Abstract
Background
Our previous studies have shown that electroacupuncture (EA) can alleviate lung injury induced by limb ischemia-reperfusion, but the specific mechanism is still unclear.
Material/Methods
The animals were randomly divided into sham operation group (Sham), model group (IR), electroacupuncture group (EA), sham electroacupuncture group (SEA), and EA+luzindole group (EA+luzindole). The limb ischemia-reperfusion model was established according to previously described, the rabbits in the EA and EA+luzindole groups were given EA at ST36 and BL13 for 7 days before the model preparation and during the model implementation, however, sham EA was mainly used to stimulate the rabbits in the SEA group with shallow needling at the points 0.5 cm near ST36 and BL13. Then, 30 mg/kg of luzindole was intraperitoneally injected 30 minutes before the model preparation in the EA+luzindole group.
Results
The wet weight/dry weight (W/D) ratio, lung injury score, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and malondialdehyde (MDA) contents in the EA group at 4 hours after reperfusion were significantly lower than those in the IR, SEA, and EA+luzindole groups. The levels of serum melatonin at T0 in the EA and EA+luzindole groups were significantly higher than those in the Sham group. The levels of serum melatonin at T1 and T2 in the IR group were significantly lower than those in the Sham group. There was no significant difference in the expression levels of melatonin receptor 1 (MR-1) and MR-2 in lung tissues among the 5 groups.
Conclusions
EA could alleviate the lung injury induced by limb ischemia-reperfusion by promoting the secretion of melatonin, while having no effect on the expression of melatonin receptor in lung tissues.
MeSH Keywords: Acute Lung Injury, Electroacupuncture, Melatonin, Reperfusion Injury
Background
Limb ischemia-reperfusion is one of the most common pathophysiological conditions during the severe trauma, surgery, and lower extremity vascular embolism [1,2]. The oxidative stress reactions during the reperfusion not only lead to the second injury of the ischemic limbs, but also may cause a certain degree of damage to remote organs, the most vulnerable organ is lung [1,2].
Electroacupuncture (EA) is an extension technique of acupuncture based on traditional acupuncture combined with modern electrotherapy [3]. It has been reported that stimulation of Zusanli (ST36) could diminish the degree of lung injury, increase the antioxidant enzyme activities and suppress the extent of lipid peroxidation in lipopolysaccharide (LPS) stimulated rabbits [4]. In addition, stimulation of Feishu (BL13) was used to treat lung disease, it was found that EA on BL13 could protect against hypoxia-induced pulmonary hypertension in rats [5]. Our previous studies have shown that EA can alleviate lung injury induced by limb ischemia-reperfusion, but the specific mechanism is still unclear [6].
In addition to regulating biological rhythm, melatonin plays an important role in regulating various physiological processes, and its metabolites also show the ability to scavenge reactive oxygen species which is harmful to the body [7]. A number of studies indicated the anti-inflammatory activity of melatonin both in vivo and in vitro [8–12]. Exogenous administration of melatonin could reduce oxidative damage and suppress interleukin (IL)-6 expression which induced by LPS administration in rats [8,9]. Melatonin also decreases inflammatory cytokine (IL-6, tumor necrosis factor [TNF]-α, and IL-1β) levels and inhibits neurons apoptosis after intraventricular Klebsiella pneumoniae injection in rats [10]. Melatonin also could inhibit expression of pro-inflammatory cytokine messenger RNA (mRNA) in LPS-stimulated cells [11,12]. All of these studies demonstrate the ability of melatonin to suppress inflammatory and reduce oxidative stress in experimental inflammation. This paper mainly discussed whether melatonin is involved in EA reducing rabbit lung injury induced by limb ischemia-reperfusion.
Material and Methods
Animals and apparatus
Three-month-old New Zealand white rabbits, weighing from 2 to 2.5 kg, were provided by the experimental animal center of the Institute of Radiology, Chinese Academy of Medical Sciences. G6805-1A low frequency electronic pulse therapy apparatus was purchased from the Hua Yi Medical Instrument Co., Ltd., China.
