Abstract
Background
Liver cancer is a common cancer with high morbidity and mortality. Due to the large toxic side effects of chemotherapeutic drugs and the overexpression of multidrug resistance genes in liver cancer, no effective chemotherapeutic drug has yet been found. Therefore, the search for a highly effective, low-toxic, and safe natural anticancer therapy is a hot issue.
Material/Methods
SMMC-7721 cells (a hepatocellular carcinoma cell line) were treated with different concentrations of oleanolic acid (OA) plus autophagy inhibitor 3-methyladenine (3-MA) (3-MA+OA) or chloroquine (CQ) plus OA (CQ+OA). We used MTT and Hoechst 33258 staining methods to determine the proliferation and apoptotic effect of OA on cells. Flow cytometry was used to detect apoptosis. Mitochondrial function was assessed by measuring mitochondrial membrane potential and adenosine triphosphate (ATP) concentration. To evaluate the ability of OA on apoptosis and autophagy mechanisms on SMMC 7721 cells, the related protein expression for apoptosis, autophagy, and the autophagic pathway were detected and analyzed by western blot.
Results
OA can inhibit and induce apoptosis of SMMC-7721 in a dose-dependent manner. Compared with the control group, OA significantly reduced the intracellular mitochondrial membrane potential, and the intracellular ATP concentration was also significantly reduced. Moreover, OA reduced the expression of p-Akt and p-mTOR. The expression of p62 was decreased, and LC3-II and Beclin-1 protein expression levels increased. After inhibiting autophagy with 3-MA or CQ, compared with OA alone, cell mitochondrial membrane potential and ATP concentration were significantly reduced, cell p62 expression was reduced, and LC3-II expression was increased, apoptosis-related protein Bax protein was increased, and Bcl-2 protein was decreased, which suggested that 3-MA or CQ treatment increased OA-induced apoptosis of SMMC-7721 cells. This suggested that OA activated autophagy of SMMC-7721 cells in a protective autophagic manner.
Conclusions
The study findings suggest that OA combined with autophagy inhibitor 3-MA can better exert its anticancer effect.
MeSH Keywords: Autophagy; Carcinoma, Hepatocellular; Oleanolic Acid
Background
Hepatocellular carcinoma is the most common liver cancer, and seriously endangers human health. It ranks third in deaths caused by tumors. Many patients with liver cancer do not have the option of radical resection because their tumors are limited by size and location and a late stage diagnosis. In addition, chemotherapeutic drugs tend to make tumor cells resistant to drugs, have toxic side effects, and have poor curative effects for liver cancer. Therefore, the search for a highly effective, low-toxic and safe natural anti-cancer component is a topic that cannot be ignored in the medical community. Apoptosis can help maintain the stability of the body’s environment by clearing senescent, abnormal and damaged cells; apoptosis can participate in the body’s immune response, embryonic development, regulation of the hematopoietic system, tumor formation, and other processes. Apoptosis is still the main goal for many liver cancer treatment methods. Therefore, finding drugs that can effectively induce apoptosis of liver cancer cells is particularly important for the clinical treatment of liver cancer.
Triterpenoids are widely present in many plants and have been used in traditional medicine. Oleanolic acid (OA) is an important pentacyclic triterpenoid which is widely present in herbaceous plants such as Ligustrum lucidum, American ginseng, Forsythia, Panax notoginseng, and Ginseng. It has been used as a liver protection and anti-hepatitis drug for many years in China. In addition to its anti-hepatitis effect, its anti-tumor effect has attracted the attention of researchers in recent years. Liver cancer [1], leukemia [2], and lung cancer [3] have been used as research subjects to verify that OA has an effect on restraining tumor growth. It can inhibit the growth of various cell lines such as colon cancer, liver cancer, and bladder cancer [4,5], and increase the rate of apoptosis [6] in vitro. Scholars have explored different signaling pathways and protein expression molecules related to the mechanism of OA antitumor effects such as reflected in the inhibition of tumor cell proliferation [7], induction of tumor cell apoptosis [8], inhibition of tumor neovascularization [9], inhibition of tumor cell invasion [10], and reverse the role of tumor cells and promote tumor cell differentiation [11]. For example, the literature reports that OA can inhibit the phosphorylation of mTOR in prostate cancer PC-3 cells and breast cancer MCF-7 cells [12], and OA also inhibits PI3K expression in human leukemia HL-60 cells [13] through the PI3K-Akt-mTOR signaling pathway. In this study, the mechanism of OA-induced proliferation and apoptosis of SMMC-7721 hepatocellular carcinoma cells were explored from the perspective of autophagy and its effect on AKT protein expression. The research results will contribute to the development of clinical anti-liver cancer drugs and our understanding of the pharmacological effects of OA and provide a certain theoretical foundation for future studies.
Material and Methods
Cells and reagents
We used the following materials: hepatocellular carcinoma cell line SMMC-7721 (Shanghai Cell Bank, Chinese Academy of Sciences, China), OA (100 mg per tablet), MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay reagents (Sigma, USA); Dulbecco’s Modified Eagle Medium (DMEM), calf serum, penicillin and streptomycin (Gibco, USA); dimethyl sulfoxide (DMSO) (Amresco, USA), Hoechst 33258 Kit (Sigma-Aldrich, USA); Annexin V-PE Apoptosis Detection Kit, ATP (adenosine triphosphate) Detection Kit, JC-1 Mitochondrial Membrane Potential Detection Kit, DAPI (4,6-diamidino-2-phenylindole), Anti-fluorescent Attenuated Sealing Tablets (Shanghai Biyuntian Biotechnology Co., Ltd.), Polylysine (Wuhan Baoshide Biological Engineering Co., Ltd.), 4% paraformaldehyde, Triton X-100 (Beijing Dingguo Changsheng Biotechnology Co., Ltd.), Bcl-2 associated X protein (Bax), B-cell lymphoma-2 (Bcl-2), β-actin, and horseradish peroxidase labeled secondary antibodies (Bioworld, USA); LC3, p62 antibody, p-mTOR antibody, p-Akt antibody (Cell Signaling Technology, USA); TRITC-conjugated goat anti-rabbit IgG (Beijing Zhongshan Jinqiao Biological Company), western blot kit (Dr., Wuhan, China); 3-methyladenine (3-MA) (Sigma-Aldrich, USA); chloroquine (CQ) (Taosu Biochemical Technology Co., Ltd. Shanghai, China).
