Abstract
Background
Inflammation plays an important part in the pathogenesis of cardiogenic shock (CGS). Whether the neutrophil-lymphocyte ratio (NLR), an integrated biomarker of inflammation, is associated with the outcome of CGS patients remains unknown. This retrospective cohort study was performed to identify the utility of using NLR among patients with CGS.
Material/Methods
Data were extracted from the MIMIC database. We applied smooth curve fitting to define the NLR cutoff values. The primary outcome was 30-day mortality. Cox proportional hazards models, subgroup analysis, and receiver operator characteristic (ROC) curve analysis were performed.
Results
A total of 1470 CGS patients were extracted, among which 801 (54.5%) were men. The mean age of the population was 70.37 years. An inverse U-shaped relationship was observed between NLR and mortality in CGS patients, with the highest risk being at values ranging from 9.4 to 15. For the primary outcome of 30-day mortality, the adjusted HR (95% CI) values of the middle tertile (NLR 9.4–15) and the upper tertile (NLR >15) were 1.47 (1.14, 1.88) and 1.22 (0.94, 1.57) compared with the reference of lower tertile (NLR <9.4). ROC curve analysis showed that NLR had a more sensitive prognostic value in predicting 30-day mortality of CGS than the neutrophil or lymphocyte percentage alone (0.660 vs. 0.540, 0.549).
Conclusions
An inverse U-shaped curve was presented between NLR and the mortality of CGS. NLR seemed to be a readily available and independent prognostic biomarker for patients with CGS. The prognostic value of NLR was more sensitive than the neutrophil or lymphocyte percentage alone, but not as good as SOFA or SAPSII score.
MeSH Keywords: Immunity; Inflammation; Lymphocytes; Neutrophils; Shock, Cardiogenic
Background
Cardiogenic shock (CGS), a severe state of systemic hypoperfusion, is an urgent complication of cardiovascular diseases. Beginning with cardiac dysfunction, CGS often leads to multiple organ failure and, ultimately, death [1,2]. Although less frequent than other fatal diseases, in-hospital and overall mortality of CGS are unacceptably high. Due to the poor outcomes, finding effective prognostic indicators in CGS patients would assist physicians to make medical decisions and identify patients at high risk.
Studies have shown that inflammation plays a vital part in the development of cardiovascular diseases. As a fatal complication of cardiac diseases, CGS has also been reported to be associated with inflammation. Among inflammatory factors, neutrophils are considered to be a marker of inflammation [3]. The neutrophil-lymphocyte ratio (NLR), a recently introduced biomarker of inflammation [4,5], is combined with neutrophil and lymphocyte counts. Easily acquired from routine laboratory tests, NLR has already been used to predict the outcome of neoplastic diseases [6–9]. Recently, more studies have detected the prognostic value of NLR in cardiovascular events. One study reported that a high NLR is associated with poor prognosis in patients with acute coronary syndrome [10]. Another showed that NLR is a useful predictor of long-term mortality in patients undergoing percutaneous coronary intervention [11]. Our recent study found that NLR was an independent predictor of 30- and 90-day mortality for coronary care unit (CCU) patients [12].
No study up to now, however, has assessed the prognostic value of NLR in CGS patients. Therefore, this retrospective cohort study was performed to explore the associations between NLR and mortality in CGS patients.
Material and Methods
Data source
We extracted all data from a large, open, and free database – the Medical Information Mart for Intensive Care Database III version 1.4 (MIMIC-III v1.4). The database contains more than 50 000 patients admitted to the CCU at Beth Israel Deaconess Medical Center from 2001 to 2012 [13]. The establishment and employment of this database were approved by the Institutional Review Boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. The database de-identified all personal information to safeguard patient privacy.
Population selection criteria
From the MIMIC-III database, we just extracted the patients definitely diagnosed with CGS. The definition of CGS was determined on the basis of the Ninth Revision of International Classification of Diseases (ICD-9), coded as R57.001. These patients be over age 16 years at first admission, and the length of stay in hospital must exceed 48 h. Patients were excluded if they met any of the following conditions: (1) diagnosed with hematologic tumors, such as multiple myeloma and leukemia; (2) more than 10% of individual data is missing; (3) individual data values exceeded the mean ±3 times the standard deviation (SD).
Date extraction
Data were extracted through Structured Query Language (SQL) [14] with MySQL tools from MIMIC-III. We recorded baseline characteristics within 24 h after patients were first admitted to the ICU, including demographic parameters, basic vital signs, laboratory indicators, and scoring systems.
Demographic parameters contained age, sex, and ethnicity. Basic vital signs were temperature, heart rate, respiratory rate, mean blood pressure (MBP), systolic blood pressure (SBP), diastolic blood pressure (DBP), and percutaneous oxygen saturation (SPO2). Laboratory indicators included anion gap, serum potassium, serum sodium, serum bicarbonate, and serum glucose. Relevant comorbidities were also extracted, including coronary heart disease (CAD), atrial fibrillation (AF), congestive heart failure (CHF), pneumonia, respiratory failure, chronic liver disease, chronic renal disease, stroke, and malignancy. Clinical severity scales, including the Sequential Organ Failure Assessment (SOFA) [15] score and the Simplified Acute Physiology Score II (SAPS II) [16], were also extracted. These scores were assessed according to the published recommendations and well-accepted formulas.
NLR was defined as the ratio of the neutrophil count to the lymphocyte count. Our study began on the patients’ first admission to the ICU. The outcomes of our study were 30-, 90-, and 365-day mortality. The primary outcome was 30-day mortality.
Processing of missing values
For missing dependent variables, we did a sensitivity comparative analysis between participants with vs. without NLR data. The purpose of this sensitivity analysis was to investigate whether NLR missing is random, and whether it would bias our findings. Our results demonstrated that nearly all variables were similar in patients with available data on NLR and participants with missing data on NLR.
