Abstract
Background
Health care-associated infections most commonly result from person-to-person transmission via the hands of health care workers.
Methods
We studied the efficacy of hand hygiene agents (n = 14) following 10-second applications to reduce the level of challenge organisms (Serratia marcescens and MS2 bacteriophage) from the hands of healthy volunteers using the ASTM-E-1174-94 test method.
Results
The highest log10 reductions of S marcescens were achieved with agents containing chlorhexidine gluconate (CHG), triclosan, benzethonium chloride, and the controls, tap water alone and nonantimicrobial soap and water (episode 1 of hand hygiene, 1.60-2.01; episode 10, 1.60-3.63). Handwipes but not alcohol-based handrubs were significantly inferior from these agents after a single episode of hand hygiene, but both groups were significantly inferior after 10 episodes. After a single episode of hand hygiene, alcohol/silver iodide, CHG, triclosan, and benzethonium chloride were similar to the controls in reduction of MS2, but, in general, handwipes and alcohol-based handrubs showed significantly lower efficacy. After 10 episodes, only benzethonium chloride (1.33) performed as well as the controls (1.59-1.89) in the reduction of MS2.
Conclusions
Antimicrobial handwashing agents were the most efficacious in bacterial removal, whereas waterless agents showed variable efficacy. Alcohol-based handrubs compared with other products demonstrated better efficacy after a single episode of hand hygiene than after 10 episodes. Effective hand hygiene for high levels of viral contamination with a nonenveloped virus was best achieved by physical removal with a nonantimicrobial soap or tap water alone.
For centuries, hand hygiene has been considered an important measure in promoting both public health and good personal hygiene. With careful attention to hand hygiene, lower rates of infectious disease have been documented in diverse settings, such as health care facilities,1, 2, 3 child care centers,4, 5 and households.6, 7 Adequate hand hygiene has the potential to remove pathogenic microorganisms from the hands and disrupt person-to-person transmission of infectious diseases.
With the increased recognition of the importance of antiseptic use in health care settings, the armamentarium for hand hygiene has now expanded to include antimicrobial foams, rubs, lotions, wipes, and soaps. Although there are many published experimental studies on the efficacy of the currently available antimicrobial agents,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 few studies have compared the efficacy of multiple agents, and no study has evaluated both the bactericidal and virucidal efficacy of the agents. Comparative efficacy is difficult to extrapolate from the existing literature because these hand hygiene efficacy studies were conducted using a variety of methodologies. Furthermore, previous hand hygiene efficacy studies have tested the efficacy of hand hygiene agents for unrealistically long contact times of at least 30 seconds, whereas health care professionals have been observed in 8 out of 14 studies to wash their hands for less than 10 seconds and never exceeding 24 seconds.24 No observational data were available on the duration of hand hygiene with alcohol-based handrubs. Thus, the largest comparative efficacy study to date using a standard methodology was undertaken to test 14 different hand hygiene agents for their efficacy in the reduction of both bacteria and viruses from the hands, using the realistic hand hygiene use time of 10 seconds. This study was conducted in conjunction with an analysis of the effects of test variables on the efficacy of hand hygiene agents.25 Based on our previously published work,25 this study employed those parameters that most clearly mimicked clinical use and enhanced the validity of a human challenge study. In addition, we observed hand hygiene among health care providers with an alcohol-based handrub to determine the duration of hand hygiene under actual use conditions.
Materials and methods
Study population
Sixty-two healthy, adult, human volunteers were recruited for 70 hand hygiene efficacy evaluations for 14 different hand hygiene test agents (5 evaluations per test agent). The study was approved by the University of North Carolina School of Medicine Institutional Review Board, and written informed consent was obtained. Subjects were screened by questionnaire and physical examination for skin disorders and allergies prior to participation and excluded if they had eczema, psoriasis, any other chronic skin condition, nonintact skin, or an allergy to any active ingredients in the hand hygiene agents. For at least 7 days prior to their participation in the study, the subjects were instructed to avoid antimicrobial hand agents and were provided with a nonantimicrobial hand soap (Soft Soap Hand Soap; Colgate Palmolive Company, New York, NY) and a pair of reusable rubber gloves (Playtex Hand Saver Gloves, Platex Products, Inc., Dover, DE) to use while cleaning or washing dishes. Only a single subject was a health care worker who provided clinical care. Subjects were allowed to participate in no more than 3 evaluations during this study. An experimental evaluation is defined as a cleansing wash, followed by 10 episodes of hand contamination, hand hygiene, and efficacy evaluation following episodes 1, 3, 5, 7, and 10 ( Fig 1). The period of time between participation, called the washout period, was at least 2 weeks. Five subjects were randomly assigned to each hand hygiene agent, and testing was done in a random manner.
Fig 1.
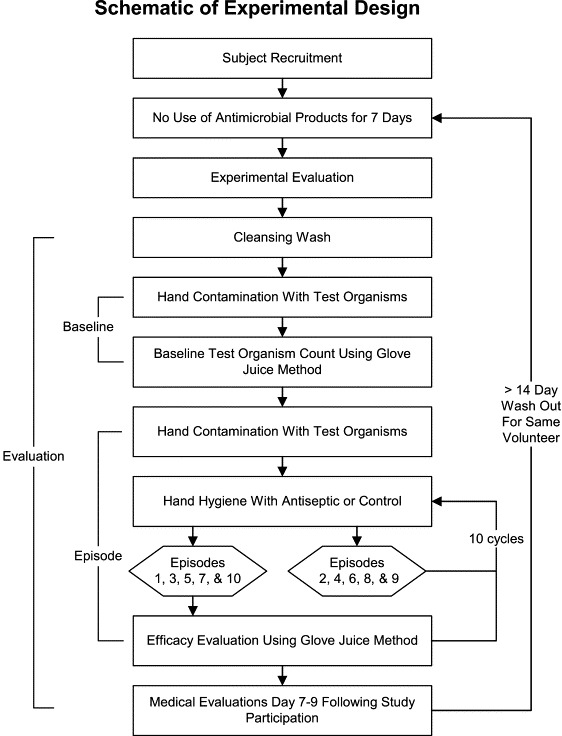
Schematic of experimental design that defines “baseline,” “episode,” and “evaluation.”
Study methods for evaluating efficacy of hand hygiene agents
The Standard Test Method for Evaluation of the Effectiveness of Healthcare Personnel Handwash Formulations26 was used to measure the comparative efficacy of various health care hand hygiene agents. Modifications to the Standard Test Method included the following: (1) All hand hygiene was performed for 10 seconds, and (2) the efficacy of hand hygiene agents was evaluated against MS2 bacteriophage in addition to S marcescens.
Study methodology is summarized as a flow diagram in Figure 1. Prior to beginning the experiment, subjects washed their hands for 10 seconds with a nonantimicrobial soap (Soft 'N Sure; Steris, St. Louis, MO) to cleanse the hands and to become familiar with the procedure. Prior to the baseline measurement and each hand hygiene, the hands were contaminated with a liquid suspension containing both ≈3 × 108 CFU/mL of S marcescens and ≈3 × 109 PFU/mL of MS2. The suspension containing the microorganisms was poured into the subjects' cupped hands and was spread over their entire hands for 45 seconds. Next, the subjects allowed their hands to air-dry for 60 seconds. For the baseline measurement, the hands were sampled immediately following this contamination and air-dry period using the glove juice method described below. The baseline measurement was used to ensure that the organisms had adhered to the hands; our data showed that the method allowed the recovery of ≈3 × 107 CFU/mL for S marcescens and ≈1 × 108 PFU/mL for MS2 on each hand. Immediately after the baseline measurement, the hands were recontaminated, air-dried for 60 seconds, cleaned with the selected hand hygiene agent for 10 seconds, rinsed for 10 seconds (if rinse was indicated per manufacturer's instructions), and then immediately sampled according to the schedule displayed in Table 1.
