Studies have shown that some patients with coronavirus disease 2019 (COVID-19) and acute hypoxaemic respiratory failure have preserved lung compliance, suggesting that processes other than alveolar damage might be involved in hypoxaemia related to COVID-19 pneumonia.1 The typical imaging features of COVID-19 pneumonia, including peripheral ground-glass opacities with or without consolidation, are also non-specific and can be seen in many other diseases.2 There has been increasing attention on microvascular thrombi as a possible explanation for the severe hypoxaemia related to COVID-19.3, 4
Dual-energy CT imaging can be used to characterise lung perfusion and is done as part of the standard protocol for imaging pulmonary embolism at our institution. Three patients with COVID-19, as confirmed by nasopharyngeal RT-PCR at our hospital, who did not have a history of smoking, asthma, chronic obstructive pulmonary disease, or other pulmonary conditions, underwent dual-energy CT imaging for elevated concentrations of D-dimer (>1000 ng/mL) and clinical suspicion of pulmonary emboli. Although no pulmonary emboli were observed in these individuals, we noted striking perfusion abnormalities that have not been previously described; in retrospect, at least nine other COVID-19 cases also shared these findings. In addition to the typical CT features of COVID-19 pneumonia,2 we observed considerable proximal and distal pulmonary vessel dilatation and tortuosity, predominately within, or surrounding, areas of lung opacities. Here, we present the first published images from dual-energy CT imaging of COVID-19 pneumonia that show profound vascular and perfusion abnormalities (figure ; appendix).
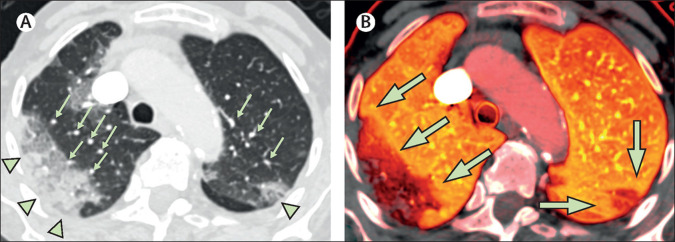
Figure.
Dual-energy CT in a patient with COVID-19 pneumonia without evidence of pulmonary emboli
Patient 1, an 87-year-old woman with a history of fever and cough for 5 days, was found on the floor of her nursing home. On admission to hospital, the patient required a non-rebreather mask with a flow rate of 15 L/min to maintain an oxygen saturation of 85%; intubation was not pursued as the patient's status was comfort measures only. (A) There is a large area of peripheral ground-glass opacity and consolidation within the right upper lobe and smaller ground-glass opacity in the posterior left upper lobe (green arrowheads), which are accompanied by dilated subsegmental vessels proximal to, and within, the opacities (green arrows). (B) The accompanying image of pulmonary blood volume shows corresponding wedge-shaped areas of decreased perfusion within the upper lobes, with a peripheral halo of higher perfusion (green arrows). COVID-19=coronavirus disease 2019.
Three major findings from dual-energy CT were observed on the images of pulmonary blood volume perfusion: preferentially increased perfusion of the lungs proximal to areas of lung opacity, decreased areas of peripheral perfusion corresponding to peripheral lung opacities, and a halo of increased perfusion surrounding peripheral areas of consolidation. The observed pulmonary vascular dilation might be due to relative failure of normal, physiological hypoxic pulmonary vasoconstriction in the setting of overactivation of a regional vasodilatation cascade as part of a dysfunctional and diffuse inflammatory process. Additionally, the mosaic perfusion pattern did not correspond to findings of bronchial wall thickening or secretions, making airway disease as the main underlying cause of hypoxaemia unlikely. Therefore, these perfusion abnormalities, combined with the pulmonary vascular dilation we observed, are suggestive of intrapulmonary shunting toward areas where gas exchange is impaired, resulting in a worsening ventilation–perfusion mismatch and clinical hypoxia. Although peripheral opacities with hypoperfusion can be seen in pulmonary infarction, no pulmonary emboli were observed in any of the studies, and segmental increased perfusion to areas of infarction would be very atypical. Furthermore, a peripheral halo of increased perfusion has not been described in pulmonary infarction, but has been described once previously in a case of bacterial pneumonia.5 However, blood and sputum cultures were negative in the three patients with COVID-19 at our hospital and did not suggest bacterial coinfection. It might be possible that the inflammatory response to COVID-19 resembles more of a bacterial infection than a viral infection. Overall, the combination of these imaging findings is novel for COVID-19 pneumonia.
Treatment for acute respiratory failure in patients with COVID-19 is challenging in part because of little understanding of the underlying pathophysiology. Our findings are atypical for acute respiratory distress syndrome or thrombotic vascular disease and point to a possible central role for previously underappreciated pulmonary vascular shunting. More detailed assessments of vascular and perfusion changes in patients with COVID-19 are urgently needed.
Acknowledgments
BPL reports royalties from Elsevier as a textbook author and editor, outside the submitted work. AW reports personal fees from Bayer Pharmaceuticals, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. published online March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernheim A, Mei X, Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 doi: 10.1148/radiol.2020200463. published online Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok FA, Kruip MJHA, van der Meer NJM. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. published online April 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otrakji A, Digumarthy SR, Lo Gullo R, Flores EJ, Shepard JA, Kalra MK. Dual-energy CT: spectrum of thoracic abnormalities. Radiographics. 2016;36:38–52. doi: 10.1148/rg.2016150081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.