For missing covariates, we used multiple multivariate imputations. Our purpose was to maximize statistical power and minimize bias that might occur when covariates with missing data were excluded from data analyses. We created 5 imputed datasets with chained equations using a Mice software package. In addition, we used sensitivity analysis to identify whether the created complete data were significantly different from pre-imputation data. Our findings demonstrated that the created complete data showed no significant difference from raw data. Therefore, all results of our multivariable analyses were based on the imputed datasets and were combined with Rubin’s rules.
Statistical analysis
Baseline characteristics were presented according to the cutoffs of NLR level. Categorical data were expressed as frequency (percentage) and continuous data as mean (SD). Comparisons between groups were performed by chi-square test [17] or Fisher’s exact test [18] for categorical data and the variance analysis or the Kruskal-Wallis test [19] for continuous data.
Cox proportional hazards modeling [20] was performed to analyze the associations between NLR and outcomes. Every outcome was respectively analyzed according to the cutoffs of NLR level derived with curve fitting methods. The first tertile or quartile was used as the reference. The results were shown as hazard ratios (HRs) with 95% confidence intervals (CIs). Multivariate analyses were applied to further identify the relationship between NLR and mortality. The confounding factors were selected depending on their associations with outcomes or the presence of more than 10% mutations [21]. In model I, covariates of age, sex, and ethnicity were adjusted. In model II, we adjusted further for heart rate, SBP, DBP, respiratory rate, SPO2, serum bicarbonate, serum potassium, SCr, BUN, hematocrit, CAD, AF, CHF, respiratory failure, chronic renal disease, chronic liver disease, malignancy, SOFA score, and SAPSII score.
Subgroup analyses were performed to evaluate the consistency of the association between NLR and 30-day mortality.
To further test the predictive value of NLR, we performed receiver operator characteristic (ROC) curve analysis for the 30-day mortality based on the neutrophil percentage, the lymphocyte percentage, NLR, SOFA score, and SAPSII score.
We regarded a two-tailed P value <0.05 as indicating a statistically significant difference. EmpowerStats version 2.17.8 (http://www.empowerstats.com/cn/) and R software version 3.42 were used for statistical analysis.
Results
Baseline characteristics
According to the specific criteria, a total of 1470 CGS patients were included in our study. The flow chart of the included population is shown in Figure 1. The baseline characteristics of the study population were shown in Table 1. Among the included patients, 801 (54.5%) were men and most (69.9%) were white. The mean (SD) age of the population was 70.37 (13.5) years.
Figure 1.
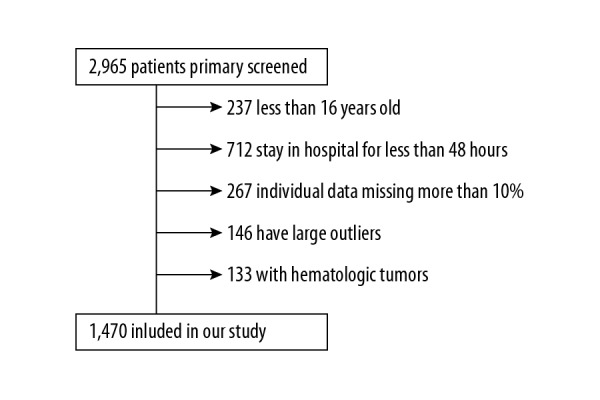
Flow chart of the population included in the study.
Table 1.
Baseline characteristics of the study population.
| Neutrophil-lymphocyte ratio | P value | |||
|---|---|---|---|---|
| <9.4 | 9.4–15 | >15 | ||
| N | 893 | 282 | 295 | |
| Age | 69.7±13.3 | 70.0±13.5 | 71.4±13.8 | 0.050 |
| Sex, n(%) | 0.004 | |||
| Female | 431 (48.3) | 104 (36.9) | 134 (45.4) | |
| Male | 462 (51.7) | 178 (63.1) | 161 (54.6) | |
| Ethnicity, n (%) | 0.003 | |||
| White | 614 (68.8) | 195 (69.1) | 218 (73.9) | |
| Black | 120 (13.4) | 20 (7.1) | 24 (8.1) | |
| Other | 159 (17.8) | 67 (23.8) | 53 (18.0) | |
| LOS_ICU | 5.8±6.8 | 7.5±9.5 | 8.6±11.5 | <0.001 |
| Vital signs | ||||
| Heart rate, beats/min | 85.1±16.4 | 89.7±15.9 | 85.3±17.0 | <0.001 |
| SBP, mmHg | 107.7±15.5 | 106.3±15.4 | 107.1±14.5 | 0.352 |
| DBP, mmHg | 57.2±9.9 | 57.3±10.5 | 56.3±9.5 | 0.374 |
| MBP, mmHg | 73.1±10.1 | 73.4±10.8 | 73.0±9.9 | 0.796 |
| Respiratory rate, beats/minute | 19.8±4.1 | 20.8±4.0 | 19.8±4.1 | <0.001 |
| Temperature, °C | 36.7±0.8 | 36.7±0.9 | 36.6±0.8 | 0.228 |
| SPO2, % | 96.7±4.1 | 96.2±4.7 | 97.1±3.0 | 0.006 |
| Laboratory parameters | ||||
| Anion gap, mmol/l | 18.1±5.0 | 18.7±4.8 | 18.4±5.2 | 0.070 |
| Serum bicarbonate, mmol/l | 25.1±5.3 | 24.1±4.2 | 24.0±4.7 | <0.001 |
| Serum sodium, mmol/l | 139.2±4.5 | 139.4±4.5 | 139.6±5.6 | 0.349 |