Table 1.
Schedule of contaminations and washes
| Contamination | Hand hygiene | Recovery | |
|---|---|---|---|
| Nonantimicrobial cleansing wash | No | Yes | No |
| Baseline | Yes | No | Yes |
| Episode 1 | Yes | Yes | Yes |
| Episode 2 | Yes | Yes | No |
| Episode 3 | Yes | Yes | Yes |
| Episode 4 | Yes | Yes | No |
| Episode 5 | Yes | Yes | Yes |
| Episode 6 | Yes | Yes | No |
| Episode 7 | Yes | Yes | Yes |
| Episode 8 | Yes | Yes | No |
| Episode 9 | Yes | Yes | No |
| Episode 10 | Yes | Yes | Yes |
The glove juice method, used to sample the hands for remaining organisms, consisted of placing each hand into a large-size, nonsterile latex glove filled with 75 mL of a sampling and neutralizing solution and massaging the gloved hand for 30 seconds. Nonsterile gloves were chosen because we had previously shown no difference in recovery or contamination with the use of nonsterile gloves as compared with a sterile glass flask.25 After the hand massage, 5 mL glove juice was aseptically retrieved from each glove, serially diluted, and assayed by the spread plate technique (S marcescens) and by the double-agar layer technique (MS2) on tryptic soy agar plates (Remel, Lenexa, KS).27 At the end of the experiment, the subjects washed their hands extensively with 4% chlorhexidine gluconate (CHG) followed by 95% ethanol.
Test organisms
The stock preparation of the bacterial test organism S marcescens (ATCC 14756) was stored frozen in skim milk at −80°C. One liter of tryptic soy broth (Becton, Dickinson, and Company, Sparks, MD) was inoculated from a subculture of the frozen stock grown on tryptic soy agar and was incubated for 24 ± 4 hours at 25°C. The culture was stirred vigorously prior to removing 5-mL aliquots for inoculation onto the hands, and each aliquot was vortexed prior to application to the hands. Serial dilutions of the S marcescens suspension were assayed using the spread plate technique (100 μL per plate) at the beginning and end of each experiment. The suspension was used for no more than 4 hours.
As a surrogate for a nonenveloped human virus, MS2 (ATCC 15597-B1) was chosen for its stability and resemblance to clinically important families of viruses, including the picornaviridae and caliciviridae families. A stock preparation was made using plaque purification, propagation in host Escherichia coli C3000 bacterial cells (ATCC 15597), and chloroform extraction of infected cell lysate for final purification.28 Aliquots of MS2 were titered using the double-agar layer technique and then stored in phosphate buffer solution (pH 7.5) containing 20% (vol/vol) glycerol at −80°C. Fifty microliters of MS2 were added to the 5-mL aliquots of S marcescens and vortexed prior to inoculation. The host bacterial strain for the MS2 assay, E coli C3000, was stored at −80°C in tryptic soy broth containing 20% (vol/vol) glycerol. To prepare the E coli C3000 for use in the bacteriophage assay, 30 mL tryptic soy broth were inoculated with the stock preparation of E coli C3000 and incubated overnight at 37°C with constant rotary shaking at approximately 150 RPM.
Solution preparation
Both the Butterfield's phosphate buffer (KH2PO4 in water) diluent and the sampling solution were prepared according to the description in the ASTM E 1174-94. In addition, a neutralizing solution (Tween 80, lecithin, sodium oleate, sodium thiosulfate, proteose peptone, tryptone)29 was added to the sampling solution to quench the antimicrobial action of the hand hygiene agent applied to the hands; this neutralizing solution was validated using ASTM E 1054-91.30
Hand hygiene test agents
The test agents displayed in Table 2 were used for the 10-second hand hygiene applications. Triclosan was not tested against MS2 because triclosan damaged the confluent layer of E coli host cells. All hand hygiene agents were purchased from commercial sources with the exception of Surfacine, which was provided by the manufacturer (Intelligent Biocides, Tyngsborough, MA). All hand hygiene agents were used at room temperature, and the temperature of the tap water used for hand hygiene and rinsing was adjusted to 40°C ± 3°C. The lotion, gel, and liquid soaps were dispensed into 3-mL aliquots using sterile syringes; the foam was dispensed by weight as 3-g aliquots, and 1 wipe was used per hand hygiene application. Controls consisted of a nonantimicrobial soap with water and tap water alone. Three-milliliter aliquots of each control agent were dispensed onto the hands, and the subjects rubbed their hands in a similar fashion as with a hand hygiene agent. Rinsing with tap water was only performed with handwashing products and with controls.
Table 2.
Hand hygiene test agents
| Active ingredient | Form | Method of application | Brand name | Manufacturer |
|---|---|---|---|---|
| 60% Ethyl alcohol | Gel | Waterless handrub | Prevacare | Johnson and Johnson, Arlington, TX |
| 61% Ethyl alcohol | Lotion | Waterless handrub | Avagard | 3M Healthcare, St. Paul, MN |
| 61% Ethyl alcohol and 1% CHG | Lotion | Waterless handrub | Avagard | 3M Healthcare, St. Paul, MN |
| 62% Ethyl alcohol | Foam | Waterless handrub | Alcare | Steris, St. Louis, MO |
| 70% Ethyl alcohol and 0.005% silver iodide | Gel | Waterless handrub | Surfacine | Intelligent Biocides, Tyngsborough, MA |
| 0.5% Parachlorometaxylenol and 40% SD alcohol | Wipe 256 cm2 | Waterless handwipe | Sanidex | Professional Disposables, Inc, Orangeburg, NY |
| 0.4% Benzalkonium chloride | Wipe, 296 cm2 | Waterless, handwipe | Wash and Dri | First Brands, Danbury, CT |
| 0.75% CHG | Liquid | Handwash | PrimaKare | Steris, St. Louis, MO |
| 2% CHG | Liquid | Handwash | Bactoshield | Steris, St. Louis, MO |
| 4% CHG | Liquid | Handwash | Bactoshield | Steris, St. Louis, MO |
| 1% Triclosan | Liquid | Handwash | Prevacare | Johnson and Johnson, Arlington, TX |
| 0.2% Benzethonium chloride | Liquid | Handwash | Pure Cleanse | Puresoft Solutions, Newfields, NH |
| Control: Nonantimicrobial soap | Liquid | Handwash | Soft 'N Sure | Steris, St. Louis, MO |
| Control: Tap water | Liquid | Handwash | N/A | N/A |
Observation of health care personnel for determining duration of hand hygiene with an alcohol-based handrub
Direct observations of hand hygiene, as practiced by health care professionals in our institution, were undertaken by trained infection control professionals. Subjects were unaware that they were being observed. No demographic information or subject identifiers were obtained. The type of hand hygiene (chlorhexidine wash or alcohol-based handrub) and duration of hand hygiene were recorded on a data abstraction form.
