Abstract
Since the discovery of ocular dominance plasticity, neuroscientists have understood that changes in visual experience during a discrete developmental time, the critical period, trigger robust changes in the visual cortex. State-of-the-art tools used to probe connectivity with cell-type-specific resolution have expanded the understanding of circuit changes underlying experience-dependent plasticity. Here, we review the visual circuitry of the mouse, describing projections from retina to thalamus, between thalamus and cortex, and within cortex. We discuss how visual circuit development leads to precise connectivity and identify synaptic loci, which can be altered by activity or experience. Plasticity extends to visual features beyond ocular dominance, involving subcortical and cortical regions, and connections between cortical inhibitory interneurons. Experience-dependent plasticity contributes to the alignment of networks spanning retina to thalamus to cortex. Disruption of this plasticity may underlie aberrant sensory processing in some neurodevelopmental disorders.
Vision is so inseparable from our conception of the world that “seeing is believing.” Sight is crucial to recognize friends, find savory food, and plan movement. While some aspects of the visual system form in utero, visual circuits have significant spontaneous activity- and experience-dependent reorganization early in life. Developmental refinement is robust to perturbations and small errors, imparting uniqueness based on experience without an exhaustive molecular code specifying each connection.
Developmental circuit changes occur at specific time points. The best-known changes occur during sensitive periods (SPs) and critical periods (CPs). SPs are developmental time windows when neuronal circuits exhibit experience-dependent modification. Such changes are easily evoked and of greater magnitude during the SP, but can be evoked to a smaller extent at other ages. CPs are a subset of SPs, distinguished by a strict temporal onset and closure and the fact that experience during the period is required for subsequent normal connectivity and function (Voss, 2013). This change is not reversible except during the CP.
The early developmental CP was described by Hubel and Wiesel. In binocular visual cortex, responsiveness to visual input from a deprived eye is attenuated by weeks of monocular deprivation (MD). This change in ocular dominance (OD), called ocular dominance plasticity (ODP), only occurs for deprivation early in development. Moreover, the circuit changes include a reduction in acuity in the deprived eye, called amblyopia. While changes in ODP and acuity can be reversed during the CP, recovery is not possible once the CP closes.
Growing evidence suggests that ODP is not entirely extinguished by the CP closure. Substantial adult plasticity outside of the CP can be activated, often with stimulus paradigms distinct from those effective during the CP (Espinosa and Stryker, 2012; Fong et al., 2016; Hensch and Quinlan, 2018; Sato and Stryker, 2008). Thus, ODP is perhaps more accurately defined as occurring during an SP. This ambiguity highlights the difficulty in distinguishing between CP and SP, especially as more sensitive tools to monitor function are developed. Different synaptic loci along the visual pathway can vary in their sensitivity to experience (Kang et al., 2013), and a change in one synapse that may exhibit reversible plasticity can have an enduring effect on overall function because of its influence on downstream synapses that change irreversibly. Homeostatic responses can also be activated to compensate for changes in one synapse, but not another synapse (Whitt et al., 2014). In the end, the visual circuit is indelibly altered by experience. Therefore, for the purpose of this review, we define CPs as a window of time during which there is a distinct onset of plasticity in response to experience that is followed by a clear diminishing responsiveness to the same stimuli.
CPs in the Mouse Visual System
Since Hubel and Wiesel, ODP has served as a robust model for studying CP plasticity. Changes in ODP following MD can be measured by comparing the neuronal response (by single unit recordings, visual evoked responses [VEPs], or in vivo imaging) to visual stimulation of each eye. These straightforward measurements have made ODP a canonical experimental system. More recently, tools enabling the measurement of other visual features such as orientation preference suggest that many visual features show plasticity, occasionally with different rules. This implies that the plasticity of traditionally monocular circuitry, such as thalamic relay cells, may show developmentally regulated plasticity in features unrelated to OD shifts.
Considerable effort has been devoted to understanding the underlying circuit and molecular basis of CP plasticity (Fagiolini et al., 2004). The means to label, monitor, and manipulate these circuit components independently is crucial to understand the basic visual circuit and to identify the loci where plasticity results in synaptic changes. Thus, the mouse has been a powerful experimental model. Despite a smaller binocular zone and lower visual acuity (Fagiolini et al., 1994), mice exhibit an MD response similar to that of primates and cats. This ODP has a clearly defined CP between postnatal day 19 (P19) and P32 (Gordon and Stryker, 1996). Mice offer the possibility of genetic access to defined neuron populations during ODP, including subsets of excitatory (Gerfen et al., 2013) and inhibitory neurons (Taniguchi et al., 2011). Such genetic approaches allow targeted recording, optical manipulation (Madisen et al., 2012), and calcium indicators to monitor activity (Chen et al., 2013).
We review advances in circuit changes underlying experience-dependent plasticity, focusing on the mouse visual system. CPs across other species, adult plasticity, and molecular mechanisms are reviewed elsewhere (Hensch and Quinlan, 2018; Kiorpes, 2015). We refer to work in other sensory cortical areas, extrapolating to the visual cortex. Studies have revealed that CPs apply to more than simply OD. With a variety of manipulations of visual experience and quantifying changes in receptive field (RF) properties, including orientation, luminance, and spatial and temporal frequency tuning, findings suggest the rules for plasticity in these features may differ from those for ODP. These studies also demonstrate that subcortical areas, including the thalamus, show experience-dependent changes.
Visual System Circuitry Underlying Experience-Dependent Plasticity
To understand where and how visual system plasticity is instantiated, a detailed system map is needed, including long-range connections between major brain structures (Figures 1 and 2) and the connections of specific cell types (Figures 3 and 4). This is especially true in the neocortex, which contains many distinct cell types, organized in layers, with multiple cell types per layer. A near-complete census of mouse cell types (Sugino et al., 2019; Tasic et al., 2018) promises to identify the full range of molecular subtypes. This provides a parts list for the local circuit. Given a map of visual cortical circuitry, can we identify the connections that are profoundly altered during plasticity? Our aim is to place the temporal order of circuit changes in context, assessing whether there is a hierarchy such that plasticity occurs earlier or in a different way for circuit nodes in thalamus and thalamocortical (TC) afferents compared to the intracortical circuitry of L4 and L2/3.
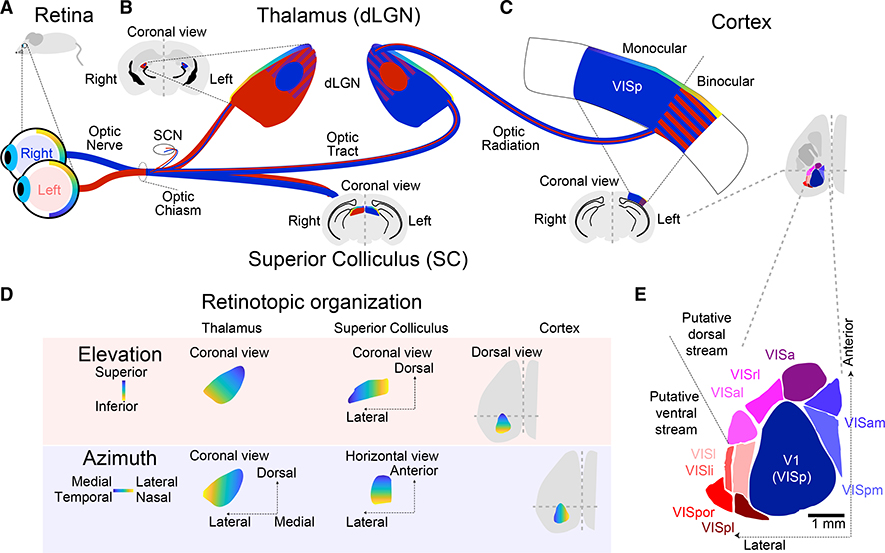
Figure 1. Ascending Pathways to Visual Cortex.
(A) Visual information enters the brain via the retina. Retinotopy is represented by the yellow-blue gradient, corresponding to (D). Output from the eyes is shown as red (left) and blue (right). (B) Output via the optic nerve projects to targets in the SCN, SC, and dLGN. Insets show SC and dLGN in coronal brain sections. Output crosses the midline to represent a given visual field in the contralateral thalamus and cortex. The right dLGN receives strong input from the left eye in rodents, whose eyes have reduced visual field overlap compared to binocular species such as primates. Retinal projections segregate into eye-specific layers (or zones) in adult dLGN. These then project to the cortex. Locations of neurons with binocular RFs are marked with red and blue stripes outside the ipsilateral patch.
(C) Mouse primary visual cortex (V1 or VISp) is predominantly monocular, with a smaller binocular field.
(D) Retinotopic organization in elevation and azimuth in dLGN (left), SC (center), and cortex (right) is preserved (Piscopo et al., 2013).
(E) Higher-order visual cortex in mice includes 9 areas adjacent to V1. V1(VISp), primary visual area; VISa, anterior area; VISal, anterolateral visual area; VISam, anteromedial visual area; VISl, lateral visual area (also LM in some papers); VISli, laterointermediate area; VISpl, posterolateral visual area; VISpm, posteromedial visual area; VISpor, postrhinal area; VISrl, rostrolateral visual area. Scale bar: 1 mm.
Structures in (A)–(E) not to relative scale.
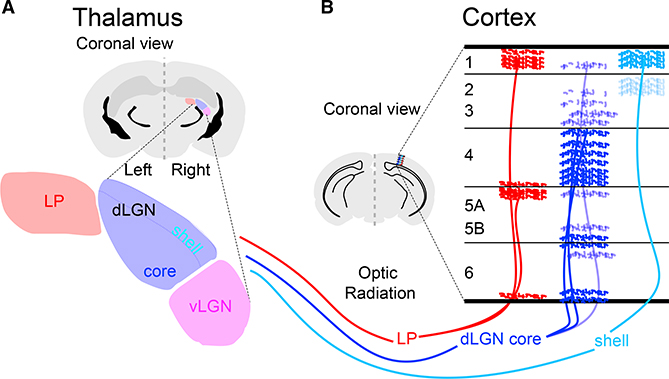
Figure 2. Thalamocortical (TC) Organization.
(A) Visual thalamus includes dorsal and ventral subdivisions (dLGN and vLGN, blue and purple). Core and shell areas of dLGN receive inputs from distinct RGC cell types. Higher-order visual thalamus includes LP (red).
(B) Thalamic axons in V1 arborize in distinct cortical laminae.