| Serum potassium, mmol/l | 4.9±1.0 | 5.0±1.0 | 5.0±1.1 | 0.213 |
| Serum chloride, mmol/l | 104.7±6.5 | 105.7±6.3 | 105.9±7.7 | 0.011 |
| Serum glucose, mg/dl | 203.2±112.9 | 233.7±142.7 | 220.5±101.0 | <0.001 |
| BUN, mg/dl | 41.8±26.9 | 39.1±25.5 | 43.5±28.6 | 0.132 |
| SCr, mg/dl | 2.2±2.0 | 2.0±1.4 | 2.3±2.2 | 0.205 |
| Hematocrit | 36.0±6.4 | 36.9±6.2 | 35.9±5.7 | 0.042 |
| Hemoglobin, g/dl | 11.8±2.2 | 12.2±2.2 | 11.7±1.9 | 0.017 |
| Platelet count, 109/l | 252.9±122.0 | 277.5±121.2 | 270.0±122.4 | <0.001 |
| WBC count, 109/l | 13.2±6.8 | 15.8±6.7 | 17.1±8.4 | <0.001 |
| Neutrophil,% | 72.8±13.4 | 86.0±5.7 | 89.4±6.1 | <0.001 |
| Lymphocyte,% | 18.9±11.4 | 7.2±1.0 | 4.1±1.1 | <0.001 |
| Comorbidities, n (%) | ||||
| CAD | 455 (51.0) | 159 (56.4) | 171 (58.0) | 0.060 |
| CHF | 459 (51.4) | 113 (40.1) | 121 (41.0) | <0.001 |
| AF | 444 (49.7) | 119 (42.2) | 149 (50.5) | 0.064 |
| Stroke | 46 (5.2) | 8 (2.8) | 16 (5.4) | 0.236 |
| Pneumonia | 242 (27.1) | 90 (31.9) | 96 (32.5) | 0.105 |
| Respiratory failure | 353 (39.5) | 114 (40.4) | 144 (48.8) | 0.018 |
| Chronic liver disease | 26 (2.9) | 3 (1.1) | 12 (4.1) | 0.072 |
| Chronic renal disease | 282 (31.6) | 66 (23.4) | 68 (23.1) | 0.002 |
| Malignancy | 94 (10.5) | 30 (10.6) | 31 (10.5) | 0.998 |
| Vasoactive drug, n (%) | 549 (61.5) | 191 (67.7) | 213 (72.2) | 0.002 |
| Scoring systems, mean (Q1–Q3) | ||||
| SOFA | 6.0±3.5 | 6.3±3.3 | 6.6±3.7 | 0.069 |
| SAPSII | 42.8±14.5 | 44.9±13.6 | 46.7±15.3 | <0.001 |
N – number; LOS_ICU – length of stay in intensive care unit; SBP – systolic blood pressure; DBP – diastolic blood pressure; MBP – mean blood pressure; SPO2 – percutaneous oxygen saturation; BUN – blood urea nitrogen; SCr – serum creatinine; WBC – white blood cell; CAD – coronary heart disease; CHF – congestive heart failure; AF – atrial fibrillation; SOFA – Sequential Organ Failure Assessment; SAPSII – Simplified Acute Physiology Score. Normally distributed data are presented as the mean±SD, non-normally distributed data are presented as median (IQR) and categorical variables are presented as n (%).
Based on the 30-day mortality according to the curve-fitting method (Figure 2), we divided all included patients into 3 groups: 893 in the low-NLR group (<9.4), 282 in the middle-NLR group (9.4–15), and 295 in the high NLR group (>15). In the middle-NLR group, most patients were male and had elevated heart rate, respiratory rate, serum glucose, hematocrit, hemoglobin, and platelet count.
Figure 2.

Fitting curve between NLR and log RR for 30-day mortality.
NLR levels and mortality
The results for 30-, 90- and 365-day mortality across groups of NLR levels are presented in Table 2. We observed an inverse U-shaped relationship between NLR and mortality in CGS patients.
Table 2.
HRs (95% CIs) for mortality across groups of Neutrophil-lymphocyte ratio.
| Non-adjusted | Model I | Model II | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| 30-day mortality | ||||||
| Tertiles | ||||||
| <9.4 | 1.0 | 1.0 | 1.0 | |||
| 9.4–15 | 1.61 (1.27, 2.04) | 0.0001 | 1.56 (1.23, 1.99) | 0.0003 | 1.47 (1.14, 1.88) | 0.0026 |
| >15 | 1.38 (1.08, 1.77) | 0.0089 | 1.29 (1.01, 1.65) | 0.0387 | 1.22 (0.94, 1.57) | 0.1292 |
| Quintiles | ||||||
| 0–3.8 | 1.0 | 1.0 | 1.0 | |||
| 3.8–6.2 | 0.88 (0.63, 1.24) | 0.4646 | 0.79 (0.56, 1.12) | 0.1878 | 1.02 (0.70, 1.48) | 0.9187 |
| 6.2–9.3 | 1.02 (0.74, 1.40) | 0.9183 | 0.95 (0.69, 1.32) | 0.7656 | 1.07 (0.76, 1.51) | 0.6927 |
| 9.3–14.8 | 1.50 (1.11, 2.03) | 0.0086 | 1.38 (1.01, 1.87) | 0.0408 | 1.44 (1.04, 2.00) | 0.0275 |
| 14.8–48.8 | 1.33 (0.98, 1.81) | 0.0679 | 1.17 (0.86, 1.60) | 0.3139 | 1.25 (0.89, 1.74) | 0.1965 |
| 90-day mortality | ||||||
| Tertiles | ||||||
| <9.4 | 1.0 | 1.0 | 1.0 | |||
| 9.4–15 | 1.63 (1.33, 2.00) | <0.0001 | 1.59 (1.29, 1.96) | <0.0001 | 1.47 (1.19, 1.82) | 0.0004 |
| >15 | 1.31 (1.05, 1.62) | 0.0150 | 1.22 (0.99, 1.52) | 0.0668 | 1.10 (0.88, 1.37) | 0.3981 |
| Quintiles | ||||||
| 0–3.8 | 1.0 | 1.0 | 1.0 | |||
| 3.8–6.2 | 0.86 (0.64, 1.16) | 0.3220 | 0.78 (0.58, 1.05) | 0.1041 | 0.93 (0.68, 1.27) | 0.6417 |
| 6.2–9.3 | 1.03 (0.78, 1.36) | 0.8239 | 0.98 (0.74, 1.30) | 0.8976 | 1.07 (0.80, 1.43) | 0.6337 |
| 9.3–14.8 | 1.50 (1.16, 1.95) | 0.0022 | 1.39 (1.07, 1.81) | 0.0136 | 1.41 (1.07, 1.85) | 0.0152 |