Data analysis
After 24 hours of incubation at 37°C, MS2 plaques in the confluent E coli layer were enumerated and used to calculate the number of MS2 plaque forming units per milliliter (PFU/mL). After 48 hours of incubation at 25°C, red-pigmented colonies of S marcescens were enumerated and used to calculate the number of S marcescens colony forming units per milliliter (CFU/mL). Log reduction was determined by calculating the difference between the log10 of the baseline measurement (PFU/mL or CFU/mL) and the log10 of the measurement following each hand hygiene episode (PFU/mL or CFU/mL).
This was a repeated measures design, with subjects nested within product (ie, 5 subjects per product), 10 hand hygiene episodes per subject, 2 hands per episode, and 2 measurements (ie, duplicate plate counts) per hand. S marcescens and MS2 were analyzed separately. One record per hand hygiene episode was created by averaging the 2 measures per hand and then by averaging the results of the left and right hand. Using the data at episodes 1 and 10, we performed a 1-way analysis of variance on each product. Here, contrasts were used (1) to assess whether products within the same category (ie, method of application) produced similar results and (2) to assess whether product categories differed. Using the data from all available episodes, we disaggregated the data by product and then used analysis of covariance methodology to implement a repeated measures analysis for each product. The predictors were subject (ie, to account for the repeated measures component of the design) and episodes (ie, operationalized as a continuous variable). The resulting slope coefficient for episodes estimates the change in the log10 number of organisms associated with each subsequent hand hygiene episode. Because each of the comparisons presented here was of independent interest, no formal adjustment was made for multiple comparisons. However, exact P values are provided for readers desiring to make such adjustments. The 95% confidence intervals provided in the Figures were calculated using Excel (Excel 97; Microsoft, Bellevue, WA).
Results
Efficacy of hand hygiene agents against S marcescens
The relative efficacy of various hand hygiene agents is displayed in Table 3, Table 4. After episode 1, the hand hygiene agents that were most efficacious were agents containing chlorhexidine (H-J) and triclosan (K) with approximately a 2 log10 reduction of S marcescens ( Fig 2). Both the nonantimicrobial control and the tap water control showed similar log10 reductions of S marcescens. Alcohol-based handrubs alone (A-C) or combined with CHG (D) or silver iodide (E), and benzethonium chloride (L), demonstrated somewhat lower log10 reductions of 1.5 to 1.78. Hand hygiene wipes (F,G) achieved a log10 reduction <0.6. There was no statistical difference within groups for alcohol-based handrubs (A-E), hand hygiene wipes (F,G), handwashing agents (H-L), and controls (M,N). Alcohol-based handrubs were inferior to handwashing agents (P < .01) and controls (P < .01) but were superior to hand hygiene wipes (P < .0001).
Table 3.
Log reductions of Serratia marcescens∗
| Agent | Episode1 | Episode 3 | Episode 5 | Episode 7 | Episode 10 |
|---|---|---|---|---|---|
| 60% Ethyl alcohol | 1.15 (0.75, 1.55) | 1.14 (0.68, 1.59) | 1.02 (0.40, 1.63) | 0.78 (0.21, 1.35) | 0.42 (0.01, 0.83) |
| 61% Ethyl alcohol | 1.55 (0.89, 2.20) | 1.54 (0.81, 2.27) | 1.54 (1.00, 2.08) | 1.39 (0.62, 2.15) | 1.35 (0.66, 2.03) |
| 62% Ethyl alcohol | 1.51 (1.19, 1.83) | 1.15 (0.78, 1.53) | 0.92 (0.26, 1.57) | 0.82 (0.28, 1.37) | 0.67 (0.23, 1.12) |
| 61% Ethyl alcohol/1% CHG | 1.74 (1.39, 2.09) | 1.58 (1.27, 1.89) | 1.46 (1.10, 1.83) | 1.37 (0.86, 1.88) | 1.08 (0.55, 1.61) |
| 70% Ethyl alcohol/0.005% silver iodide | 1.78 (1.25, 2.31) | 1.52 (0.90, 2.15) | 1.40 (0.82, 1.98) | 1.38 (0.71, 2.05) | 1.07 (0.52, 1.62) |
| 0.5% Parachlorometaxylenol/40% SD alcohol | 0.57 (0.35, 0.80) | 0.68 (0.43, 0.94) | 0.64 (0.39, 0.90) | 0.62 (0.31, 0.93) | 0.84 (0.52, 1.17) |
| 0.4% Benzalkonium chloride | 0.25 (0.13, 0.36) | 0.07 (−0.08, 0.23) | 0.04 (−0.07, 0.14) | −0.01 (−0.19, 0.18) | 0.01 (−0.18, 0.20) |
| 0.75% Chlorhexidine gluconate | 1.98 (1.68, 2.27) | 2.63 (2.46, 2.81) | 2.78 (2.48, 3.08) | 2.66 (2.52, 2.80) | 3.04 (2.75, 3.33) |
| 2% Chlorhexidine Gluconate | 2.01 (1.91, 2.10) | 2.63 (2.43, 2.83) | 2.78 (2.44, 3.11) | 2.81 (2.37, 3.25) | 3.63 (3.08, 4.18) |
| 4% Chlorhexidine gluconate | 1.89 (1.63, 2.16) | 2.72 (2.47, 2.98) | 2.41 (1.88, 2.94) | 2.75 (2.40, 3.09) | 3.14 (2.40, 3.89) |
| 1% Triclosan | 1.90 (1.50, 2.29) | 2.24 (1.85, 2.62) | 2.13 (1.73, 2.53) | 2.19 (1.88, 2.49) | 2.49 (1.77, 3.21) |
| 0.2% Benzethonium chloride | 1.60 (1.40, 1.79) | 1.88 (1.56, 2.20) | 1.91 (1.66, 2.16) | 1.92 (1.58, 2.25) | 1.98 (1.77, 2.19) |
| Control: Nonantimicrobial soap | 1.87 (1.55, 2.19) | 1.73 (1.38, 2.08) | 1.66 (1.29, 2.02) | 1.56 (1.17, 1.96) | 1.60 (1.26, 1.95) |
| Control: Tap water | 2.00 (1.80, 2.19) | 1.78 (1.66, 1.91) | 1.69 (1.55, 1.83) | 1.71 (1.55, 1.86) | 1.68 (1.55, 1.81) |
95% Confidence intervals are shown in parentheses.
Table 4.