Instruments
We used the following equipment: SK3c cell incubator (Sanyo Corporation, Japan); microplate reader (SpectraMaxM5, Molecular Device, USA); Gel Imaging System (UVP, USA), FACS420 Flow Cytometer (Becton-Dickinson, USA), Inverted fluorescence microscope (Olympus CKX71, Japan), and LB942 Microplate Luminometer (Berthold Technologics, Germany).
Measurement indicators and methods
Detection of OA on the proliferation of SMMC-7721 cells by MTT assay
SMMC-7721 cells were inoculated into culture flasks with a final serum concentration of 10% in DMEM and incubated in saturated humidity (37°C, 5% CO2). Logarithmic growth phase cells were taken and digested to prepare single cell suspensions. The cells were seeded in 96-well culture plates at 200 μL/well. After cultured for 24 hours, medium was aspirated and different concentrations of OA (15, 30, and 60 μmol/L) were added at 200 μL/well. Five replicate wells were set for each concentration group. An equal volume of culture solution was added to the blank control group. After 24 hours of culture, 20 μL of 5 g/L MTT solution was added and incubated in a 37°C incubator for 4 hours. The medium was aspirated, 150 μL DMSO per well was added, shaken with a shaker, and after the color in the well was uniform, place in a microplate reader and the optical density (OD) of each well was measured (measurement wavelength 490 nm); the cell survival rate was calculated.
Detection of OA on the apoptosis of SMMC-7721 cells through immunofluorescence Hoechst 33258/PI staining
The logarithmic growth phase of SMMC-7721 cells were seeded at a concentration of about 1 x 105 cells/mL in a 6-well plate. After the cell fusion rate reached 80%, 15, 30, and 60 μmol/L OA was added. After 24 hours of treatment, the cells were stained according to Hoechst 33258 staining apoptosis kit. After staining, the samples were placed under a fluorescent microscope to observe and take photos. Apoptotic cells were densely condensed and nucleated.
Annexin V-PE method to detect apoptosis
The cells in the logarithmic growth phase were cultured in culture solution containing 15, 30, and 60 μmol/L OA for 24 hours, and then the cells were collected, centrifuge at 1000 g for 5 minutes, the supernatant discarded and the cells collect and gently resuspend in phosphate-buffered saline (PBS) and counted. We took 50 000–100 000 resuspended cells, centrifuge at 1000 g for 5 minutes, discarded the supernatant, and added 195 μL of Annexin V-PE binding solution to gently resuspend the cells. Then we added 5 μL Annexin V-PE and mixed gently. Cells were incubated at room temperature (20–25ºC) in the dark for 10–20 minutes before placing in an ice bath. Cells were resuspended 2–3 times during incubation to improve the labeling effect. Flow cytometry was performed immediately, and Annexin V-PE showed red fluorescence.
Detection of cell mitochondrial dysfunction
The JC-1 method for detecting mitochondrial membrane potential
Cells in the logarithmic growth phase were inoculate onto a 6-well culture plate with 1×106 cells per well. After treating the cells with different concentrations of OA for 24 hours, the cells were trypsinize. We adjusted the cell concentration to 1×1012/L with PBS, added 0.5 mL mitochondrial membrane potential detection fluorescent dye (JC-1) staining working solution (1x), mixed by inverting and incubated at 37ºC for 20 minutes; then we discarded the supernatant. After resuspending the cells with an appropriate amount of JC-1 staining buffer (1×), we placed 150 uL cell suspension in a black 96-well plate, and analyze the absorbance of the JC-1 polymer and JC-1 monomer by flow cytometry (JC-1 monomer has an excitation wavelength of 490 nm and an emission wavelength of 530 nm; JC-1 polymer has an excitation wavelength of 525 nm and an emission wavelength of 590 nm.) The mitochondrial membrane potential was expressed as a ratio of fluorescence intensity.
Detection of ATP level
After culturing cells with OA at 15, 30, and 60 μmol/L for 24 hours, the cells were lysed by adding 200 μL lysate, and then centrifuged at 1200 g for 10 minutes to obtain the supernatant. We drew the standard curve and prepared the ATP detection working solution, then added 100 μL detection working solution to each well. We used a microplate luminescence detector (560 nm) for detection after 3–5 minutes at room temperature. Finally, the concentration of ATP was converted to nmol/mg protein.
Apoptosis, autophagy, and autophagic pathway-related protein expression were detected by western blotting
The cells were collected after incubation with 15, 30, and 60 μmol/L OA for 24 hours, and lysate was added to extract the total protein. We used the bicinchoninic acid (BCA) method to quantify the protein level. The total protein was separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membrane. The membrane was placed in a 5% skimmed milk powder solution for blocking, placed on a horizontal shaker at 80 rpm, and after 2 hours, the PVDF film was removed, rinsed in TBST (tris-buffered saline and Tween) solution, and washed twice for 10 minutes each time. We added the primary antibody (dilution ratio 1: 1000; β-actin dilution ratio 1: 3000) to the antibody incubation box and incubated at 4°C in a closed bag overnight. After the primary antibody was incubated, the PVDF film was washed on the horizontal shaker 3 times with TBST buffer for 10 minutes each time. We then added the secondary antibody (dilution ratio 1: 1000) solution and incubate 2 hours at room temperature on a shaker at 80 rpm. After the secondary antibody incubation, we washed the PVDF membrane with 10 mL of TBST buffer 3 times, 10 minutes each time. The PVDF membrane was treated with ECL (enhanced chemiluminescence) kit and the protein bands were detected. The relative content of a certain target protein was expressed as the ratio of the integral OD of the target protein to the internal reference.
LC3-II expression were detected with immunofluorescence analysis
To measure LC3-II expression, cells were divided into control groups and OA (15, 30, and 60 μmol/L OA) groups. The cell suspension was added dropwise at a density of 1×105 cells per well on a special glass slide of a lysine-coated 6-well plate and supplemented with medium. When 60% confluence was reached, a blank control and OA intervention were performed. After 24 hours, we discarded the original culture solution, washed 3 times with pre-chilled PBS for 5 minutes, and then fixed the cells with 4% paraformaldehyde for 30 minutes. The fixative was discarded and washed 5 minutes 3 time with PBS, and permeabilized with 0.2% Triton X-100 at room temperature for 20 minutes. After washing 5 minutes 3 times with PBS, we added 5% bovine serum albumin (BSA) and blocked at room temperature for 30 minutes, then removed the section, shook off the blocking solution, diluted the primary antibody LC3-II (1: 100), added the primary antibody dropwise, covered the section specimen, and incubated in a wet box at 4°C overnight. Then we removed the specimen from the wet box the next day and rewarmed it at room temperature for 1 hour. We washed the specimen for 5 minutes 3 times in PBS, then added a fluorophore-labeled secondary antibody (TRITC-goat anti-rabbit IgG, 1:100 dilution) in a wet box, and incubated at 37°C for 1 hour in the dark. The specimen was washed for 5 minutes 3 times in PBS, then we added DAPI and incubated for 5 minutes in the dark. We washed the specimens for 5 minutes 3 times in PBS, then covered the slides with glycerol containing anti-fluorescence extinguishing substance under dark conditions, and immediately observed and collected the images (200×, scale bars: 100 μm) under a fluorescence microscope.