| 14.8–48.8 | 1.25 (0.96, 1.64) | 0.1021 | 1.11 (0.85, 1.46) | 0.4407 | 1.09 (0.82, 1.45) | 0.5413 |
| 365-day mortality | ||||||
| Tertiles | ||||||
| <9.4 | 1.0 | 1.0 | 1.0 | |||
| 9.4–15 | 1.31 (1.08, 1.58) | 0.0053 | 1.29 (1.07, 1.56) | 0.0083 | 1.24 (1.02, 1.51) | 0.0286 |
| >15 | 1.18 (0.98, 1.42) | 0.0836 | 1.11 (0.92, 1.34) | 0.2856 | 0.99 (0.82, 1.21) | 0.9449 |
| Quintiles | ||||||
| 0–3.8 | 1.0 | 1.0 | 1.0 | |||
| 3.8–6.2 | 0.97 (0.76, 1.24) | 0.8152 | 0.88 (0.69, 1.13) | 0.3189 | 1.00 (0.77, 1.30) | 0.9821 |
| 6.2–9.3 | 1.14 (0.91, 1.44) | 0.2547 | 1.09 (0.86, 1.37) | 0.4863 | 1.19 (0.93, 1.52) | 0.1633 |
| 9.3–14.8 | 1.28 (1.02, 1.62) | 0.0366 | 1.20 (0.95, 1.52) | 0.1287 | 1.24 (0.97, 1.59) | 0.0823 |
| 14.8–48.8 | 1.21 (0.96, 1.53) | 0.1069 | 1.08 (0.85, 1.37) | 0.5223 | 1.04 (0.81, 1.33) | 0.7405 |
HR – hazard ratio; CI – confidence interval. Models I and II were derived from Cox proportional hazards regression models: model I covariates were adjusted for age; gender; ethnicity; model II covariates were adjusted for age; gender; ethnicity; heart rate; SBP – respiratory rate; SPO2; serum bicarbonate; serum potassium; SCr; BUN; hematocrit; CAD; CHF; AF; chronic liver disease; chronic renal disease; respiratory failure; malignancy; SOFA; SAPSII.
For the primary outcome of 30-day mortality, when NLR level was divided into tertiles, the HR (95% CI) values of the middle tertile (NLR 9.4–15) and the upper tertile (NLR >15) were 1.61 (1.27, 2.04) and 1.38 (1.08, 1.77), respectively, compared with the reference of lower tertile (NLR <9.4), and the middle tertile had higher HR than the upper tertile. When adjusted for covariates of age, sex, and ethnicity in model I, the adjusted HR (95% CI) values showed NLR of 9.4–15 and >15 were 1.56 (1.23, 1.99) and 1.29 (1.01, 1.65), and the middle tertile still had higher HR than the upper tertile. After further adjustment for heart rate, SBP, respiratory rate, and other confounding factors in model II, the adjusted HR (95% CI) of the middle tertile [1.47 (1.14, 1.88)] was still statistically significant. The adjusted HR (95% CI) of the upper tertile [1.22 (0.94, 1.57)], however, showed no statistical significance. When dividing the NLR level into quintiles, the trend among NLR quintiles was significant in the non-adjusted model and model I, but not in model II. Only the HR (95% CI) values given an NLR of 9.3–14.8 were statistically significant compared to the reference [non-adjusted model: 1.50 (1.11, 2.03), model I: 1.38 (1.01, 1.87), model II: 1.44 (1.04, 2.00)].
The results of the secondary outcomes of 90-day and 365-day mortality were not statistically significant. In tertile analysis, the adjusted HR (95% CI) values for 90-day mortality given an NLR of 9.4–15 and >15 were 1.47 (1.19, 1.82) and 1.10 (0.88, 1.37) in model II, while those for 365-day mortality were 1.24 (1.02, 1.51) and 0.99 (0.82, 1.21). In quintile analysis, the adjusted HR (95% CI) value given an NLR of 9.3–14.8 was 1.41 (1.07, 1.85) for 90-day mortality, while it was insignificant for 365-day mortality [1.24 (0.97, 1.59)].
Subgroup analyses
We performed subgroup analyses to identify the consistency of association between NLR and 30-day mortality in CGS patients. For all the factors presented in Table 3, statistically significant interactions were only found in the following: serum glucose (p=0.0461), CHF (p=0.0371), respiratory failure (p=0.0303), chronic liver disease (p=0.0114), and vasoactive drug (p=0.0364). Among them, the subgroups of chronic liver disease had the strongest interaction. Patients without the history of chronic liver disease showed a significantly higher risk of 30-day mortality for NLR 9.4–15 [HR 1.60 (95% CI 1.25, 2.04)] and NLR >15 [HR 1.29 (95% CI 1.00, 1.66)]. For patients with history of chronic liver disease, however, HR (95% CI) for NLR 9.4–15 was invalid and for NLR >15 was of nonsignificant [0.31 (0.07, 1.34)]. In addition, patients with respiratory failure had a lower 30-day mortality risk for NLR 9.4–15 [HR 1.07 (95% CI 0.74, 1.55) vs. HR 2.05 (95% CI 1.48, 2.83)] and for NLR >15 [HR 1.09 (95% CI 0.78, 1.51) vs. HR 1.40 (95% CI 0.97, 2.02)]. For the subgroup of vasoactive drug, CHF, and serum glucose, the statistical significance was relatively weak. All results are presented in Table 3.
Table 3.