Log reductions of MS2 bacteriophage∗
| Agent | Episode1 | Episode 3 | Episode 5 | Episode 7 | Episode 10 |
|---|---|---|---|---|---|
| 60% Ethyl alcohol | −0.15 (−0.40, 0.09) | −0.39 (−0.60, −0.17) | −0.44 (−0.52, −0.37) | −0.65 (−0.95, −0.35) | −0.67 (−1.06, −0.29) |
| 61% Ethyl alcohol | −0.08 (−0.31, −0.14) | −0.24 (−0.40, −0.09) | −0.31 (−0.52, −0.11) | −0.52 (−0.67, −0.36) | −0.59 (−0.80, −0.38) |
| 62% Ethyl alcohol | −0.26 (−0.66, 0.15) | −0.48 (−1.04, 0.08) | −0.61 (−1.18, −0.03) | −0.61 (−1.14, −0.07) | −0.71 (−1.34, −0.08) |
| 61% Ethyl alcohol/1% CHG | −0.03 (−0.19, 0.13) | −0.34 (−0.54, −0.14) | −0.61 (−0.78, −0.44) | −0.60 (−0.76, −0.44) | −0.87 (−1.23, −0.50) |
| 70% Ethyl alcohol/0.005% silver iodide | 0.96 (0.58, 1.34) | 0.51 (0.17, 0.84) | 0.53 (0.37, 0.69) | 0.42 (−0.04, 0.87) | 0.18 (−0.15, 0.50) |
| 0.5% Parachlorometaxylenol/40% SD alcohol | 0.21 (0.08, 0.35) | 0.00 (−0.13, 0.13) | −0.13 (−0.31, 0.06) | −0.16 (−0.33, 0.01) | −0.23 (−0.34, −0.12) |
| 0.4% Benzalkonium chloride | 0.23 (−0.11, 0.58) | −0.07 (−0.33, 0.19) | −0.17 (−0.49, 0.14) | −0.38 (−0.62, −0.13) | −0.46 (−0.75, −0.18) |
| 0.75% Chlorhexidine gluconate | 2.10 (1.91, 2.29) | 0.91 (0.79, 1.03) | 0.79 (0.48, 1.10) | 0.81 (0.66, 0.96) | 0.77 (0.32, 1.22) |
| 2% Chlorhexidine gluconate | 1.38 (1.11, 1.65) | 0.64 (0.51, 0.78) | 0.60 (0.33, 0.87) | 0.59 (0.55, 0.64) | 0.30 (0.13, 0.47) |
| 4% Chlorhexidine gluconate | 1.35 (0.70, 2.01) | 0.77 (0.41, 1.13) | 0.71 (0.34, 1.08) | 0.57 (0.24, 0.90) | 0.30 (−0.20, 0.79) |
| 0.2% Benzethonium chloride | 1.92 (1.67, 2.17) | 1.61 (1.39, 1.84) | 1.53 (1.24, 1.81) | 1.48 (1.21, 1.74) | 1.33 (1.00, 1.66) |
| Control: Nonantimicrobial soap | 1.85 (1.41, 2.28) | 1.77 (1.50, 2.03) | 2.03 (1.51, 2.56) | 1.54 (1.06, 2.01) | 1.59 (1.17, 2.02) |
| Control: Tap water | 2.56 (2.26, 2.86) | 2.24 (1.86, 2.61) | 2.25 (1.92, 2.58) | 2.06 (1.79, 2.33) | 1.89 (1.65, 2.13) |
95% Confidence intervals are shown in parentheses.
Fig 2.
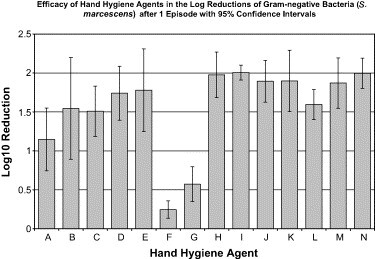
Efficacy of hand hygiene agents in the log reductions of gram-negative bacteria (S marcescens) after 1 episode, with 95% confidence intervals. Hand hygiene agents tested were as follows: (A) 60% ethyl alcohol (n = 5); (B) 61% ethyl alcohol (n = 5); (C) 62% ethyl alcohol (n = 5); (D) 61% ethyl alcohol/1% CHG (n = 5); (E) 70% ethyl alcohol/0.005% silver iodide (n = 5); (F) 0.4% benzalkonium chloride (n = 5); (G) 0.5% PCMX/40% SD alcohol (n = 5); (H) 0.75% chlorhexidine gluconate (n = 5); (I) 2% chlorhexidine gluconate (n = 5); (J) 4% chlorhexidine gluconate (n = 5); (K) 1% triclosan (n = 5); (L) 0.2% benzethonium chloride (n = 5); (M) nonantimicrobial control (n = 5); (N) tap water control (n = 5).
After episode 10 ( Fig 3), the hand hygiene agents that were the most efficacious (1.98-3.63 log10 reductions) in the reduction of S marcescens on the hands were the handwashing agents (ie, liquids containing CHG, triclosan, or benzethonium chloride) (Table 3). By the tenth episode, these agents were all significantly more efficacious than the alcohol-based handrubs (P < .0001) and waterless handwipes (P < .0001). Within the waterless handrub agents, the maximum difference in log reduction was 0.93 (P = .0702), and, within the waterless handwipe agents, the maximum difference in log reduction was 0.84 (P = .0179). Among the handwashing agents, the CHG agents were more efficacious than the agents containing triclosan (P = .0070) or benzethonium chloride (P = .0001). The triclosan agent was more efficacious (P = .0060) than either the nonantimicrobial soap or the tap water alone. Over the 10 episodes, improved efficacy in bacterial reductions was demonstrated by the CHG handwashing agents (slope coefficients, 0.096 to 0.158; P = .0001), triclosan (slope coefficient, 0.052; P = .0056), and benzethonium chloride (slope coefficient, 0.035; P = .0005). Decreased efficacy trends in reduction of S marcescens on the hands across multiple episodes was observed with the 0.4% benzalkonium chloride waterless handwipe (slope coefficient, −0.024; P = .0002) and all of the waterless handrubs (slope coefficient −0.088 to −0.070; P = .0001 to P = .0005), except the 61% ethyl alcohol.
Fig 3.
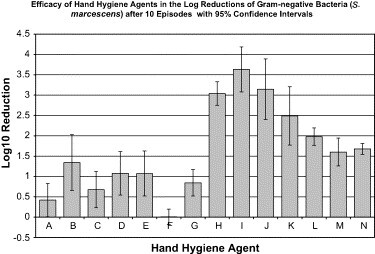
Efficacy of hand hygiene agents in the log reductions of gram-negative bacteria (S marcescens) after 10 episodes, with 95% confidence intervals. Hand hygiene agents tested were as follows: (A) 60% ethyl alcohol (n = 5); (B) 61% ethyl alcohol (n = 5); (C) 62% ethyl alcohol (n = 5); (D) 61% ethyl alcohol/1% CHG (n = 5); (E) 70% ethyl alcohol/0.005% silver iodide (n = 5); (F) 0.4% benzalkonium chloride (n = 5); (G) 0.5% PCMX/40% SD alcohol (n = 5); (H) 0.75% chlorhexidine gluconate (n = 5); (I) 2% chlorhexidine gluconate (n = 5); (J) 4% chlorhexidine gluconate (n = 5); (K) 1% triclosan (n = 5); (L) 0.2% benzethonium chloride (n = 5); (M) nonantimicrobial control (n = 5); (N) tap water control (n = 5).
Efficacy of hand hygiene agents against MS2
After a single episode, all handwashing agents and controls demonstrated a log10 reduction of 1.35 to 2.56 ( Fig 4). The alcohol-based handrub containing ethyl alcohol and silver iodide resulted in a statistically significant reduction of approximately 1 log10. All other agents except PCMX, which included alcohol-based handrubs and wipes failed to demonstrate a statistically significant reduction of MS2. The controls demonstrated statistically improved reduction of MS2 compared with alcohol-based handrubs (P < .0001), hand hygiene wipes (P < .0001), and handwashing agents (P < .01). Handwashing agents were superior to both alcohol-based handrubs (P < .0001) and hand hygiene wipes (P < .0001).
Fig 4.
