Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December, 2019, in Wuhan, China, and has spread all over the world. This virus is responsible for a range of clinical manifestations in adults and children, ranging from mild respiratory symptoms to severe acute respiratory distress syndrome (ARDS) with clinical and radiological signs of severe bilateral pneumonia.1 Mortality rates range between 0·12% in the paediatric population and 14·8% in individuals aged 80 years and older.2
There is no current definitive data regarding the clinical features, morbidity, and mortality of coronavirus disease 19 (COVID-19) in pregnant women. The question of vertical transmission has been raised and, thus far, the literature available (albeit with small groups of mothers infected with COVID-19) has not provided evidence of vertical transmission. Obstetricians, neonatologists, and infectious disease specialists have therefore focused on improving and optimising prevention methods of this highly infectious virus' horizontal spread in all areas of care, including labour and delivery units, maternity wards, neonatal wards, and intensive care units. We report the case of an extremely preterm infant with COVID-19.
A female neonate at 26 gestational weeks plus 4 days was born in the Cliniques Universitaires Saint Luc, a tertiary level hospital in Brussels, Belgium, on March 1, 2020. The mother had been referred from a peripheral hospital for pre-eclampsia and suspected cholecystitis, which was being managed with intravenous antibiotic therapy. On admission, the mother's C-reactive protein (CRP) concentration was 39 mg/L (normal value <5 mg/L), and she had normal white blood cell (10·69 × 109/L) and platelet counts (259 × 109/L). During hospitalisation, the mother developed HELLP (haemolysis, elevated liver enzymes, and low platelet count) syndrome and intramuscular corticosteroids were administered for fetal pulmonary maturation. The neonate was delivered by caesarean section 48 h later. There were no signs of preterm labour.
The neonate was born weighing 960 g (75th percentile) with an Apgar score of 5 at 1 min, 8 at 5 min, and 8 at 10 min. She was transferred to the neonatal intensive care unit (NICU) on non-invasive intermittent positive pressure ventilation and received surfactant therapy (200 mg/kg) by less invasive surfactant administration procedure. Despite clinical stability and a fractional concentration of oxygen in inspired air (FiO2) that remained 21%, 12 h after the less invasive surfactant administration procedure she developed a pneumothorax requiring drainage. She had a haemodynamically significant patent ductus arteriosus that was successfully closed after three doses of intravenous ibuprofen. She remained stable on non-invasive intermittent positive pressure ventilation and her chest drain was removed at day 5, after which she was switched to continuous positive airway pressure. The infant was in a closed incubator throughout her admission.
The day following the caesarean section, the mother developed fever despite ongoing antibiotic therapy. Her CRP increased to 85 mg/L and subsequently to 214 mg/L 24 h later. Her antibiotic regimen was changed on the basis of a positive urine culture. The mother always wore a surgical mask while visiting her infant in the NICU, as she felt ill with fever and a cough. She was discharged home on day 5. She had skin-to-skin contact with her infant for the first and only time on day 6. Because of continued fever and shortness of breath, she was referred to the emergency room, where on further enquiry she revealed she had been coughing since the caesarean section 6 days before. A chest radiograph showed signs of bilateral pneumonia and her nasopharyngeal swab resulted positive for SARS-CoV-2 (day 7 post delivery). The PCR method used was COVID-19 genesig real-time PCR (rtPCR) assay (Primerdesign, Chandler's Ford, UK), targeting the RNA dependent RNA polymerase gene. The amplification was performed on the LightCycler 480 instrument (Roche Diagnostics, Mannheim, Germany).3 The mother had no known COVID-19 contacts and no travel history. Following the positive diagnosis, the mother stayed at home in quarantine and was not permitted to enter the hospital or NICU until she was both asymptomatic and her nasopharyngeal swab tested negative.