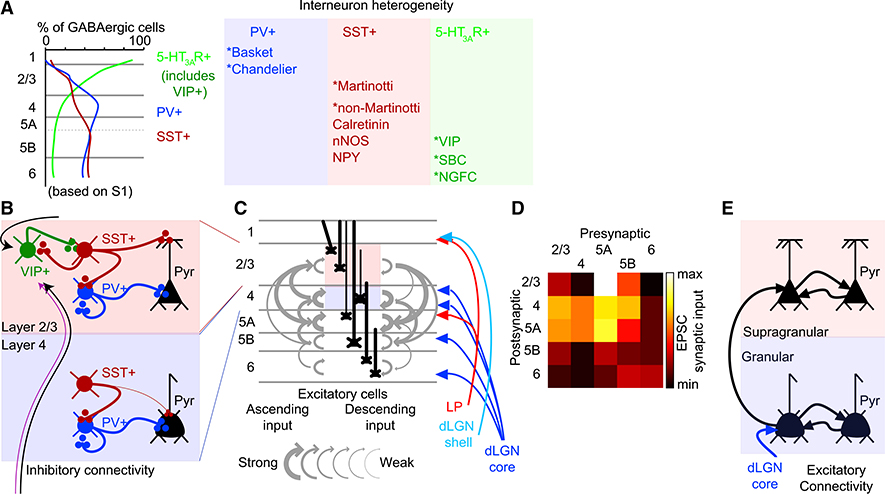
Figure 3. Inhibitory and Excitatory Local Connectivity in Cortex.
(A) Inhibitory interneurons vary in their laminar distribution across the cortex. 5-HT3AR+ neurons are concentrated in L1 and L2/3, including the VIP+ interneurons. PV+ and SST+ interneurons are present across all of the layers, except L1 (after Lee et al., 2010; Xu et al., 2010). Subtypes are highlighted in the table at right.
(B) In L2/3, bipolar VIP+ interneurons disinhibit the cortex by specifically inhibiting SST+ neurons. SST+ cells tonically inhibit PV+ neurons and pyramidal (Pyr) neuron apical dendrites. Tonic inhibition is released when VIP+ neurons become active (Pfeffer et al., 2013). Input to VIP neurons (black and magenta arrows) may vary across cortical areas. Local inhibitory circuits differ in L4, where SST+ interneurons also inhibit PV+ cells, but VIP+ neurons are less abundant.
(C and D) Major excitatory connections of the granular and supragranular visual cortex. The local excitatory circuit includes connections from excitatory cells in a given layer to those in nearby layers (Xu et al., 2016). Ascending (left) and descending projections (right), with thicknesses proportional to excitatory connection strength. Laminae targeted by thalamic input shown in blue and red. Strength is shown in the illustration (C) and as a connectivity matrix (D), with pre- and postsynaptic layers labeled.
(E) Local excitatory connectivity in L2/3 and L4.
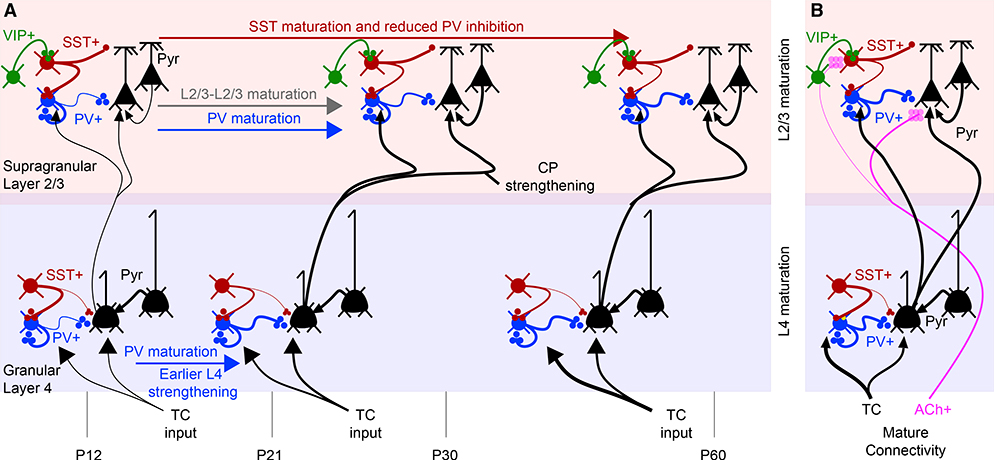
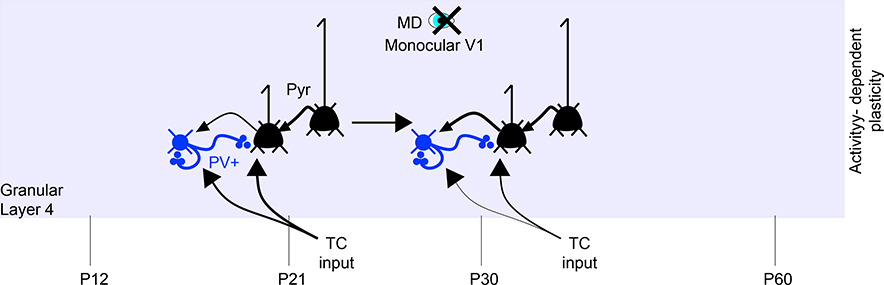
Figure 4. Developmental Changes in Cortical Connectivity Near the Critical Period (CP).
(A) For granular and supragranular layers, developmental changes in the connectivity of local circuits are illustrated. Excitatory neurons are illustrated in black. PV+ (blue), SST+ (burgundy), and VIP+ (green) inputs are color coded, along with neuromodulatory inputs such as acetylcholine (purple). Excitatory TC inputs are shown in black. Developmental age and time are annotated at the bottom. L4 changes in interneuron connectivity precede L2/3 changes (Jiang et al., 2010), with PV maturation in L2/3 correlated in time to CP onset and closure (Fagiolini et al., 2004). L2/3 connectivity matures during this period (Cossell et al., 2015; Ko et al., 2013). SST+ connectivity to PV+ neurons, initially strong, grows weaker during this time and loses sensitivity to cholinergic modulation (Yaeger et al., 2019).
(B) Mature connectivity is summarized.
The Thalamus
The visual pathway of mammals begins in the retina, with retinal ganglion cell (RGC) projections that target a number of subcortical regions, including the superior colliculus (SC), dorsal lateral geniculate nucleus (dLGN), and suprachiasmatic nucleus (SCN; Figures 1A–1C). Blue and red in the figure illustrate projections corresponding to ipsilateral and contralateral eyes, with eye-specific segregation in mouse dLGN. In the mouse, the ipsilateral projection to dLGN is a small rounded patch surrounded by a larger area of contralateral input. Some binocular overlap in the mouse dLGN also exists (Figure 1B; Howarth et al., 2014).
The dLGN (Figure 2) can be subdivided into a core and shell region, with the core projecting principally to layer 4 (L4) of primary visual cortex (V1, also VISp in the reference atlas; Kuan et al., 2015). The shell largely targets L1 (Cruz-Martín et al., 2014). Higher-order visual thalamus, called the lateral posterior nucleus (LP) in mice (similar to the pulvinar of primates), projects strongly to L1, with some input to deeper layers, including L5A. The laminar structure of LP output may vary across visual areas (Bennett et al., 2019).
Two classes of dLGN neurons are excitatory TC neurons and intrinsic GABAergic interneurons, in which the former outnumber the latter ∼4:1. Intrinsic interneurons make dendrodendritic and axodendritic inhibitory connections onto TC neurons and other dLGN interneurons (triads; Bickford et al., 2010; Morgan et al., 2016; Rafols and Valverde, 1973). These two cell types receive excitatory RGC and corticothalamic (CT) inputs as well as inhibitory inputs from thalamic reticular neurons (TRNs). TC neurons, also called relay neurons, project to the cortex, sending collaterals to TRN. Interneurons in the thalamic reticular nucleus are parvalbumin positive (PV+), whereas intrinsic interneurons are largely PV−, although detailed characterization is not complete (Kalish et al., 2018). CT inputs, in turn, also send collaterals to TRNs. This circuit integrates, modifies, and relays visual information passing from the retina to V1.
Experience-Dependent Plasticity in the Thalamus
In the cat, studies revealed much more plasticity in cortical than in dLGN circuits (Wiesel and Hubel, 1963a). MD leads to the shrinkage of TC neuron somata in dLGN, but to little change in OD (Sherman and Spear, 1982; Wiesel and Hubel, 1963b). MD also has little effect on eye-specific segregation in mice (Guzik-Kornacka et al., 2016). These studies led to the view that CP plasticity was predominantly a cortical phenomenon.
In contrast to the cat, studies have uncovered more plasticity in the mouse thalamus than previously recognized. The traditional view divides dLGN into distinct eye-specific territories, with a predominantly contralateral area and a much smaller ipsilateral region. However, in vivo recordings from dorsomedial dLGN show a significant number of TC neurons that are responsive to binocular stimulation, indicating that convergent retinal inputs from both eyes are present in adult mice (Howarth et al., 2014). These findings suggest that some TC neurons in the ipsilateral patch extend dendrites into the contralateral dLGN layer (Krahe et al., 2011). Moreover, MD during the developmental window corresponding to the OD cortical CP described below (∼P18–P32) shifts the relative TC neuron response magnitude evoked by the contralateral and ipsilateral eye. This thalamic plasticity depends on the GABAA receptor α1 subunit (Sommeijer et al., 2017). Future studies are needed to reveal whether the potential for thalamic OD plasticity onsets at eye opening or during the cortical CP, and whether thalamic OD shifts are reversible. Notably, TC boutons terminating in L4 of binocular V1 show changes in OD following MD, even in adult mice (Jaepel et al., 2017; Rose and Bonhoeffer, 2018). The fact that plasticity in TC responses persists in adult mice while those in cortical neurons diminish in response to MD suggests that some synaptic mechanisms for the closure of the OD CP exist downstream of the thalamus.
Studies using dark rearing rather than MD demonstrated a previously unrecognized CP in the thalamus. Changes in visual experience alter the number and strength of retinal inputs innervating a given TC neuron beginning at P20. Similar changes in vision before ∼P20 or after P30 fail to alter retinogeniculate convergence (Hooks and Chen, 2006, 2008). The timing of this retinothalamic CP overlaps with that of the cortical CP (Gordon and Stryker, 1996), suggesting that cortical and thalamic changes are interrelated. Changes in L6 CT activity during this developmental window can regulate the number of retinal inputs onto TC neurons, even without visual deprivation (Thompson et al., 2016). These studies suggest that experience sculpts both cortical and subcortical circuits and that CT interactions are necessary for the proper formation of sensory circuits. One role of such plasticity may be to align the RF tuning of feedforward and feedback information in the thalamus.
The TC Circuit
The dLGN in turn projects to the visual cortex, with the monocular zone covering the largest territory, medial to a smaller binocular zone (Figure 1C). The mouse primary visual cortex contains retinotopically organized neurons that respond to visual stimuli of different orientations and directions. As in other sensory cortices, a major thalamorecipient layer, L4, is the middle layer of the cortex. This layer is often called granular due to the dense packing of neurons in L4; the layers superficial to it toward pia are called supragranular (L1 and L2/3), and the deeper layers are infragranular (L5 and L6).
Development of the TC Circuit
Ascending and descending projections connect the thalamus and cortex. The strongest projection from dLGN connects most to L4 following early development (see below; Catalano et al., 1991; Ghosh et al., 1990). This input also arborizes near the L5/6 boundary, and thus may also directly excite some deep-layer neurons (Morgenstern et al., 2016).