Study design
To maintain the serum melatonin under stable levels, all rabbits were fed under conditions of 12 hours of light (Illuminance: 500 Lx) during the day and 12 hours of complete darkness (Illuminance: 0–5 Lx) at night for 1 week before the experiment. Fifty New Zealand white rabbits were randomly divided into 5 groups: sham operation (Sham), model (IR), electroacupuncture (EA), sham electroacupuncture (SEA), and EA+uzindole (EA+luzindole) (n=10). Time to start the experiment was 8: 00 a.m. After intraperitoneal injection of pentobarbital 30 mg/kg (Sigma, Merck Life Science (Shanghai) Co., Ltd.), the rabbit was fixed in the supine position on a special rabbit platform. Incision was made in the triangle area of femoral artery on both hind limbs, and the femoral artery were clamped near the inguinal ligament for 3 hours to induce the ischemic injury, then the clamps were removed to make the limb subject to 4 hours reperfusion. In the Sham group, the femoral artery was isolated but not clamped. In the EA+luzindole group, 30 mg/kg melatonin receptor antagonist luzindole (Sigma-Aldrich, USA) was injected intraperitoneally 30 minutes before the model preparation [13]. During the experiment, 1.5 mL−1/kg−1/hour saline [Baxter (China), China)] was continuous infused via the right internal jugular vein.
EA pretreatment
Referring to the “animal acupuncture acupoint atlas” formulated by the acupuncture and Moxibustion Research Society of the Chinese Acupuncture Association, the bilateral Zusanli point (ST36, which is located between the tibia and fibular approximately 5 mm lateral to the anterior tubercle of the tibia) and the Feishu point (BL13, which is located between T3 and T4 of the spine approximately 1.5 cm lateral to the midline) [14]. Special rabbit box was used to expose the needle site. After local disinfection, acupuncture needles (Huatuo, 0.3×25 mm, China) were directly inserted 4–6 mm into the skin, then G6805-1A low frequency electronic pulse therapy instrument was connected to the needles for continuous stimulation [14]. EA parameters were as follows: dense wave, frequency 2/15 Hz, stimulus current 1–2 mA. The needles were maintained in place for 30 minutes per day. The EA group and the EA+luzindole group were given EA stimulation for 7 days before the model preparation and during model implementation.
Treatment of sham acupuncture group
The sham EA was mainly used to stimulate the rabbits with shallow needling (the depth of needling insert into the skin was 3 mm) at points 0.5 cm from ST36 and BL13. The remaining needling parameters were the same as those in the EA group.
Sample collection
Venous blood samples were collected at 3 time points: the beginning of model establishment (T0), limb ischemia for 3 hours (T1), and limb reperfusion for 4 hours (T2). The changes of serum melatonin were detected in other groups at corresponding time. The rabbits were then killed by common carotid artery bloodletting and bilateral lung tissues were retained. The wet weight/dry weight (W/D) ratio was measured in the upper lobe of the right lung. Some left lung tissues were fixed with 10% paraformaldehyde. Some left lung tissues were frozen in liquid nitrogen and stored in refrigerator at −80°C.
Collection of bronchoalveolar lavage fluid (BALF)
In the separated experiment, the bronchoalveolar lavage was carried out at the end of the experiment by injecting 10 mL saline into the tracheal tube. After repeated lavage, BALF was collected in the centrifugal tube and centrifuged for 10 minutes at 2500 rpm. The supernatant was collected and stored at −70°C.
Measurement of W/D ratio in lung tissue
The right upper lobe tissue was taken, and superfluous water was removed, then the lung was weighed as wet weight (W), then subsequently dried in an electrothermal constant temperature drying oven at 80°C for 72 hours. The dry weight (D) was weighed at constant weight and the W/D ratio of the lung tissue was calculated.
Lung injury score
The left upper lobe was placed in 10% formaldehyde solution and routinely processed into paraffin sections (4–5 mm). Sections were used for hematoxylin and eosin staining. Pulmonary injury score was assessed with reference to the literature [15].
Electron microscopic observation of lung tissue
After fixing the lung tissue specimens, they were treated by dyeing, dehydration, embedding, osmosis, and aggregation in turn. The specimens were cut into sections with thickness of about 90 nm under the ultra-thin slice machine. Then the sections were stained with uranium acetate. The ultrastructural changes of lung tissue were observed under transmission electron microscopy. At the same time, image analysis was carried out using ImageJ software. Mitochondrial membrane lines were drawn by hand tools. We counted the number of mitochondria and calculated the cross-sectional area of each mitochondria in the picture to evaluate the morphological changes of mitochondria [16] (the cross-sectional area of mitochondria became larger after mitochondrial swelling).
Detection of IL-6, TNF-α, and IL-1β in BALF by enzyme-linked immunosorbent assay (ELISA)
The separated supernatant of BALF was used according to the instructions of IL-6, TNF-α, and IL-1β ELISA kit (R & D, USA). The absorbance values at 450 nm were measured, and the standard curves were drawn according to the absorbance values of the standard samples. The contents of IL-6, TNF-α, and IL-1β in lung tissues were calculated.
Changes of superoxide dismutase (SOD) activity and malondialdehyde (MDA) content in BALF
The activity of superoxide dismutase (SOD) was determined by xanthine oxidase method and the content of malondialdehyde (MDA) was determined by thiobarbituric acid method. All steps were determined strictly according to the operation steps of the kit (R & D, USA).