Subgroup analysis of the associations between the NLR and 30-day mortality.
| N | Stratification of NLR | P for interaction | |||
|---|---|---|---|---|---|
| <9.4 | 9.4–15 | >15 | |||
| Vital signs | |||||
| Heart rate, beats/min | 0.4379 | ||||
| 45.27–84.79 | 728 | 1.0 | 1.77 (1.17, 2.69)** | 1.38 (0.94, 2.02) | |
| 84.79–141 | 736 | 1.0 | 1.33 (0.99, 1.80) | 1.21 (0.87, 1.68) | |
| SBP, mmHg | 0.9420 | ||||
| 50.86–105.80 | 731 | 1.0 | 1.41 (1.03, 1.92)* | 1.05 (0.77, 1.43) | |
| 105.80–173.85 | 732 | 1.0 | 1.93 (1.30, 2.85)** | 1.56 (1.03, 2.37)* | |
| DBP, mmHg | 0.4359 | ||||
| 17–56.26 | 730 | 1.0 | 1.68 (1.21, 2.33)** | 1.28 (0.92, 1.79) | |
| 56.26–95.97 | 733 | 1.0 | 1.48 (1.03, 2.12)* | 1.27 (0.87, 1.84) | |
| MBP, mmHg | 0.5308 | ||||
| 37.92–72.18 | 728 | 1.0 | 1.73 (1.27, 2.36)# | 1.25 (0.91, 1.71) | |
| 72.18–130.69 | 736 | 1.0 | 1.43 (0.97, 2.11) | 1.29 (0.87, 1.93) | |
| Respiratory rate, beats/min | 0.4965 | ||||
| 11.44–19.49 | 729 | 1.0 | 1.82 (1.23, 2.69)** | 1.30 (0.89, 1.88) | |
| 19.49–40.5 | 735 | 1.0 | 1.35 (0.99, 1.84) | 1.27 (0.91, 1.77) | |
| Temperature, °C | 0.4824 | ||||
| 32.2–36.69 | 714 | 1.0 | 1.96 (1.41, 2.71)# | 1.52 (1.10, 2.09)* | |
| 36.69–39.72 | 715 | 1.0 | 1.39 (0.96, 2.03) | 1.05 (0.71, 1.57) | |
| SPO2, % | 0.1605 | ||||
| 41.92–97.43 | 727 | 1.0 | 1.96 (1.44, 2.66)# | 1.37 (0.96, 1.94) | |
| 97.43–100 | 737 | 1.0 | 1.15 (0.77, 1.72) | 1.27 (0.89, 1.80) | |
| Laboratory parameters | |||||
| Anion gap, mmol/l | 0.9491 | ||||
| 5–16 | 581 | 1.0 | 1.50 (0.90, 2.52) | 1.35 (0.83, 2.18) | |
| 17–44 | 877 | 1.0 | 1.53 (1.16, 2.00)** | 1.25 (0.94, 1.66) | |
| Bicarbonate, mmol/l | 0.2634 | ||||
| 9–24 | 740 | 1.0 | 1.30 (0.95, 1.77) | 1.25 (0.93, 1.68) | |
| 25–47 | 725 | 1.0 | 1.94 (1.32, 2.85)# | 1.18 (0.76, 1.83) | |
| Serum sodium, mmol/l | 0.3463 | ||||
| 108–138 | 596 | 1.0 | 1.44 (0.99, 2.08) | 1.36 (0.94, 1.95) | |
| 139–167 | 873 | 1.0 | 1.67 (1.21, 2.30)** | 1.26 (0.91, 1.76) | |
| Serum potassium, mmol/l | 0.2768 | ||||
| 3–4.6 | 684 | 1.0 | 1.99 (1.38, 2.88)# | 1.35 (0.91, 1.99) | |
| 4.7–11.4 | 786 | 1.0 | 1.28 (0.93, 1.77) | 1.23 (0.90, 1.69) | |
| Serum chloride, mmol/l | 0.7914 | ||||
| 67–104 | 680 | 1.0 | 1.60 (1.11, 2.29)* | 1.45 (1.02, 2.07)* | |
| 105–138 | 786 | 1.0 | 1.57 (1.13, 2.17)** | 1.13 (0.80, 1.58) | |
| Serum glucose, mg/dl | 0.0461* | ||||
| 42–180 | 732 | 1.0 | 1.70 (1.15, 2.50)** | 1.27 (0.85, 1.89) | |
| 181–1075 | 737 | 1.0 | 1.44 (1.06, 1.97)* | 1.24 (0.91, 1.69) | |
| BUN, mg/dl | 0.1493 | ||||
| 4–33 | 728 | 1.0 | 1.63 (1.10, 2.42)* | 0.79 (0.49, 1.28) | |
| 34–204 | 742 | 1.0 | 1.60 (1.18, 2.18)** | 1.74 (1.30, 2.32)# | |
| SCr, mg/dl | 0.9255 | ||||
| 0.2–1.5 | 708 | 1.0 | 1.57 (1.02, 2.42)* | 1.05 (0.67, 1.62) | |
| 1.6–29.6 | 762 | 1.0 | 1.56 (1.16, 2.09)** | 1.47 (1.09, 1.98)* | |
| Hematocrit | 0.7410 | ||||
| 19.6–35.3 | 728 | 1.0 | 1.78 (1.24, 2.56)** | 1.58 (1.12, 2.24)** | |
| 35.4–63 | 739 | 1.0 | 1.37 (0.99, 1.89) | 1.09 (0.77, 1.55) | |
| Hemoglobin, g/dl | 0.7578 | ||||
| 6.6–11.5 | 716 | 1.0 | 1.56 (1.07, 2.27)* | 1.51 (1.07, 2.14)* | |
| 11.6–21 | 749 | 1.0 | 1.49 (1.08, 2.05)* | 1.10 (0.78, 1.57) | |
| Platelet count, 109/l | 0.9401 | ||||
| 21–237 | 726 | 1.0 | 1.26 (0.87, 1.82) | 1.39 (0.99, 1.94) | |
| 238–1219 | 741 | 1.0 | 1.91 (1.37, 2.64)# | 1.24 (0.87, 1.77) | |
| WBC count, 109/l | 0.4974 | ||||
| 0.1–13.5 | 728 | 1.0 | 1.54 (1.01, 2.37)* | 1.14 (0.72, 1.80) | |
| 13.6–253.4 | 737 | 1.0 | 1.30 (0.96, 1.74) | 1.10 (0.82, 1.47) | |
| Comorbidities | |||||
| CAD | 0.6145 | ||||
| No | 685 | 1.0 | 1.45 (1.01, 2.09)* | 1.39 (0.97, 1.99) | |
| Yes | 785 | 1.0 | 1.77 (1.27, 2.47)# | 1.28 (0.91, 1.79) | |
| CHF | 0.0371* | ||||
| No | 777 | 1.0 | 1.20 (0.88, 1.65) | 1.03 (0.75, 1.42) | |