TC afferents reach the cortex by embryonic day 13 (E13) in mice, invading the subplate by E14, and growing radially into the cortex at E15 (Auladell et al., 2000). In contrast, L6 CT axons arrive in the visual thalamus by P0.5, but do not finish innervating dLGN until P14.5. Therefore, the visual TC loop is not fully connected until approximately eye opening at P12–P14 (Jacobs et al., 2007; Seabrook et al., 2013), 1 week before the onset of the cortical CP. Influences of visual experience on the development and plasticity of the TC circuit are therefore an underappreciated contributor to CP plasticity.
A Transient Circuit Develops Before the CP
The circuit organization described in Figures 3 and 4 is the configuration upon which cortical CP plasticity occurs in V1, although the details of how synaptic weights throughout the circuit change before, during, and after the CP are still a topic of intense investigation. Studies of the somatosensory cortex (S1) suggest that transiently formed TC circuits involving different inhibitory neurons in the cortex exist early in development. There are three main classes of cortical interneurons: those expressing PV, somatostatin (SST), and the 5-HT3A receptor (5-HT3AR) (Figure 3A; Lee et al., 2010). Each class contains further subtypes (Tremblay et al., 2016). PV+ neurons are mainly fast-spiking interneurons targeting pyramidal (Pyr) neuron somata. SST+ interneurons include low threshold spiking (LTS) interneurons, especially Martinotti cells, whose axons target Pyr neuron dendrites and arborize extensively in L1. SST+ neurons are heterogeneous, expressing a range of markers (calretinin, neuronal nitric oxide synthase [nNOS], and neuropeptide Y [NPY]). SST+ neurons in L4 of V1 are mostly Martinotti cells, while the same layer in S1 contains mainly non-Martinotti SST+ neurons, suggesting regional differences in SST+ subtypes (Scala et al., 2019). 5-HT3AR+ neurons include two major groups: Lamp5+ interneurons and vasopressin intestinal peptide (VIP)+, an important subset that targets SST+ neurons (Tremblay et al., 2016).
Studies using rabies virus labeling, laser scanning photostimulation, and optogenetics in S1 showed that SST+ interneurons act as an intermediate between TC inputs and PV interneurons. This transient circuit is thought to act as a developmental scaffold to establish the mature synaptic network (Marques-Smith et al., 2016; Tuncdemir et al., 2016), like the circuit formed by V1 subplate neurons that is essential for the proper formation of functional architecture (Ghosh et al., 1990; McConnell et al., 1989). In the first postnatal week, TC axons innervate SST+ neurons more strongly than PV neurons (Tuncdemir et al., 2016). Over the second postnatal week, there is an activity-dependent shift in TC innervation in which TC inputs onto SST interneurons weaken, while those onto PV neurons strengthen (Chattopadhyaya et al., 2004). Intracortical connections between SST → PV and SST → Pyr neurons, which are of comparable strength in the first postnatal week, diverge in the third postnatal week. While SST → Pyr synapses strengthen, those onto PV neurons weaken, and disruption of the SST circuit prevents PV circuit maturation (Butt et al., 2017). Whether a similar transient inhibitory scaffold exists in the developing visual cortex and how it relates to CP plasticity is not known.
The TC Circuit during the CP
By the third week, TC axons in L4 innervate fast spiking interneurons (PV+ interneurons), LTS neurons (SST+ interneurons), and excitatory Pyr neurons in V1 (Figure 4; Gouwens et al., 2019; Scala et al., 2019). In the mature circuit of V1 and S1, the excitatory TC drive is stronger onto PV neurons than onto SST+ interneurons or Pyr neurons (Cruikshank et al., 2010; Miska et al., 2018). TC synaptic responses onto PV and Pyr neurons also differ in short-term synaptic plasticity—synaptic depression is greater at TC inputs onto PV (Cruikshank et al., 2010). Thus, TC activation leads to stronger evoked spiking of PV neurons when compared to Pyr neurons and feedforward inhibition from L4 PV interneurons. Despite the asymmetry in TC drive, comparison of the TC-evoked excitatory postsynaptic current (EPSC) to the disynaptic inhibitory postsynaptic potential (IPSC) from the same cell shows that the excitation-to-inhibition (E/I) balance is comparable for PV and Pyr neurons (Miska et al., 2018).
The Effects of Sensory Experience on the TC Circuit
How does MD during the CP change the dynamics of the TC circuit? Closure of one eye is believed to reduce visually evoked activity from RGC to TC neurons. However, in vivo dLGN recordings from awake mice revealed that MD does not decrease the average spike rate of TC neurons innervated by the deprived eye (Linden et al., 2009). Instead, MD results in a decorrelation between relay neurons. Decorrelated TC inputs converging onto a given L4 neuron therefore contribute to the previously reported long-term depression of TC → Pyr synapse with deprivation (Kirkwood et al., 1996). Whether long-term depression of this synapse fully accounts for the reduced cortical responsiveness is still a focus of investigation (Crozier et al., 2007; Frenkel and Bear, 2004; Heynen et al., 2003).
Optogenetic tools have revealed distinct changes in the TC versus intracortical circuitry to deprivation in mice. By optically activating specific cell types in both the thalamus and L4 of monocular V1, Turrigiano and colleagues demonstrated that the deprivation of one eye depresses the strength of TC inputs onto PV cells to a greater degree than TC inputs onto Pyr cells (Figure 5). The E/I balance for synaptic responses to thalamic stimulation increases due to a shift toward excitation. In contrast, selective optogenetic activation of intracortical synapses revealed a decrease in E/I ratio due to an increase in the strength of Pyr → PV synapses that, in turn, enhances inhibitory drive onto L4 Pyr neurons (Maffei et al., 2006; Miska et al., 2018). Thus, MD leads to a divergence of the E/I ratios in TC and intracortical circuits driven by the deprived eye. As intracortical excitatory synapses outnumber TC synapses, the net shift in the E/I ratio from both circuits may lead to a greater degree of inhibition in L4 than depression of the TC input alone (Miska et al., 2018).
Figure 5. Developmental Changes in L4 Cortical Connectivity.
Effect of visual deprivation (MD in monocular V1) on excitatory connectivity in L4. TC inputs to both PV+ and Pyr neurons weaken, while within-layer excitatory connections strengthen.
Studies in cats and mice reveal that TC axon arbors remodel in response to MD almost 1 week after functional changes in V1 are detected (Antonini et al., 1999; Antonini and Stryker, 1993), which is consistent with a dissociation between structure and function described in other sensory circuits (Hong et al., 2014; Oberlaender et al., 2012). In the cat, functional changes in L2/3 (within 1 day of MD [1d-MD]) precede that in L4 (3d-MD), supporting the idea that feedback from intracortical circuits influences TC axon morphology and connectivity (Trachtenberg and Stryker, 2001; Trachtenberg et al., 2000). In contrast, simultaneous recordings from L2/3 and L4 in mice show reduced visually evoked potentials in both layers by 1d-MD (Liu et al., 2008). The difference in the sequence of laminar circuit changes may be due to a compressed developmental window in mice or other species differences such as distinct intracortical circuitry or homeostatic mechanisms. However, both species share a common theme involving circuit feedback during experience-dependent plasticity, whether it be between laminae or between cortex and thalamus (Thompson et al., 2016). Future studies will continue to expand on this detailed account of complex circuit dynamics. The assessment of other aspects of intracortical circuits, including the effects of MD on PV → PV and PV → SST+ neurons within L4, as well as connections between L4 and more superficial layers of V1 (Xue et al., 2014), will also advance our understanding of CP plasticity.
Intracortical Connections and Their Plasticity
Local Excitatory Connectivity
Plasticity in V1 during the CP has been attributed to changes in intracortical connectivity (Figure 4). Intracortical excitatory connection strength has been mapped in mouse V1, identifying intralaminar and translaminar pathways (Xu et al., 2016). These are similar to the major pathways in S1 (Lefort et al., 2009) and can be quantitatively compared across motor and sensory cortical areas (S1, S2, and M1; Hooks et al., 2011; Weiler et al., 2008). For supragranular connections, the major connections are L4 → L2/3 and L2/3 → L2/3. For infragranular connections, these are the reciprocal L2/3 → L5, and L5 → L2/3 connections (Figure 3).
Development and Plasticity of Local Excitatory Connections
CP changes include long-term reweighting of synaptic connections, but not all affected circuit loci are known. The CP shift likely includes changes in inhibitory circuitry, regulation of intrinsic excitability, and alteration of excitatory inputs, including L4 to L2/3 and within L2/3 connectivity. Some studies also investigate changes in short-term plasticity, such as synaptic facilitation and depression (Maffei et al., 2006). The temporal dynamics of intracortical excitatory connections reach maturity in L4 near CP onset, when TC inputs are already mature (Miao et al., 2016).
As L2/3 neurons are approachable, especially for in vivo imaging, their strong interconnectivity and its plasticity has made within-L2/3 excitatory connectivity a natural target for study. Small-scale networks (Yoshimura et al., 2005) of excitatory neurons exist within L2/3 neurons sharing similar orientation. Local connectivity is twice as likely for L2/3 excitatory neurons sharing the same orientation preference (Ko et al., 2011), and these connections are stronger (Cossell et al., 2015).
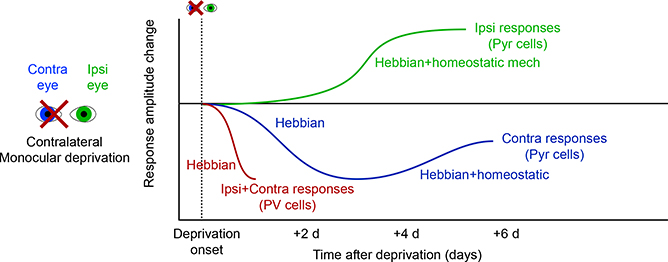
What about plasticity during deprivation? The time course of responsiveness changes in excitatory neurons suggests a multiphasic response to MD (Figure 6; Frenkel and Bear, 2004). Initially, L2/3 responses are rapidly reduced to the deprived eye (over a time course of days), with responses to the nondeprived eye strengthened more slowly (Mrsic-Flogel et al., 2007). Early weakening of the deprived contralateral eye inputs is due to Hebbian long-term depression (LTD). A slower phase of input strengthening from the non-deprived eye follows, along with a slight strengthening of deprived eye inputs, due to a mix of homeostatic and Hebbian mechanisms (Espinosa and Stryker, 2012). These mechanisms focus on changes in excitatory input (inhibitory connections are discussed below). Supporting this, MD in rats causes a biphasic shift in miniature EPSC (mEPSC) amplitude, which matches the time course of initial weakening (over 1–2 days), followed by strengthening (over 6 days) of synaptic inputs (Lambo and Turrigiano, 2013). The source of the mEPSC inputs could be L2/3 or L4 inputs, as these two sources are the strongest excitatory connections to L2/3 cells. Furthermore, intrinsic excitability of L2/3 pyramids contributes to this strengthening (Lambo and Turrigiano, 2013).
