster and bacteria, mostly following forced association between the two species. How do Drosophila normally perceive and respond to the presence of Ecc is unknown. Using a fly feeding two-choice assay and video tracking, we show that Drosophila are first attracted but then repulsed by an Ecc-contaminated solution. The initial attractive phase is dependent on the olfactory Gr63a and Gαq proteins, whereas the second repulsive phase requires a functional gustatory system. Genetic manipulations and calcium imaging indicate that bitter neurons and gustatory receptors Gr66a and Gr33a are needed for the aversive phase and that the neuropeptide leukokinin is also involved. We also demonstrate that these behaviors are independent of the NF-κB cascade that controls some of the immune, metabolic, and behavioral responses to bacteria.
Subject Areas: Neuroscience, Behavioral Neuroscience, Sensory Neuroscience
Graphical Abstract
Highlights
-
•
Starved flies are first attracted and then repulsed by Erwinia-contaminated solution
-
•
Flies' attraction to Erwinia is mediated by the olfactory receptor Gr63a and Gαq
-
•
Flies repulsion' to Erwinia requires Gr66a neurons and the leukokinin neuropeptide
-
•
Feeding on Erwinia-contaminated solution potentiates bitter neurons of starved flies
Neuroscience; Behavioral Neuroscience; Sensory Neuroscience
Introduction
In nature, animals, including insects, thrive in an environment with constant exposure to microorganisms. Over the evolution times, insects have established various types of associations with microorganisms that may be classified as mutualistic, commensalistic, saprophytic, or parasitic (Douglas, 2014). Besides, some insects are major vectors of plant and animal diseases, spreading microorganisms throughout animal and plant kingdoms. In all these cases, insects must sample their contaminated environment to identify the type of microorganisms in which they are in contact and to engage in adapted behaviors. As for other biological processes that need to be molecularly characterized, identification of the players and the mechanisms that insects use to perceive microorganisms and to react adequately is greatly facilitated by the use of model organisms. Over the last 30 years, Drosophila has emerged as a very powerful model for modeling the interactions between bacteria and insects (Buchon et al., 2014) (Bergman et al., 2017, Capo et al., 2016, Lee and Lee, 2014, Lestradet et al., 2014). These studies, which allowed the discovery of fundamental immune mechanisms regulating invertebrate-bacteria interactions, relied on a relatively small number of bacteria species, including Escherichia coli (E. coli), Pseudomonas entomophila, Serratia marcescens, and Erwinia carotovora carotovora (Ecc) (Acosta Muniz et al., 2007, Basset et al., 2000, Bosco-Drayon et al., 2012, Buchon et al., 2009, Haller et al., 2014, Nehme et al., 2007, Quevillon-Cheruel et al., 2009, Royet et al., 2011, Vodovar et al., 2005). The present study focuses on one of them, Ecc also named Pectinobacterium carotovorum. Naturally transmitted by insects, this phytopathogenic organism that causes soft rot in fruits has been intensively used to decipher the mechanisms that control host-pathogen interactions in the Drosophila model (Buchon et al., 2014). When used to orally infect Drosophila larvae or adults, Ecc is not only able to trigger a local immune response in the gut epithelium but also a systemic activation of one the NF-κB signaling cascade, named IMD, in immunocompetent organs bathing in the hemolymph (Basset et al., 2000, Vodovar et al., 2005). This characteristic, which reflects the ability of gut-born Ecc-derived peptidoglycan to cross the gut epithelium and reach the hemolymph, has been used to study the impact that gut microbiota can exert on the host (Charroux et al., 2018, Kurz et al., 2017). Indeed, detection of gut-derived PeptidoGlycaN (PGN) by receptors belonging to the peptidoglycan recognition protein family (PGRP) has a very strong influence on many aspects of host metabolism and also on fly behavior (Royet et al., 2011). However, in all previous studies, wherein the impact of Ecc on Drosophila larvae or adult has been tested, animals are orally infected following forced oral association with Ecc for few to many hours. This protocol, which is non-natural, could induce biases in the interpretation of the obtained results. We therefore decided to analyze the behavior of D. melanogaster adults confronted with food sources contaminated by Ecc. To do so, we developed a two-choice feeding assay where flies have the possibility to feed either on an axenic solution or on Ecc-contaminated media. Using time-lapse video recording, we show that D. melanogaster adult flies display a robust stereotyped behavior. They are first attracted by the bacterial solution that they ingest, before showing, after a long period only, a strong aversion toward Ecc and deciding to move away to feed on the axenic solution. Our results show that if olfactory cues and, more specifically, the Gr63a receptor participate in the initial attraction phase, the subsequent aversion phase relies on gustatory neurons expressing Gr66a receptors. We also show that aversion to Ecc requires the Gr66a and Gr33a bitter receptors as well as the neuropeptide leukokinin (Lk) but does not involve the chemosensory cation channel and lipopolysaccharide (LPS) receptor TrpA1 or the IMD innate immune pathway.
Results
Using Time-Lapse Video Recording to Study Fly Feeding Choice
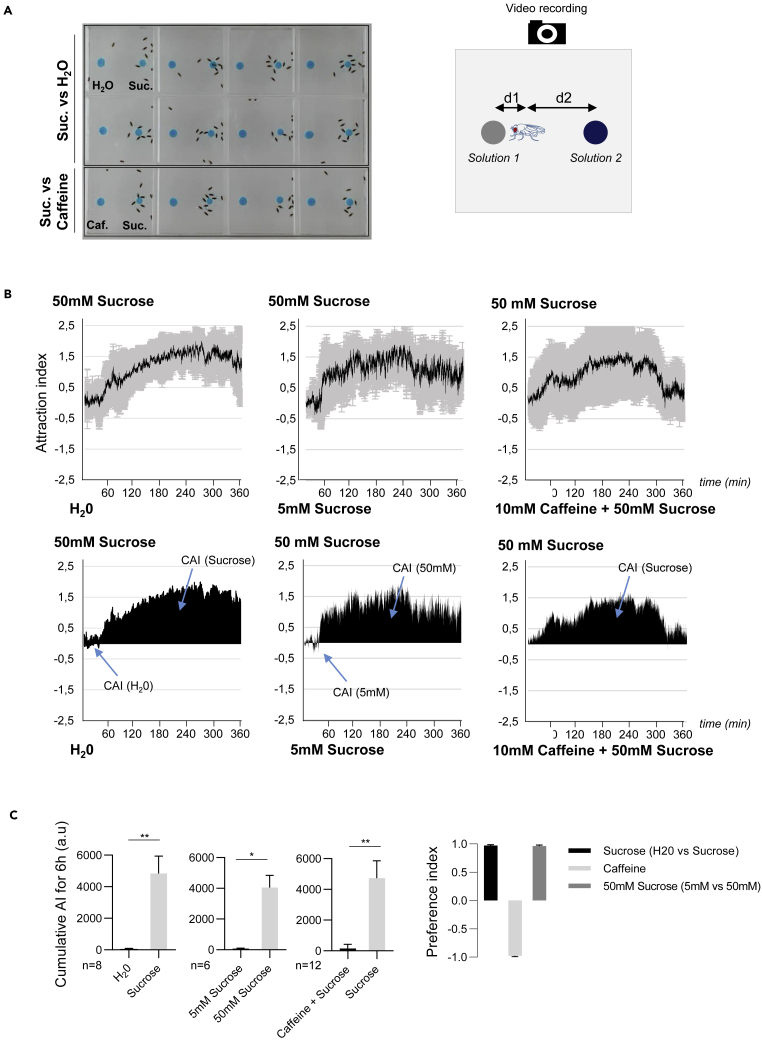
To investigate the impact that a contamination by Ecc has on adult fly feeding behavior, we developed an experimental setup to video record flies' movements during a two-choice feeding experiment. This apparatus can record in live the feeding behavior of multiple flies in arenas simultaneously (See Methods). Experiments were performed with 10 or 20 females per arena that were starved overnight. Before adding flies into the arenas, two drops of feeding solution were positioned at a precise distance from each side of the arena (Figure 1A). To track flies' movements, we mounted a webcam on top of the multi-arena apparatus. One image was acquired every 5 s during a period of 6 h, and the movies were created by combining 10 images per second.
Figure 1.
Drosophila Females Were Attracted by Sucrose and Avoid Caffeine
(A) (Left) Still frame taken during the video recording (t = 240 min) of an experiment with 12 arenas, each containing 10 females and two drops of feeding solution (in blue). The top 8 arenas contain one drop of H2O (left) and one drop of 50 mM sucrose (right). The bottom 4 arenas contain one drop of 50 mM sucrose + 10 mM caffeine (left) and one drop of 50 mM sucrose (right). (Right) The drawing illustrates the 2 distances (d1 and d2) measured at every time frame of the video (see main text).
(B) Flies displayed a strong preference for sucrose and aversion to caffeine + sucrose. (Top) Per graphs: Kinetics of the attraction index (AI) for sucrose in a sucrose versus H2O experiment (left), in a 50 mM sucrose versus 5 mM sucrose experiment (middle), or in a sucrose versus sucrose + caffeine experiment (right). The black lines and the gray zones correspond, respectively, to the mean and the standard deviation. (Bottom) Cumulative attraction index (CAI) area for each specified solution (arrows) and its distribution over time. For simplicity, only the mean value of the CAI obtained with multiple replicates is shown in black.
(C) Histograms built with the CAI values from (B) showing that flies have a strong and statistically significant preference for sucrose versus H2O and for 50 mM sucrose versus 5 mM sucrose and a strong aversion for a mixture of caffeine + sucrose versus sucrose only. ∗p < 0.05 and ∗∗p < 0.01. Wilcoxon matched-pairs signed rank test, Two-tailed p value. Error bars correspond to standard deviation. The preference indexes for sucrose, for 50 mM sucrose, and for caffeine are calculated with the CAI values from C. n indicates the number of independent experimental replicates. a.u.: arbitrary unit. Data are represented as mean ± SD.