| Yes | 693 | 1.0 | 2.18 (1.49, 3.18)# | 1.64 (1.12, 2.39)* | |
| AF | 0.7238 | ||||
| No | 758 | 1.0 | 1.67 (1.20, 2.33)** | 1.23 (0.85, 1.78) | |
| Yes | 712 | 1.0 | 1.47 (1.03, 2.09)* | 1.34 (0.96, 1.87) | |
| Stroke | 0.6901 | ||||
| No | 1400 | 1.0 | 1.55 (1.22, 1.98)# | 1.32 (1.03, 1.69)* | |
| Yes | 70 | 1.0 | 1.50 (0.28, 8.03) | 0.69 (0.14, 3.34) | |
| Pneumonia | 0.1523 | ||||
| No | 1042 | 1.0 | 1.56 (1.15, 2.11)** | 1.52 (1.13, 2.05)** | |
| Yes | 428 | 1.0 | 1.65 (1.10, 2.48)* | 0.95 (0.61, 1.47) | |
| Respiratory failure | 0.0303* | ||||
| No | 859 | 1.0 | 2.05 (1.48, 2.83)# | 1.40 (0.97, 2.02) | |
| Yes | 611 | 1.0 | 1.07 (0.74, 1.55) | 1.09 (0.78, 1.51) | |
| Chronic liver disease | 0.0114* | ||||
| No | 1429 | 1.0 | 1.60 (1.25, 2.04)# | 1.29 (1.00, 1.66)* | |
| Yes | 41 | 1.0 | N* | 0.31 (0.07, 1.34) | |
| Chronic renal disease | 0.8592 | ||||
| No | 1054 | 1.0 | 1.49 (1.12, 1.99)** | 1.24 (0.93, 1.66) | |
| Yes | 416 | 1.0 | 1.72 (1.10, 2.70)* | 1.40 (0.89, 2.23) | |
| Malignancy | 0.1726 | ||||
| No | 1315 | 1.0 | 1.70 (1.31, 2.20)# | 1.40 (1.07, 1.82)* | |
| Yes | 155 | 1.0 | 0.94 (0.46, 1.92) | 0.81 (0.40, 1.64) | |
| Vasoactive drug | 0.0364* | ||||
| No | 517 | 1.0 | 2.77 (1.65, 4.66)# | 1.17 (0.59, 2.32) | |
| Yes | 953 | 1.0 | 1.26 (0.96, 1.66) | 1.17 (0.90, 1.52) | |
| Score systems | |||||
| SOFA | 0.5176 | ||||
| 0–5 | 689 | 1.0 | 1.93 (1.22, 3.06)** | 1.26 (0.76, 2.08) | |
| 6–21 | 781 | 1.0 | 1.40 (1.05, 1.85)* | 1.22 (0.92, 1.61) | |
| SAPSII | 0.8502 | ||||
| 10–41 | 688 | 1.0 | 1.59 (0.94, 2.70) | 1.56 (0.92, 2.63) | |
| 42–110 | 782 | 1.0 | 1.40 (1.06, 1.84)* | 1.14 (0.86, 1.50) | |
SBP – systolic blood pressure; DBP – diastolic blood pressure; MBP – mean blood pressure; SPO2 – percutaneous oxygen saturation; BUN – blood urea nitrogen; SCr – serum creatinine; WBC – white blood cell; CAD – coronary heart disease; CHF – congestive heart failure; AF – atrial fibrillation; SOFA – Sequential Organ Failure Assessment; SAPSII – Simplified Acute Physiology Score.
P value:
P<0.05;
P<0.01;
P<0.001.
ROC curve analysis
ROC curve analysis was performed to assess the potential prognostic value of NLR in CGS patients (Figure 3). Compared with the neutrophil or lymphocyte percentage alone, NLR was more sensitive in predicting 30-day mortality of CGS (0.660 vs. 0.540, 0.549). The C statistic for NLR, however, was lower than that of SOFA or SAPSII scores (0.660 vs. 0.707, 0.749).
Figure 3.

ROC curve for 30-day mortality of CGS patients.
Discussion
We found that NLR was associated in an inverse U-shaped pattern with mortality among patients with CGS. NLR, serving as a readily available biomarker of systemic inflammation, has already been reported to predict the prognosis of various diseases, especially neoplastic diseases. Recently, however, several studies also investigated the value of NLR in predicting the survival of patients with cardiovascular diseases. Ghaffari et al. [22] evaluated the prognostic value of total neutrophil count and NLR in a small cohort of patients with ST-segment elevation myocardial infarction (STEMI), finding that increased neutrophil count was correlated with higher in-hospital mortality, but the association between NLR and survival in these patients was not significant. Gul et al. [23] investigated the correlation between NLR and mortality in STEMI patients thrombolysed with streptokinase. They concluded that a high NLR predicted a higher in-hospital complication rate and 30-day mortality in these patients. However, they used 4.50 as the cutoff point to divide NLR values into only 2 groups, and the scale of the study was small. Angkananard et al. [24] performed a systematic review and meta-analysis, showing that high NLR was associated with CAD, ACS, stroke, and composite cardiovascular events.