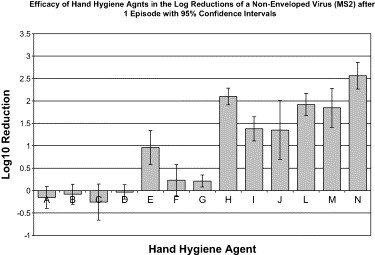
Efficacy of hand hygiene agents in the log reductions of a nonenveloped virus (MS2) after 1 episode, with 95% confidence intervals. Hand hygiene agents tested were as follows: (A) 60% ethyl alcohol (n = 5); (B) 61% ethyl alcohol (n = 5); (C) 62% ethyl alcohol (n = 4); (D) 61% ethyl alcohol/1% CHG (n = 5); (E) 70% ethyl alcohol/0.005% silver iodide (n = 5); (F) 0.4% benzalkonium chloride (n = 5); (G) 0.5% PCMX/40% SD alcohol (n = 4); (H) 0.75% chlorhexidine gluconate (n = 3); (I) 2% chlorhexidine gluconate (n = 2); (J) 4% chlorhexidine gluconate (n = 3); (K) 1% triclosan (not tested); (L) 0.2% benzethonium chloride (n = 5); (M) nonantimicrobial control (n = 5); (N) tap water control (n = 4).
After episode 10 ( Fig 5), the greatest reductions (1.33-1.89 log10 reductions) of MS2 on the hands were achieved with a handwashing agent containing benzethonium chloride, the nonantimicrobial control soap, and the tap water alone (Table 4). CHG was statistically less efficacious than benzethonium chloride (P = .0002) or the soap and the tap water controls (P = .0001). Furthermore, CHG showed a significantly decreased efficacy over the 10 episodes (slope coefficient, −0.118 to −0.098; P = .0001 to P = .0103). Over the ten episodes, every waterless handrub (slope coefficient, −0.086 to −0.045; P = .0001 to P = .0025) and waterless handwipe agent (slope coefficient, −0.075 to −0.046; P = .0001) showed a negative trend in log10 reductions in the reduction of MS2 from the hands. These results indicate a progressive accumulation of test microbes on the hands (ie, decreased efficacy of hand hygiene agents).
Fig 5.
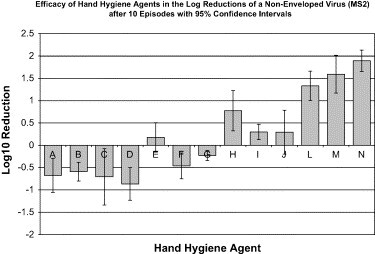
Efficacy of hand hygiene agents in the log reductions of a nonenveloped virus (MS2) after 10 episodes, with 95% confidence intervals. Hand hygiene agents tested were as follows: (A) 60% ethyl alcohol (n = 5); (B) 61% ethyl alcohol (n = 5); (C) 62% ethyl alcohol (n = 4); (D) 61% ethyl alcohol/1% CHG (n = 5); (E) 70% ethyl alcohol/0.005% silver iodide (n = 5); (F) 0.4% benzalkonium chloride (n = 5); (G) 0.5% PCMX/40% SD alcohol (n = 4); (H) 0.75% chlorhexidine gluconate (n = 3); (I) 2% chlorhexidine gluconate (n = 2); (J) 4% chlorhexidine gluconate (n = 3); (K) 1% triclosan (not tested); (L) 0.2% benzethonium chloride (n = 5); (M) nonantimicrobial control (n = 5); (N) tap water control (n = 4).
Observation of health care personnel for determining duration of hand hygiene with an alcohol-based handrub
Fifty episodes of hand hygiene with an alcohol-based handrub were observed for health care personnel working in an intensive care unit. The mean time of application (rubbing product onto hands) was 11.6 seconds (SD ± 7.0) with a median time of 10 seconds (range, 2-45 seconds).
Discussion
Health care-associated infections rank in the top 5 causes of death, with an estimated 90,000 deaths each year in the United States.31 Cross transmission has been estimated to cause 40% of nosocomial infections.32 The most common pathogens involved in these health care-associated infections are aerobic gram-negative bacteria (E coli, Pseudomonas) and aerobic gram-positive bacteria (S aureus, coagulase-negative Staphylococcus). Although the impact of viral infections remains incompletely defined,33 epidemics because of influenza,34 respiratory syncytial virus (RSV),35 rotavirus,36 adenovirus,37 noroviruses,38 hepatitis A virus,39 and coronaviruses40 are well described in the health care setting.
Hand hygiene has been repeatedly shown to reduce the level of transient microorganisms on the hands.41, 42 More recently, hand hygiene has been demonstrated to reduce the incidence of health care-associated infections.2, 3, 43, 44 For these reasons, the Centers for Disease Control and Prevention (CDC) and professional organizations recommend hand antisepsis as a key measure for reducing the incidence and impact of health care-associated infections.24 However, many studies have demonstrated that, on average, only 40% of health care workers' contacts with patients result in appropriate hand hygiene.24 Barriers to appropriate hand hygiene have been reported to be (1) inaccessibility of hand hygiene supplies, (2) skin irritation from hand hygiene agents, (3) an inadequate amount of time for hand hygiene, (4) interference with patient care, (5) lack of knowledge of the guidelines, and (6) lack of information on the importance of hand hygiene. Therefore, recent guidelines promote the use of alcohol-based handrubs that are easily accessible.
A variety of hand hygiene agents are now available, with different active ingredients and application methods. Common active ingredients include CHG, triclosan, and alcohols. Typical ways of using these agents include detergents (nonantimicrobial soap) used with water, antimicrobial soap used with water, waterless alcohol-based handrubs, and waterless handwipes, which are disposable papers impregnated with antimicrobial agents. The FDA's standard method used to evaluate the efficacy of these agents is similar to the ASTM-E 1174-94, which involves inoculation of hands with a standardized suspension of S marcescens and hand hygiene with a specified volume of a test agent. Studies using at least a 30-second exposure time have shown high levels of reduction of transient microorganisms with many hand hygiene agents, including both antimicrobial handwashing agents and alcohol-based handrubs.9, 10, 45, 46 A recent summary of the literature noted that of 22 studies that assessed the efficacy of hand hygiene agents in reducing the counts of viable bacteria on the hands, only 3 used an exposure time as short as 15 seconds.24 However, in 14 observational studies of health care workers, hand hygiene has averaged approximately 12.6 seconds.24 Our observations of hand hygiene with an alcohol-based handrub demonstrated a similar duration of use (mean, 11.6 seconds; median, 10 seconds) as previously demonstrated for handwashing agents. We have previously shown that the efficacy of hand hygiene with one alcohol rub (62% ethyl alcohol) was similar when comparing rubbing for 10 seconds with rubbing until dry (>2 minutes) for episodes 3 to 7; for episode 1, rubbing for 10 seconds was more efficacious, and, for episode 10, rubbing until dry was more efficacious.25 Overall, no consistent benefit was demonstrated for rubbing until dry.25 Based on the duration of hand hygiene with handwashing agents reported in the literature and hand hygiene with an alcohol product demonstrated in our observations, we undertook a comprehensive comparative trial of plain soap and tap water vs antimicrobial-based soaps and handrubs at a realistic exposure time of 10 seconds. We have previously evaluated the methodologic factors that may alter the efficacy of hand hygiene.25
Our data showed that, for the reduction of gram-negative bacteria from the hands after a single episode, CHG and triclosan handwashing agents achieved the highest reductions of S marcescens. However, benzethonium chloride handwashing agent and alcohol-based handrubs, although achieving slightly lower log10 reductions, were not statistically different from the levels achieved by CHG and handwashing agents. Handwipes that contained benzalkonium chloride or PCMX/alcohol were significantly inferior to all other agents tested. No agents were significantly superior to nonantimicrobial or tap water controls.