We started with the assumption that flies attracted by a feeding solution should spend more time close to it than to the other solution. Such a behavior was observed when flies were given the choice between a drop of 50 mM sucrose and a drop of water (Figure 1A and Video S1). Most of the time (from roughly t = 1 h until t = 6 h), the flies stayed in close vicinity to the sucrose solution. As a consequence of fly feeding, the size of the drop progressively diminished (Video S1). We analyzed and quantified this behavior over time by calculating an attraction index (AI) for each time frame as follows. The distance of each of the 10 females from droplet 1 (d1) and droplet 2 (d2) was measured every 5 s, and the AI was calculated as the log2 ratio of the average of distances d1 divided by the average of distances d2. The d2 attraction will be translated into a positive index, and the d1, by a negative one. We then calculated a cumulative AI (CAI) corresponding to the area between the AI curve and the abscissa axis for x = 0, which represents the absolute preference of the flies for each of the two feeding solutions. We then could calculate the preference index (PI) for the solution 1 as follows: PI (solution 1) = (CAI solution 1)-(CAI solution 2)/(CAI solution 1)+(CAI solution 2). An example of such an analysis is shown in Figures 1A–1C, where flies were given the choice between water (solution 1) and a 50 mM sucrose solution (solution 2). The AI calculated for eight experimental replicates showed that during the first 45 min, flies were not preferentially positioned close to any of the two solutions. From then on, the flies got closer to the sucrose solution until the end of the movie (Video S1). Hence, once the choice has been made, it stayed robust and was not alterable over time. The CAI for sucrose (>4,000 a.u.) and the PI for sucrose of 0.97 indicated that flies had a clear preference for sucrose over water, as we could have expected for a gustatory attractive substance. Note that during the 6 h movie, we observed a reduction in the size of the feeding solution drop chosen by the flies as well as a progressive appearance of small deposits around the flies corresponding very likely to feces (Video S1).
Video recording of an experiment with 12 arenas, each containing 10 wild-type females and two drops of feeding solution (in blue). The top 8 arenas contain one drop of H2O (right) and one drop of 50 mM sucrose (left). The bottom 4 arenas contain one drop of 50 mM sucrose + 10 mM caffeine (right) and one drop of 50 mM sucrose (left).
We then tested our device with flies that were given the choice between sucrose solutions, one at 5 mM and the other at 50 mM (Figures 1B and 1C). The AI curve displayed in Figure 1B showed that flies were more attracted by the highly concentrated sucrose solution. No obvious choice was made when the flies were given the choice between two equimolar sucrose solutions (Figures 1A and 1B). When the flies were given the choice between a sweet (50 mM sucrose) and a bitter solution (50 mM sucrose +10 mM caffeine) (Video S1) the aversion of the bitter solution started less than 30 min after the beginning of the experiment and lasted for the next five and a half hours. Once again, flies made a robust and permanent choice to avoid caffeine with a CAI for sucrose over 4,500 a.u. and a PI for caffeine of −0.98 (Figures 1B and 1C).
D. melanogaster Are First Attracted by Ecc but Then Repulsed by It
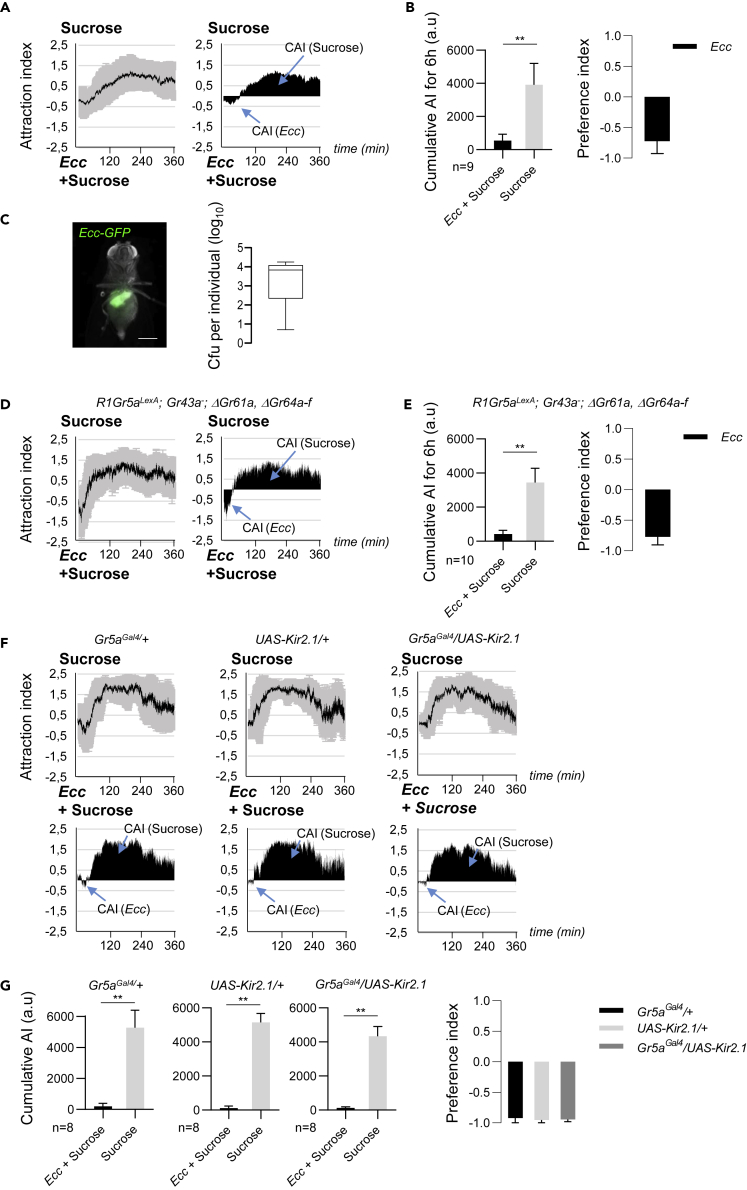
We next explored the behavior of adult females in the presence of two feeding solutions, one containing Ecc bacteria in 50 mM sucrose and the other 50 mM sucrose only. Collected data revealed that flies displayed a two-step stereotyped behavior. They were first attracted by the contaminated solution before moving away from it and staying close to the sucrose solution permanently (Video S2). The CAI for Ecc was lower (552 a.u. +/− 381 SD) than for sucrose (3,918 a.u. +/− 1291 SD), and the preference index for Ecc was negative (−0.72 ± 0.2 SEM) (Figures 2A and 2B). This indicated that flies displayed a global aversion toward Ecc. However, the video tracking helpfully revealed the presence of two distinct phases throughout the experiment. Flies were first attracted by the Ecc solution (for approximately 60 min), whereas they were later preferentially found in the proximity to the sucrose solution. Identical results were obtained when positions of sucrose and Ecc solutions were randomized showing that there was no directional bias (Figures S1C and S1D). A similar biphasic curve was obtained using males, indicative of an absence of sex-biased comportment of D. melanogaster toward Ecc (Figures 1E and 1F).
Figure 2.
D. melanogaster Adults Were First Attracted by Ecc before Being Repelled by It
(A and B) Adult females displayed a two-step behavior when given the choice between an Ecc-contaminated sucrose solution and a sucrose-only solution. (A) (Left) Kinetics of the AI for sucrose in a sucrose versus sucrose + Ecc experiment. (Right) CAI area for each specified solution (arrows) and its distribution over time. (B) (Left) histograms built with the CAI values from A. ∗∗p value < 0.01. Wilcoxon matched-pairs signed rank test, two-tailed p value. Error bars correspond to standard deviation. The preference index for Ecc is calculated with the CAI values from (B).
(C) Flies ingested bacteria during the initial phase. Picture: ventral view of an adult female sampled at t = 90 min and showing Ecc-GFP bacteria accumulating in the digestive tract. Scale bar, 0.5 mm. Graph: Boxplot of colony-forming unit (CFU) analysis of individual flies sampled at t = 90 min.
(D and E) Sugar-blind flies of the R1Gr5aLexa; Gr43a-; ΔGr61a, ΔGr64a-f genotype displayed a two-step behavior. (D) (Left) Kinetics of the AI for sucrose in a sucrose versus sucrose + Ecc experiment. (Right) CAI area for each specified solution (arrows) and its distribution over time.
(E) Histograms built with the CAI values from (D). ∗∗p value < 0.01. Wilcoxon matched-pairs signed rank test, Two-tailed p value. Error bars correspond to standard deviation. The preference index for Ecc is calculated with the CAI values from (D).
(F and G) Adult females expressing UAS-Kir2.1 in sweet neurons using Gr5aGal4 displayed aversion to Ecc. (F) (Top) Kinetics of the AI for sucrose. (Bottom) CAI area for each specified solution (arrows) and its distribution over time. (G) Histograms built with the CAI values from (F). ∗∗p value < 0.01. Wilcoxon matched-pairs signed rank test, Two-tailed p value. Error bars correspond to standard deviation. The preference indexes for Ecc are calculated with the CAI from (F). For (A and D) left graphs and (F) top graphs, the black lines and the gray lines correspond, respectively, to the mean and the standard deviation, and for right graphs in (A and D) and bottom graphs in (F), solely the mean value of the CAI obtained with multiple replicates is shown in black. n indicates the number of experimental replicates. a.u.: arbitrary unit. Data are represented as mean ± SD.
Video recording of an experiment with 6 arenas, each containing around 15 females and two drops of feeding solution (in blue). Each arena contains one drop of a mixture of Ecc + 50 mM sucrose (left) and one drop of 50 mM sucrose (right). The top 3 arenas contain wild-type CantonS flies and the bottom 3 arenas contain w1118 flies.