Effects of autophagy inhibitors on apoptosis and autophagy induced by OA in SMMC-7721 cells
SMMC-7721 cells were divided into 4 groups: 1) control group (without any treatment), 2) OA group (cells treated with 30 μmol/L OA alone for 24 hours), 3) 3-MA+OA group (pre-treatment of the cells for 12 hours with 2 mmol/L 3-MA, then treated with 30 μmol/L OA for 24 hours), and 4) CQ+OA group (pre-treatment of cells with 6.25 umol/L CQ for 12 hours, and then treated with 30 μmol/L OA for 24 hours). Cell proliferation activity, apoptosis, mitochondrial membrane potential, ATP content, and major proteins of autophagy and apoptosis were measured according to the test methods described.
Statistical analysis
The test data was statistically analyzed using SPSS 17.0, Excel, and Imagine ProPrus61 image processing software. Data were expressed by mean±standard deviation (x±s). One-way analysis of variance and SNK-q test were used to perform multiple group and pairwise comparisons, respectively. P<0.05 was the difference considered statistically significant.
Results
Effect of OA on proliferation of SMMC-7721 cells
After MTT assay, the inhibitory rates of 15, 30, and 60 μmol/L OA on SMMC-7721 cells were 26.91±4.37%, 52.65±4.35%, and 86.59±4.12%, respectively, and the inhibition rates of the blank control cells was 3.54% ± 0.12%. There were statistically significant differences among the groups by analysis of variance, suggesting that OA inhibited the proliferation of SMMC-7721 cells in a dose-response relationship.
Effect of OA on apoptosis of SMMC-7721 cells
Hoechst 33258/PI is a specific apoptotic cell fluorescent stain that binds to chromatin in the nucleus. Changes in the nucleus can be observed under a fluorescent microscope. As shown in Figure 1, the normal control cells grew well, the cell nucleus was intact, and the chromatin was evenly distributed. Twenty-four hours after OA treatment, apoptotic cells increased, and nucleoplasm condensed and formed apoptotic bodies. With the increase of OA concentration, the staining and fluorescence of cells increased, and apoptotic cells gradually increased.
Figure 1.
Nuclear morphology of SMMC-7721 cells after oleanolic acid treatment.
As shown in Figure 2, apoptosis rates of SMMC-7721 cells treated by OA at 15, 30, and 60 μmol/L were 15.91±3.37%, 22.65±3.35%, and 31.12±3.12%, respectively, by Annexin V-PE method. The apoptosis rate of cells in the control group was 7.15±0.23%. There were statistically significant differences among the groups by analysis of variance. Compared with the control group, the differences were statistically significant, suggesting that OA can significantly promote the apoptosis of SMMC-7721 cells.
Figure 2.
Apoptosis rate of SMMC-7721 cells after oleanolic acid treatment.
Effect of OA on mitochondrial dysfunction of SMMC-7721 cells
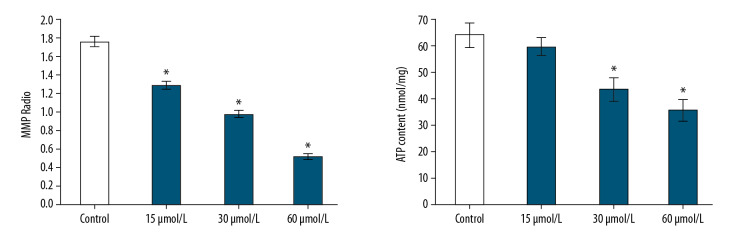
To evaluate mitochondrial dysfunction of SMMC-7721 cells, this study used 2 independent parameters: mitochondrial membrane potential and adenosine-50-triphosphate (ATP) levels. The results showed that the mitochondrial membrane potential ratio (OD polymer/OD monomer) of the control group cells was 1.76±0.054. The mitochondrial membrane potential ratio of SMMC-7721 cells caused by oleanolic acid at 15, 30, and 60 μmol/L was 1.29±0.037, 0.98±0.035, and 0.52±0.029, respectively. At the same time, the ATP content (nmol/mg) of SMMC-7721 cells decreased, the ATP content of cell with 15, 30, and 60 μmol/L OA was 58.65±3.35%, 42.91±4.37, and 35.12±4.12%, respectively, and the ATP content of control cells was 62.91±4.37; there was a statistically significant difference (P<0.05) (Figure 3).
Figure 3.
Effect of oleanolic acid on mitochondrial dysfunction of SMMC-7721 cells. * P<0.05 versus the control group; n=6 in each group.
Effect of OA on expression of apoptosis and autophagy-related proteins in SMMC-7721 cells
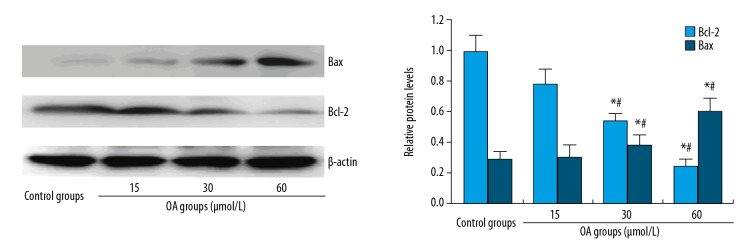
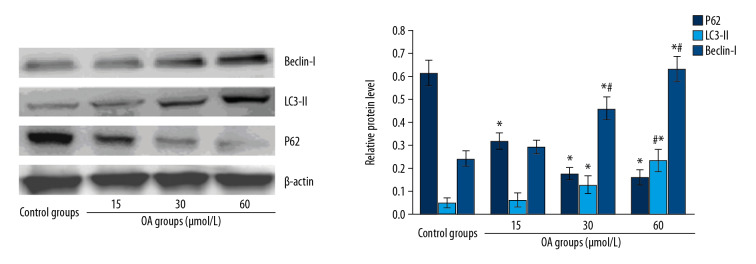
After human hepatocellular carcinoma SMMC-7721 cells were treated with OA for 24 hours, and apoptosis-associated proteins and autophagy-related proteins were detected by western blot. The results are shown in Figures 4 and 5. The expression of Bcl-2 anti-apoptotic protein in SMMC-7721 cells gradually decreased, while expression of Bax pro-apoptotic protein increased gradually with the increase of OA dose. The expression of p62 decreased, and LC3-II and Beclin-1 protein gradually increased. Compared with the control group, the difference was significant, suggesting that OA may promote SMMC 7721 apoptosis and autophagy in a dose-dependent manner.