Changes of expression of melatonin receptor 1 (MR-1) and MR-2 in lung tissue
The total protein was extracted according to the instructions of the protein extraction kit (Thermo, USA). After centrifugation, the supernatant was taken to determine the protein concentration (Thermo, USA). After gel electrophoresis, transferred to membrane and blocking, 10 μg of protein was incubated overnight at 4°C with polyclonal rabbit antibodies against melatonin receptor 1 (MR-1) (1: 800, Biorbyt, UK) or MR-2 (1: 300, Biorbyt, UK). After washing with TBST (tris-buffered saline and Tween), blots were incubated at 37°C for 1 hour with the horseradish peroxidase-conjugated goat anti-rabbit IgG (1: 2000, Biorbyt, UK). Quantity One image analysis software was used to analyze the expression level of MR-1 and MR-2 protein by the ratio of target protein to β-actin.
Statistical analysis
Values are expressed as mean standard deviation (SD) or median (range). Maximum and minimum possible lung injury scores were 1 and 0, respectively. The counting data were expressed in the form of mean±standard error of the mean (M±SEM). The ALI scores were analyzed using nonparametric test by the Kruskal-Wallis rank test followed by the Dunnett test. The other data were analyzed using parametric test by one-way ANOVA followed by the Tukey-Kramer post hoc test. P<0.05 was deemed statistically significant.
Results
EA could alleviate limb ischemia-reperfusion induced lung injury
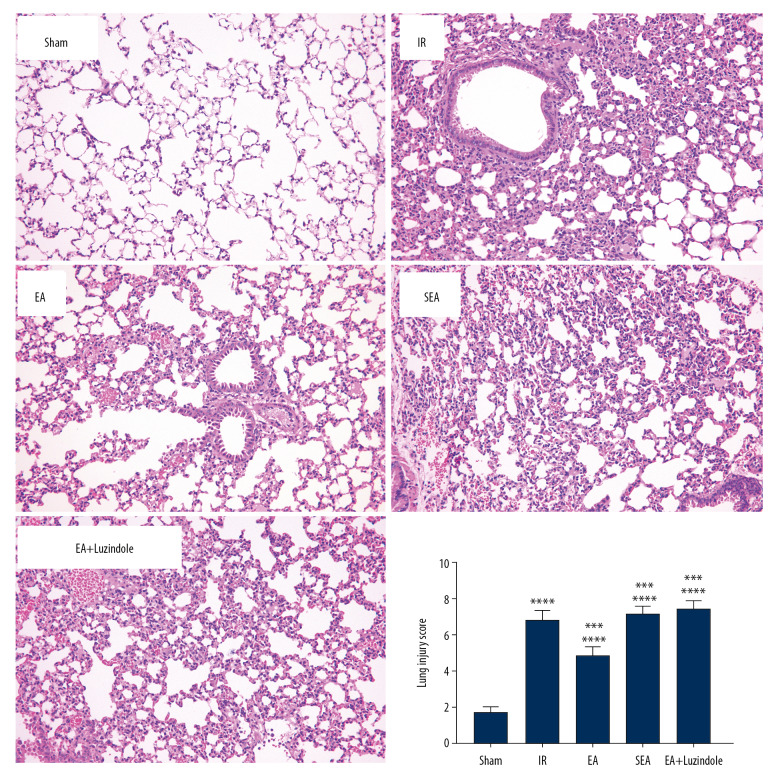
Compared with the Sham group, extensive edema, inflammatory cell infiltration, and hemorrhage were observed in the alveoli and interstitium in the IR, EA, SEA, and EA+luzindole groups at 4 hours after reperfusion (*** P<0.001,** P<0.01). Compared with the IR group, the degree of edema, inflammatory cell infiltration, and hemorrhage in lung tissue of rabbits in the EA group were alleviated, and lung injury scores were significantly reduced, suggesting that EA pretreatment could alleviate limb ischemia-reperfusion induced lung injury (# P<0.05). In addition, the lung injury score in the EA group was significantly lower than those in the EA+luzindole and SEA groups (* P<0.05), suggesting that the degree of alleviation of lung injury by EA was not obvious after the administration of melatonin receptor inhibitor (Figure 1).
Figure 1.
Comparison of lung injury scores in different groups. All data were expressed as mean (min, max) (n=10), compared with the Sham group, *** P<0.001; compared with the IR group, # P<0.05; compared with the EA group,* P<0.05. IR – model group; EA – electroacupuncture.