Figure 6. Time Course of Synaptic Changes Following Visual Deprivation.
Timeline of changes during short periods around MD. Initial firing rates of excitatory L2/3 neurons fall for the deprived contralateral eye on a short timescale, but partially recover over 1 week (Frenkel and Bear, 2004; Mrsic-Flogel et al., 2007). The initial loss of responsiveness is attributed to Hebbian mechanisms, but the slower recovery involves some homeostatic changes as well. In contrast, non-deprived eye responses strengthen more slowly. The transient rapid change is associated with the loss of excitation to PV+ interneurons, which occurs as rapidly as 1 day and is associated with the loss of excitatory synaptic input to these cells (Kuhlman et al., 2013).
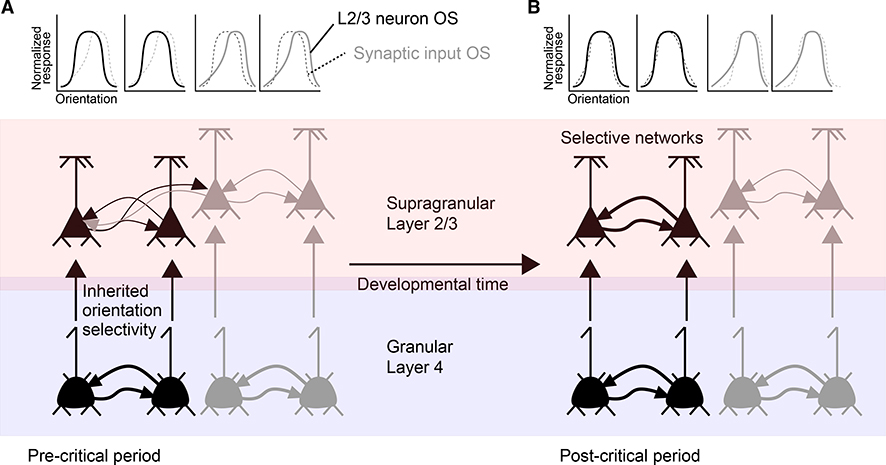
As the OD CP coincides with development of intralaminar L2/3 connectivity, maturation of this circuit is a potential contributor to CP plasticity (Figure 7). Before CP onset, L2/3 neurons show tuned responses at eye opening (Ko et al., 2013), but similarly tuned L2/3 neurons are not preferentially connected to each other at P13–P15. By the middle of the CP (P22–P26), however, L2/3 neurons of similar orientations have enhanced connectivity (Cossell et al., 2015). Thus, early in development, L2/3 orientation selectivity is likely inherited from L4 inputs. Later, selective connectivity within L2/3 develops via plasticity, with increases in the connection probability of similarly tuned cells and the elimination of connections between differently tuned ones (Ko et al., 2013). Remarkably, this process is not disrupted by visual deprivation (Ko et al., 2014); MD results in a seemingly normal pattern of orientation selectivity and connectivity. This suggests that spontaneous activity suffices for this developmental change, perhaps without CP plasticity for orientation selectivity. Binocular matching, however, is disrupted by visual deprivation (see below). It will be interesting to know whether prolonged dark rearing results in a regression of matching in tuned neurons. That this reorganization occurs during the CP for ODP suggests that many circuit changes occur contemporaneously. Other paradigms evoke L2/3 plasticity even in older mice, suggesting that some cortical plasticity exists outside the CP for ODP. Associating grating orientation with reward induces RF changes in excitatory (Poort et al., 2015) and inhibitory cells in mice after the traditional CP (Khan et al., 2018). However, it is unclear how the sensitivity to learned associations changes developmentally.
Figure 7. Functional Consequences of L2/3 CP Plasticity.
(A) In early development, L2/3 Pyr neurons inherit their orientation selectivity (OS) from L4 inputs, not intralaminar L2/3 inputs. OS tuning curve is shown as solid lines above each L2/3 cell, with dotted lines reflecting heterogeneous OS preference of other L2/3 inputs.
(B) During development, L2/3 neurons resolve into smaller L2/3 networks preferentially sharing OS preference (black and gray networks). This process occurs with developmental time, even during visual deprivation (Ko et al., 2014).
Interneuron Connectivity in Local Circuitry
Inhibitory GABAergic interneurons are ∼10%–15% of all cortical neurons (Peters and Kara, 1985; Tremblay et al., 2016), outnumbered by excitatory cells in all cortical layers, except in the cell-poor L1. Local excitatory inputs to L2/3 interneurons suggest that GABAergic cells integrate inputs from most neighboring Pyr cells in the mouse (Bock et al., 2011; Hofer et al., 2011). Consistent with this, L2/3 interneurons of all classes have reduced orientation selectivity compared to nearby Pyr cells (Kerlin et al., 2010). The first interneuron Cre-driver mouse, PV-Cre (Hippenmeyer et al., 2005), enabled the manipulation of PV+ interneurons, showing that L2/3 PV+ interneurons are broadly tuned and PV+ firing modulation bidirectionally altered Pyr neuron firing rates, but they did not affect the orientation tuning width (Atallah et al., 2012; Wilson et al., 2012). Thus, the PV+ neuron function is not to shape the orientation selectivity of L2/3 pyramids. Similarly, both SST+ and PV+ interneurons are relatively non-selective in the excitatory neurons they inhibit, targeting most neighboring Pyr neurons with high probability (Fino et al., 2013), typically neurons whose somata are in the same cortical layer (Kätzel et al., 2011). In other mammals, in which cortical orientation selectivity is locally organized, interneuron tuning may shape Pyr cell tuning by specifically suppressing non-preferred responses (Wilson et al., 2017, 2018). Alternatively, precise weighting of specific connections may be achieved by reweighting inhibitory synapses from PV+ neurons. Developmentally, visual cortex PV+ interneurons show plastic connections in response to activity changes in their targets, while SST+ neurons do not (Xue et al., 2014).
The elucidation of cell-type-specific rules for local interneuron connectivity is of great interest, especially in their connectivity to other interneurons. A network for disinhibiting cortex has been demonstrated across many cortical areas (Figure 3). VIP+ interneurons specifically inhibit SST+ neurons (Pfeffer et al., 2013). Since SST+ interneurons are often tonically active, this transiently inactivates network inhibition, freeing the targets of SST+ inhibition to fire. These targets are both PV+ interneurons as well as Pyr neurons (Figure 3B). The net effect of VIP+-mediated disinhibition is an increase in cortical responsiveness (Fu et al., 2014). The disinhibitory VIP+ circuit motif seems to be present across the cortex, with examples in auditory, somatosensory, and frontal cortex (Lee et al., 2013; Pi et al., 2013). However, the input that activates VIP+ interneurons varies across cortical areas. In the mature visual cortex, running results in an increase in cortical gain (Niell and Stryker, 2010), mediated by a brainstem circuit activating cholinergic inputs in the basal forebrain (Lee et al., 2014). Cholinergic input is thus proposed as one source of VIP+ interneuron activation in V1. The VIP+ network may be engaged by glutamatergic inputs in other areas, such as motor cortex activation of VIP+ neurons in S1 during whisker movement (Lee et al., 2013). In auditory areas, running decreases responsiveness. It will be interesting to see how this circuit explains disinhibition in the infragranular cortex, where somata of VIP+ neurons are much less prevalent, but their axons may be present and regulate inhibition and disinhibition (Yu et al., 2019). Furthermore, differing subtypes of SST+ neurons, such as apical dendrite targeting Martinotti cells in V1 L4 and nearby S1 L4 SST+ non-Martinotti cells (Scala et al., 2019), suggest different disinhibitory rules across areas.
Plasticity of Intracortical Connections in Supragranular Layers
Changes in inhibitory circuitry are a major mechanism of CP plasticity. The development of cortical inhibition plays an important role in triggering the onset of CP plasticity in V1 (Hensch, 2005). The development of GABAergic circuitry precedes CP onset, with inhibitory inputs to excitatory neurons strengthening in L4 and L2/3 after eye opening. Consistent with sequential maturation along the ascending circuit, L4 GABAergic inputs strengthen during the third postnatal week (∼P12–P21), while L2/3 inputs strengthen later (Jiang et al., 2010) (Figure 4). The development of GABAergic connections is halted by dark rearing (Huang et al., 1999), and their strengthening is needed to trigger the onset of the CP for OD plasticity (Cynader et al., 1976). PV+ basket cells have been proposed to be the circuit component whose maturation triggers CP plasticity for ODP, based on the manipulation of specific GABAA receptor subunits (Fagiolini et al., 2004).
PV+ cells are well situated to regulate rapid changes in Pyr neuron excitability, thus explaining changes in V1 responsiveness as rapidly as after 1d-MD during the CP (Figure 6). Local excitatory inputs to Pyr neurons are unchanged, but responsiveness of L2/3 PV+ interneurons is reduced with a dramatic loss of excitatory L4/L5A input (Kuhlman et al., 2013). Such inhibitory circuit changes seem crucial for plasticity, as manipulation of PV+ neuron firing, such as by inhibitory designer receptors exclusively activated by designer drugs (DREADD) expression targeted to these cells, can restore CP plasticity at ages after the typical CP into adulthood (∼P85; Harauzov et al., 2010; Kuhlman et al., 2013). This implies that the CP closure is due to increased PV+ activity (but see Hoseini et al., 2019). The change in PV+ input is due to deprivation and not competition between open and closed eye inputs, since PV+ responsiveness is reduced during binocular deprivation as well (Feese et al., 2018). In the short term, 1-d MD results in the reduction of PV+-mediated feedforward inhibition due to reduced PV+ neuron firing. For longer manipulations, the reduction in the excitatory neuron firing rate can result in reduced PV+ but not SST+ IPSC amplitude (Xue et al., 2014). It is worth noting, however, that the activity manipulations used to achieve this were chronic from in utero expression to the time of the CP opening period (∼P17–P23). Thus, interneuron plasticity due to targeted manipulations during the CP may differ.
SST+ interneurons also integrate into the cortical circuit at the same developmental time (Figure 4). SST+ cell inhibition of PV+ interneurons is already strong in L2/3 by the CP and weakens over development after P28 (Yaeger et al., 2019). SST+ neurons also show strong connectivity with PV+ cells and pyramids in the infragranular layers (Butt et al., 2017; Tuncdemir et al., 2016). In both of these layers, a developmental strengthening of glutamatergic input (local L2/3 and L4 input to L2/3 pyramids; TC input to L5 pyramids) occurs concurrently with a reduction in SST+-mediated inhibition. Such SST+-mediated inhibition of PV+ cells, along with apical dendritic inhibition, may be required for CP plasticity. Suppression of SST+ neuron activity during the CP (assumed to increase PV+ neuron activity) blocks RF development of excitatory cells (Yaeger et al., 2019).