Flies' Attraction to Ecc Is Mediated by the Gr63a and Gαq
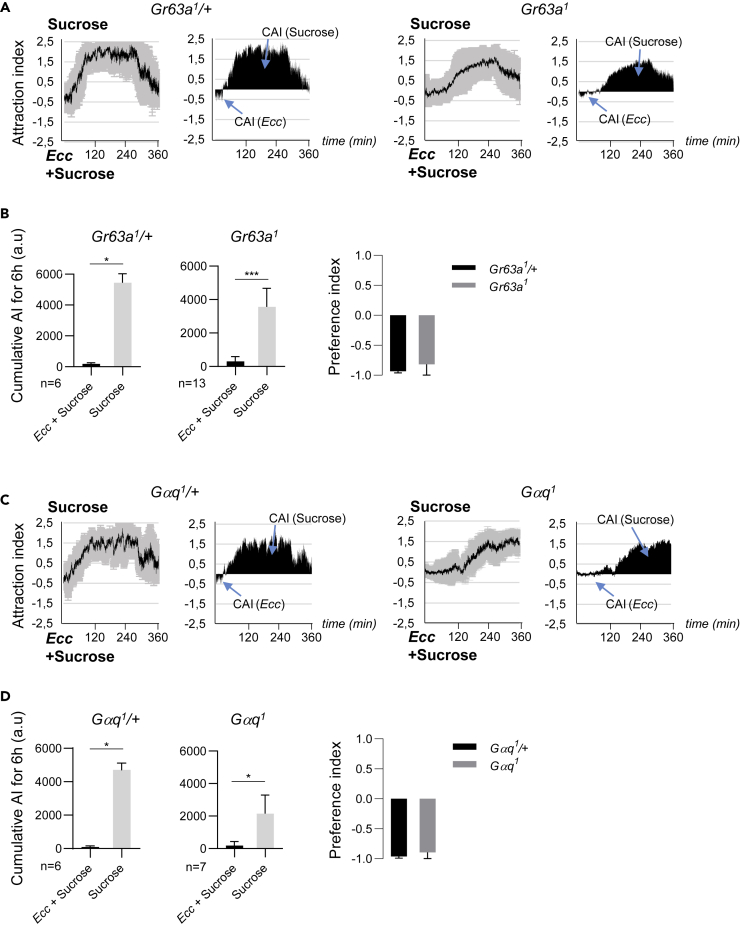
We then tested which of the sensory system(s) controls the initial attractive phase. As flies were first attracted by bacteria, we tested whether the gustatory sweet pathway, known to be involved in fly's attractiveness, was involved. For this purpose we took advantage of a “sugar-blind” strain in which all nine sugar gustatory receptor genes are deleted (Yavuz et al., 2014). R1Gr5aLexA; Gr43a-; ΔGr61a,ΔGr64a-f flies showed the same behavior as control flies demonstrating that the sweet pathway is not involved in the initial attraction phase to Ecc (Figures 2D and 2E). This result was confirmed using Gr5a-Gal4; UAS-Kir2.1 flies in which the sweet neurons are inactivated (Figures 2F and 2G). From previous studies, we hypothesized that flies could be attracted by the odors emanating from the bacterial solution (Kapsetaki et al., 2014, Stensmyr et al., 2012). To test this hypothesis, we analyzed the contributions of members of the odorant receptor (Or) gene family using flies lacking the obligate Or co-receptor Orco. Orco mutant flies behaved as wild-type controls when given the choice between sucrose and Ecc solutions (Figures S2A and S2B). We finally tested the putative implication of an inhibition of the CO2-sensing neurons in the attraction phase. Previous work has indeed shown that D. melanogaster can sense CO2 via a heterodimeric receptor composed of two members of the gustatory receptor family Gr21a and Gr63a (Jones et al., 2007, Kwon et al., 2007). Although CO2 is normally repulsive to adult flies, compounds such as polyamines are attractive by antagonizing this Gr63a/Gr21a-dependent CO2 repulsion (MacWilliam et al., 2018, Turner and Ray, 2009). The attractiveness of polyamines such as spermidine is therefore lost in Gr63a or Gr21a mutants. We found that whereas heterozygous Gr63a mutants behaved as wild-type flies, Gr63a homozygous mutants were no longer attracted by Ecc (Figures 3A and 3B). Interestingly, this lack of attractiveness for Ecc was associated with a delay in the establishment of the repulsive phase (see later). Similar phenotypes, including a loss of the initial attraction and a delay of the subsequent repulsion, were observed using mutants for the G protein (Gαq) that transduces the GR63a/Gr21a receptor signal (Figures 3C and 3D) (Yao and Carlson, 2010). These results demonstrate that the attraction of flies toward Ecc is independent of the sweet gustatory pathway and of the Orco-mediated olfactory pathways but requires GR63a and Gαq. It remains to be demonstrated whether Gr63a and Gαq are acting in the same cells and therefore in a linear pathway.
Figure 3.
Flies Attraction to Ecc Is Mediated by Gr63a and Gαq
(A and B) Flies homozygous for the loss-of-function allele Gr63a1 displayed no attraction to Ecc, and a delayed aversion to the bacteria, whereas control Gr63a1/+ behaved normally. (A) (Left graphs) Kinetics of the AI for sucrose. (Right graphs) CAI area for each specified solution (arrows) and its distribution over time. (B) Histograms built with the CAI values from (D). ∗p < 0.05 and ∗∗∗p < 0.001. Wilcoxon matched-pairs signed rank test, two-tailed p value. Error bars correspond to standard deviation. The preference indexes for Ecc are calculated with the CAI values from (B).
(C and D) Flies homozygous for the hypomorphic allele Gαq1 displayed reduced attraction to Ecc, whereas control Gαq1/+ showed a usual one. (C) (Left) Kinetics of the AI for sucrose. (Right) CAI area for each specified solution (arrows) and its distribution over time. (D) (Right) Histograms built with the CAI values from (D). ∗p < 0.05. Wilcoxon matched-pairs signed rank test, two-tailed p value. Error bars correspond to standard deviation. The preference indexes for Ecc are calculated with the CAI values from (D). For (A and C) (left graphs) the black lines and the gray lines correspond, respectively, to the mean and the standard deviation, and (right graphs) solely the mean value of the CAI obtained with multiple replicates is shown in black. n indicates the number of experimental replicates. a.u.: arbitrary unit. Data are represented as mean ± SD.
Ecc Aversion Is Not due to Medium Modification
As the repulsive phase required a long time to be established, we wondered whether flies have ingested the bacteria-containing solution during the initial phase, before aversion is established. To address this question, we performed a two-choice feeding assay using Ecc-GFP fluorescent bacteria. After 90 minutes of having been deposited into the arenas, all the flies displayed a fluorescent crop, demonstrating they had indeed ingested bacteria (Figure 2C). When the same flies were plated for colony-forming unit (CFU) quantification, we found that all flies have eaten live bacteria (6.9 × 108 CFU/fly on average). In conclusion, Ecc ingestion preceded the fly's choice to move away and feed onto the bacterial free sucrose solution.
As aversion to Ecc is taking place after a 60- to 90-min latency period, we asked whether Ecc could metabolize the attractive sucrose solution and transform it into an aversive one via, for example, the release of aversive metabolic by-products. To test this hypothesis, we measured the attraction of Ecc bacteria pre-incubated 2 h with sucrose before being deposited into the arena. If indeed an incubation period of Ecc with sucrose was required to transform it from an attractant to an aversive solution, one might expect the pre-incubated solution to be aversive immediately without any latency. The attractive phase was not only still present when flies were put in the presence of a pre-incubated medium but also lasted longer than with the non-pre-incubated solution (Figures S2C and 2D). The aversion was therefore neither due to an Ecc-mediated sucrose modification nor due to an alteration of Ecc when put in the presence of sucrose.
D. melanogaster Behavior toward Ecc Is Independent of the IMD/NF-κB Pathway and LPS
In D. melanogaster, DiaminoPimelic Acid (DAP)-type peptidoglycan containing bacteria, such as Ecc, are sensed by pattern recognition receptors belonging to the PGRP family (Royet et al., 2011). Direct recognition of bacterial cell wall-derived PGN by either membrane-associated (PGRP-LC) or cytosolic (PGRP-LE) proteins activates the IMD signaling pathway (Charroux et al., 2018). This leads to the nuclear translocation of the NF-κB transcription factor Relish, a step required for the transcriptional activation of a set of immune effectors and regulators (Kleino and Silverman, 2014). For some bacteria such as Ecc, gut-born peptidoglycan can cross the gut epithelium and reach the circulating hemolymph where it gets in contact with remote tissues and organs in which it activates immune signaling (Basset et al., 2000, Bosco-Drayon et al., 2012). As bacteria can be found in the gut lumen within 1 h of the experiment, and because PGN can affect host signaling in a short period, we asked whether internal sensing of PGN was a required step to mediate the delayed aversive behavior.