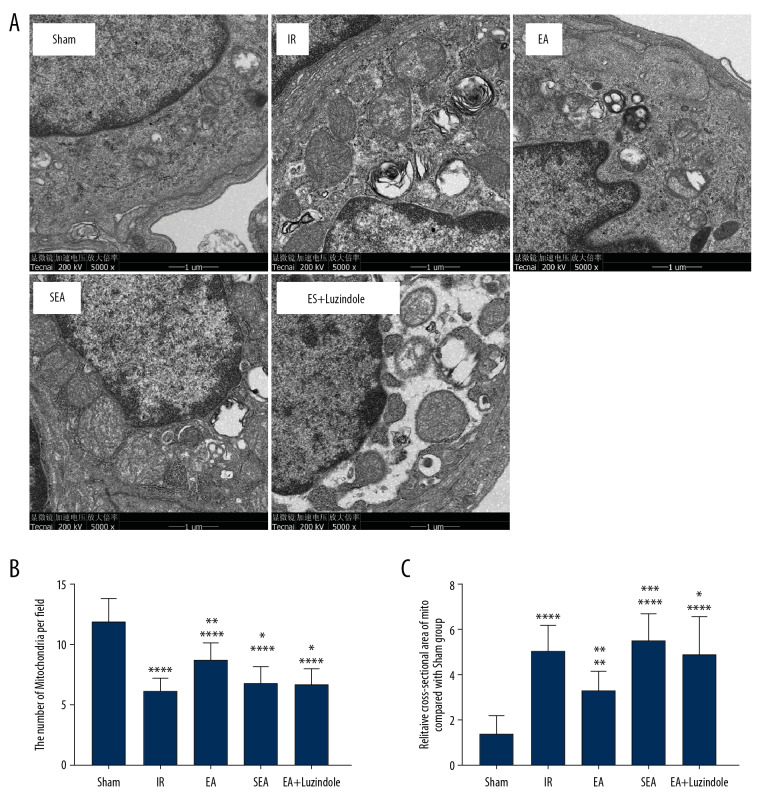
Further electron microscopic examination showed that the mitochondria of lung tissue in the IR, EA, SEA, and EA+luzindole groups were significantly swollen, vacuolated, accompanied by the breakage, and with disappearance of mitochondrial ridge. Compared with the IR group, the swelling degree of mitochondria in the EA group was reduced. Compared with the EA group, the degree of mitochondrial swelling in the EA+luzindole group was still more obvious. By analyzing the number and cross-sectional area of mitochondria in different visual fields, we found that compared with the Sham group, the number of mitochondria in the unit visual field of lung tissue cells significant decreased, while cross-sectional area of mitochondria increased dramatically in the IR, SEA, and EA+luzindol group (**** P<0.0001,** P<0.01); compared with the IR group, the number and cross-sectional area of mitochondria in unit visual field of lung tissue cells in the EA group increased significantly (all ** P<0.01). The number and cross-sectional area of mitochondria in the EA+luzindole and SEA groups decreased significantly and increased significantly compared with the EA group (*** P<0.001,* P<0.05) (Figure 2).
Figure 2.
The results of transmission electron microscopy of lung tissue in different groups. (A) Transmission electron microscopy of lung tissue in different groups. (B, C) Quantitative analysis of changes in mitochondrial number and cross-sectional area. All data are mean±SEM (n=10), compared with the Sham group, **** P<0.0001,** P<0.01; compared with the IR group, ** P<0.01; compared with the EA group,*** P<0.001, * P<0.05. SEM – standard error of the mean; IR – model group; EA – electroacupuncture.
The changes of W/D in lung tissues
The W/D ratio of lung tissue in IR, EA, SEA, and EA+luzindole groups was significantly higher than that in the Sham group (**** P<0.0001,* P<0.05). The W/D ratio of lung tissue in the EA group was significantly lower than that in the IR group (**** P<0.0001). Compared with the EA group, the W/D ratio in the EA+luzindole and SEA group was significantly higher (*** P<0.001,** P<0.01) (Figure 3).
Figure 3.
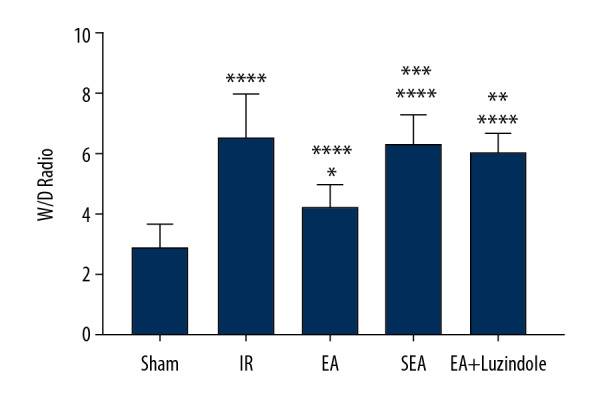
Changes of W/D values in different groups. All data are mean±SEM (n=10), compared with the Sham group, **** P<0.0001, * P<0.05; compared with the IR group, **** P<0.0001; compared with the EA group,*** P<0.001, ** P<0.01. W/D – wet weight/dry weight; SEM – standard error of the mean.