It remains unclear why NLR has such a significant prognostic value in cardiovascular diseases. Previous studies have attributed poor outcomes to several possible mechanisms, of which inflammation was the most important. As a severe complication of cardiovascular diseases, CGS is also associated with systemic inflammation. Various inflammatory mediators have been reported to play an important role in CGS, including blood cells [25], enzymes [26], cytokines [27], and complement [28]. Neutrophils and lymphocytes are well known to be potential biomarkers of inflammation and they have also been studied regarding the generation and development of CGS. Inspired by these results, we speculated that NLR could also predict the outcomes in CGS patients, as in other populations.
In our study, NLR shows an inverse U-shaped relationship with mortality among patients with CGS. The highest mortality in these patients occurred in those with NLR values of 9.4 to 15. Extremely high NLR values did not show a statistically significant difference in mortality risk compared to the reference. Several studies showed that the neutrophil count increased with occurrence of systemic inflammation, while the lymphocyte count, which indicates a state of weak immunity, was inversely correlated with inflammation [29–31]. During the early stages of inflammation, the shortage of circulating neutrophils can cause difficulties in effective innate responses [32]. Overwhelming activation of neutrophils, however, is known to cause tissue damage by adhesion to the blood vessel walls [33]. Host immunodeficiency and increased microcirculation damage can both result in poor outcomes in patients. In addition, Kim et al. [34] reported that the activation of innate T cells can worsen critical diseases by regulating harmful inflammatory responses, and the possible mechanisms mentioned above may help explain the association between NLR and CGS mortality. Further research is needed to clarify the mechanism involved.
The mortality rate of CGS is extremely high, which may be influenced by a few factors, such as basic vital signs (e.g., DBP [35] and MBP [36]), laboratory parameters (e.g., serum bicarbonate levels [37]), cardiac power index [5], vasopressor support [4], clinical severity scales (e.g., SAPSII [37]), and other comorbidities. In our subgroup analyses, taking 30-day mortality as an example, statistical significance was observed for the following factors: chronic liver disease, respiratory failure, vasoactive drug, CHF, and serum glucose. Patients without a history of chronic liver disease showed a higher risk of 30-day mortality. However, the sample size of these patients was quite small. Patients with respiratory failure showed a lower 30-day mortality risk. The improved survival might be related to the assisted ventilation strategies used. For patients treated with vasoactive drugs, the improved outcome might be associated with the drug itself. For patients with a history of CHF, chronic cardiac degeneration can result in a poor prognosis. In the subgroup of serum glucose, patients with lower serum glucose showed higher 30-day mortality risk. Serum glucose is the body’s main energy source, and lack of energy may help explain the increased death rate. The underlying mechanisms between these factors and the prognosis of CGS need further investigation.
As a combination of neutrophils count and lymphocytes count, NLR has been reported to have greater risk-predictive value than either of them alone, which is consistent with the results of our ROC analysis. Horne et al. [38] concluded that among high neutrophil count, low lymphocyte counts, and NLR, the greatest risk predictive value of mortality in patients with or at high risk for CAD is given by NLR, which increased the hazard by 2.2-fold. Papa et al. [39] found that cardiac mortality is closely associated with NLR, which is the expression of relative balance between neutrophilia and lymphopenia. In addition, we also found that the prognostic value of NLR was not as good as SOFA score or SAPSII. As clinical severity scores, SOFA score and SAPSII have already been used in critically ill patients. However, these 2 scores are composed of multiple indicators and the evaluation process takes time. Although the prognostic value is inferior to either of these scores, NLR is readily available and can play a role in rapid clinical evaluation.
This is the first study to assess the prognostic value of NLR among CGS patients. We used smooth curve fitting to define the cutoff values, applied Cox proportional hazards models to evaluate the association, and performed subgroup analyses to confirm this association. Our study inevitably has limitations. Firstly, it was a retrospective observational study at a single center. The biases inherent in this type of study should not be ignored and further studies based on multiple centers are needed. Secondly, the sample size small, and larger studies are needed. Furthermore, NLR was measured only on first admission to the ICU, and dynamic evaluation of NLR during the ICU stay could have produced different results.
Conclusions
An inverse U-shaped curve was presented between NLR and the mortality of CGS. NLR is a readily available and independent prognostic biomarker for patients with CGS. The prognostic value of NLR was more sensitive than the neutrophil or lymphocyte percentage alone, but not as good as SOFA or SAPSII score. However, further prospective studies are required.