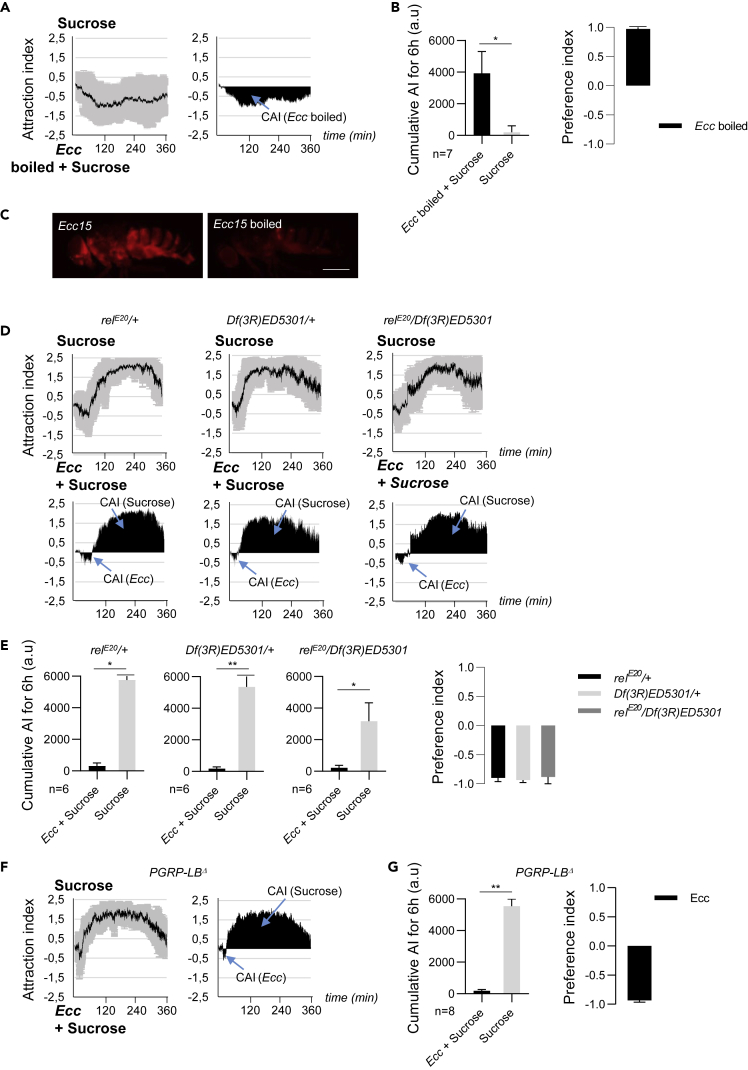
We addressed this issue in two ways. First, we performed an experiment using heat-killed Ecc bacteria that no longer activated the gut local and the fat body systemic NF-κB responses (Basset et al., 2000 and Figure 4C). Although flies remained attracted to heat-killed bacteria, they no longer escaped from them and even fed on them during the 6 h that the experiment lasted (Figures 4A and 4B). This result indicated that either the IMD pathway activation is required to establish the aversive behavior, but not the attraction, and/or that the putative bitter substance(s) produced by Ecc is (are) heat sensitive. To directly test the implication of the NF-κΒ activation in establishing the aversion toward Ecc, we performed the experiment using live Ecc and flies null mutant for the Relish transactivator. The results indicated that relishE20 mutant flies behaved as controls, showing the stereotyped two-phase compartment with an initial attraction to Ecc followed by a constant aversion to it (Figures 4D and 4E). Finally, to exclude the possibility that bacterial PGN affects the flies' feeding behavior independently of IMD signaling activation, we examined the feeding behavior of fly mutant for the amidase PGRP-LB, an enzyme that cleaves PGN fragments into non-immunogenic neuropeptides (Zaidman-Remy et al., 2006). We found no substantial differences between wild-type and flies mutant for the PGRP-LBΔ null allele in our experimental setup (Figures 4F and 4G). Altogether, these results suggested that neither bacterial PGN nor the NF-κB signaling contributes to the feeding choice regarding Ecc and that bacteria have to be alive to be aversive for D. melanogaster. As previous work has reported a gustatory-mediated avoidance of bacterial LPS via the TrpA1 channel, we asked whether LPS could also mediate the second aversive phase that we observed with Ecc (Soldano et al., 2016). Two results let us believe that LPS is not the aversive molecule that repulses flies when in contact with Ecc. First, as mentioned in previous sections, E. coli whose cell wall is also composed of LPS was not repulsive for flies. Second, we found that flies mutant for TrpA1 showed the same biphasic behavior toward Ecc that controls flies that carry a functional TrpA1 receptor (Figures S2E and S2F).
Figure 4.
Ecc Aversion Does Not Require the IMD/NF-κB Signaling Pathway
(A and B) Heat-killed Ecc is no longer aversive to adult females. (A) (Top) Kinetics of the AI for sucrose when flies were given the choice between a heat-killed Ecc + sucrose versus sucrose-only solution. (Bottom) CAI area for each specified solution (arrows) and its distribution over time. (B) (Left): Histograms built with the CAI values from (A). ∗p < 0.05. Wilcoxon matched-pairs signed rank test, two-tailed p value. Error bars correspond to standard deviation. The reference index for boiled Ecc calculated with the CAI values from (A).
(C) PGRP-LB, Diptericin-Cherry flies fed for 24 h with either fresh (left) or boiled Ecc (right). Scale bar, 0.5 mm.
(D and E) Relish mutant flies behaved like controls. (D) (Top) Kinetics of the AI for sucrose when relE20/Df(3R)ED5301 flies and control relE20/+ and Df(3R)ED5301/+ flies were given the choice between a Ecc + sucrose solution versus sucrose only. (Bottom) CAI area for each of the specified solution (arrows) and its distribution over time. (E) Histograms built with the CAI values from (D). ∗p < 0.05 and ∗∗p < 0.01. Wilcoxon matched-pairs signed rank test, two-tailed p value. Error bars correspond to standard deviation. The preference index for Ecc is calculated with the CAI values from (D).
(F and G) Flies mutant for the amidase PGRP-LB displayed a normal two-step behavior regarding Ecc. (F) (Right) Kinetics of the AI for sucrose when PGRP-LBΔ flies were given the choice between an Ecc + sucrose solution versus sucrose only. (Left) CAI area for each specified solution (arrows) and its distribution over time. (G) Histograms built with the CAI values from (F). ∗∗p < 0.01. Wilcoxon matched-pairs signed rank test, two-tailed p value. Error bars correspond to standard deviation. The preference index for Ecc is calculated with the CAI values from (F). For (A and F) left graphs and (D) top graphs, the black lines and the gray lines correspond, respectively, to the mean and the standard deviation, and for (A and D) right graphs and (C) bottom graphs, solely the mean value of the CAI obtained with multiple replicates is shown in black. n indicates the number of experimental replicates. a.u.: arbitrary unit. Data are represented as mean ± SD.
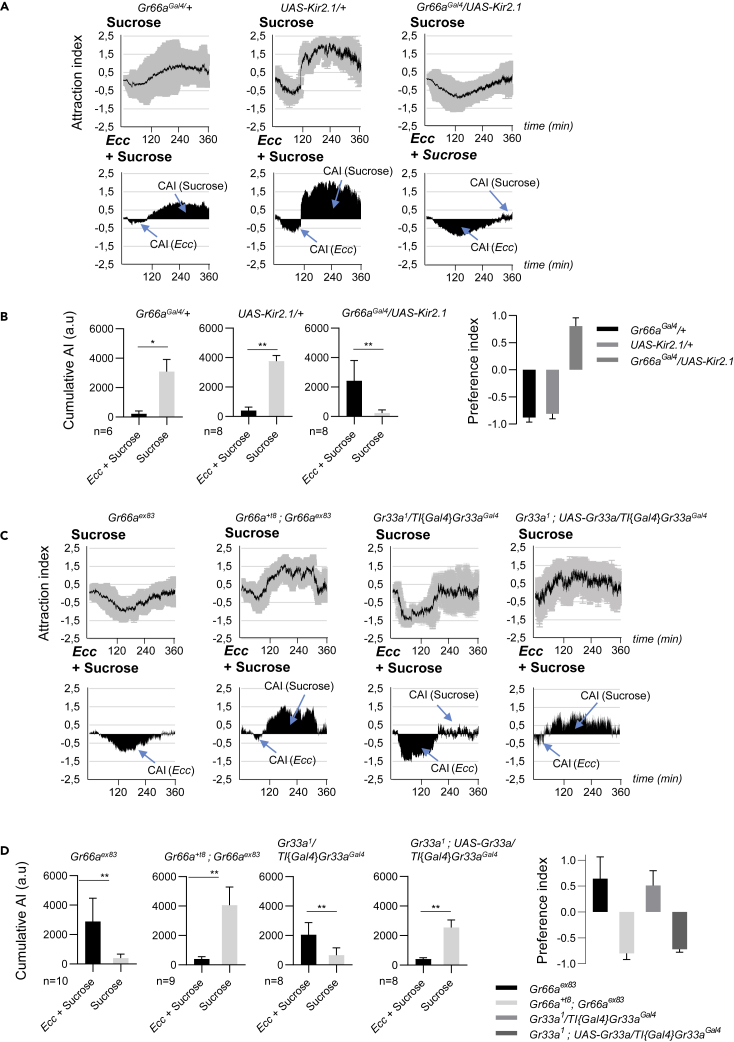
Bitter Neurons and Bitter Gustatory Receptors Gr66a and Gr33a Are Required for the Aversion to Ecc
In D. melanogaster, detection of non-volatile repellents is mediated by dedicated gustatory receptors such as Gr66a, Gr33a, or Gr32a, expressed by a set of gustatory receptor neurons (Scott, 2018). We found that inactivation of bitter Gr66a neurons by expressing Kir2.1, an inwardly rectifying K+ channel, abolished the repugnance to Ecc without affecting the initial attractive phase (Figures 5A and 5B) (Shim et al., 2015). Flies expressing UAS-Kir2.1 under the control of Gr66aGal4 did not show any aversion to Ecc but were instead constantly feeding on the bacteria-containing solution until there was no more bacterial solution to feed on (Figures 5A and 5B). Furthermore, we found that the gustatory receptors Gr66a and Gr33a were both required for the aversion to Ecc (Moon et al., 2009; Video S3). Flies mutant for any of the two receptors displayed abnormal behavior, as they stayed close to the Ecc drop during most of the time with a high cumulative AI for Ecc (Figures 5C and 5D).
Figure 5.
Bitter Neurons and Bitter Gustatory Receptors Gr66a and Gr33a Are Required for the Aversion to Ecc
(A and B) Adult females expressing UAS-Kir2.1 in bitter neurons using Gr66aGal4 displayed no aversion to Ecc, whereas control UAS-Kir2.1/+ or Gr66Gal4/+ did. (A) (Top) Kinetics of the AI for sucrose. (Bottom) CAI area for each specified solution (arrows) and its distribution over time. (B) Histograms built with the CAI values from (A). ∗p < 0.05 and ∗∗p < 0.01. Wilcoxon matched-pairs signed rank test, two-tailed p value. Error bars correspond to standard deviation. The preference index for Ecc is calculated with the CAI from (B).