After multiple (10) hand hygiene episodes, the CHG handwashing agent was the most efficacious, followed by triclosan, benzethonium chloride, nonantimicrobial soap, and tap water alone. Although the alcohol-based handrubs were as efficacious as the handwashing agents after the first episode, these handrubs were significantly less efficacious over repeated hand hygiene episodes and when compared with the handwashing agents. A recent publication reported that alcohol-based hand hygiene agents applied for 15 to 30 seconds achieved a 4- to 7-log10 reduction in test bacteria using a different methodology (EN1500) that employs the fingertip method.46 In addition, these investigators used products containing much higher concentrations of ethanol (≥80%) than were present in our test products. The lowered efficacy of alcohol-based products in our study may be related to the shorter exposure time and specific composition of the alcohol hand hygiene agents tested (eg, concentration of alcohol, type of alcohol, other active ingredients, inert ingredients, and emollients). The decreasing efficacy of alcohol demonstrated in our study after episode 10 is most likely due to the lack of persistent antimicrobial effect of alcohols24 and the progressive accumulation of organisms on the hands following repeated episodes of contamination.
Improved compliance with hand hygiene using alcohol-based handrubs has been demonstrated in many studies.3, 47, 48, 49, 50 It would be difficult, if not impossible, to make handwashing sinks as readily accessible as waterless handrub agents. Therefore, the use of alcohol-based handrubs will continue to be an important addition to our existing infection control armamentarium to improve hand hygiene compliance and at those locations at which sinks are not available. Furthermore, the exact reduction of transient microorganisms required to prevent cross transmission is unknown, and it is likely that even a 90% reduction achieved by alcohol-based handrubs along with improved compliance will decrease the incidence of health care-associated infections.51, 52 However, given the trend of a reduced efficacy of alcohol-based handrubs with multiple episodes, it is prudent to recommend traditional hand hygiene with an antiseptic agent or a nonantimicrobial soap periodically throughout the day.
The morphology of the MS2 bacteriophage closely resembles nonenveloped, hydrophilic viruses that are relatively resistant to disinfectants and antiseptics. Nosocomial outbreaks have been reported because of nonenveloped viruses, including noroviruses,38 hepatitis A,39 and others.53 Similarly, day care center outbreaks have been reported because of noroviruses54 and hepatitis A.55 By evaluating the efficacy of hand hygiene agents with a surrogate (MS2) of clinically important nonenveloped viruses, hand hygiene agents might be selected that are efficacious in the reduction of both bacteria and viruses. The bacteriophage MS2 has been demonstrated to have similar susceptibility to alcohols, organic acids, and alkalis as poliovirus.56 MS2 has previously been used in hand decontamination studies because it is an excellent surrogate for human enteroviruses, such as polio, which are known to be transmitted by hand contact.56
In our study, after a single episode, products containing alcohol and silver, CHG, triclosan, and benzethonium chloride all showed significant reductions of MS2; however, none was superior to nonantimicrobial or tap water controls. Alcohol-based handrubs alone or combined with CHG did not demonstrate any significant reduction in MS2. After 10 episodes, every waterless agent showed low efficacy (<0.18 log10 reduction of MS2) and significantly decreasing reductions over the 10 episodes. These results are consistent with the literature, which reports that hydrophilic viruses are more resistant than lipophilic viruses to inactivation by alcohols.57, 58 In addition, other studies conducted in vivo, which assessed viral reduction using MS2 as a surrogate, showed a log10 reduction of 2.1 with a 110-second contact time with 50% ethanol (pH 11.5),56 an ≈0.5 log10 reduction with a 60-second contact time with 70% ethanol,23 an ≈0.3 log10 reduction with a 60-second contact time with 0.5% CHG/70% isopropanol,23 an ≈0 log10 reduction with a 60-second contact time with 4% CHG, 23 and a 2.29 log10 reduction with a 30-second contact time with plain soap.12 These studies are comparable with our results when accounting for methodologic differences in contact time, volume of agent used, and concentration of agents. Of all the hand hygiene agents, the most efficacious at reducing MS2 was the handwashing with tap water alone, followed by the nonantimicrobial soap handwash, and the 0.2% benzethonium chloride handwash. Our data support the proposition that reduction of a nonenveloped virus was achieved by physical removal rather than inactivation. Our data do not provide an explanation why chlorhexidine performed less well than soap and water in removing MS2. The most likely explanation is that chlorhexidine enhanced viral adherence to human skin leading to a lower reduction of the virus.
In conclusion, our study shows that, at a short exposure time of 10 seconds, all agents with the exception of handwipes and a 60% ethyl alcohol handrub performed similar to nonantimicrobial and tap water controls with reductions of 1.15 to 2.01 log10 of Serratia marcescens. After 10 episodes, which evaluates the efficacy of agents following multiple episodes of contamination, handwashing agents with 0.75% CHG, 2% CHG, 4% CHG, 1% triclosan, 0.2% benzethonium chloride, nonantimicrobial soap handwash, and tap water alone were efficacious (≥1.5 log10) in reduction of bacteria. Our data demonstrate that short contact times are effective in reducing transient hand flora, and, therefore, the future focus of hand hygiene can be on improving the compliance rather than increasing the duration of hand hygiene. Because use of a shorter duration of hand hygiene is likely to improve compliance, greater compliance should then lead to a reduction in health care-associated infections. Alcohol-based handrubs and wipes were generally ineffective in demonstrating significant virus reduction from the hands either after a single episode or multiple episodes of hand hygiene. Although viruses are a less common cause of health care-associated infections than are bacteria,33 in situations in which infection with viruses is likely (eg, gastroenteritis because of norovirus or hepatitis A infections), the use of soap and water washes should be considered.
Acknowledgments
The authors thank Lawrence K. Mandelkehr for assisting in creating graphics and Tina Adams, Becky Brooks, Vickie Brown, Brenda Featherstone, and Irene Kittrell for conducting hand hygiene observations.
Chapel Hill and Durham, North Carolina
Footnotes
Supported by the UNC Health Care System and the NC Statewide Program in Infection Control and Epidemiology.