Figure 4.
Apoptosis-related protein expression levels in SMMC-7721 cell treated by oleanolic acid. * P<0.05 versus the control group; # P<0.05 versus 15 μmol/L OA groups; n=6 in each group.
Figure 5.
Autophagy-related protein expression levels in SMMC-7721 cell treated by oleanolic acid. * P<0.05 versus the control group; # P<0.05 versus 15 μmol/L OA groups; n=6 in each group.
ImageJ was used to quantify the protein and the OD ratio of the target protein to β-actin is used to indicate the relative expression of the target protein (Table 1). After statistical analysis, compared with the control and 15 μmol/L OA group, the expression of Bcl-2 decreased and Bax and the autophagy marker proteins LC3-II and Beclin-1 increased significantly in the 30 and 60 μmol/L OA group (P<0.05). The expression of autophagic substrate p62 decreased with the increase of OA dose; the difference was statistically significant (P<0.05).
Table 1.
Relative protein level of apoptosis or autophagy-related protein in SMMC-7721 cells.
| Groups | Control | 15 μmol/L OA | 30 μmol/L OA | 60 μmol/L OA |
|---|---|---|---|---|
| Bc1-2 | 0.985±0.102 | 0.773±0.098 | 0.531±0.042# | 0.241±0.039# |
| Bax | 0.289±0.045 | 0.297±0.082 | 0.370±0.079# | 0.604±0.087# |
| p62 | 0.605±0.072 | 0.292±0.052* | 0.145±0.032# | 0.112±0.004# |
| LC3-II | 0.047±0.011 | 0.068±0.008 | 0.137±0.007# | 0.211±0.009# |
| Beclin-1 | 0.223±0.009 | 0.271±0.032 | 0.471±0.054# | 0.603±0.062# |
P<0.05 versus the control group;
P<0.05 versus 15 μmol/L oleanolic acid groups; n=6 in each group.
Effect of OA on LC3-II levels of SMMC-7721 cells with immunofluorescence assay
Immunofluorescence cytochemical staining results (Figure 6) showed that OA can stimulate autophagy of SMMC-7721 cells. Compared with the control group, with the increase of OA dose, the intensity of red fluorescent staining of LC3-II expression in cells increased after OA treatment of cells. This shows that autophagy-induced protein LC3-II significantly increase.
Figure 6.
LC3-II levels of SMMC-7721 cells after oleanolic acid treatment with the immunofluorescence assay.
Effect of OA on autophagy pathway in SMMC-7721 cells
By examining the expression of p-Akt (phosphorylated Akt) protein and p-mTOR (phosphorylated mTOR) protein, the effect of OA on Akt/mTOR activity of autophagy-related signaling pathway in SMMC-7721 cells was further explored. Western blot results (Figure 7) showed that p-mTOR protein level in the 15 μmol/L OA group was not significantly different from that in the control group, while the p-mTOR expression in the 30 and 60 μmol/L OA groups decreased significantly; the p-Akt protein level gradually decreased with increasing dosage of OA. Quantitative analysis of proteins was performed using ImageJ software. The relative levels of p-Akt in the SMMC-7721 cells at 15, 30, and 60 μmol/L OA were 0.738±0.086, 0.251±0.031, and 0.080 ± 0.011, respectively, while the expression level of p-Akt at the control group was 0.967±0.102. The p-Akt expression levels in 30 and 60 μmol/L OA groups had statistically significant differences (P<0.05); the relative expression levels of p-mTOR were similar to p-Akt. The expression of p-mTOR in the 30 and 60 μmol/L OA groups was 0.175±0.047 and 0.060±0.009, respectively, which was obviously lower than the control group (0.817±0.098) and the 15 μmol/L OA group (0.776±0.054) (P<0.05). This result suggests that OA inhibits the activity of Akt/mTOR autophagy signaling pathway in hepatoma SMMC-7721 cells.
Figure 7.
p-Akt and p-mTOR expression levels in SMMC-7721 cell treated by oleanolic acid. * P<0.05 versus the control group; # P<0.05 versus 15 μmol/L OA groups; n=6 in each group.
Effects of autophagy inhibitors on the proliferation of SMMC-7721 cells induced by OA
The results of MTT experiments showed that the cell inhibition rate of the control group was 3.85±0.32%, and the inhibition rate of OA (30 μmol/L) treated cells was significantly increased to 50.25±4.65%. The inhibition rate of cells in the 3-MA+OA and CQ+OA groups was 68.52±4.78% and 70.23±5.21%, respectively. Compared with the control group, the difference was significant (P<0.05). Compared with the OA group alone, the cell viability of the 3-MA+OA and CQ+OA groups was significantly reduced (P<0.05), but there was no statistical difference between the 3-MA+OA and CQ+OA groups. The results suggest that the combination of autophagy-specific inhibitor 3-MA or CQ with OA enhances the proliferation inhibition effect on SMMC-7721 cells.
Effects of autophagy inhibitors on apoptosis induced by OA in SMMC-7721 cells
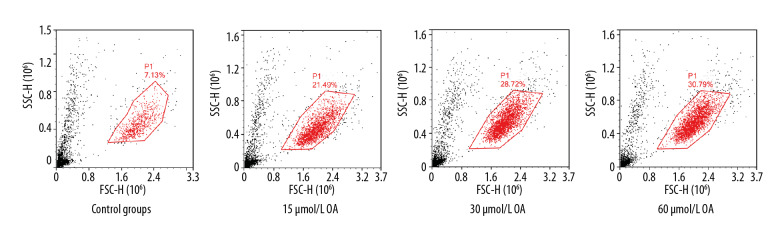
By Annexin V-PE method (as shown Figure 8), the apoptosis rates of SMMC-7721 cells in OA group, 3-MA+OA group, and CQ+OA group were 20.65±4.12%, 28.72±3.96%, and 30.63±4.23% respectively. The apoptotic rate of the control cells was 7.15±0.23%; there were statistically significant differences among the groups by analysis of variance (P<0.05). Compared with the OA group alone, the apoptosis of the 3-MA+OA and CQ+OA groups increased, and the difference was significant (P<0.05), and there was no statistical difference between the 3-MA+OA and CQ+OA groups (P>0.05). The results suggest that 3-MA or CQ, an autophagy-specific inhibitor, can interact with OA and enhance the apoptosis effect on SMMC-7721 cells.