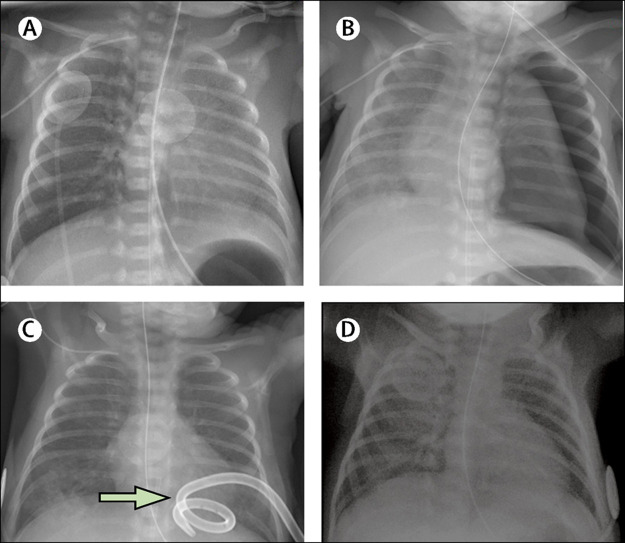
A nasopharyngeal swab was done on the infant as soon as the mother's results were known (day 7 of life) and she was placed in isolation with nursing and medical staff following strict aerosol, droplet, and contact precautions. She had received expressed breast milk before the diagnosis, in which a SARS-CoV-2 RT-PCR test on a maternal milk sample was negative. She had no feeding intolerance nor gastrointestinal symptoms such as diarrhoea. Total parenteral nutrition was discontinued and the central line was removed at day 14, when full enteral feedings were reached. The chest radiographs showed no signs of parenchymal infiltrates during the first week of life, and repeat imaging on day 17 showed non-specific bilateral streaky infiltrates (figure ). On day 21, she remained stable on continuous positive airway pressure at 5 cm with FiO2 21% and was receiving caffeine for apnoea prevention. No temperature instability was noted before or after testing positive for SARS-CoV-2, and she showed no clinical or biochemical signs of sepsis, with a maximal CRP value of 5·1 mg/L on day 5. Her white blood cell count was normal at birth but decreased to 3·64 × 109 cells per L (normal 7·3–16·6) on day 5, with a lymphopenia of 1·34 × 109 cells per L (normal 2·5–10·0). Her white blood cell count subsequently normalised and her platelet count remained normal. She showed neither clinical nor laboratory signs of hepatitis. Considering her stable condition, we did not consider the need for any additional treatments targeted at COVID-19. Head ultrasounds done at day 4 and day 28 of life were healthy. Screening for retinopathy of prematurity by fundoscopy showed signs of stage 2. The SARS-CoV-2 rtPCR on her nasopharyngeal swab was positive 7 days after the initial positive test, and tested negative after 14 days. The mother tested negative only after 21 days. The neonate is still in the unit waiting to reach term gestational age.
Figure.
Radiographic findings showing the development of pulmonary disease
Radiographies taken at birth (A), at day 2 (B), at day 5 with an arrow pointing at pigtail chest drain (C), and at day 14 (D).
Various articles have described cohorts of pregnant women with COVID-19 and the demographic information and outcomes of their infants. Zhu and colleagues4 describe the outcomes of ten neonates born to mothers with COVID-19 infection. The four term and six preterm infants (between 31 and 35 weeks) tested negative for SARS-CoV-2. Two of the neonates were critically ill in the NICU and one died because of a presumably unrelated cause. Similarly, Li and colleagues5 describe a mother who tested positive for SARS-CoV-2 and gave birth by caesarean section at 35 gestational weeks to a well appearing neonate who tested negative and remained asymptomatic. Another report describes a woman who was 30 weeks pregnant delivering a 1·83 kg infant by caesarean section, who is considered to be the most premature and smallest neonate born to a mother testing positive for COVID-19 reported so far.6 Amniotic fluid, placenta, cord blood, and nasopharyngeal swab from the neonate tested negative for SARS-CoV-2. Chen and colleagues7 analysed the placentas after delivery of three mothers affected by COVID-19 in the third trimester of pregnancy. All neonatal nasopharyngeal swabs and placentas tested negative. Another study reports four mothers at term with confirmed COVID-19 during the third trimester.8 All women delivered neonates who were well and asymptomatic, three of whom tested negative for the virus (one neonate was not tested because parental consent was denied). In a report by Chen and colleagues9 nine women testing positive for SARS-CoV-2 delivered healthy neonates beyond 36 weeks. Cord blood, amniotic fluid, breastmilk, and infants' nasopharyngeal swabs tested negative in the six neonates tested. All samples had been collected at the time of delivery, strongly supporting the absence of vertical transmission. At the time of writing, the shortest time for SARS-CoV-2 positivity in an infant after delivery was 30 h. The infant was born by caesarean section to a mother with confirmed COVID-19, raising the question of vertical transmission. Amniotic fluid, cord blood, and the placenta were unfortunately not analysed.10
The studies published thus far have not shown vertical transmission and the mode of transmission in our case is most likely to be horizontal. Histological and microbiological analysis of the placenta is currently in process; however, maternal breastmilk tested negative, presumably excluding breastfeeding as the route of transmission. The neonate was delivered by caesarean section and remained in a closed incubator with standard NICU precautions, as COVID-19 was not suspected or diagnosed in the mother until 7 days post partum. The mother wore a surgical mask at all times while present in the NICU, given her yet unidentified febrile illness. Following the COVID-19 diagnosis of mother and infant, the NICU staff and other neonates and parents who had been in the vicinity of, or in direct contact with, the affected mother and neonate were either tested for SARS-CoV-2 or monitored for 14 days. Although we presume horizontal transmission from the mother to the infant, it is vital to consider all potential modes including vertical or congenital transmission, and perinatal or postnatal transmission via aerosol, droplet, and direct contact. This mother–infant pair was diagnosed with COVID-19 in the first stage of the Belgian pandemic, when approximately 250 nationwide cases were confirmed at the time and only two adult patients tested positive and were admitted in our hospital. Therefore, it seems unlikely that the infant was infected by nosocomial spread of aerosolised virus or by an infected health-care worker. There was a single nurse in the NICU who, despite being asymptomatic, tested positive 2 days after the mother–infant diagnosis.