EA decreased the production of proinflammatory cytokines
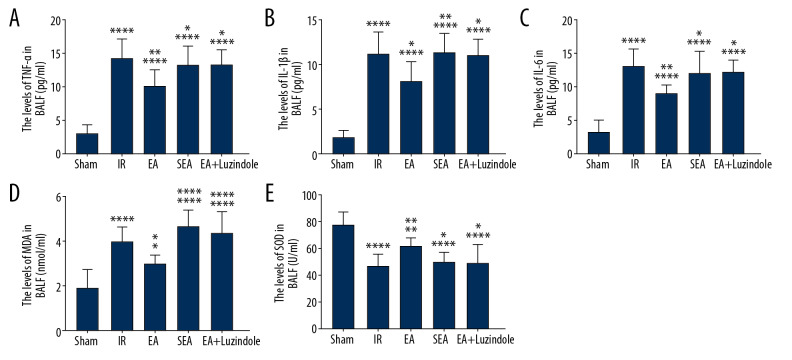
The ELISA results showed that the levels of TNF-α, IL-1β, and IL-6 in the IR, EA, SEA, and EA+luzindole groups at 4 hours after reperfusion were significantly higher than those in the Sham group (**** P<0.0001), while TNF-α, IL-1β, and IL-6 levels in the EA were significantly lower than those in the IR (** P<0.01, * P<0.05), TNF-α, IL-1β, and IL-6 levels in the EA+luzindole and SEA groups were significantly higher than those in the EA group (**P<0.01, * P<0.05). There was no significant difference in the contents of TNF-α, IL-1β, and IL-6 among the IR, SEA, and EA+luzindole groups (Figure 4A–4C).
Figure 4.
Changes of inflammatory factors and oxidative stress in different groups. (A–C) TNF-α, IL-1β, and IL-6 levels in different groups, compared with the Sham group, **** P<0.0001; compared with the IR group,** P<0.01, * P<0.05; compared with EA group, * P<0.05, ** P<0.01. (D, E) MDA content and SOD activity in different groups, compared with Sham group, **** P<0.0001, ** P<0.01, * P<0.05; compared with the IR group, ** P<0.01, * P<0.05; compared with the EA group, * P<0.05, **** P<0.0001. All data are mean±SEM (n=10). TNF – tumor necrosis factor; IL – interleukin; IR – model group; MDA – malondialdehyde; SOD – superoxide dismutase; SEM – standard error of the mean.
EA attenuates the oxidative stress induced by limb ischemia-reperfusion
Compared with the Sham group, the MDA content in the IR, EA, SEA, and EA+luzindole groups increased significantly, while the SOD activity decreased significantly (**** P<0.0001, ** P<0.01, * P<0.05); compared with the IR group, the MDA content in EA group decreased significantly, while SOD activity increased (**P<0.01, * P<0.05); compared with the EA group, the MDA content in the SEA and EA+luzindole groups increased significantly, however, SOD activity decreased significantly (**** P<0.0001, * P<0.05) (Figure 4D, 4E).
EA, not the sham EA, enhanced the secretion of melatonin
The results showed that serum melatonin levels in the EA and EA+luzindole groups at T0 time point were significantly higher than those in Sham group (* P<0.05), but there was no significant change in serum melatonin levels at T0 time point among the Sham, IR, and SEA groups; serum melatonin levels in the IR and SEA groups at T1 and T2 time point were significantly lower than those in the Sham group (* P<0.05); compared with EA group, serum melatonin levels in the IR and SEA groups were significantly lower at different time points (# P<0.05). There was no significant difference in serum melatonin between the EA and EA+luzindole groups at the same time points (Figure 5).
Figure 5.