Figure 8.
Effect of autophagy inhibitors on apoptosis of SMMC-7721 cells induced by oleanolic acid.
Effect of autophagy inhibitors on mitochondrial dysfunction of SMMC-7721 cells induced by OA
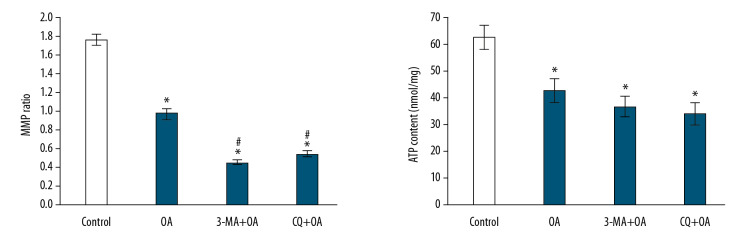
The mitochondrial membrane potential ratios of SMMC-7721 cells in the OA group, 3-MA+OA group, and CQ+OA group were 0.98±0.035, 0.45±0.018, 0.54±0.025, respectively, and the control group’s membrane potential ratio was 1.76±0.054. The ATP levels (nmol/mg) of SMMC-7721 cells in the OA group, 3-MA+OA group, and CQ+OA group were 42.91±4.37, 36.86±3.65, and 34.26±3.98, respectively, and the control group had ATP of 62.91±4.37. There were statistical significance differences among the groups by analysis of variance, as shown in Figure 9).
Figure 9.
Effect of autophagy inhibitors on mitochondrial dysfunction of SMMC-7721 cells induced by oleanolic acid. * P<0.05 versus the control group; # P<0.05 versus the oleanolic acid group; n=6 in each group.
Effects of autophagy inhibitors on autophagy and apoptosis-related protein expression of SMMC-7721 cells induced by OA
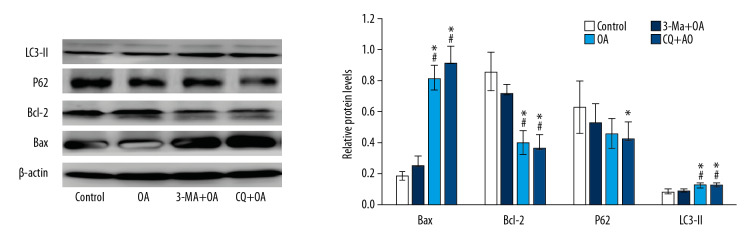
Western blot results showed (Figure 10) that compared with the OA group, the 3-MA and CQ groups inhibited autophagy, the expression of p62 decreased, the expression of LC3-II increased, the expression of apoptosis-related protein Bax increased, and the expression of Bcl-2 decreased. This suggested that 3-MA and CQ treatment increased OA-induced SMMC-7721 cell apoptosis.
Figure 10.
Apoptosis, autophagy-related protein expression in SMMC-7721 cell treated by oleanolic acid and 3-MA or CQ. * P<0.05 versus the control group; # P<0.05 versus the oleanolic acid group; n=6 in each group.
Discussion
Oleanolic acid (OA) and its derivatives not only have anti-oxidation, anti-inflammatory, and antiviral effects [14,15], but also have been proven to induce apoptosis of different types of cancer cells through various pathways [16–18]. In this experiment, SMMC-7721 cells were treated with different doses of OA, and MTT assay showed that OA can significantly inhibit the proliferation of SMMC-7721. After Hoechst 33258 staining, immunofluorescence assay also found OA induced apoptosis in SMMC-7721 hepatoma cells with a dose-dependent relationship.
Apoptosis is a complex regulatory mechanism in which organisms maintain their health and homeostasis. This process involves the participation of a series of genes [19]. Bcl-2 and Bax are considered classical anti-apoptotic and pro-apoptotic members, respectively. Bcl-2 protein is mainly distributed on the mitochondrial outer membrane, endoplasmic reticulum membrane surface, and nuclear membrane. It can inhibit apoptosis by participating in inhibiting the transmembrane transport of calcium ions, inhibiting the release of cytochrome C, and apoptosis-inducing factor. Bax protein, on the other hand, has an anti-apoptotic effect that antagonizes Bcl-2. Bax is an important component of the mitochondrial apoptotic pathway of cells, and it cooperates with Bcl-2 to cause changes in mitochondrial membrane permeability, make transmembrane potential disappear, release cytochrome C, and apoptotic protease activation factors. So, the overexpression of Bax promotes apoptosis. Our study results showed that OA can promote the expression of Bax protein in SMMC-7721 cells, and downregulate the expression of anti-apoptotic protein Bcl-2 and reduce the formation of dimer of Bc1-2 and Bax, suggesting that OA can promote apoptosis through the Bax protein-mediated gene pathway.
Autophagy, or “self-phagocytosis”, is an important regulatory mechanism for cell growth, development, maturation and death, and is closely related to the occurrence and development of a variety of human diseases, including tumors. The relationship between autophagy and tumors has been a hot topic in recent years. It has been found that autophagy can be found associated with the action of some anticancer drugs, such as rapamycin, resveratrol, and tamoxifen, suggesting that autophagy may be a potential anticancer mechanism. The effect of autophagy is often determined by detecting autophagy-related markers, including LC3 and Beclin-1. LC3 plays an important role in autophagy. LC3 exists in 2 forms, LC3-I and LC3-II. Among them, LC3-I is an inactive form. After autophagy begins, LC3-I will enzymatically dissolve a small peptide, transforming into LC3-II and then aggregate to the autophagosome membrane. Therefore, the level of LC3-II reflects the degree of autophagy to a certain extent, and it is a specific protein marker for intracellular autophagy level. Beclin-1 gene is also a mammal-specific gene involved in autophagy [20]. Beclin-1 gene mainly participates in the formation of complexes with type III PI3K and regulates autophagic activity. Besides, p62 is a protein that accumulates on autophagosomes and can interact with LC3II to guide autophagosome degradation. Therefore, p62 is an index for evaluating autophagosome degradation [21]. Therefore, p62 can also reflect autophagic activity, and p62 protein is negatively correlated with the level of autophagy. In this experiment, western blot showed that when the concentration of OA increased, the expression level of autophagic substrate p62 decreased, and the autophagy marker protein LC3-II and the Beclin-1 protein were gradually increased. Therefore, the experimental results showed that when OA acts on tumor cells, autophagy can be induced in SMMC-7721 hepatoma cells, and the autophagy level gradually increases with the increase of OA concentration. Combined with the aforementioned inhibitory proliferation effect of SMMC-7721 tumor cells, this indicates that OA inhibits tumor growth through autophagy.