This case strongly influenced the need for subsequent rapid action. Work groups were formed and, together with the obstetric team, antenatal, labour, maternity, and neonatal wards all dedicated to COVID-19 were established in secure isolated environments. Clear protocols and guidelines were developed for the management of all neonates born to mothers testing positive for SARS-CoV-2.
This case also highlighted the relative ease of the presumed horizontal transmission, even within a controlled NICU. We therefore set up hospital and national guidelines for not only the peripartum period, but also stringent screening and management for all parents and staff entering the NICU. Key guidelines in our unit include no visitors being allowed in the NICU and only one parent at any given time permitted to be at the infant's bedside. Before entering the NICU, all parents and staff are screened with verbal questions regarding fever and respiratory symptoms, and more recently, temperatures have been measured on entering the NICU. The wearing of surgical masks is obligatory for all parents and staff. Personal protective equipment is only worn in the specific area of the ward that is dedicated to COVID-19. Skin-to-skin contact is still permitted in the NICU as all mothers are screened for COVID-19 and those who test positive stay in a unit dedicated to COVID-19 or at home until they subsequently test negative. Contamination of breastmilk and other bodily fluids needs to be studied further, but our current policy is to allow the use of expressed breastmilk. Clear guidelines need to be followed with regard to all aspects of this process.
Since this first case, there have been five further deliveries in our hospital to mothers with COVID-19. All neonates tested negative and only one required NICU admission because of unrelated reasons. This Case Report suggests that neonates infected with SARS-CoV-2 (even if extremely preterm) might not necessarily be susceptible to severe disease with clinically significant or major morbidity.
As the number of people affected by COVID-19 continues to rapidly increase worldwide, the importance of containing the spread of SARS-CoV-2 is vital. Our case highlights the need for clear local and national guidelines and protocols that maximise aerosol, droplet, and contact precautions to prevent horizontal and nosocomial transmission. These guidelines not only encompass infants born to mothers with COVID-19, but also all staff and parents entering the NICU. The risk benefits of parental access and skin-to-skin contact should be carefully balanced, and in our unit there is no current contraindication to the use of maternal breastmilk. Neonatal cases of SARS-CoV-2 infection should be thoroughly documented, followed, and reported to create a database for neonatal COVID-19. These data will help to fill the knowledge gaps on the incidence, severity, and prognosis and will assist with the development of further rational, evidence-based management guidelines.
Contributors
FP and KC wrote the report. OD revised and added intellectual content. CaH, BVG, and CoH proofread the Case Report. OC and DVDL proofread the Case Report and assisted with infection control measures and guidelines after the diagnosis of mother and infant. Written consent for publication was obtained from the patient.
Declaration of interests
We declare no competing interests.
References
- 1.Zhu N, Zhang D, Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohrabi C, Alsafi Z, O'Neill N. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corman VM, Landt O, Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu H, Wang L, Fang C. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Zhao R, Zheng S. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020 doi: 10.3201/eid2606.200287. published online March 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Zhou Z, Zhang J, Zhu F, Tang Y, Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa200. published online Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Huang B, Luo DJ. [Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases] Zhonghua Bing Li Xue Za Zhi. 2020 doi: 10.3760/cma.j.cn112151-20200225-00138. published online March 1. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Peng H, Wang L. Infants born to mothers with a new coronavirus (COVID-19) Front Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Guo J, Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niu Y, Yue H. Wuhan Tongji Hospital diagnoses first case of neonatal infection with new coronavirus (in Chinese) Feb 5, 2020. http://society.people.com.cn/n1/2020/0205/c1008-31572959.html