(C and D) The gustatory receptors Gr66a and Gr33a are required for the aversion to Ecc. Gr66ex83 or Gr33a1/TI{Gal4}Gr33aGal4 mutant flies, but not rescue Gr66+t8; Gr66ex83 or Gr33a1, UAS-Gr33a/TI{Gal4}Gr33aGal4 flies, displayed attraction but no aversion to Ecc when given the choice between an Ecc-contaminated sucrose solution versus sucrose only. (C) (Top) Kinetics of the AI for sucrose. (Bottom) CAI area for each specified solution (arrows) and its distribution over time. (D) Histograms built with the CAI values from (C). ∗∗p < 0.01. Wilcoxon matched-pairs signed rank test, two-tailed p value. Error bars correspond to standard deviation. The preference indexes for Ecc are calculated with the CAI values from (E). For (A and C) (top), the black lines and the gray lines correspond, respectively, to the mean and the standard deviation, and for bottom graphs, solely the mean value of the CAI obtained with multiple replicates is shown in black. n indicates the number of experimental replicates. a.u.: arbitrary unit. Data are represented as mean ± SD.
Video recording of an experiment with 6 arenas, each containing 20 females and two drops of feeding solution (in blue). Each arena contains one drop of a mixture of Ecc + 50 mM sucrose (left) and one drop of 50 mM sucrose (right). The top 3 arenas contain flies mutant for the loss-of-function allele Gr66aex83 and the bottom 3 arenas contain flies of genotype Gr66a+t8 ; Gr66aex83, which are rescued for the Gr66aex83 mutant allele.
Having shown that Gr66a-expressing neurons are functionally required for Ecc avoidance, we wondered whether this effect was mediated by direct activation of these neurons by bacteria. To do so, we took advantage of the Ca-LexA (calcium-dependent nuclear import of LexA) technique that indirectly assesses Ca+ release in neurons that express the mLexA-VP16-NFAT fusion protein (Masuyama et al., 2012). When LexAop-CD8-GFP-2A-CD8-GFP; UAS-mLexA-VP16-NFAT, lexAop-rCD2-GFP/Gr66aGal4 flies were fed 4 days with an Ecc-contaminated solution, activation of the Ca-LexA reporter was detected in the sub-esophageal zone (SEZ) of the central brain, where peripheral nervous system (PNS) bitter neuron projections are found (Figure S3A). Flies fed with E. coli, boiled Ecc, or sucrose only showed weak or no Ca-LexA activation in that brain region (Figure S3A). No Ca-LexA signal was observed in LkGal4, LexAop-CD8-GFP-2A-CD8-GFP; UAS-mLexA-VP16-NFAT, lexAop-rCD2-GFP Ecc-fed flies, showing the specificity of Ca-LexA results (Figure S3B). Altogether, these experiments indicated that flies are able to sense the presence of Ecc via bitter gustatory neurons. They also showed that these neurons are necessary to trigger an avoidance behavior toward these bacteria.
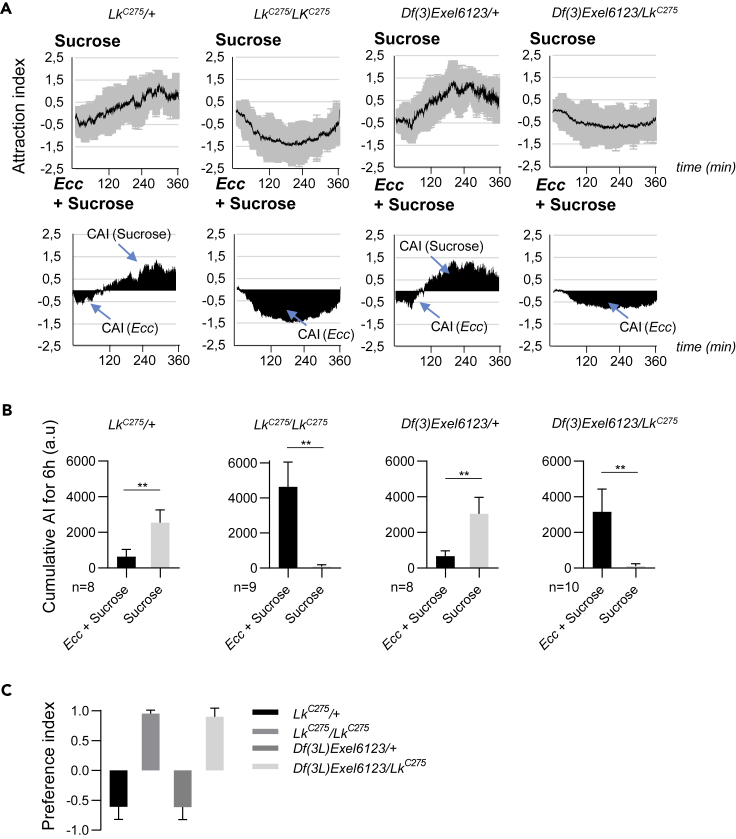
The Neuropeptide Leukokinin Is Required for the Aversive Perception of Ecc
Given that Ecc avoidance by adult flies was not immediate and occurred only after flies had ingested the bacteria-contaminated solution, we wondered which mechanism could contribute to this delayed Ecc-induced behavior. Neuropeptides, which are known to influence neuronal activities at a relatively low timescale (seconds to hours) compared with neurotransmitters (milliseconds), are good candidates to mediate the switch from attraction to aversion. Lk has been shown to modify the feeding behavior toward sucrose from attraction to aversion in the mosquito (Kwon et al., 2016). We, therefore, asked whether D. melanogaster Lk could be involved in the switch of fly behavior from attraction to repulsion when in contact with Ecc. Flies homozygotes for the hypomorphic allele LkC275 or transheterozygotes LkC275/Df(3L)Exel6123 were no longer avoiding the feeding solution contaminated by Ecc (Figures 6A–6C and Video S4), whereas control flies still did. This aberrant behavior of LkC275 mutant flies was rescued by expressing UAS-Lk under the control of the LkGal4 driver (Figures S4A–S4C). These results demonstrated that the production of the neuropeptide Lk, by LkGal4-expressing cells, is required for optimal avoidance of Ecc.
Figure 6.
The Neuropeptide Leukokinin Is Required for the Aversive Perception of Ecc
(A and B) Flies homozygous for the hypomorphic allele LkC275 or transheterozygotes LkC275/Df(3L) Exel6123 no longer avoided the feeding solution contaminated by Ecc, whereas control flies (LkC275/+ and Df(3L) Exel6123/+) did, when females were given the choice between an Ecc-contaminated sucrose solution versus sucrose only. (A) (Top) Kinetics of the AI for sucrose. (Bottom) CAI area for each specified solution (arrows) and its distribution over time. (B) Histograms built with the CAI values from (A). ∗∗p < 0.01. Wilcoxon matched-pairs signed rank test, two-tailed p value. Error bars correspond to standard deviation.
(C) Preference indexes for Ecc calculated with the CAI from (B). For (A) top graphs, the black lines and the gray lines correspond, respectively, to the mean and the standard deviation, and for bottom graphs, solely the mean value of the CAI obtained with multiple replicates is shown in black. n indicates the number of experimental replicates. a.u.: arbitrary unit. Data are represented as mean ± SD.
Video recording of an experiment with 6 arenas, each containing around 15 females and two drops of feeding solution (in blue). Each arena contains one drop of a mixture of Ecc + 50 mM sucrose (left) and one drop of 50 mM sucrose (right). The top 2 arenas (starting from the left) contain transheterozygous mutant flies LKC275/Df(3)Exel6123. The last top right arena and the first bottom arena (starting from the left) contain control flies LKC275/+. The last 2 bottom arenas contain control flies Df(3)Exel6123/+.
We found that LkGal4 is expressed in the adult central nervous system (Figure S5A), with some cells sending projections to the SEZ where gustatory information relay might occur. Thus, because Gr66aGal4- and LkGal4-expressing cells were both required, although at a different level, for the Ecc gustatory repellent phenotype, we asked whether these two populations of cells share some common cells by focusing on the SEZ region of the central brain. Using an intersectional expression approach, we found that none of the Gr66aLexa-positive axons correspond to the LkGal4 ones (Figure S5B). However, we observed that the LkGal4 projections localized in the vicinity of axonal projections of bitter gustatory neurons labeled by Gr32aLexa (Figure S5A).