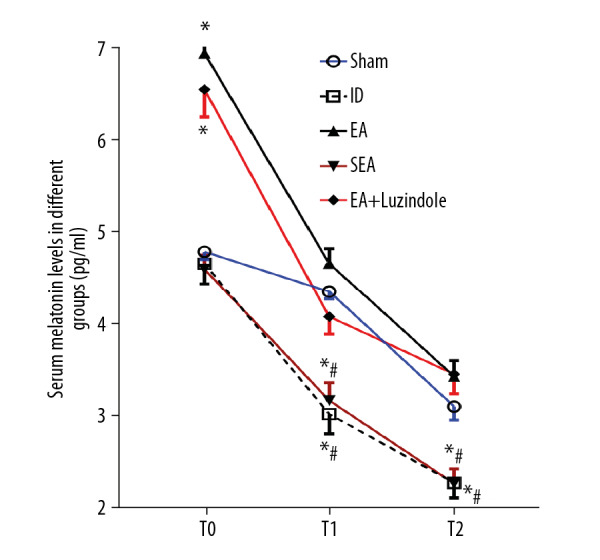
EA, not the sham EA, enhanced the secretion of melatonin. All data are mean±SEM (n=6), compared with the Sham group, * P<0.05; compared with the EA group, # P<0.01. EA – electroacupuncture; SEM – standard error of the mean.
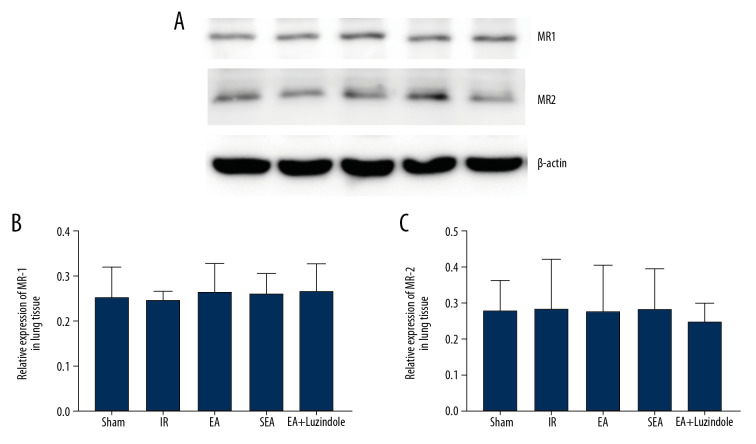
EA had no effect on the expressions of MR-1 and MR-2 in lung tissue
The results showed that there was no significant difference in the relative expression levels of MR-1 and MR-2 in lung tissue among the 5 groups at 4 hours of reperfusion (Figure 6).
Figure 6.
(A–C) The relative expression levels of MR-1 and MR-2 in lung tissue among the 5 groups. All data are mean±SEM (n=3). MR-1 – melatonin receptor 1; MR-2 – melatonin receptor 2; SEM – standard error of the mean.
Discussion
The pathogenesis of lung injury caused by limb ischemia-reperfusion is complex. Reperfusion could lead to the production of a large number of oxygen free radicals and reactive oxygen species, and oxidative stress injury induced by limb ischemia-reperfusion is also considered an important mechanism of limb ischemia-reperfusion-induced lung injury [1,17]. It has been shown that TNF-α and IL-6 play an important role in organ damage pathogenesis induced by local and distant effects of limb ischemia-reperfusion [18]. IL-6 levels were shown to increase in correlation with TNF-α in studies dealing with lower extremity ischemia-reperfusion models [18]. It has been suggested that ischemia-reperfusion related inflammatory response can be seen with serum TNF-α and IL-6 levels and that there is a positive correlation between this increase and distant organ injuries [19]. As an important pro-inflammatory cytokine, IL-1β can promote the release of a variety of inflammatory factors and chemotactic inflammatory cells into the alveolar cavity, damage the alveolar capillary barrier, reduce the function of fluid transport, and cause pulmonary edema [20,21]. Serum IL-1β begins to rise in the early stage of acute lung injury and is closely related to the prognosis of patients [20]. So, in this study, we detected the levels of TNF-α, IL-6, and IL-1β to evaluate the lung injury induced by the limb ischemia-reperfusion.
EA stimulation can protect organs by reducing the production of oxygen free radicals and reducing anti-oxidative stress [22]. ST36 belongs to the Foot Yangming Stomach Meridian, and BL13 belongs to the Foot Taiyang Bladder Meridian. Studies have shown that EA at ST36 and BL13 could alleviate LPS-induced lung injury, and its mechanism may be related to its anti-oxidative stress and anti-inflammatory effects [4,14]. It has been shown that EA could prevent organ dysfunction induced by limb ischemia-reperfusion. Chen et al. [23] suggested that EA pretreatment could prevent cognitive impairment induced by limb ischemia-reperfusion via inhibition of microglial activation and attenuation of oxidative stress in rats. Our latest study showed that EA could attenuate limb ischemia-reperfusion induced lung injury by inhibition of pro-inflammatory cytokine response and oxidative stress through activating the p38 MAPK-mediated Nrf2/HO-1 pathway [6].