Autophagy-related signal transduction pathways are intricate and complex. At present, there are several important related signal pathways such as mTOR, PI3K/AKT, ROS, and NF-κB. Studies have shown that PI3K/AKT/mTOR autophagy-related signaling pathways are abnormally active in tumor cells [22]. It has been reported in the literature that some antitumor drugs can induce autophagy by inhibiting this pathway [23–25]. A study on hydrogen sulfide showed that hydrogen sulfide could induce autophagy and apoptosis of hepatocellular carcinoma cell lines Hep G2 and HLE by inhibiting the PI3K/Akt/mTOR signal transduction pathway, and inhibiting liver cancer cell proliferation and migration [26]. In a study of a histone acetyltransferase P300/CBP-associated factor (PCAF), it was found that PCAF could also promote the autophagy of liver cancer cells by inhibiting the PI3K/Akt/mTOR signal transduction pathway and inhibiting the growth of liver cancer cells [27]. Furthermore, anti-apoptotic proteins BC1-2 and BCI-XI, which can bind to Beclin-1 and inhibit Beclin-1 activity, can also regulate autophagy activity and have important implications for cell survival [28]. The experimental results showed that OA can reduce the expression p-Akt, p-mTOR of autophagy pathway proteins, while downregulating the expression of Bcl-2, enhancing Beclin-1 expression and promoting autophagic cells. In summary, these results suggest that OA may induce the activation of SMMC-7721 hepatoma cellautophagy by inhibiting Akt/mTOR signaling pathway.
At different stages of tumor development, the role of autophagy has changed dramatically. In the early stage, inhibition of autophagy leads to the continuous growth of tumor cells. At this time, autophagy exerts a tumor-suppressing effect [29]. When tumor cells continue to divide and proliferate, cancer cells use autophagy mechanisms to counteract external adverse factors. At this time, autophagy plays a role in promoting the growth and survival of cancer cells [30]. On the other hand, regulation of autophagy has become a new direction in the current treatment of cancer [31]. In recent years, more and more autophagy inhibitors have been used in the treatment of tumors. Most scholars believe that autophagy inhibitors can enhance the anti-tumor effect [32,33]. Studies have shown that autophagy inhibitors can work synergistically with chemotherapy and radiotherapy to enhance the lethality of tumor cells. Schonewolf et al. found that the autophagy inhibitor CQ can significantly increase the sensitivity HT-29 cells to 5-FU and radiotherapy [34]. Liang et al. also found that autophagy inhibition enhances the sensitivity of breast cancer cells to radiotherapy [35]. Research by Wu et al. [36] also showed that autophagy inhibitors 3-MA and chloroquine (CQ) can effectively enhance the effect of bortezomib on inducing apoptosis, thereby inhibit lung cancer cell proliferation. The specific effect of autophagy on hepatocellular carcinoma is related to various factors such as tumor growth stage, microenvironment, gene polymorphism, and other external stimuli [37]. Some researchers have studied the autophagy of liver cancer cells. Ding et al. [38] found that the expression of autophagy-related protein Beclin-1 in liver cancer tissues was significantly lower than in surrounding normal tissues, and the expression level of Beclin-1 was related to the malignant degree of liver cancer. Ren et al. [39] found that autophagy can inhibit the proliferation of Hep G2 cells, and autophagy is not conducive to the survival of Hep G2 cells. Glaaer et al. [40] applied PEG-modified arginine deiminase to break down arginine in the environment, creating an arginine-deficient environment, which can promote cell autophagy and lead to liver cancer cell death. Studies have shown that autophagy inhibitors 3-MA and chloroquine can inhibit the growth of liver cancer tumors [41]. Therefore, we investigated whether the OA-induced autophagy is a cytoprotective response or leads to the cell death process. We introduced the autophagy-specific blocker 3-MA and CQ to prevent autophagy. In this experiment, SMMC-7721 cells were incubated with a culture solution containing 2 mmol/L 3-MA or 6.25 umol/L CQ for 12 hours, and then treated with 30 μmol/L OA. The results showed that 3-MA or CQ significantly enhanced the inhibition of proliferation of hepatoma SMMC-7721 cells by OA. Since the anti-apoptotic proteins and pro-apoptotic proteins in the Bcl-2 family can determine the cell’s life and death. 3-MA or CQ can further enhance the expression changes of these 2 proteins. After treatment with 3-MA or CQ and OA, the expression of Bcl-2 further decreased, while Bax protein further increased. Therefore, we speculated that 3-MA or CQ inhibited autophagy and increased apoptosis caused by OA. These data indicate that OA activates the protective autophagy of SMMC-7721 cells to adapt to the severe environment and protect SMMC-7721 cells from death, while inhibition of protective autophagy can increase anti-tumor effect of OA.
Metabolic changes are one of the hallmarks of tumor cells, and redox signaling pathways play an important role in tumor cell formation, metabolism, and response to radiotherapy and chemotherapy [42]. Mitochondria are one of the important sources of intracellular oxidative stress and are involved in the maintenance of redox balance, which is essential for cell survival. Mitochondrial dysfunction is characterized by the breakdown of mitochondrial membrane which is manifested by a decrease in membrane potential and an increase in membrane permeability. When tumor cells are exposed to external harmful factors, the mitochondrial membrane potential is attacked first, and then a series of apoptotic signals are triggered, and the anti-apoptotic pathway is inhibited, which leads to inhibition of tumor cell proliferation, activation of apoptosis, and cell death At the same time, it is accompanied by the activation of cell autophagy to reduce the damage caused by oxidative stress to tumor cells [43]. Studies have shown that during the initial stage of apoptosis, mitochondrial membrane potential will decrease rapidly, and the changes may be earlier than the eversion of the cell membrane, phosphatidylserine eversion, activation of cysteine proteinase caspase-3, and changes in cell morphology. Once the mitochondrial membrane potential decreases, it indicates that the cell has entered an irreversible apoptotic process, and it is proven that the decrease in mitochondrial membrane potential is a characteristic event in the process of apoptosis [44]. In addition, mitochondria are the cell’s energy supply factory and can efficiently use ATP. Cancer cells need to consume large amounts of ATP as energy supply. Therefore, any disruption in this supply could lead to tumor cell apoptosis. More and more studies have shown that inhibition of autophagy can lead to mitochondrial dysfunction, which in turn induces cell death [45–46]. In this study, we found that OA inhibits autophagy of SMMC-7721 cells accompanied by mitochondrial dysfunction, and that inhibition of autophagy increases OA-induced mitochondrial dysfunction. These results indicate that in liver cancer cell SMMC-772, changes in autophagy can cause cell mitochondrial dysfunction, and OA causes apoptosis by inhibiting SMMC-7721 cells autophagy.