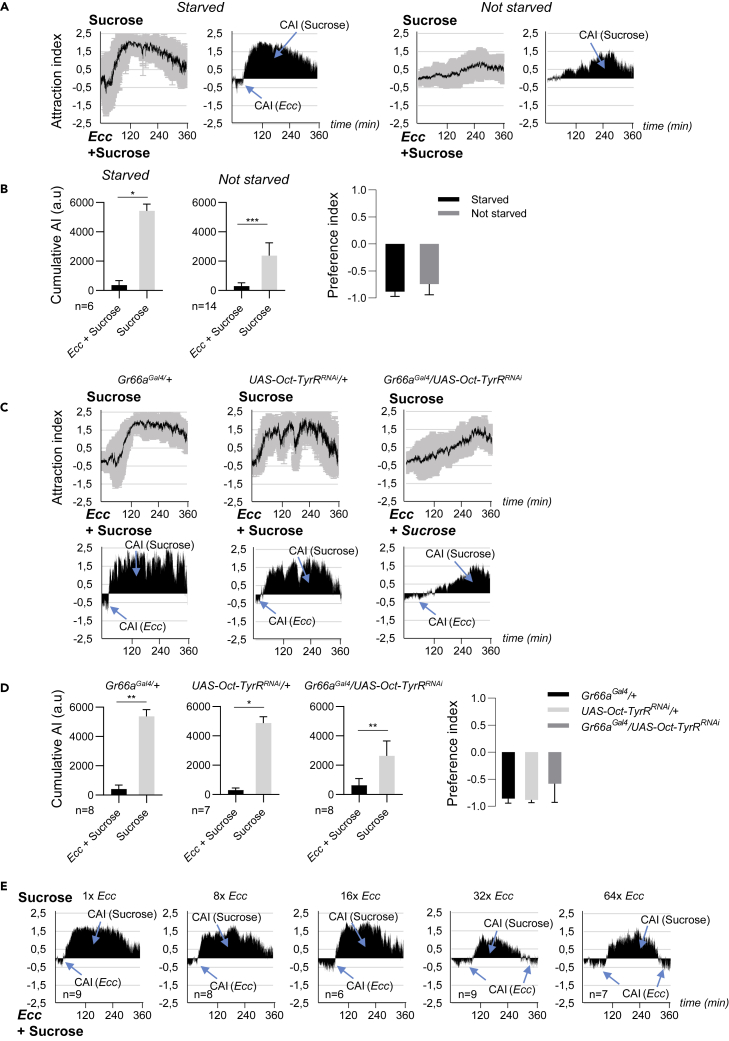
The Latency before Repulsion Requires Depotentiation of Bitter Taste
As for most of the tests used to analyze fly behavior upon feeding, our experimental paradigm requires that we used starved flies. However, nutrient deprivation can lead to dramatic changes in feeding behavior, including acceptance of foods that are normally rejected. Bitter substances are more acceptable, and sweet molecules less attractive for starved than for fed flies (Freeman and Dahanukar, 2015, LeDue et al., 2016). As we used starved flies in our experiments, we asked whether the attractive and/or the aversion phases were dependent on the fly feeding status. To appreciate the influence of starvation to the results of our test, we performed it with non-starved flies (Figures 7A and 7B). In this case, the attraction phase was completely lost and the repulsive phase was very slowly and progressively established. This behavioral shift could depend in part on reciprocal sensitization and desensitization of sweet and bitter tastes (Inagaki et al., 2014, LeDue et al., 2016). Good candidates to mediate this effect are the neuropeptides NeuroPeptide F (NPF) that control reciprocal changes in sweet and bitter sensitivity during starvation. dNPF+ neurons promote increased sugar sensitivity, whereas sNPF neurons promote decreased bitter sensitivity (Inagaki et al., 2014). However, as sNPF mutant flies behaved as controls in our behavior test, we excluded sNPF implication in Ecc perception (Figures S6A and S6B). Previous works have also identified a set of neurons (named OA-VL) in which starvation induces a reduction of octopamine production leading to Gr66a bitter taste neuron depotentiation (LeDue et al., 2016). Consistent with this model, artificial silencing of octopamine and/or tyramine activity in these neurons induces a starvation-like reduction in bitter sensory neuron output. To test if the attraction of starved flies to Ecc was also dependent by this OA-VL module, we recorded the behavior of flies in which the octopamine receptor Oct-TyrR was genetically down-regulated in Gr66a neurons. In contrast to parental strains that behaved as controls, progeny having reduced octopamine signaling in bitter neurons were attracted by Ecc (Figures 7C and 7D). However, in this case the attraction phase lasted longer than for control flies, and hence the repulsive phase started later. These results suggested a model in which starved flies that have highly depotentialized bitter neurons do not perceive the “bitterness” of Ecc and fed on it. They prefer Ecc to sucrose only because the bacteria solution has probably a higher nutrient value for them than sucrose-only solution. Once the flies are fed by bacteria, their starvation status progressively decreases together with bitter neuron depotentiation. In bacteria-fed flies, the bitter neurons are no longer silenced and progressively sense the bitterness of Ecc, and hence flies begin to avoid it. To further test this hypothesis, we performed the experiments with progressively diluted Ecc solutions. We hypothesized that diluted Ecc solutions will be less nutritive than concentrated ones. If such, bitter neuron potentiation should take longer and the attraction phase length should last longer with diluted Ecc solutions. Our results showed that the more diluted the bacterial solution is, the longer the duration of the attraction phase lasted and the more the repulsive phase was delayed. For the highest diluted Ecc solutions (32× and 64×), the repulsive phase was even shorter followed by a novel attractive phase (Figure 7E). These data suggest that Ecc has a higher nutrient value for the flies than sucrose only. For the highly diluted bacterial solutions, this nutrient value is not strong enough to maintain the flies in a fed status for the entire experiment. After a certain time, the flies enter a novel phase of starvation and are therefore again attracted by Ecc.
Figure 7.
Starvation and Depotentiation of Bitter Neurons Delay Aversion to Ecc
(A and B) Non-starved flies displayed reduced attraction and aversion to Ecc. (A) (Left graphs) Kinetics of the AI for sucrose when starved flies or non-starved flies were given the choice between an Ecc-contaminated sucrose solution versus sucrose only. (Right graphs) CAI area for each specified solution (arrows) and its distribution over time. (B) Histograms built with the CAI values from (A). ∗p < 0.05 and ∗∗∗p < 0.001. Wilcoxon matched-pairs signed rank test, two-tailed p value. Error bars correspond to standard deviation. The preference indexes for Ecc are calculated with the CAI values from (A). For (A) left graphs and (C) top graphs, the black lines and the gray lines correspond, respectively, to the mean and the standard deviation, and for (A) left graphs and (C) bottom graphs, solely the mean value of the CAI obtained with multiple replicates is shown in black.
(C and D) Adult females expressing UAS-Oct-TyrRRNAi in bitter neurons using Gr66aGal4 displayed prolonged attraction and delayed aversion to Ecc, whereas control UAS- Oct-TyrRRNAi/+ or Gr66Gal4/+ do not. (C) (Top) Kinetics of the AI for sucrose. (Bottom) CAI area for each specified solution (arrows) and its distribution over time. (D) Histograms built with the CAI values from (C). ∗p < 0.05 and ∗∗p < 0.01. Wilcoxon matched-pairs signed rank test, two-tailed p value. Error bars correspond to standard deviation. The preference indexes for Ecc are calculated with the CAI from (D).
(E) Increased attraction to Ecc and delayed repulsion to Ecc when using diluted bacteria solutions. CAI area for each specified solution (arrows) and its distribution over time when flies are given the choice between an Ecc-contaminated sucrose solution of the indicated dilution (1× to 64×) versus sucrose only. For (A) left graphs and (C) top graphs, the black lines and the gray lines correspond, respectively, to the mean and the standard deviation, and for (A) right graphs and (C) bottom graphs, solely the mean value of the CAI obtained with multiple replicates is shown in black. n indicates the number of experimental replicates. a.u.: arbitrary unit. Data are represented as mean ± SD.
Behaviors toward Ecc Are Bacterial and Fly Species Specific
To analyze the universality of the above-described phenomena, we tested the fly feeding behavior toward sucrose solution contaminated with other DAP-type PGN, such E. coli, Lactobacillus plantarum, or Acetobacter pomorum the latter two being commensal bacteria that have been shown to colonize Drosophila gut (Sharon et al., 2011, Storelli et al., 2011). Both E. coli and L. plantarum species were clearly attractive for female flies, PI (E. coli) = 0.89 ± 0.11 SD et PI (L. plantarum) = 0.94 ± 0.16 SD, whereas A. pomorum was equally preferred (PI (A. pomorum) = -0.02 ± 0.32 SD) but with a long attraction phase. The second aversive phase observed with Ecc was not present with the three species (Figures S7A–S7F). This showed that although flies are attracted by bacteria in general, the subsequent aversive phase is specific to Ecc.
We next wanted to test whether the stereotyped behavior toward Ecc was conserved among different Drosophilidae. Interestingly, whereas the biphasic behavior was also observed for Drosophila biarmipes, it was not when its closely related species Drosophila suzukii was used for the tow-choice assay (Figures S8A–S8C). Indeed, we observed that Ecc was not aversive and only slightly attractive for D. suzukii. A similar pattern was observed for Drosophila ananassae. The absence of yet available genetic tools in D. biarmipes prevented us to test whether the cues and sensory systems at play to mediate this bacteria-fly interaction are the same in D. biarmipes and D. melanogaster, two phylogenetically distant species.
Discussion
Our results demonstrate that Ecc is perceived as bitter by the flies that are therefore repulsed by it. This repulsive phase, which takes around 1 h to be established, depends on Gr66a-positive neurons (Figure 8). One bacterial product candidate to trigger the repulsive behavior is the cell wall LPS, which has been shown to be perceived as bitter by D. melanogaster in a TRPA1-dependent manner (Soldano et al., 2016). However, we do not believe that, in our assay, LPS is the bitter substance. Indeed, E. coli whose cell wall also contains LPS was not repulsive to flies. In addition, TrpA1 mutant flies, which are supposed to be LPS insensitive, were as repulsed as control flies by Ecc. The use of live bacteria that are probably sensed via multiple cues instead of an LPS solution as in the Soldano et al. studies might explain these discrepancies. Besides, as the structure of LPS and its recognition by ad hoc pattern recognition receptor(s) are highly bacterial species dependent, different bacterial LPS might trigger different responses via different receptors. It should also be noted that the repulsive phase is Orco-independent indicating that Geosmin and phenol, two olfactory cues previously shown to mediate bacteria avoidance, are probably not involved in this behavior (Mansourian et al., 2016, Stensmyr et al., 2012).