Besides regulating body rhythm, melatonin plays an important role in regulating inflammation. Pre-intraperitoneal administration of melatonin can inhibit oxidative damage in CLP rats and reduce myocardial damage in sepsis rats [24]. In addition, melatonin could reduce LPS-induced inflammatory response, and effectively antagonize lung tissue damage caused by lipid peroxidation in septic rats, and its effect was positively correlated with its dose [8]. Melatonin was also found to reduce the degree of renal injury in rats with renal ischemia-reperfusion injury, reduce the level of serum urea nitrogen and creatinine, MDA content, and increase the activity of mitochondria and SOD in kidney, and the effect was still positively correlated with the dose [25]. It has been reported that EA can regulate the circadian rhythm of sleep-wake and melatonin in rats with insomnia [26]. It has also been reported that chronic stress could decrease melatonin content, while EA could enhance the content of melatonin in a chronic stress depression rat model, which might be one of the effect mechanisms of EA on anti-depression [27]. However, we have little knowledge about the changes of serum melatonin levels and the effects of EA. In this study, we evaluated the effects of EA on serum melatonin levels. If melatonin was involved in the protection effects of EA against limb ischemia-reperfusion induced lung injury, then EA pretreatment could significantly increase the basic level of melatonin in rabbits, alleviate oxidative stress and inflammation caused by limbs ischemia-reperfusion, and then improve the degree of lung injury. However, we found that administration of melatonin receptor antagonist could antagonize the protection effect of EA, indicating that melatonin mainly plays a protective role through melatonin receptors, but it does not affect the secretion of melatonin. Interestingly, sham EA pretreatment had no effect on the level of serum melatonin, suggesting that only stimulation on the real acupoints not the sham acupoints could enhance the secretion of melatonin.
Conclusions
Thus, we concluded that EA pretreatment at ST36 and BL13 could promote the release of melatonin in a rabbit model and reduce the degree of lung injury and inflammation after limb ischemia-reperfusion, while EA had no effect on the expression of melatonin receptor in lung tissues.
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by key projects of Tianjin Natural Science Foundation (18JCZDJC35400), National Natural Science Foundation of China (81772106), and Health and Family Planning Commission of Binhai New Area, Science and Technology Joint Research Project (2015BWKL003)
References
- 1.Takhtfooladi HA, Hesaraki S, Razmara F, et al. Effects of N-acetylcysteine and pentoxifylline on remote lung injury in a rat model of hind-limb ischemia/reperfusion injury. J Bras. 2016;42(1):9–14. doi: 10.1590/S1806-37562016000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao YR, Wang D, Liu Y, et al. The PI3K/Akt, p38MAPK, and JAK2/STAT3 signaling pathways mediate the protection of SO2 against acute lung injury induced by limb ischemia/reperfusion in rats. J Physiol Sci. 2016;66(3):229–39. doi: 10.1007/s12576-015-0418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Liu Y, Xu H, et al. Effect of electroacupuncture on urinary leakage among women with stress urinary incontinence: A randomized clinical trial. JAMA. 2017;317(24):2493–501. doi: 10.1001/jama.2017.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu JB, Shi J, Zhang Y, et al. Electroacupuncture ameliorates acute renal injury in lipopolysaccharide-stimulated rabbits via induction of HO-1 through the PI3K/Akt/Nrf2 Pathways. PLoS One. 2015;10(11):e0141622. doi: 10.1371/journal.pone.0141622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Sun M, Ye J, et al. The mechanism of acupuncture in treating essential hypertension: A narrative review. Int J Hypertens. 2019;2019 doi: 10.1155/2019/8676490. 8676490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong LR, Kan YX, Lian Y, et al. Electroacupuncture attenuates limb ischemia-reperfusion-induced lung injury via p38 mitogen-activated protein kinase-nuclear factor erythroid-2-related factor-2/heme oxygenase pathway. J Surg Res. 2020;246:170–81. doi: 10.1016/j.jss.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Mańka S, Majewska E. Immunoregulatory action of melatonin. The mechanism of action and the effect on inflammatory cells. Postepy Hig Med Dosw (Online) 2016;70(0):1059–67. doi: 10.5604/17322693.1221001. [DOI] [PubMed] [Google Scholar]