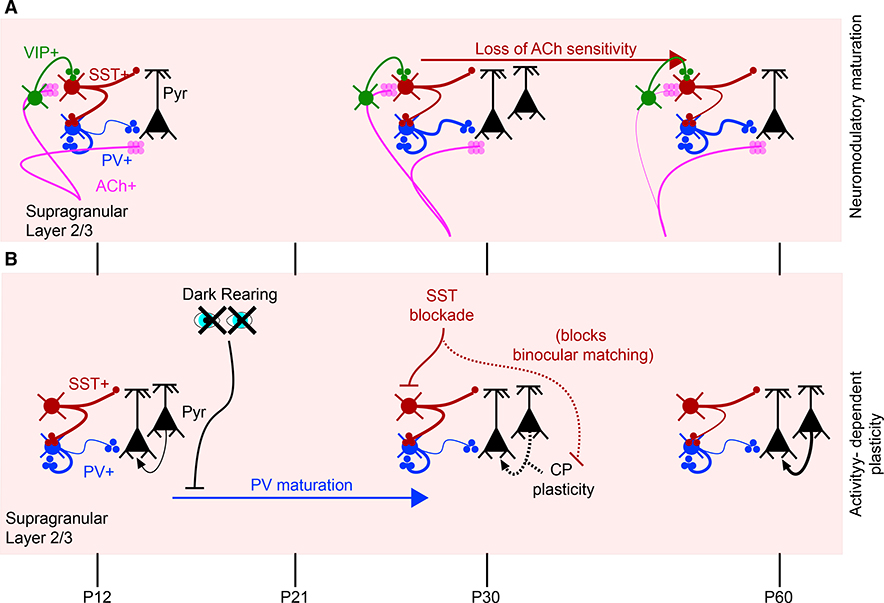
Basal forebrain cholinergic inputs also regulate CP closure through the modulation of inhibitory circuits. The Lynx1 gene, an endogenous antagonist of nicotinic acetylcholine receptors (AChRs), increases in expression in V1 PV+ neurons over development, suggesting that some circuit components in V1 developmentally lose responsiveness to cholinergic input. Knockout (KO) of Lynx1 prevents closure of the CP (Morishita et al., 2010). More recently, activation of excitatory AChR projections onto SST+ neurons was shown to disynaptically decrease PV neuron activity early in development. This second form of cholinergic modulation is lost in adulthood, leading to increased PV neuron activity, thus implicating developmental reduction of the cholinergic modulation of SST+ neurons in CP closure (Figure 8; Yaeger et al., 2019).
Figure 8. Developmental Changes in L2/3 Cortical Connectivity.
(A) Timeline summarizes the developmental changes in the cholinergic innervation of L2/3.
(B) Timeline emphasizing the effect of visual deprivation on PV and SST maturation. Manipulations such as dark rearing modulate CP onset by preventing PV maturation. Within-L2/3 excitatory connectivity is emphasized as a site of slow plasticity (over days; see Figure 7) for ODP and binocular matching. SST blockade prevents binocular matching.
In addition to SST+-mediated inhibition, a descending L1 input from non-VIP-expressing 5-HT3AR+ interneurons regulates PV+ excitability. Blockade of this input abolishes auditory cortex CP plasticity (Takesian et al., 2018). This may explain the distinct effects of AChR modulation on PV+ versus SST+ neurons. While developmental changes may help explain CP closure, cholinergeric input during arousal remains effective at modulating the cortical state in adulthood (Fu et al., 2014; Niell and Stryker, 2010). Thus, it has been proposed that the cholinergic pathway could be a target for future drug development in the treatment of diseases such as amblyopia, in which prolongation of cortical plasticity may contribute to recovery.
Themes in Cortical Plasticity
Reweighting of intralaminar L2/3 connections seems to be a major contributor to CP plasticity of L2/3 excitatory neurons. The circuit mechanisms underlying these changes implicate PV+ interneurons as a key node in determining whether plasticity is possible, as cortical plasticity does not occur before PV+ connections have begun to mature (CP onset) nor after PV+ connectivity is fully mature (CP closure). Once the CP is open, mechanisms regulating PV+ interneuron excitability may gate whether plasticity continues. Inhibitory circuits that target PV+ cells may be manipulated to block or permit CP plasticity (Takesian et al., 2018; Yaeger et al., 2019). Furthermore, mechanisms for altering excitation to PV+ neurons also regulate rapid CP changes (Kuhlman et al., 2013). Since mature PV+ inhibition reduces or prevents plasticity, one may hypothesize that a targeted reduction of PV+ inhibition in the adult would be helpful in restoring plasticity, if runaway excitation could be avoided. If this is how CP plasticity is regulated, and a second, later window of CP plasticity was evoked, then it would address whether such changes were indeed a one-time event, or whether plasticity can be reopened multiple times. Transplantation of immature GABAergic neurons into adult V1, even when their activity is blocked, seems to make this possible (Hoseini et al., 2019; Southwell et al., 2010).
How inhibitory development triggers the opening of the CP is not straightforward. Without data, it seems that if disinhibition is helpful for CP plasticity at ages ∼P20–P30, then the lack of inhibition before the development of PV+ interneurons should also be helpful in enhancing plasticity. However, the data suggest that dark rearing and other manipulations that disrupt the development of PV+ interneuron-mediated inhibition also delay CP onset. Perhaps a minimum level of perisomatic inhibition is permissive for circuit changes, or perhaps a concert of other changes linked to PV+ maturation occur that support the initiation of the CP? One model proposes that without a sufficient level of PV-mediated inhibition, it may be difficult to differentiate excitatory inputs in a noisy background, while higher levels of inhibition assist in enhancing the signal-to-noise ratio and thus select specific inputs for plasticity (Toyoizumi et al., 2013).
Distinct Roles for TC and Intracortical Circuits of the Visual System
The two most commonly used in vivo assays for CP plasticity, OD and spatial acuity, are assumed to track with each other. However, conditions that dissociate plasticity in TC and intracortical circuits suggest that their underlying mechanisms are distinct (Liu et al., 2008). Chronic visual deprivation from birth halts the onset of OD plasticity as assayed by single-unit recordings across all cortical lamina. However, the development of spatial acuity, measured by in vivo VEP responses around the granular layer, is slowed significantly but does eventually reach adult levels (Kang et al., 2013). Mismatch in the maturation of the TC and intracortical circuits may underlie the observation that while the initial development of orientation selectivity is normal, tuning is then degraded over time in dark-reared mice (Wang et al., 2010). The loss of the extracellular matrix protein NARP, an extracellular lectin that accumulates at excitatory synapses onto PV interneurons, results in reduced inhibition and the failure to express ODP. However, experience-dependent regulation of spatial acuity (VEP responses) is intact (Gu et al., 2013). In addition, the Nogo-66 receptor (ngr1), a myelin-associated protein, plays a role in limiting OD plasticity, and deletion of the protein in the adult brain leads to recovery of this plasticity and improved acuity in amblyopic mice. Selectively deleting ngr1 in excitatory cortical neurons leads to the recovery of OD but not visual acuity. In contrast, deleting the protein from TC neurons recovers acuity, but not OD plasticity (Stephany et al., 2018). These findings suggest that TC and corticocortical connections contribute to different features of visual circuit plasticity.
The Purpose of CPs
While many studies have identified molecular and circuit-based mechanisms underlying the opening and closing of CPs, the fundamental purpose for this developmental window has also drawn attention. Vision is encoded in many information lines that represent different RF features. Accumulating data support the idea that CPs allow for the precise matching of these information lines within and across brain areas during developmental fine-tuning of the neuronal network. For example, CT feedback from V1 to dLGN, which develops relatively late, should ideally match the retinotopy and feature selectivity of feedforward inputs from retina converging onto a given TC neuron. Fine-tuning of the retinal-thalamo-cortical circuit may occur during the retinogeniculate CP, when changes in experience can rewire afferent retinal inputs (Thompson et al., 2016).
Another canonical example of alignment during the CP is fine-tuning of binocular matching in mice. Similar to other species, orientation preference of inputs from the contralateral and ipsilateral eye converging on a given binocular neuron in V1 appear random early in development, but are well matched in adult mice (Crair et al., 1998; Hubel and Wiesel, 1962; Wang et al., 2010). With age, the difference in orientation tuning of inputs driven by the two eyes decreases (Gu and Cang, 2016; Wang et al., 2010). This decrease occurs without experience, but the final refinement of binocular matching occurs during the CP and involves the late developmental refinement of synaptic circuits driven by the ipsilateral eye to match the orientation tuning of those driven by the contralateral eye. Binocular matching is disrupted by MD or dark rearing between P20 and P30, but not at older ages. The restoration of normal visual experience after the CP does not fully rescue these defects (Wang et al., 2010). Notably, TC inputs serving the two eyes become substantially matched before the CP, followed by matching of intracortical circuits. The sequential timing suggests that, when compared to TC circuits, binocular matching in intracortical circuits may be more sensitive to experience during the CP (Gu and Cang, 2016; Ko et al., 2014). Supporting this idea, inhibitory circuits between SST and PV neurons in L2/3 rewire during the CP and contribute to binocular matching (Yaeger et al., 2019). Visual deprivation paradigms that shift OD during the CP are also associated with decreased binocular matching of orientation preference. This plasticity cannot be entirely explained by on-off subregion correspondence (Sarnaik et al., 2014), and the deficits induced by aberrant experience can be rescued by environmental enrichment (Levine et al., 2017).
If the purpose of CPs is to fine-tune information flow such that bottom-up, top-down, and intralaminar inputs to neurons match in their preferences for OD, orientation selectivity, or other features, then it is worth asking why the visual system uses this method of developmental plasticity instead of a fully predetermined molecular mechanism. In normal development, fine-tuning is needed, even in systems in which molecular gradients accomplish much in the service of retinotopy and somatotopy. Perhaps the molecular code is insufficient to also instantiate ocular preference and orientation and direction selectivity. An activity-dependent approach allows the circuit to form, even when the number of elements is not predetermined (as seems to be the case for mammals but not all invertebrates). Closure or reduction of plasticity at near-adult ages may confer an advantage by reducing the cost of constant circuit refinement after development in typical animals. However, since different cortical areas specialize for different sensory, motor, and cognitive functions, there may be temporally different CPs using potentially similar mechanisms to open and close the peak periods of cortical responsiveness to change. This accounts for the accent-free acquisition of new languages and the ability to learn new motor skills in adolescence, for example, the stereotypically poor risk management of teenagers learning to drive—all changes occurring later than the visual cortex CP.
CPs and Neurodevelopmental Disorders: NDDs as Synaptopathies
CPs are times when synaptic circuits are sensitive to environmental changes, but these periods can also be windows when aberrant experience leads to permanent disruption in neuronal function. Supporting this idea are findings that children raised in orphanages who are deprived of normal social, cognitive, and emotional stimulation show developmental delays and reduced intellectual abilities when compared to children raised in families (Nelson et al., 2007). The younger that an orphan is placed with a foster family, the more likely it is that the child can recover his or her cognitive skills. Identifying the circuits disrupted in environmentally deprived children is a topic of active research. One network implicated in autism spectrum disorders (ASD) is face recognition (Weigelt et al., 2012). In non-human primates raised without exposure to faces, higher-order cortical face processing is impaired (Arcaro et al., 2017). These examples highlight how abnormal experience can derail cognitive and social development in an otherwise healthy brain.