Conclusions
OA can significantly inhibit the growth of liver cancer SMMC-7721 cells and induce autophagy and apoptosis. Interfering with mitochondrial function of SMMC-7721 cells may be one of the mechanisms. Autophagy activation decreased the toxicity of OA on SMMC-7721 cells. Blocking OA-induced autophagy by 3-MA can significantly enhance the growth inhibition and apoptosis of SMMC-7721 cells induced by OA. This study suggests that the combination of autophagy inhibitor 3-MA or CQ and OA can exert its anti-cancer effect better, which suggest OA could play in role in the clinical treatment of liver cancer.
Footnotes
Availability of data and materials
The data are available from the corresponding author.
Conflict of interest
None.
Source of support: This work was supported by National Natural Science Foundation (No. 21665015)
References
- 1.Yan SL, Huang CY, Wu ST, et al. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol In Vitro. 2010;24(3):842–48. doi: 10.1016/j.tiv.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Rawendra RD, Lin PY, Chang CD, et al. Potentiation of acute promyelocytic leukemia cell differentiation and prevention of leukemia development in mice by oleanolic acid. Anticancer Res. 2015;35(12):6583–90. [PubMed] [Google Scholar]
- 3.Lucio KA, da Graca Rocha G, Moncao-Ribeiro LC, et al. Oleanolic acid initiates apoptosis in non-small cell lung cancer cell lines and reduces metastasis of a B16F10 melanoma model in vivo. PLoS One. 2011;6(12):e28596. doi: 10.1371/journal.pone.0028596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Lin J, Sun G, et al. Oleanolic acid inhibits colorectal cancer angiogenesis in vivo and in vitro via suppression of STAT3 and Hedgehog pathways. Mol Med Rep. 2016;13(6):5276–82. doi: 10.3892/mmr.2016.5171. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Song Q, Hu D, et al. Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3 K/Akt/Mtor and ROS-dependent pathway. Korean J Physiol Pharmacol. 2016;20(3):237–43. doi: 10.4196/kjpp.2016.20.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling L, Jian-long Z, Jian-gang W. [Oleanolic acid induces G2/M phase arrest and apoptosis in human hepatocellular carcinoma Bel-7402 cells]. Zhongguo Zhong Yao Za Zhi. 2015;40(24):4897–902. [in Chinese] [PubMed] [Google Scholar]
- 7.Zhou R, Zhang Z, Zhao L, et al. Inhibition of mTOR signaling by oleanolic acid contributes to its anti-tumor activity in osteosarcoma cells. J Orthop Res. 2011;29(6):846–52. doi: 10.1002/jor.21311. [DOI] [PubMed] [Google Scholar]
- 8.Shyu MH, Kao TC, Yen GC. Oleanolic acid and ursolic acid induce apoptosis in HuH7 human hepatocellular carcinoma cells through a mitochondrial-development pathway and downregulation of XIAP. J Agric Food Chem. 2010;58(10):6110–18. doi: 10.1021/jf100574j. [DOI] [PubMed] [Google Scholar]
- 9.Lin CC, Huang VY, Mong MC, et al. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J Agric Food Chem. 2011;59(2):755–62. doi: 10.1021/jf103904b. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Liu M, Li D. Oleanolic acid suppresses the proliferation of lung carcinoma cells by miR-122/CyclinG1/MEF2D axis. Mol Cell Biochem. 2015;400(1–2):1–7. doi: 10.1007/s11010-014-2228-7. [DOI] [PubMed] [Google Scholar]
- 11.Žiberna L, Šamec D, Mocan A, et al. Oleanolic acid alters multiple cell signaling pathways: Implication in cancer prevention and therapy. Int J Mol Sci. 2017;18(3) doi: 10.3390/ijms18030643. pii: E643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Wu N, Ma LN, et al. Oleanolic acid suppresses aerobic glycolysis in cancer cells by switching pyruvate kinase type misoforms. PLoS One. 2014;9:e91606. doi: 10.1371/journal.pone.0091606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma W, Wang DD, Li L, et al. Caveolin-1 plays a key role in the oleanolic acid-induced apoptosis of HL-60 cells. Oncol Rep. 2014;32(1):293–301. doi: 10.3892/or.2014.3177. [DOI] [PubMed] [Google Scholar]
- 14.Lin C, Wen X, Sun H. Oleanolic acid derivatives for pharmaceutical use: A patent review. Expert Opin Ther Pat. 2016;26(6):643–55. doi: 10.1080/13543776.2016.1182988. [DOI] [PubMed] [Google Scholar]
- 15.Yan M, Min-lin M, Nan G, et al. Ursolic acid induces apoptosis of prostate cancer cells via the PI3K/Akt/mTOR pathway. Am J Chin Med. 2015;43(7):1–16. doi: 10.1142/S0192415X15500834. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Ryu HG, Lee J, et al. Ursolic acid exerts anti-cancer activity by suppressing vaccinia- related kinase 1-mediated damage repair in lung cancer cells. Sci Rep. 2015;(5):1–12. doi: 10.1038/srep14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Huang KY, Ling Y, et al. Discovery of an oleanolic acid/hederagenin-nitric oxide donor hybrid as an EGFR tyrosine kinase inhibitor for non-small-cell lung cancer. J Nat Prod. 2019;82(11):3065–73. doi: 10.1021/acs.jnatprod.9b00659. [DOI] [PubMed] [Google Scholar]
- 18.Liu CM, Huang JY, Sheng LX, et al. Synthesis and antitumor activity of fluorouracil–oleanolic acid/ursolicacid/glycyrrhetinic acid conjugates. Medchemcomm. 2019;10(8):1370–78. doi: 10.1039/c9md00246d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang M, Yang XM, Yin DH, et al. Beclin-1 enhances cisplatin-induced apoptosis via Bcl-2-modulated autophagy in laryngeal carcinoma cells Hep-2. Neoplasma. 2018;65(1):42–48. doi: 10.4149/neo_2018_161102N528. [DOI] [PubMed] [Google Scholar]
- 20.Cohen-Kaplan V, Ciechanover A, Livneh I. p62 at the crossroad of the ubiquitin-proteasome system and autophagy. Oncotarget. 2016;7(51):83833–34. doi: 10.18632/oncotarget.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Fan J, Wang S, et al. Targeting CD47 and autophagy elicited enhanced antitumor effects in non-small cell lung cancer. Cancer Immunol Res. 2017;5(5):363–75. doi: 10.1158/2326-6066.CIR-16-0398. [DOI] [PubMed] [Google Scholar]