- 8.Hu W, Deng C, Ma Z, et al. Utilizing melatonin to combat bacterial infections and septic injury. Br J Pharmacol. 2017;174(9):754–68. doi: 10.1111/bph.13751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Li F, Su X, et al. Melatonin prevents lung injury by regulating apelin 13 to improve mitochondrial dysfunction. Exp Mol Med. 2019;51(7):73. doi: 10.1038/s12276-019-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, Ji H, Wang Y, et al. Melatonin alleviates radiation-induced lung injury via regulation of miR-30e/NLRP3 Axis. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/4087298. 4087298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu GM, Tan W. Melatonin inhibits lipopolysaccharide-induced inflammation and oxidative stress in cultured mouse mammary tissue. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/8597159. 8597159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu GM, Kubota H, Okita M, et al. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS One. 2017;12(5):e0178525. doi: 10.1371/journal.pone.0178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong YH, Hua N, Zang X, et al. Melatonin ameliorates Aβ1-42 -induced Alzheimer’s cognitive deficits in mouse model. J Pharm Pharmacol. 2018;70(1):70–80. doi: 10.1111/jphp.12830. [DOI] [PubMed] [Google Scholar]
- 14.Yu JB, Dong SA, Luo XQ, et al. Role of HO-1 in protective effect of electro-acupuncture against endotoxin shock-induced acute lung injury in rabbits. Exp Biol Med (Maywood) 2013;238(6):705–12. doi: 10.1177/1535370213489487. [DOI] [PubMed] [Google Scholar]
- 15.Pinto EF, Santos RS, Antunes MA, et al. Static and dynamic transpulmonary driving pressures affect lung and diaphragm injury during pressure-controlled versus pressure-support ventilation in experimental mild lung injury in rats. Anesthesiology. 2020;132(2):307–20. doi: 10.1097/ALN.0000000000003060. [DOI] [PubMed] [Google Scholar]
- 16.Bassot A, Chauvin MA, Bendridi N, et al. Regulation of mitochondria-associated membranes (MAMs) by NO/sGC/PKG participates in the control of hepatic insulin response. Cells. 2019;8(11):1319. doi: 10.3390/cells8111319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye Y, Shan Y, Bao C, et al. Ginsenoside Rg1 protects against hind-limb ischemia reperfusion induced lung injury via NF-κB/COX-2 signaling pathway. Int Immunopharmacol. 2018;60:96–103. doi: 10.1016/j.intimp.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 18.Küçükebe ÖB, Özzeybek D, Abdullayev R, et al. Effect of dexmedetomidine on acute lung injury in experimental ischemia-reperfusion model. Braz J Anesthesiol. 2017;67(2):139–46. doi: 10.1016/j.bjane.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Leurcharusmee P, Sawaddiruk P, Punjasawadwong Y, et al. The possible pathophysiological outcomes and mechanisms of tourniquet-induced ischemia-reperfusion injury during total knee arthroplasty. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/8087598. 8087598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Störmann P, Lustenberger T, Relja B, et al. Role of biomarkers in acute traumatic lung injury. Injury. 2017;48(11):2400–6. doi: 10.1016/j.injury.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 21.Herrero R, Sanchez G, Lorente JA. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann Transl Med. 2018;6(2):32. doi: 10.21037/atm.2017.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Yi T, Long M, et al. Electro-acupuncture at Zusanli acupoint (ST36) suppresses inflammation in allergic contact dermatitis via triggering local IL-10 production and inhibiting p38 MAPK activation. Inflammation. 2017;40(4):1351–64. doi: 10.1007/s10753-017-0578-5. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Zhou J, Li J, et al. Electroacupuncture pretreatment prevents cognitive impairment induced by limb ischemia-reperfusion via inhibition of microglial activation and attenuation of oxidative stress in rats. Brain Res. 2012;1432:36–45. doi: 10.1016/j.brainres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang WX, He BM, Wu Y, et al. Melatonin protects against sepsis-induced cardiac dysfunction by regulating apoptosis and autophagy via activation of SIRT1 in mice. Life Sci. 2019;217:8–15. doi: 10.1016/j.lfs.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 25.Chen DQ, Feng YL, Chen L, et al. Poricoic acid A enhances melatonin inhibition of AKI-to-CKD transition by regulating Gas6/AxlNFκB/Nrf2 axis. Free Radic Biol Med. 2019;134:484–97. doi: 10.1016/j.freeradbiomed.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 26.Zheng XN, Wu XF, Guo X, et al. [Manual acupuncture stimulation of paired acupoints can relieve sleep disorder possibly by upregulating pineal melatonin protein and its receptor mRNA levels in the suprachiasmatic nucleus in insomnia rats]. Zhen Ci Yan Jiu. 2018;43(6):360–64. doi: 10.13702/j.1000-0607.170409. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 27.Yao HJ, Song HT, Mo YP, et al. [Effects of electroacupuncture on circadian rhythm of temperature and melatonin in depression rats model induced by chronic stress]. Zhongguo Zhen Jiu. 2014;34(7):685–89. [in Chinese] [PubMed] [Google Scholar]