The majority of neurodevelopmental disorders (NDDs), including ASD and schizophrenia, occur in the setting of a normal environment. A disruption in CP refinement is potentially the underlying pathophysiology. That is, abnormal plasticity responses during the CP may lead to improper consolidation of synaptic circuits. In this model, experience-dependent plasticity of synaptic circuits described in sensory systems is likely generalizable to other regions of the brain, including the limbic system.
Studies from many labs have focused on monogenetic mouse models of NDDs to identify defects in specific circuits. Many of these genes have also been associated with CP plasticity. Studies have revealed a disruption in the incorporation of sensory experience into circuits. For example, the Fmr1 KO mouse line, a model for the most common intellectual disability disorder for males, fragile X, exhibits an abnormal CP response. Rather than normal depression of cortical responses to MD (Figure 6), these animals exhibit a potentiation of the open eye response, a feature that is typically only seen with longer periods of MD in wild-type (WT) mice (Dölen et al., 2007). Disrupted mGluR5 signaling in Fmr1 KO mice is thought to underlie this abnormal response to sensory experience, although the synaptic locus is not clear. Abnormally increased dendritic spine density has been observed in L3 of V1 (Dölen and Bear, 2008), implicating the disruption of excitatory connections, likely from L4 and within L2/3 inputs. Moreover, sensory-dependent plasticity in the somatosensory system, such as exposure of Fmr1 KO mice to an enriched environment, does not lead to the normal increase in spine density seen in WT mice (Arroyo et al., 2019).
The Ube3a KO mouse is a model of Angelman syndrome, an NDD characterized by recurrent seizures and microcephaly. Maturation of synaptic connections onto L2/3 neurons is abnormal, and both L4 to L2/3-evoked long-term potentiation (LTP) and LTD are impaired in these mice (Yashiro et al., 2009). Curiously, late-onset dark rearing from P30 to P40 restores LTD in the mutants. These abnormal synaptic responses to visual experience likely contribute to the attenuated OD plasticity and diminished orientation selectivity in Ube3a KO mice (Sato and Stryker, 2010; Wallace et al., 2017; Yashiro et al., 2009). In other NDD mouse models, there is accelerated maturation of PV interneurons. Mecp2 KO mice is a model for Rett syndrome, an NDD notable for relatively normal initial development until ∼18 months of age, followed by the regression of cognitive and language skills (Zoghbi and Bear, 2012). Studies of Mecp2 KO mice show that the increase in PV interneuron maturation corresponds to a precocious onset and ending of OD plasticity (Durand et al., 2012; Krishnan et al., 2015). Defects in cholinergic modulation of cortical circuits have also been described in a number of NDD mouse models (Artoni et al., 2019), raising the possibility that certain synaptic loci can be differentially affected. A shift in the timing of plasticity of one synapse is likely detrimental to normal brain development as different layers of the cortex have distinct windows of sensitivity to visual experience. Therefore, disruption of the development of one synapse or microcircuit can lead to progressively discoordinated development of intracortical or TC circuits.
Many ASD mouse models have defects in synaptic function and structure, in particular, brain regions and circuits (Lee et al., 2017; Luo et al., 2018; Zoghbi and Bear, 2012), yet the drive to find a unifying theme that links different NDDs continues. One common feature that has emerged is the finding of E/I imbalance in the sensory cortex (LeBlanc and Fagiolini, 2011; Lee et al., 2017; Nelson and Valakh, 2015). This finding fits well with another autism model first proposed by Rubenstein and Merzenich (2003), arguing that E/I imbalance toward hyperexcitability could lead to a “noisy” cortex, reducing information processing and enhancing the incidence of seizures. The model is also consistent with the recent demonstration that acute manipulation of E/I balance in the prefrontal cortex leads to the disruption of normal social behavior (Yizhar et al., 2011). However, depending on the ASD mouse model, and even specific synapses studied, disruption of the E/I balance shifts in opposite directions (Dani and Nelson, 2009; Durand et al., 2012; Gogolla et al., 2009). Whether the disrupted E/I balance is causal for the disorder or a compensatory response is still debated (Nelson and Valakh, 2015). Supporting the latter possibility, a recent electrophysiological study systematically examined the feedforward circuitry from L4 to L2/3 of several different ASD models. These mouse models exhibit an increased E/I ratio in L4 of the S1, yet the firing rates of L2/3 Pyr neurons were unchanged between mutant and WT littermates (Antoine et al., 2019). However, while homeostatic plasticity appears robust at the L4-to-L2/3 synapse in these models, it is possible that it is disrupted in other feedforward synapses, as has been described for Mecp2 and Fmr1 KO mice (Blackman et al., 2012; Soden and Chen, 2010). Infragranular circuits have not been assessed to the same degree as supragranular circuits and thus their involvement is unknown.
Disruption of thalamic circuits has also been associated with NDDs. Both the Ptched1 (patched-domain containing protein 1) gene and the Cacna1 gene, which encodes a low-voltage activated calcium channel (T-type calcium channel), are highly enriched in the thalamus and are associated with the risk of ASD (for review, see Halassa and Kastner, 2017; Krol et al., 2018). In the visual thalamus of Mecp2 KO mice, initial synaptic development in the visual thalamus is relatively normal, but then becomes abnormal during the experience-dependent period, and changes in sensory experience no longer elicit a similar response in synaptic connectivity (Noutel et al., 2011). As proper interactions between cortex and thalamus are necessary for the development of normal cognition, one can speculate that focal defects in synaptic circuits of cortex or thalamus by the loss of Mecp2 may lead to the progressive regression of sensory processing during sensory CP (Durand et al., 2012; Thompson et al., 2016).
Even disruption of peripheral sensory circuits can lead to permanent alterations in both behavior and sensory perception. In a remarkable study, investigators selectively deleted individual ASD-associated genes Mecp2 or Gababr3 (β3 subunit of the GABAA receptor) from the dorsal root ganglion and trigeminal somatosensory neurons (Orefice et al., 2016). Disruption of these genes, whether during development or in adulthood, leads to abnormal tactile discrimination and hypersensitivity to light touch. However, abnormal sensory perception during development was associated with disrupted social behavior and heightened anxiety, whereas new-onset disruption in the adult did not affect behavior. Therefore, abnormal sensory processing during CPs of social behavior led to indelible changes in behavior. This study reveals how individuals exposed to abnormal sensory experience, as in the case of orphans and individuals with genetic defects leading to abnormal development of sensory circuits, can share similar clinical presentations. The consequences of abnormal sensory experience and disrupted mechanisms underlying CP plasticity may converge on a common synaptic circuit pathophysiology associated with NDDs.
Conclusions
Over the past 13 years (Hooks and Chen, 2007), studies taking advantage of cell-type-specific Cre lines combined with tools to activate, suppress, and monitor neurons have led to a deeper understanding of the local cortical circuitry and the rules underlying experience-dependent construction of the visual system. Themes emerging from these studies include the existence of transient developmental circuits involving different types of inhibitory neurons. These circuit motifs, reminiscent of the role of subplate neurons in TC circuit development, appear to serve as a scaffold for the maturation of intracortical circuits. Studies have also uncovered an experience-dependent process of matching specific tuning features that contribute to CP plasticity. Such matching may occur not only within cortex but also between cortex and thalamus. Finally, studies have uncovered a surprising degree of plasticity in the thalamus that may contribute to downstream changes in cortical circuitry. We have a better understanding of the molecular mechanisms by which inhibitory circuits can regulate the closure of the CP, but how they trigger the onset of the CP remains a compelling question. A more detailed understanding of specific mechanisms that are unique to intracortical and TC circuits and that distinguish experience-dependent plasticity during development from that during adulthood will be essential for harnessing CP plasticity to repair NDDs and degenerative disorders. Identifying the interactions between different circuits underlying image- and non-image-forming vision, including the SC, will also be important for understanding the basis of visual behaviors. With the range of techniques for targeted circuit manipulations, including the means to manipulate specific Pyr cell types, the advances of the next decade in cortical plasticity should be quite exciting indeed.
ACKNOWLEDGMENTS
The authors thank Gord Fishell, Alison Barth, Takao Hensch, Michela Fagiolini, Qiufen Jiang, Celeste-Elise Stephany, Taehyeon Kim, and Staci Sorenson for insightful comments on the figures and manuscript. This work was supported by a Brain and Behavior Research Foundation NARSAD Young Investigator Award (to B.M.H.) and by NIH/National Institute of Neurological Disorders and Stroke (NINDS) R01 NS103993 (to B.M.H.), NIH/NINDS NS095959 (to C.C.), NIH/National Eye Institute (NEI) EY013613 (to C.C.), and NIH U54 HD090255 (to C.C.).