References
- 1.Semmelweis I. Etiology, concept, and prophylaxis of childbed fever. In: Carter K.C., editor. 1st ed. The University of Wisconsin Press; Madison, WI: 1983. [Google Scholar]
- 2.Larson E.L., Early E., Cloonan P., Sugrue S., Parides M. An organizational climate intervention associated with increased handwashing and decreased nosocomial infections. Behav Med. 2000;26:14–22. doi: 10.1080/08964280009595749. [DOI] [PubMed] [Google Scholar]
- 3.Pittet D., Hugonnet S., Harbarth S., Mourouga P., Sauvan V., Touveneau S. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Lancet. 2000;356:1307–1312. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]
- 4.Niffenegger J.P. Proper handwashing promotes wellness in child care. J Pediatr Health Care. 1997;11:26–31. doi: 10.1016/s0891-5245(97)90141-3. [DOI] [PubMed] [Google Scholar]
- 5.Roberts L., Jorm L., Patel M., Smith W., Douglas R.M., McGilchrist C. Effect of infection control measures on the frequency of diarrheal episodes in child care: a randomized, controlled trial. Pediatrics. 2000;105:743–746. doi: 10.1542/peds.105.4.743. [DOI] [PubMed] [Google Scholar]
- 6.Shahid N.S., Greenough W.B., Samadi A.R., Huq M.I., Rahman N. Hand washing with soap reduces diarrhoea and spread of bacterial pathogens in a Bangladesh village. J Diarrhoeal Dis Res. 1996;14:85–89. [PubMed] [Google Scholar]
- 7.Wilson J.M., Chandler G.N., Muslihatun, Jamiluddin Hand-washing reduces diarrhoea episodes: a study in Lombok, Indonesia. Trans R Soc Trop Med Hyg. 1991;85:819–821. doi: 10.1016/0035-9203(91)90468-e. [DOI] [PubMed] [Google Scholar]
- 8.Aly R., Maibach H. Comparative study on the antimicrobial effect of 0.5% chlorhexidine gluconate an 70% isopropyl alcohol on the normal flora of the hands. Appl Environ Microbiol. 1979;37:610–613. doi: 10.1128/aem.37.3.610-613.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayliffe G.A.J., Babb J.R., Davies J.G., Lilly H.A. Hand disinfection: a comparison of various agents in laboratory and ward studies. J Hosp Infect. 1988;11:226–243. doi: 10.1016/0195-6701(88)90101-6. [DOI] [PubMed] [Google Scholar]
- 10.Bartzokas C.A., Gibson M.F., Graham R., Pinder D.C. A comparison of triclosan and chlorhexidine preparations with 60% isopropyl alcohol for hygienic hand disinfection. J Hosp Infect. 1983;4:245–255. doi: 10.1016/0195-6701(83)90025-7. [DOI] [PubMed] [Google Scholar]
- 11.Butz A.M., Laughon B.E., Gullette D.L., Larson E.L. Alcohol-impregnated wipes as an alternative in hand hygiene. Am J Infect Control. 1990;18:70–76. doi: 10.1016/0196-6553(90)90084-6. [DOI] [PubMed] [Google Scholar]
- 12.Davies J.G., Babb J.R., Bradley C.R., Ayliffe G.A.J. Preliminary study of test methods to assess the virucidal activity of skin disinfectants using poliovirus and bacteriophages. J Hosp Infect. 1993;25:125–131. doi: 10.1016/0195-6701(93)90103-7. [DOI] [PubMed] [Google Scholar]
- 13.Eggers H.J. Experiments on antiviral activity of hand disinfectants: some theoretical and practical considerations. Zbl Bakt. 1990;273:36–51. doi: 10.1016/s0934-8840(11)80238-0. [DOI] [PubMed] [Google Scholar]
- 14.Kampf G., Jarosch R., Rüden H. Limited effectiveness of chlorhexidine based hand disinfectants against methicillin-resistant Staphylococcus aureus (MRSA) J Hosp Infect. 1998;38:297–303. doi: 10.1016/s0195-6701(98)90078-0. [DOI] [PubMed] [Google Scholar]
- 15.Larson E.L., Aiello A.E., Bastyr J., Lyle C., Stahl J., Cronquist A. Assessment of two hand hygiene regimens for intensive care unit personnel. Crit Care Med. 2001;29:944–951. doi: 10.1097/00003246-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Larson E.L., Laughon B.E. Comparison of four antiseptic products containing chlorhexidine gluconate. Antimicrob Agents Chemother. 1987;31:1572–1574. doi: 10.1128/aac.31.10.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson E.L., Eke P.I., Laughon B.E. Efficacy of alcohol-based hand rinses under frequent-use conditions. Antimicrob Agents Chemother. 1986;30:542–544. doi: 10.1128/aac.30.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minakuchi K., Yamamoto Y., Matsunaga K., Hayata M., Yasuda T., Katsuno Y. The antiseptic effect of a quick drying rubbing type povidone-iodine alcoholic disinfectant solution. Postgrad Med J. 1993;69:S23–S26. [PubMed] [Google Scholar]
- 19.Rosenberg A., Alatary S.D., Peterson A.F. Safety and efficacy of the antiseptic chlorhexidine gluconate. Surg Gynecol Obstet. 1976;143:789–792. [PubMed] [Google Scholar]
- 20.Schürmann W., Eggers H.J. An experimental study on the epidemiology of enteroviruses: water and soap washing of poliovirus 1–contaminated hands, its effectiveness and kinetics. Med Microbiol Immunol. 1985;174:221–236. doi: 10.1007/BF02124807. [DOI] [PubMed] [Google Scholar]
- 21.Schürmann W., Eggers H.J. Antiviral activity of an alcoholic hand disinfectant: comparison of the in vitro suspension test with in vivo experiments on hands, and on individual fingertips. Antivir Res. 1983;3:25–41. doi: 10.1016/0166-3542(83)90012-8. [DOI] [PubMed] [Google Scholar]
- 22.Ulrich J.A. Clinical study comparing hibistat (0.5% chlorhexidine gluconate in 70% isopropyl alcohol) and betadine surgical scrub (7.5% povidone-iodine) for efficacy against experimental contamination of human skin. Curr Ther Res. 1982;31:27–30. [Google Scholar]
- 23.Woolwine J.D., Gerberding J.L. Effect of testing method on apparent activities of antiviral disinfectants and antiseptics. Antimicrob Agents Chemother. 1995;39:921–923. doi: 10.1128/aac.39.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyce J.M., Pittet D. Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1–S46. doi: 10.1067/mic.2002.130391. [DOI] [PubMed] [Google Scholar]
- 25.Sickbert-Bennett E.E., Weber D.J., Gergen-Teague M.F., Rutala W.A. The effects of test variables on the efficacy on hand hygiene agents. Am J Infect Control. 2004;32:69–83. doi: 10.1016/j.ajic.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 26.American Society for Testing Materials . American Society Testing Materials; West Conshohocken, PA: 1994. Standard test method for evaluation of healthcare personnel handwash formulation. [Google Scholar]
- 27.Adams M.H. Interscience Publishers, Inc; New York: 1959. Bacteriophages. [Google Scholar]
- 28.Shin G.-A., Sobsey M.D. Reduction of Norwalk virus, poliovirus 1, and bacteriophage MS2 by ozone disinfection of water. Appl Environ Microbiol. 2003;69:3975–3978. doi: 10.1128/AEM.69.7.3975-3978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benson L., Bush L., LeBlanc D. Importance of neutralizers in the stripping fluid in a simulated healthcare personnel handwash. Infect Control Hosp Epidemiol. 1990;11:595–599. doi: 10.1086/646101. [DOI] [PubMed] [Google Scholar]