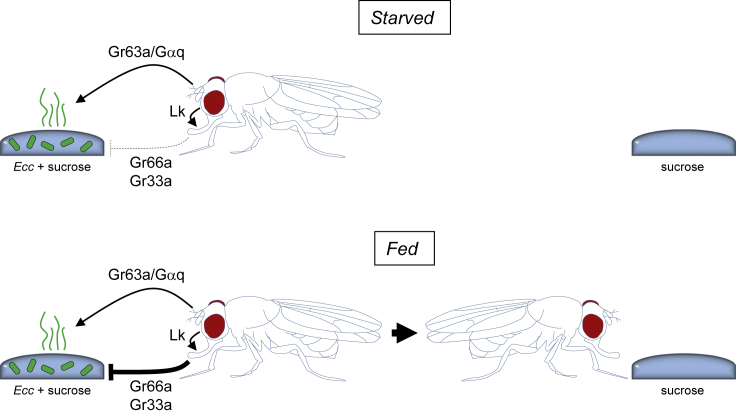
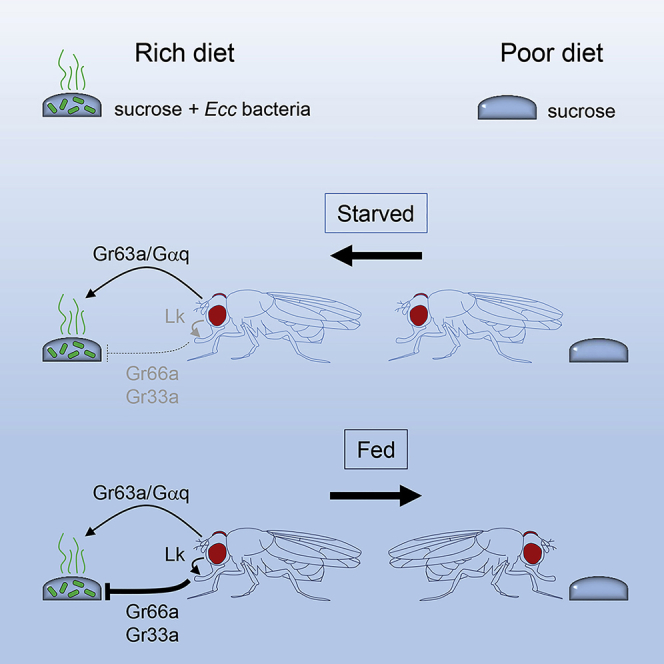
Figure 8.
Model for the Role of the Olfactory and Gustatory Modalities in the Biphasic Behavior of Adult Flies in Response to Ecc
Starved flies are first attracted by odors emanating from the Ecc-contaminated solution. This step requires both the olfactory receptor Gr63a, expressed by the CO2-sensing neurons hosted by the antenna, and the Gαq1 transducer. Starved flies have reduced bitter sensitivity due to Gr66a/Gr33a-expressing neuron depotentiation. Feeding on the sucrose + Ecc-contaminated solution induces the re-potentiation of bitter neurons. No longer potentiated, the bitter neurons established an aversive behavior toward Ecc. The gustatory receptors Gr66a and Gr33a, expressed by the bitter neurons of the labellum and of the tarsae, are required for this second phase. The neuropeptide Lk expressed by the central nervous system is also required for optimal aversion.
The data obtained with bitter Gr mutant and Ca-LexA suggested that flies can sense bacteria bitterness after being in contact with them. One puzzling result of this study is the latency of around 1 h that is required for the repulsive phase to be consolidated. A few reasons let us hypothesize that this phase requires that bacteria are internally sensed by the flies and that bacteria-derived PGN could be the mediator of the effect. First, in contrast to E. coli and L. plantarum, which were not repulsive in our assay, gut-associated Ecc was shown to release PGN that can reach fly blood where it interacts with both immune and neuronal tissues. The ability of Ecc to activate fly immunity, to modify fly egg-laying behavior, and to be perceived as bitter are all dependent on using live bacteria because they are abolished when Ecc is heat killed (Basset et al., 2000, Kurz et al., 2017). Finally, at the time Ecc was avoided by the flies, it was present in the intestinal tract. One could propose that internal sensing of gut-born Ecc PGN translocated to the hemolymph could explain the 1-h delay between attraction and repulsion. However, our data using flies mutant for the NF-κB transcription factor Relish downstream of the main gram-negative bacteria immune cascade IMD indicate that PGN sensing that mediates both immune and behavioral responses to bacteria is involved neither in the attractive nor in the repulsive phase. Consistently, PGRP-LBΔ mutant in which both immune and behavior responses to Ecc are exacerbated presented the same comportment as controls when given the choice between sucrose and Ecc.
Our results demonstrate that non-starved flies are more repulsed by Ecc let us propose another model to explain the delayed response. In starved flies, reduced octopamine signaling promotes depotentiation of bitter neurons. Ecc produce bitter substances that are not sensed by the bitter pathway because of its depotentiation. As flies feed on Ecc, their starvation status is progressively decreased, bitter neuron depotentiation is lost, and Ecc bitterness is perceived again. These data suggest that bacteria can be a source of food for the flies, which is consistent with previous reports (Yamada et al., 2015). The fact that the other bacterial species tested are attractive but not repulsive for the flies suggests that they also are a source of food but do not produce substances that are bitter for the flies.
Our data showed that, in the presence of Ecc, D. melanogaster were attracted to the bacteria-contaminated solution. The fact that Orco minus flies still preferred Ecc-contaminated over sucrose solution suggested that other odors/Rc complexes were implicated in this attractive phase or other sensory modalities such as gustation contributed to the initial attraction (Becher et al., 2018). Ionotropic receptors that sense odors in an Orco-independent manner are good candidates to mediate the effect (Gomez-Diaz et al., 2018). Although we do not know what is the nature of the attractive substance produced by Ecc, our results clearly demonstrate that this attraction mediates its effects via the CO2 receptor Gr63a and the Gαq transducer. Our results suggest that, as it has been shown for spermidine, Ecc produces a yet unknown compound that inhibits the CO2 receptor neurons and that inhibition of this avoidance pathway is necessary for attraction toward Ecc.
The present work demonstrated that the behavior of different Drosophila species toward Ecc is not generic but rather species specific. D. melanogaster is a vector for this potato blackleg bacterium by transmitting it from contaminated to healthy plants (Czajkowski et al., 2015). It would be interesting to know whether D. biarmipes, which presented a similar behavioral profile, is also a vector for Ecc. It is also clear that the persistent presence of Ecc in D. melanogaster had deleterious effects on the host, some of them being mediated by the PGN/NF-κB module (Lee and Ferrandon, 2011). This biphasic mode of interaction with Ecc, with an initial attractive phase rapidly followed by a repulsive one, would allow D. melanogaster-mediated Ecc dispersion on plants without affecting the integrity of the host due to an overprolonged contact with the bacteria.
Limitations of the Study
Although we have shown that Ecc is perceived as bitter by D. melanogaster, we have not identified the exact compound(s) that repulse the flies. Similarly, we have not uncovered what initially attracted starved flies to the Ecc-contaminated solution. If our data speak for a role of Gr66a bitter neurons and Lk in D. melanogaster aversion toward Ecc, we are missing a putative functional link between them. Finally, we do not know whether GR63a and Gαq1 act in a linear signaling pathway to control D. melanogaster attraction to Ecc.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Julien Royet (Julien.royet@univ-amu.fr).
Materials Availability
All unique/stable reagents generated in this study will be made available on request.
Data and Code Availability
The original/source data are available from the lead contact on request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Sabine Peslier for technical help. This work was supported by (ANR-11-LABX-0054) (Investissements d'Avenir–Labex INFORM), ANR BACNEURODRO (ANR-17-CE16-0023-01) and ANR PEPTIMET (ANR-18-CE15-0018-02), Equipe Fondation pour la Recherche Médicale (EQU201903007783), and l’Institut Universitaire de France to J.R.
Author Contributions
Conceptualization, B.C., F.D., and J.R.; Methodology, B.C. and F.D.; Investigation, B.C.; Formal analysis, B.C. and F.D.; Writing – Original Draft, B.C. and J.R.; Writing – Review & Editing, B.C. and J.R.; Funding Acquisition, J.R.; Supervision, B.C. and J.R.
Declaration of Interests
The authors declare no competing interests.
Published: June 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101152.
Contributor Information
Bernard Charroux, Email: bernard.charroux@univ-amu.fr.
Julien Royet, Email: julien.royet@univ-amu.fr.