REFERENCES
- Antoine MW, Langberg T, Schnepel P, and Feldman DE (2019). Increased Excitation-Inhibition Ratio Stabilizes Synapse and Circuit Excitability in Four Autism Mouse Models. Neuron 101, 648–661.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, and Stryker MP (1993). Rapid remodeling of axonal arbors in the visual cortex. Science 260, 1819–1821. [DOI] [PubMed] [Google Scholar]
- Antonini A, Fagiolini M, and Stryker MP (1999). Anatomical correlates of functional plasticity in mouse visual cortex. J. Neurosci. 19, 4388–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro MJ, Schade PF, Vincent JL, Ponce CR, and Livingstone MS (2017). Seeing faces is necessary for face-domain formation. Nat. Neurosci. 20, 1404–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo ED, Fiole D, Mantri SS, Huang C, and Portera-Cailliau C (2019). Dendritic Spines in Early Postnatal Fragile X Mice Are Insensitive to Novel Sensory Experience. J. Neurosci. 39, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artoni P, Piffer A, Vinci V, LeBlanc J, Nelson CA, Hensch TK, and Fagiolini M (2019). Deep learning of spontaneous arousal fluctuations detects early cholinergic defects across neurodevelopmental mouse models and patients. Proc. Natl. Acad. Sci. USA. 10.1073/201820847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Bruns W, Carandini M, and Scanziani M (2012). Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73, 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auladell C, Pérez-Sust P, Supèr H, and Soriano E (2000). The early development of thalamocortical and corticothalamic projections in the mouse. Anat. Embryol. (Berl.) 201, 169–179. [DOI] [PubMed] [Google Scholar]
- Bennett C, Gale SD, Garrett ME, Newton ML, Callaway EM, Murphy GJ, and Olsen SR (2019). Higher-Order Thalamic Circuits Channel Parallel Streams of Visual Information in Mice. Neuron 102, 477–492.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME, Slusarczyk A, Dilger EK, Krahe TE, Kucuk C, and Guido W (2010). Synaptic development of the mouse dorsal lateral geniculate nucleus. J. Comp. Neurol. 518, 622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman MP, Djukic B, Nelson SB, and Turrigiano GG (2012). A critical and cell-autonomous role for MeCP2 in synaptic scaling up. J. Neurosci. 32, 13529–13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock DD, Lee WC, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, and Reid RC (2011). Network anatomy and in vivo physiology of visual cortical neurons. Nature 471, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Stacey JA, Teramoto Y, and Vagnoni C (2017). A role for GABAergic interneuron diversity in circuit development and plasticity of the neonatal cerebral cortex. Curr. Opin. Neurobiol. 43, 149–155. [DOI] [PubMed] [Google Scholar]
- Catalano SM, Robertson RT, and Killackey HP (1991). Early ingrowth of thalamocortical afferents to the neocortex of the prenatal rat. Proc. Natl. Acad. Sci. USA 88, 2999–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, and Huang ZJ (2004). Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J. Neurosci. 24, 9598–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossell L, Iacaruso MF, Muir DR, Houlton R, Sader EN, Ko H, Hofer SB, and Mrsic-Flogel TD (2015). Functional organization of excitatory synaptic strength in primary visual cortex. Nature 518, 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, and Stryker MP (1998). The role of visual experience in the development of columns in cat visual cortex. Science 279, 566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RA, Wang Y, Liu CH, and Bear MF (2007). Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc. Natl. Acad. Sci. USA 104, 1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, and Connors BW (2010). Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65, 230–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martín A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A, and Huberman AD (2014). A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M, Berman N, and Hein A (1976). Recovery of function in cat visual cortex following prolonged deprivation. Exp. Brain Res. 25, 139–156. [DOI] [PubMed] [Google Scholar]
- Dani VS, and Nelson SB (2009). Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J. Neurosci. 29, 11263–11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, and Bear MF (2008). Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J. Physiol. 586, 1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, and Bear MF (2007). Correction of fragile X syndrome in mice. Neuron 56, 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Patrizi A, Quast KB, Hachigian L, Pavlyuk R, Saxena A, Carninci P, Hensch TK, and Fagiolini M (2012). NMDA receptor regulation prevents regression of visual cortical function in the absence of Mecp2. Neuron 76, 1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, and Stryker MP (2012). Development and plasticity of the primary visual cortex. Neuron 75, 230–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, and Maffei L (1994). Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 34, 709–720. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Löw K, Möhler H, Rudolph U, and Hensch TK (2004). Specific GABAA circuits for visual cortical plasticity. Science 303, 1681–1683. [DOI] [PubMed] [Google Scholar]
- Feese BD, Pafundo DE, Schmehl MN, and Kuhlman SJ (2018). Binocular deprivation induces both age-dependent and age-independent forms of plasticity in parvalbumin inhibitory neuron visual response properties. J. Neurophysiol. 119, 738–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Packer AM, and Yuste R (2013). The logic of inhibitory connectivity in the neocortex. Neuroscientist 19, 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong MF, Mitchell DE, Duffy KR, and Bear MF (2016). Rapid recovery from the effects of early monocular deprivation is enabled by temporary inactivation of the retinas. Proc Natl Acad Sci U S A 113, 14139–14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, and Bear MF (2004). How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron 44, 917–923. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, and Stryker MP (2014). A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, and Heintz N (2013). GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80, 1368–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, and Shatz CJ (1990). Requirement for subplate neurons in the formation of thalamocortical connections. Nature 347, 179–181. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, and Hensch TK (2009). Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J. Neurodev. Disord. 1, 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, and Stryker MP (1996). Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J. Neurosci. 16, 3274–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouwens NW, Sorensen SA, Berg J, Lee C, Jarsky T, Ting J, Sunkin SM, Feng D, Anastassiou CA, Barkan E, et al. (2019). Classification of electrophysiological and morphological neuron types in the mouse visual cortex. Nat. Neurosci. 22, 1182–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, and Cang J (2016). Binocular matching of thalamocortical and intracortical circuits in the mouse visual cortex. eLife 5, e22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Huang S, Chang MC, Worley P, Kirkwood A, and Quinlan EM (2013). Obligatory role for the immediate early gene NARP in critical period plasticity. Neuron 79, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik-Kornacka A, van der Bourg A, Vajda F, Joly S, Christ F, Schwab ME, and Pernet V (2016). Nogo-A deletion increases the plasticity of the optokinetic response and changes retinal projection organization in the adult mouse visual system. Brain Struct. Funct. 221, 317–329. [DOI] [PubMed] [Google Scholar]
- Halassa MM, and Kastner S (2017). Thalamic functions in distributed cognitive control. Nat. Neurosci. 20, 1669–1679. [DOI] [PubMed] [Google Scholar]
- Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, Viegi A, Berardi N, and Maffei L (2010). Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J. Neurosci. 30, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK (2005). Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888. [DOI] [PubMed] [Google Scholar]
- Hensch TK, and Quinlan EM (2018). Critical periods in amblyopia. Vis. Neurosci. 35, E014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, and Bear MF (2003). Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat. Neurosci. 6, 854–862. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, and Arber S (2005). A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 3, e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SB, Ko H, Pichler B, Vogelstein J, Ros H, Zeng H, Lein E, Lesica NA, and Mrsic-Flogel TD (2011). Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat. Neurosci. 14, 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Park S, Litvina EY, Morales J, Sanes JR, and Chen C (2014). Refinement of the retinogeniculate synapse by bouton clustering. Neuron 84, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, and Chen C (2006). Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 52, 281–291. [DOI] [PubMed] [Google Scholar]
- Hooks BM, and Chen C (2007). Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron 56, 312–326. [DOI] [PubMed] [Google Scholar]
- Hooks BM, and Chen C (2008). Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. J. Neurosci. 28, 4807–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Hires SA, Zhang YX, Huber D, Petreanu L, Svoboda K, and Shepherd GM (2011). Laminar analysis of excitatory local circuits in vibrissal motor and sensory cortical areas. PLoS Biol. 9, e1000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoseini MS, Rakela B, Flores-Ramirez Q, Hasenstaub AR, Alvarez-Buylla A, and Stryker MP (2019). Transplanted Cells Are Essential for the Induction But Not the Expression of Cortical Plasticity. J. Neurosci. 39, 7529–7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth M, Walmsley L, and Brown TM (2014). Binocular integration in the mouse lateral geniculate nuclei. Curr. Biol. 24, 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, and Tonegawa S (1999). BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755. [DOI] [PubMed] [Google Scholar]
- Hubel DH, and Wiesel TN (1962). Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. 160, 106–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EC, Campagnoni C, Kampf K, Reyes SD, Kalra V, Handley V, Xie YY, Hong-Hu Y, Spreur V, Fisher RS, and Campagnoni AT (2007). Visualization of corticofugal projections during early cortical development in a tau-GFP-transgenic mouse. Eur. J. Neurosci. 25, 17–30. [DOI] [PubMed] [Google Scholar]
- Jaepel J, Hu€bener M, Bonhoeffer T, and Rose T (2017). Lateral geniculate neurons projecting to primary visual cortex show ocular dominance plasticity in adult mice. Nat. Neurosci. 20, 1708–1714. [DOI] [PubMed] [Google Scholar]
- Jiang B, Sohya K, Sarihi A, Yanagawa Y, and Tsumoto T (2010). Laminar-specific maturation of GABAergic transmission and susceptibility to visual deprivation are related to endocannabinoid sensitivity in mouse visual cortex. J. Neurosci. 30, 14261–14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish BT, Cheadle L, Hrvatin S, Nagy MA, Rivera S, Crow M, Gillis J, Kirchner R, and Greenberg ME (2018). Single-cell transcriptomics of the developing lateral geniculate nucleus reveals insights into circuit assembly and refinement. Proc. Natl. Acad. Sci. USA 115, E1051–E1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E, Durand S, LeBlanc JJ, Hensch TK, Chen C, and Fagiolini M (2013). Visual acuity development and plasticity in the absence of sensory experience. J. Neurosci. 33, 17789–17796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kätzel D, Zemelman BV, Buetfering C, Wölfel M, and Miesenböck G (2011). The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nat. Neurosci. 14, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlin AM, Andermann ML, Berezovskii VK, and Reid RC (2010). Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67, 858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AG, Poort J, Chadwick A, Blot A, Sahani M, Mrsic-Flogel TD, and Hofer SB (2018). Distinct learning-induced changes in stimulus selectivity and interactions of GABAergic interneuron classes in visual cortex. Nat. Neurosci. 21, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L (2015). Visual development in primates: neural mechanisms and critical periods. Dev. Neurobiol. 75, 1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, and Bear MF (1996). Experience-dependent modification of synaptic plasticity in visual cortex. Nature 381, 526–528. [DOI] [PubMed] [Google Scholar]