- 30.American Society for Testing Materials . American Society Testing Materials; West Conshohocken, PA: 1991. Practices for evaluation inactivators of antimicrobial agents used in disinfectant, sanitizer, antiseptic or preserved products. [Google Scholar]
- 31.Burke J.P. Infection control–a problem for patient safety. N Engl J Med. 2003;348:651–656. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 32.Weist K., Pollege K., Schulz I., Rüden H., Gastmeier P. How many nosocomial infections are associated with cross-transmission: a prospective cohort study in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2002;23:127–132. doi: 10.1086/502021. [DOI] [PubMed] [Google Scholar]
- 33.Valenti W.M., Menegus M.A., Hall C.B., Pincus P.H., Douglas R.G. Nosocomial viral infections: I. epidemiology and significance. Infect Control. 1980;1:33–37. doi: 10.1017/s0195941700052371. [DOI] [PubMed] [Google Scholar]
- 34.Slinger R., Dennis P. Nosocomial influenza at a canadian pediatric hospital from 1995 to 1999: opportunities for prevention. Infect Control Hosp Epidemiol. 2002;23:627–629. doi: 10.1086/501985. [DOI] [PubMed] [Google Scholar]
- 35.Heerens A.T., Marshall D.D., Bose C.L. Nosocomial respiratory syncytial virus: a threat in the modern neonatal intensie care unit. J Perinatol. 2002;22:306–307. doi: 10.1038/sj.jp.7210696. [DOI] [PubMed] [Google Scholar]
- 36.Widdowson M.A., van Doornum G.J., van der Poel W.H., de Boer A.S., van de Heide R., Mahdi U. An outbreak of diarrhea in a neonatal medium care unit caused by a novel strain of rotavirus: investigation using both epidemiologic and microbiological methods. Infect Control Hosp Epidemiol. 2002;23:665–670. doi: 10.1086/501991. [DOI] [PubMed] [Google Scholar]
- 37.Caspari G., Wassill H. Nosocomial virus infections in the eye. Dev Ophthalmol. 2002;33:207–209. doi: 10.1159/000065918. [DOI] [PubMed] [Google Scholar]
- 38.McCall J., Smithson R. Rapid response and strict control measures can contain a hospital outbreak of Norwalk-like virus. Commun Dis Public Health. 2002;5:243–246. [PubMed] [Google Scholar]
- 39.Petrosillo N., Raffaele B., Martini L., Nicastri E., Nurra G., Anzidei G. A nosocomial and occupational cluster of hepatitis a virus infection in a pediatric ward. Infect Control Hosp Epidemiol. 2002;23:343–345. doi: 10.1086/502064. [DOI] [PubMed] [Google Scholar]
- 40.Wong T., Lee C., Tam W., Lau J., Yu T., Lui S. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis. 2004;10:269–276. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaragoza M., Sallés M., Gomez J., Bayas J.M., Trilla A. Handwashing with soap or alcoholic solutions: a randomized clinical trial of its effectiveness. Am J Infect Control. 1999;27:258–261. doi: 10.1053/ic.1999.v27.a97622. [DOI] [PubMed] [Google Scholar]
- 42.Marena C., Lodol L., Zecca M., Bulgheroni A., Carretto E., Maserati R. Assessment of handwashing practices with chemical and microbiologic methods: preliminary results from a prospective crossover study. Am J Infect Control. 2002;30:334–340. doi: 10.1067/mic.2002.125809. [DOI] [PubMed] [Google Scholar]
- 43.Fendler E.J., Ali Y., Hammond B.S., Lyons M.K., Kelley M.B., Vowell N.A. The impact of alcohol hand sanitizer use on infection rates in an extended care facility. Am J Infect Control. 2002;30:226–233. doi: 10.1067/mic.2002.120129. [DOI] [PubMed] [Google Scholar]
- 44.Doebbeling B.N., Stanley G.L., Sheetz C.T., Pfaller M.A., Houston A.K., Annis L. Comparative efficacy of alternative hand-washing agents in reducing nosocomial infections in intensive care units. N Engl J Med. 1992;327:88–93. doi: 10.1056/NEJM199207093270205. [DOI] [PubMed] [Google Scholar]
- 45.Ayliffe G.A.J., Babb J.R., Quoraishi A.H. A test for “hygienic” hand disinfection. J Clin Pathol. 1978;31:923–928. doi: 10.1136/jcp.31.10.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dharan S., Hugonnet S., Sax H., Pittet D. Comparison of waterless hand antisepsis agents at short application times: raising the flag of concern. Infect Control Hosp Epidemiol. 2003;24:160–164. doi: 10.1086/502182. [DOI] [PubMed] [Google Scholar]
- 47.Graham M. Frequency and duration of handwashing in and intensive care unit. Am J Infect Control. 1990;18:77–80. doi: 10.1016/0196-6553(90)90085-7. [DOI] [PubMed] [Google Scholar]
- 48.Bischoff W.E., Reynolds T.M., Sessler C.N., Edmond M.B., Wenzel R.P. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med. 2000;160:1017–1021. doi: 10.1001/archinte.160.7.1017. [DOI] [PubMed] [Google Scholar]
- 49.Mody L., McNeil S.A., Sun R., Bradley S.F., Kauffman C.A. Introduction of a waterless alcohol-based handrub in a long-term-care facility. Infect Control Hosp Epidemiol. 2003;24:165–171. doi: 10.1086/502185. [DOI] [PubMed] [Google Scholar]
- 50.Hugonnet S., Perneger T.V., Pittet D. Alcohol-based handrub improves compliance with hand hygiene in intensive care units. Arch Intern Med. 2002;162:1037–1050. doi: 10.1001/archinte.162.9.1037. [DOI] [PubMed] [Google Scholar]
- 51.McDonald L.C. Hand hygiene in the new millennium: drawing the distinction between efficacy and effectiveness. Infect Control Hosp Epidemiol. 2003;24:157–159. doi: 10.1086/502183. [DOI] [PubMed] [Google Scholar]
- 52.Boyce J.M., Larson E.L., Weinstein R.A. Alcohol-based hand gels and hand hygiene in hospitals. Lancet. 2002;360:1509–1510. doi: 10.1016/S0140-6736(02)11442-5. [DOI] [PubMed] [Google Scholar]
- 53.Sattar S.A., Springthorpe V.S., Tetro J., Vashon R., Keswick B. Hygienic hand antiseptics: should they not have activity and label claims against viruses. Am J Infect Control. 2002;30:355–372. doi: 10.1067/mic.2002.124532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gotz H., de J.B., Lindback J., Parment P.A., Hedlund K.O., Torven M. Epidemiological investigation of a food-borne gastroenteritis outbreak caused by norwalk-like virus in 30 day-care centres. Scand J Infect Dis. 2002;34:115–121. doi: 10.1080/00365540110080133. [DOI] [PubMed] [Google Scholar]
- 55.Chitambar S.D., Chadha M.S., Yeolekar L.R., Arankalle V.A. Hepatitis A in day care centre. Indian J Pediatr. 1996;63:781–783. doi: 10.1007/BF02730929. [DOI] [PubMed] [Google Scholar]
- 56.Klein M., DeForest A. Antiviral action of germicides. Soap and Chemical Specialties. 1963;39:70–72. 95-97. [Google Scholar]
- 57.Klein M., DeForest A. Principles of viral inactivity. In: Block S.S., editor. Disinfection, Sterilization, and Preservation. 3rd ed. Lea & Febiger; Philadelphia: 1983. pp. 422–434. [Google Scholar]
- 58.Jones M.V., Bellamy K., Alcock R., Hudson R. The use of bacteriophage MS2 as a model system to evaluate virucidal hand disinfectants. J Hosp Infect. 1991;17:279–285. doi: 10.1016/0195-6701(91)90272-a. [DOI] [PubMed] [Google Scholar]