Supplemental Information
References
- Acosta Muniz C., Jaillard D., Lemaitre B., Boccard F. Erwinia carotovora Evf antagonizes the elimination of bacteria in the gut of Drosophila larvae. Cell. Microbiol. 2007;9:106–119. doi: 10.1111/j.1462-5822.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- Basset A., Khush R.S., Braun A., Gardan L., Boccard F., Hoffmann J.A., Lemaitre B. The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl. Acad. Sci. U S A. 2000;97:3376–3381. doi: 10.1073/pnas.070357597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher P.G., Lebreton S., Wallin E.A., Hedenstrom E., Borrero F., Bengtsson M., Joerger V., Witzgall P. The scent of the fly. J. Chem. Ecol. 2018;44:431–435. doi: 10.1007/s10886-018-0950-4. [DOI] [PubMed] [Google Scholar]
- Bergman P., Seyedoleslami Esfahani S., Engstrom Y. Drosophila as a model for human diseases-focus on innate immunity in barrier epithelia. Curr. Top. Dev. Biol. 2017;121:29–81. doi: 10.1016/bs.ctdb.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Bosco-Drayon V., Poidevin M., Boneca I.G., Narbonne-Reveau K., Royet J., Charroux B. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host Microbe. 2012;12:153–165. doi: 10.1016/j.chom.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Buchon N., Broderick N.A., Poidevin M., Pradervand S., Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Buchon N., Silverman N., Cherry S. Immunity in Drosophila melanogaster--from microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014;14:796–810. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo F., Charroux B., Royet J. Bacteria sensing mechanisms in Drosophila gut: local and systemic consequences. Dev. Comp. Immunol. 2016;64:11–21. doi: 10.1016/j.dci.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Charroux B., Capo F., Kurz C.L., Peslier S., Chaduli D., Viallat-Lieutaud A., Royet J. Cytosolic and secreted peptidoglycan-degrading enzymes in Drosophila respectively control local and systemic immune responses to microbiota. Cell Host Microbe. 2018;23:215–228.e4. doi: 10.1016/j.chom.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Czajkowski R., Perombelon M., Jafra S., Lojkowska E., Potrykus M., van der Wolf J., Sledz W. Detection, identification and differentiation of Pectobacterium and Dickeya species causing potato blackleg and tuber soft rot: a review. Ann. Appl. Biol. 2015;166:18–38. doi: 10.1111/aab.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A.E. The molecular basis of bacterial-insect symbiosis. J. Mol. Biol. 2014;426:3830–3837. doi: 10.1016/j.jmb.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E.G., Dahanukar A. Molecular neurobiology of Drosophila taste. Curr. Opin. Neurobiol. 2015;34:140–148. doi: 10.1016/j.conb.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Diaz C., Martin F., Garcia-Fernandez J.M., Alcorta E. The two main olfactory receptor families in Drosophila, ORs and IRs: a comparative approach. Front. Cell. Neurosci. 2018;12:253. doi: 10.3389/fncel.2018.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S., Limmer S., Ferrandon D. Assessing Pseudomonas virulence with a nonmammalian host: Drosophila melanogaster. Methods Mol. Biol. 2014;1149:723–740. doi: 10.1007/978-1-4939-0473-0_56. [DOI] [PubMed] [Google Scholar]
- Inagaki H.K., Panse K.M., Anderson D.J. Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila. Neuron. 2014;84:806–820. doi: 10.1016/j.neuron.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W.D., Cayirlioglu P., Kadow I.G., Vosshall L.B. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Kapsetaki S.E., Tzelepis I., Avgousti K., Livadaras I., Garantonakis N., Varikou K., Apidianakis Y. The bacterial metabolite 2-aminoacetophenone promotes association of pathogenic bacteria with flies. Nat. Commun. 2014;5:4401. doi: 10.1038/ncomms5401. [DOI] [PubMed] [Google Scholar]
- Kleino A., Silverman N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Comp. Immunol. 2014;42:25–35. doi: 10.1016/j.dci.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz C.L., Charroux B., Chaduli D., Viallat-Lieutaud A., Royet J. Peptidoglycan sensing by octopaminergic neurons modulates Drosophila oviposition. Elife. 2017;6:e21937. doi: 10.7554/eLife.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Ali Agha M., Smith R.C., Nachman R.J., Marion-Poll F., Pietrantonio P.V. Leucokinin mimetic elicits aversive behavior in mosquito Aedes aegypti (L.) and inhibits the sugar taste neuron. Proc. Natl. Acad. Sci. U S A. 2016;113:6880–6885. doi: 10.1073/pnas.1520404113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J.Y., Dahanukar A., Weiss L.A., Carlson J.R. The molecular basis of CO2 reception in Drosophila. Proc. Natl. Acad. Sci. U S A. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDue E.E., Mann K., Koch E., Chu B., Dakin R., Gordon M.D. Starvation-induced depotentiation of bitter taste in Drosophila. Curr. Biol. 2016;26:2854–2861. doi: 10.1016/j.cub.2016.08.028. [DOI] [PubMed] [Google Scholar]
- Lee K.A., Lee W.J. Drosophila as a model for intestinal dysbiosis and chronic inflammatory diseases. Dev. Comp. Immunol. 2014;42:102–110. doi: 10.1016/j.dci.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Lee K.Z., Ferrandon D. Negative regulation of immune responses on the fly. EMBO J. 2011;30:988–990. doi: 10.1038/emboj.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestradet M., Lee K.Z., Ferrandon D. Drosophila as a model for intestinal infections. Methods Mol. Biol. 2014;1197:11–40. doi: 10.1007/978-1-4939-1261-2_2. [DOI] [PubMed] [Google Scholar]
- MacWilliam D., Kowalewski J., Kumar A., Pontrello C., Ray A. Signaling mode of the broad-spectrum conserved CO2 receptor is one of the important determinants of odor valence in Drosophila. Neuron. 2018;97:1153–1167.e4. doi: 10.1016/j.neuron.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansourian S., Corcoran J., Enjin A., Lofstedt C., Dacke M., Stensmyr M.C. Fecal-derived phenol induces egg-laying aversion in Drosophila. Curr. Biol. 2016;26:2762–2769. doi: 10.1016/j.cub.2016.07.065. [DOI] [PubMed] [Google Scholar]
- Masuyama K., Zhang Y., Rao Y., Wang J.W. Mapping neural circuits with activity-dependent nuclear import of a transcription factor. J. Neurogenet. 2012;26:89–102. doi: 10.3109/01677063.2011.642910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S.J., Lee Y., Jiao Y., Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr. Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme N.T., Liegeois S., Kele B., Giammarinaro P., Pradel E., Hoffmann J.A., Ewbank J.J., Ferrandon D. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 2007;3:e173. doi: 10.1371/journal.ppat.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevillon-Cheruel S., Leulliot N., Muniz C.A., Vincent M., Gallay J., Argentini M., Cornu D., Boccard F., Lemaitre B., van Tilbeurgh H. Evf, a virulence factor produced by the Drosophila pathogen Erwinia carotovora, is an S-palmitoylated protein with a new fold that binds to lipid vesicles. J. Biol. Chem. 2009;284:3552–3562. doi: 10.1074/jbc.M808334200. [DOI] [PubMed] [Google Scholar]
- Royet J., Gupta D., Dziarski R. Peptidoglycan recognition proteins: modulators of the microbiome and inflammation. Nat. Rev. Immunol. 2011;11:837–851. doi: 10.1038/nri3089. [DOI] [PubMed] [Google Scholar]
- Scott K. Gustatory processing in Drosophila melanogaster. Annu. Rev. Entomol. 2018;63:15–30. doi: 10.1146/annurev-ento-020117-043331. [DOI] [PubMed] [Google Scholar]
- Sharon G., Segal D., Zilber-Rosenberg I., Rosenberg E. Symbiotic bacteria are responsible for diet-induced mating preference in Drosophila melanogaster, providing support for the hologenome concept of evolution. Gut Microbes. 2011;2:190–192. doi: 10.4161/gmic.2.3.16103. [DOI] [PubMed] [Google Scholar]
- Shim J., Lee Y., Jeong Y.T., Kim Y., Lee M.G., Montell C., Moon S.J. The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nat. Commun. 2015;6:8867. doi: 10.1038/ncomms9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldano A., Alpizar Y.A., Boonen B., Franco L., Lopez-Requena A., Liu G., Mora N., Yaksi E., Voets T., Vennekens R. Gustatory-mediated avoidance of bacterial lipopolysaccharides via TRPA1 activation in Drosophila. Elife. 2016;5:e13133. doi: 10.7554/eLife.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensmyr M.C., Dweck H.K., Farhan A., Ibba I., Strutz A., Mukunda L., Linz J., Grabe V., Steck K., Lavista-Llanos S. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- Storelli G., Defaye A., Erkosar B., Hols P., Royet J., Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Turner S.L., Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- Vodovar N., Vinals M., Liehl P., Basset A., Degrouard J., Spellman P., Boccard F., Lemaitre B. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc. Natl. Acad. Sci. U S A. 2005;102:11414–11419. doi: 10.1073/pnas.0502240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada R., Deshpande S.A., Bruce K.D., Mak E.M., Ja W.W. Microbes promote amino acid harvest to rescue undernutrition in Drosophila. Cell Rep. 2015;10:865–872. doi: 10.1016/j.celrep.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C.A., Carlson J.R. Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila. J. Neurosci. 2010;30:4562–4572. doi: 10.1523/JNEUROSCI.6357-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuz A., Jagge C., Slone J., Amrein H. A genetic tool kit for cellular and behavioral analyses of insect sugar receptors. Fly (Austin) 2014;8:189–196. doi: 10.1080/19336934.2015.1050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidman-Remy A., Herve M., Poidevin M., Pili-Floury S., Kim M.S., Blanot D., Oh B.H., Ueda R., Mengin-Lecreulx D., Lemaitre B. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity. 2006;24:463–473. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video recording of an experiment with 12 arenas, each containing 10 wild-type females and two drops of feeding solution (in blue). The top 8 arenas contain one drop of H2O (right) and one drop of 50 mM sucrose (left). The bottom 4 arenas contain one drop of 50 mM sucrose + 10 mM caffeine (right) and one drop of 50 mM sucrose (left).
Video recording of an experiment with 6 arenas, each containing around 15 females and two drops of feeding solution (in blue). Each arena contains one drop of a mixture of Ecc + 50 mM sucrose (left) and one drop of 50 mM sucrose (right). The top 3 arenas contain wild-type CantonS flies and the bottom 3 arenas contain w1118 flies.
Video recording of an experiment with 6 arenas, each containing 20 females and two drops of feeding solution (in blue). Each arena contains one drop of a mixture of Ecc + 50 mM sucrose (left) and one drop of 50 mM sucrose (right). The top 3 arenas contain flies mutant for the loss-of-function allele Gr66aex83 and the bottom 3 arenas contain flies of genotype Gr66a+t8 ; Gr66aex83, which are rescued for the Gr66aex83 mutant allele.
Video recording of an experiment with 6 arenas, each containing around 15 females and two drops of feeding solution (in blue). Each arena contains one drop of a mixture of Ecc + 50 mM sucrose (left) and one drop of 50 mM sucrose (right). The top 2 arenas (starting from the left) contain transheterozygous mutant flies LKC275/Df(3)Exel6123. The last top right arena and the first bottom arena (starting from the left) contain control flies LKC275/+. The last 2 bottom arenas contain control flies Df(3)Exel6123/+.
Data Availability Statement
The original/source data are available from the lead contact on request.