- Ko H, Hofer SB, Pichler B, Buchanan KA, Sjöström PJ, and Mrsic-Flogel TD (2011). Functional specificity of local synaptic connections in neocortical networks. Nature 473, 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Cossell L, Baragli C, Antolik J, Clopath C, Hofer SB, and Mrsic-Flogel TD (2013). The emergence of functional microcircuits in visual cortex. Nature 496, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Mrsic-Flogel TD, and Hofer SB (2014). Emergence of feature-specific connectivity in cortical microcircuits in the absence of visual experience. J. Neurosci. 34, 9812–9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe TE, El-Danaf RN, Dilger EK, Henderson SC, and Guido W (2011). Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. J. Neurosci. 31, 17437–17448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Wang BS, Lu J, Wang L, Maffei A, Cang J, and Huang ZJ (2015). MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proc. Natl. Acad. Sci. USA 112, E4782–E4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A, Wimmer RD, Halassa MM, and Feng G (2018). Thalamic Reticular Dysfunction as a Circuit Endophenotype in Neurodevelopmental Disorders. Neuron 98, 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan L, Li Y, Lau C, Feng D, Bernard A, Sunkin SM, Zeng H, Dang C, Hawrylycz M, and Ng L (2015). Neuroinformatics of the Allen Mouse Brain Connectivity Atlas. Methods 73, 4–17. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, and Trachtenberg JT (2013). A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 501, 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambo ME, and Turrigiano GG (2013). Synaptic and intrinsic homeostatic mechanisms cooperate to increase L2/3 pyramidal neuron excitability during a late phase of critical period plasticity. J. Neurosci. 33, 8810–8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc JJ, and Fagiolini M (2011). Autism: a “critical period” disorder? Neural Plast. 2011, 921680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, and Rudy B (2010). The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. 30, 16796–16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, and Rudy B (2013). A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 16, 1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Hoy JL, Bonci A, Wilbrecht L, Stryker MP, and Niell CM (2014). Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron 83, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Lee J, and Kim E (2017). Excitation/Inhibition Imbalance in Animal Models of Autism Spectrum Disorders. Biol. Psychiatry 81, 838–847. [DOI] [PubMed] [Google Scholar]
- Lefort S, Tomm C, Floyd Sarria JC, and Petersen CC (2009). The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron 61, 301–316. [DOI] [PubMed] [Google Scholar]
- Levine JN, Chen H, Gu Y, and Cang J (2017). Environmental Enrichment Rescues Binocular Matching of Orientation Preference in the Mouse Visual Cortex. J. Neurosci. 37, 5822–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden ML, Heynen AJ, Haslinger RH, and Bear MF (2009). Thalamic activity that drives visual cortical plasticity. Nat. Neurosci. 12, 390–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Heynen AJ, Shuler MG, and Bear MF (2008). Cannabinoid receptor blockade reveals parallel plasticity mechanisms in different layers of mouse visual cortex. Neuron 58, 340–345. [DOI] [PubMed] [Google Scholar]
- Luo J, Norris RH, Gordon SL, and Nithianantharajah J (2018). Neurodevelopmental synaptopathies: insights from behaviour in rodent models of synapse gene mutations. Prog. Neuropsychopharmacol. Biol. Psychiatry 84 (Pt B), 424–439. [DOI] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ 3rd, Gu X, Zanella S, et al. (2012). A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, and Turrigiano GG (2006). Potentiation of cortical inhibition by visual deprivation. Nature 443, 81–84. [DOI] [PubMed] [Google Scholar]
- Marques-Smith A, Lyngholm D, Kaufmann AK, Stacey JA, Hoerder-Suabedissen A, Becker EB, Wilson MC, Molnár Z, and Butt SJ (2016). A Transient Translaminar GABAergic Interneuron Circuit Connects Thalamocortical Recipient Layers in Neonatal Somatosensory Cortex. Neuron 89, 536–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, and Shatz CJ (1989). Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science 245, 978–982. [DOI] [PubMed] [Google Scholar]
- Miao Q, Yao L, Rasch MJ, Ye Q, Li X, and Zhang X (2016). Selective Maturation of Temporal Dynamics of Intracortical Excitatory Transmission at the Critical Period Onset. Cell Rep. 16, 1677–1689. [DOI] [PubMed] [Google Scholar]
- Miska NJ, Richter LM, Cary BA, Gjorgjieva J, and Turrigiano GG (2018). Sensory experience inversely regulates feedforward and feedback excitation-inhibition ratio in rodent visual cortex. eLife 7, e38846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Berger DR, Wetzel AW, and Lichtman JW (2016). The Fuzzy Logic of Network Connectivity in Mouse Visual Thalamus. Cell 165, 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern NA, Bourg J, and Petreanu L (2016). Multilaminar networks of cortical neurons integrate common inputs from sensory thalamus. Nat. Neurosci. 19, 1034–1040. [DOI] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N, and Hensch TK (2010). Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 330, 1238–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, and Hu€bener M (2007). Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron 54, 961–972. [DOI] [PubMed] [Google Scholar]
- Nelson SB, and Valakh V (2015). Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron 87, 684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA 3rd, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, and Guthrie D (2007). Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science 318, 1937–1940. [DOI] [PubMed] [Google Scholar]
- Niell CM, and Stryker MP (2010). Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noutel J, Hong YK, Leu B, Kang E, and Chen C (2011). Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice. Neuron 70, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlaender M, Ramirez A, and Bruno RM (2012). Sensory experience restructures thalamocortical axons during adulthood. Neuron 74, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orefice LL, Zimmerman AL, Chirila AM, Sleboda SJ, Head JP, and Ginty DD (2016). Peripheral Mechanosensory Neuron Dysfunction Underlies Tactile and Behavioral Deficits in Mouse Models of ASDs. Cell 166, 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, and Kara DA (1985). The neuronal composition of area 17 of rat visual cortex. I. The pyramidal cells. J. Comp. Neurol. 234, 218–241. [DOI] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, and Scanziani M (2013). Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 16, 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, and Kepecs A (2013). Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscopo DM, El-Danaf RN, Huberman AD, and Niell CM (2013). Diverse visual features encoded in mouse lateral geniculate nucleus. J. Neurosci. 33, 4642–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poort J, Khan AG, Pachitariu M, Nemri A, Orsolic I, Krupic J, Bauza M, Sahani M, Keller GB, Mrsic-Flogel TD, and Hofer SB (2015). Learning Enhances Sensory and Multiple Non-sensory Representations in Primary Visual Cortex. Neuron 86, 1478–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafols JA, and Valverde F (1973). The structure of the dorsal lateral geniculate nucleus in the mouse. A Golgi and electron microscopic study. J. Comp. Neurol. 150, 303–332. [DOI] [PubMed] [Google Scholar]
- Rose T, and Bonhoeffer T (2018). Experience-dependent plasticity in the lateral geniculate nucleus. Curr. Opin. Neurobiol. 53, 22–28. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, and Merzenich MM (2003). Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnaik R, Wang BS, and Cang J (2014). Experience-dependent and independent binocular correspondence of receptive field subregions in mouse visual cortex. Cereb. Cortex 24, 1658–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, and Stryker MP (2008). Distinctive features of adult ocular dominance plasticity. J. Neurosci. 28, 10278–10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, and Stryker MP (2010). Genomic imprinting of experience-dependent cortical plasticity by the ubiquitin ligase gene Ube3a. Proc. Natl. Acad. Sci. USA 107, 5611–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala F, Kobak D, Shan S, Bernaerts Y, Laturnus S, Cadwell CR, Hartmanis L, Froudarakis E, Castro JR, Tan ZH, et al. (2019). Layer 4 of mouse neocortex differs in cell types and circuit organization between sensory areas. Nat. Commun. 10, 4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TA, El-Danaf RN, Krahe TE, Fox MA, and Guido W (2013). Retinal input regulates the timing of corticogeniculate innervation. J. Neurosci. 33, 10085–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, and Spear PD (1982). Organization of visual pathways in normal and visually deprived cats. Physiol. Rev. 62, 738–855. [DOI] [PubMed] [Google Scholar]
- Soden ME, and Chen L (2010). Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J. Neurosci. 30, 16910–16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommeijer JP, Ahmadlou M, Saiepour MH, Seignette K, Min R, Heimel JA, and Levelt CN (2017). Thalamic inhibition regulates critical-period plasticity in visual cortex and thalamus. Nat. Neurosci. 20, 1715–1721. [DOI] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, and Gandhi SP (2010). Cortical plasticity induced by inhibitory neuron transplantation. Science 327, 1145–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephany CE, Ma X, Dorton HM, Wu J, Solomon AM, Frantz MG, Qiu S, and McGee AW (2018). Distinct Circuits for Recovery of Eye Dominance and Acuity in Murine Amblyopia. Curr. Biol. 28, 1914–1923.e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Clark E, Schulmann A, Shima Y, Wang L, Hunt DL, Hooks BM, Tränkner D, Chandrashekar J, Picard S, et al. (2019). Mapping the transcriptional diversity of genetically and anatomically defined cell populations in the mouse brain. eLife 8, e38619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Bogart LJ, Lichtman JW, and Hensch TK (2018). Inhibitory circuit gating of auditory critical-period plasticity. Nat. Neurosci. 21, 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. (2011). A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, Yao Z, Graybuck LT, Smith KA, Nguyen TN, Bertagnolli D, Goldy J, Garren E, Economo MN, Viswanathan S, et al. (2018). Shared and distinct transcriptomic cell types across neocortical areas. Nature 563, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AD, Picard N, Min L, Fagiolini M, and Chen C (2016). Cortical Feedback Regulates Feedforward Retinogeniculate Refinement. Neuron 91, 1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoizumi T, Miyamoto H, Yazaki-Sugiyama Y, Atapour N, Hensch TK, and Miller KD (2013). A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron 80, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, and Stryker MP (2001). Rapid anatomical plasticity of horizontal connections in the developing visual cortex. J. Neurosci. 21, 3476–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Trepel C, and Stryker MP (2000). Rapid extragranular plasticity in the absence of thalamocortical plasticity in the developing primary visual cortex. Science 287, 2029–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay R, Lee S, and Rudy B (2016). GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 91, 260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncdemir SN, Wamsley B, Stam FJ, Osakada F, Goulding M, Callaway EM, Rudy B, and Fishell G (2016). Early Somatostatin Interneuron Connectivity Mediates the Maturation of Deep Layer Cortical Circuits. Neuron 89, 521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss P (2013). Sensitive and critical periods in visual sensory deprivation. Front. Psychol. 4, 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ML, van Woerden GM, Elgersma Y, Smith SL, and Philpot BD (2017). Ube3a loss increases excitability and blunts orientation tuning in the visual cortex of Angelman syndrome model mice. J. Neurophysiol. 118, 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BS, Sarnaik R, and Cang J (2010). Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron 65, 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt S, Koldewyn K, and Kanwisher N (2012). Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci Biobehav. Rev. 36, 1060–1084. [DOI] [PubMed] [Google Scholar]
- Weiler N, Wood L, Yu J, Solla SA, and Shepherd GM (2008). Top-down laminar organization of the excitatory network in motor cortex. Nat. Neurosci. 11, 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt JL, Petrus E, and Lee HK (2014). Experience-dependent homeostatic synaptic plasticity in neocortex. Neuropharmacology 78, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, and Hubel DH (1963a). Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, and Hubel DH (1963b). Effects of Visual Deprivation on Morphology and Physiology of Cells in the Cats Lateral Geniculate Body. J. Neurophysiol. 26, 978–993. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Runyan CA, Wang FL, and Sur M (2012). Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DE, Smith GB, Jacob AL, Walker T, Dimidschstein J, Fishell G, and Fitzpatrick D (2017). GABAergic Neurons in Ferret Visual Cortex Participate in Functionally Specific Networks. Neuron 93, 1058–1065.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DE, Scholl B, and Fitzpatrick D (2018). Differential tuning of excitation and inhibition shapes direction selectivity in ferret visual cortex. Nature 560, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, and Callaway EM (2010). Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 518, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Olivas ND, Ikrar T, Peng T, Holmes TC, Nie Q, and Shi Y (2016). Primary visual cortex shows laminar-specific and balanced circuit organization of excitatory and inhibitory synaptic connectivity. J. Physiol. 594, 1891–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Atallah BV, and Scanziani M (2014). Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 511, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger CE, Ringach DL, and Trachtenberg JT (2019). Neuromodulatory control of localized dendritic spiking in critical period cortex. Nature 567, 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, Weinberg RJ, Ehlers MD, and Philpot BD (2009). Ube3a is required for experience-dependent maturation of the neocortex. Nat. Neurosci. 12, 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. (2011). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Dantzker JL, and Callaway EM (2005). Excitatory cortical neurons form fine-scale functional networks. Nature 433, 868–873. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu H, Agmon A, and Svoboda K (2019). Recruitment of GABAergic Interneurons in the Barrel Cortex during Active Tactile Behavior. Neuron 104, 412–427.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY, and Bear MF (2012). Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol. 4, a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]