- 22.Kim KY, Park KI, Kim SH. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int J Mol Sci. 2017;18(5) doi: 10.3390/ijms18051088. pii: E1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Huang YH, Huang FY, et al. 3′-epi-12β-hydroxyfroside, a new cardenolide, induces cytoprotective autophagy via blocking the Hsp90/Akt/mTOR axis in lung cancer cells. Theranostics. 2018;8(7):2044–60. doi: 10.7150/thno.23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Pi C, Wang G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed Pharmacother. 2018;103:699–707. doi: 10.1016/j.biopha.2018.04.072. [DOI] [PubMed] [Google Scholar]
- 25.Wang SS, Chen YH, Ning C, et al. Hydrogen sulfide promotes autophagy of hepatocellular carcinoma cells through the PI3K/Akt/mTOR signaling pathway. Cell Death Dis. 2017;8(3):e2688. doi: 10.1038/cddis.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia YL, Xu M, Dou CW, et al. P300/CBP – associated factor (PCAF) inhibits the growth of hepatocellular carcinoma by promoting cell autophagy. Cell Death Dis. 2016;7(10):e2400. doi: 10.1038/cddis.2016.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song X, Lee DH, Dilly AK, et al. Crosstalk between apoptosis and autophagy is regulated by the arginylated BiP/Beclin-1/p62 complex. Mol Cancer Res. 2018;16(7):1077–91. doi: 10.1158/1541-7786.MCR-17-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li YJ, Lei YH, Yao N, et al. Autophagy and multidrug resistance in cancer. Chin J Cancer. 2017;36(1):52. doi: 10.1186/s40880-017-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onorati AV, Dyczynski M, Ojha R, et al. Targeting autophagy in cancer. Cancer. 2018;124(16):3307–18. doi: 10.1002/cncr.31335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang T, Song X, Yang Y, et al. Autophagy and hallmarks of cancer. Crit Rev Oncog. 2018;23(5–6):247–67. doi: 10.1615/CritRevOncog.2018027913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chude CI, Amaravadi RK. Targeting autophagy in cancer: Update on clinical trials and novel inhibitors. Int J Mol Sci. 2017;18(6) doi: 10.3390/ijms18061279. pii: E1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang A, Zhao Y, Wang Y, et al. Huaier suppresses proliferative and metastatic potential of prostate cancer PC3 cells via downregulation of Lamin B1 and induction of autophagy. Oncol Rep. 2018;39(6):3055–63. doi: 10.3892/or.2018.6358. [DOI] [PubMed] [Google Scholar]
- 33.Lin SR, Fu YS, Tsai MJ, et al. Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy. Int J Mol Sci. 2017;18(7) doi: 10.3390/ijms18071412. pii: E1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schonewolf CA, Mehta M, Schiff D. Autophagy inhibition by chloroquine sensitizes HT-29 colorectal cancer cells to concurrent chemoradiation. World J Gastrointest Oncol. 2014;6(3):74–82. doi: 10.4251/wjgo.v6.i3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang DH, El-Zein R, Dave B. Autophagy inhibition to increase radiosensitization in breast cancer. J Nucl Med Radiat Ther. 2015;6(5) doi: 10.4172/2155-9619.1000254. pii: E254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu G, Li H, Ji Z, et al. Inhibition of autophagy by autophagic inhibitors enhances apoptosis induced by bortezomib in non-small cell lung cancer cells. Biotechnol Lett. 2014;36(6):1171–78. doi: 10.1007/s10529-014-1470-0. [DOI] [PubMed] [Google Scholar]
- 37.Dash S, Chava S, Chandra PK, et al. Autophagy in hepatocellular carcinomas: From pathophysiology to therapeutic response. Hepat Med. 2016;8:9–20. doi: 10.2147/HMER.S63700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding ZB, Shi YH, Zhou J, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68(22):9167–75. doi: 10.1158/0008-5472.CAN-08-1573. [DOI] [PubMed] [Google Scholar]
- 39.Ren N, Wang SM, Dong XS, et al. [Study on the effect of autophagy on the proliferation and cell cycle of HepG2 cells in liver cancer]. China Contemporary Medicine. 2012;19(11):8–9. [in Chinese] [Google Scholar]
- 40.Glaaer ES, Stone EM, Zhu C, et al. Bioengineered human arginase I with enhanced activity and stability controls hepatocellular and pancreatic carcinoma xenografts. Transl Oncol. 2011;4(3):138–46. doi: 10.1593/tlo.10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yazdani HO, Huang H, Tsug A. Autophagy: Dual response in the development of hepatocellular carcinoma. Cells. 2019;8(2):E91. doi: 10.3390/cells8020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward PS, Thompson CB. Metabolic reprogramming: A cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao W, Ye JZ, Zhao J, et al. Cadmium induces apoptosis in human embryonic kidney (HEK) 293 cells by caspase-dependent and -independent pathways acting on mitochondria. Toxicol in Vitro. 2007;21(3):343–54. doi: 10.1016/j.tiv.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Barroso G, Kably A, Carballo E, et al. Mitochondrial transmembrane potential and plasma membrane translocation of phosphatidyscrine (PS) as early apoptosis markers: Analysis of fractionated human spermatozoa. Fertil Steril. 2001;76(3):S154–55. [Google Scholar]
- 45.Hall A, Unwin R. The not so ‘mighty chondrion’: Emergence of renal diseases due to mitochondrial dysfunction. Nephron Physiology. 2007;105(1):1–10. doi: 10.1159/000096860. [DOI] [PubMed] [Google Scholar]
- 46.Hagiwara M, Yamagata K, Capaldi RA, et al. Mitochondrial dysfunction in focal segmental glomerulosclerosis of puromycin aminonucleoside nephrosis. Kidney Int. 2006;69(7):1146–52. doi: 10.1038/sj.ki.5000207. [DOI] [PubMed] [Google Scholar]