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Tewelde SZ, Liu SS, Winters ME. Cardiogenic shock. Cardiol Clin. 2018;36:53–61. doi: 10.1016/j.ccl.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 2.van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: A scientific statement from the American Heart Association. Circulation. 2017;136:e232–68. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 3.Gibson PH, Croal BL, Cuthbertson BH, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154:995–1002. doi: 10.1016/j.ahj.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 4.Geppert A, Dorninger A, Delle-Karth G, et al. Plasma concentrations of interleukin-6, organ failure, vasopressor support, and successful coronary revascularization in predicting 30-day mortality of patients with cardiogenic shock complicating acute myocardial infarction. Crit Care Med. 2006;34:2035–42. doi: 10.1097/01.CCM.0000228919.33620.D9. [DOI] [PubMed] [Google Scholar]
- 5.Wigger O, Bloechlinger S, Berger D, et al. Baseline serum bicarbonate levels independently predict short-term mortality in critically ill patients with ischaemic cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2018;7:45–52. doi: 10.1177/2048872616683526. [DOI] [PubMed] [Google Scholar]
- 6.An X, Ding PR, Wang FH, et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in nasopharyngeal carcinoma. Tumour Biol. 2011;32:317–24. doi: 10.1007/s13277-010-0124-7. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Kim SH, Han JY, et al. Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. J Cancer Res Clin Oncol. 2012;138:2009–16. doi: 10.1007/s00432-012-1281-4. [DOI] [PubMed] [Google Scholar]
- 8.Arigami T, Okumura H, Matsumoto M, et al. Analysis of the fibrinogen and neutrophil-lymphocyte ratio in esophageal squamous cell carcinoma: A promising blood marker of tumor progression and prognosis. Medicine. 2015;94:e1702. doi: 10.1097/MD.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omichi K, Cloyd JM, Yamashita S, et al. Neutrophil-to-lymphocyte ratio predicts prognosis after neoadjuvant chemotherapy and resection of intrahepatic cholangiocarcinoma. Surgery. 2017;162:752–65. doi: 10.1016/j.surg.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Tamhane UU, Aneja S, Montgomery D, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–57. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Duffy BK, Gurm HS, Rajagopal V, et al. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97:993–96. doi: 10.1016/j.amjcard.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Sun H, Que J, Peng Y, et al. The neutrophil-lymphocyte ratio: A promising predictor of mortality in coronary care unit patients – a cohort study. Int Immunopharmacol. 2019;74:105692. doi: 10.1016/j.intimp.2019.105692. [DOI] [PubMed] [Google Scholar]
- 13.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamison DC. Structured Query Language (SQL) fundamentals. Curr Protoc Bioinformatics. 2003;Chapter 9(Unit 9):2. doi: 10.1002/0471250953.bi0902s00. [DOI] [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 16.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 17.Tallarida RJ, Murray RB. In: Chi-square test, in manual of pharmacologic calculations: With computer programs. Tallarida RJ, Murray RB, editors. Springer New York; New York, NY: 1987. pp. 140–42. [Google Scholar]
- 18.Rédei GP, editor. Fisher’s exact test, in encyclopedia of genetics, genomics, proteomics and informatics. Springer Netherlands; Dordrecht: 2008. p. 690. [Google Scholar]
- 19.Dalgaard RP, editor. Introductory statistics. Springer New York; New York, NY: 2002. Analysis of variance and the Kruskal-Wallis test; pp. 111–27. [Google Scholar]
- 20.Kirch W, editor. Encyclopedia of public health. Springer Netherlands; Dordrecht: 2008. Cox proportional hazards model; p. 176. [Google Scholar]
- 21.Jaddoe VW, de Jonge LL, Hofman A, et al. First trimester fetal growth restriction and cardiovascular risk factors in school age children: Population based cohort study. BMJ. 2014;348:g14. doi: 10.1136/bmj.g14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghaffari S, Nadiri M, Pourafkari L, et al. The predictive value of total neutrophil count and neutrophil/lymphocyte ratio in predicting in-hospital mortality and complications after STEMI. J Cardiovasc Thorac Res. 2014;6:35–41. doi: 10.5681/jcvtr.2014.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gul U, Hussain S, Munir R, et al. Neutrophil lymphocyte ratio: A prognostic marker in acute ST elevation myocardial infarctio. J Coll Physicians Surg Pak. 2017;27(1):4–7. [PubMed] [Google Scholar]
- 24.Angkananard T, Anothaisintawee T, McEvoy M, et al. Neutrophil lymphocyte ratio and cardiovascular disease risk: A systematic review and meta-analysis. Biomed Res Int. 2018;2018 doi: 10.1155/2018/2703518. 2703518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagnon DR, Zhang TJ, Brand FN, et al. Hematocrit and the risk of cardiovascular disease – the Framingham study: A 34-year follow-up. Am Heart J. 1994;127:674–82. doi: 10.1016/0002-8703(94)90679-3. [DOI] [PubMed] [Google Scholar]
- 26.Dominguez-Rodriguez A, Samimi-Fard S, Abreu-Gonzalez P, et al. Prognostic value of admission myeloperoxidase levels in patients with ST-segment elevation myocardial infarction and cardiogenic shock. Am J Cardiol. 2008;101:1537–40. doi: 10.1016/j.amjcard.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 27.Prondzinsky R, Unverzagt S, Lemm H, et al. Interleukin-6, -7, -8 and -10 predict outcome in acute myocardial infarction complicated by cardiogenic shock. Clin Res Cardiol. 2012;101:375–84. doi: 10.1007/s00392-011-0403-3. [DOI] [PubMed] [Google Scholar]
- 28.Granger CB, Mahaffey KW, Weaver WD, et al. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: The COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation. 2003;108:1184–90. doi: 10.1161/01.CIR.0000087447.12918.85. [DOI] [PubMed] [Google Scholar]
- 29.Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–76. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 30.Ommen SR, Gibbons RJ, Hodge DO, et al. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997;15:812–14. doi: 10.1016/s0002-9149(96)00878-8. [DOI] [PubMed] [Google Scholar]
- 31.Bissell MG. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Yearbook of Pathology and Laboratory Medicine. 2009;2009:276–77. [Google Scholar]
- 32.Bermejo-Martín JF, Tamayo E, Ruiz G, et al. Circulating neutrophil counts and mortality in septic shock. Crit Care. 2014;2014:407. doi: 10.1186/cc13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown KA, Brain SD, Pearson JD, et al. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–69. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 34.Kim EY, Oldham WM. Innate T cells in the intensive care unit. Mol Immunol. 2019;105:213–23. doi: 10.1016/j.molimm.2018.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rigamonti F, Graf G, Merlani P, et al. The short-term prognosis of cardiogenic shock can be determined using hemodynamic variables: A retrospective cohort study. Crit Care Med. 2013;41:2484–91. doi: 10.1097/CCM.0b013e3182982ac3. [DOI] [PubMed] [Google Scholar]
- 36.Axler O. Low diastolic blood pressure as best predictor of mortality in cardiogenic shock. Crit Care Med. 2013;41:2644–47. doi: 10.1097/CCM.0b013e31829cb36e. [DOI] [PubMed] [Google Scholar]
- 37.Popovic B, Fay R, Cravoisy-Popovic A, et al. Cardiac power index, mean arterial pressure, and Simplified Acute Physiology Score II are strong predictors of survival and response to revascularization in cardiogenic shock. Shock (Augusta, GA) 2014;42:22–26. doi: 10.1097/SHK.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 38.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–43. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 39.Papa A, Emdin M, Passino C, et al. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. 2008;395:27–31. doi: 10.1016/j.cca.2008.04.019. [DOI] [PubMed] [Google Scholar]


