Summary
Background
Mortality of patients with coronavirus disease 2019 (COVID-19), acute respiratory distress syndrome (ARDS), and systemic inflammation is high. In areas of pandemic outbreak, the number of patients can exceed maximum capacity of intensive care units (ICUs), and, thus, these individuals often receive non-invasive ventilation outside of the ICU. Effective treatments for this population are needed urgently. Anakinra is a recombinant interleukin-1 receptor antagonist that might be beneficial in this patient population.
Methods
We conducted a retrospective cohort study at the San Raffaele Hospital in Milan, Italy. We included consecutive patients (aged ≥18 years) with COVID-19, moderate-to-severe ARDS, and hyperinflammation (defined as serum C-reactive protein ≥100 mg/L, ferritin ≥900 ng/mL, or both) who were managed with non-invasive ventilation outside of the ICU and who received standard treatment of 200 mg hydroxychloroquine twice a day orally and 400 mg lopinavir with 100 mg ritonavir twice a day orally. We compared survival, mechanical ventilation-free survival, changes in C-reactive protein, respiratory function, and clinical status in a cohort of patients who received additional treatment with anakinra (either 5 mg/kg twice a day intravenously [high dose] or 100 mg twice a day subcutaneously [low dose]) with a retrospective cohort of patients who did not receive anakinra (referred to as the standard treatment group). All outcomes were assessed at 21 days. This study is part of the COVID-19 Biobank study, which is registered with ClinicalTrials.gov, NCT04318366.
Findings
Between March 17 and March 27, 2020, 29 patients received high-dose intravenous anakinra, non-invasive ventilation, and standard treatment. Between March 10 and March 17, 2020, 16 patients received non-invasive ventilation and standard treatment only and comprised the comparison group for this study. A further seven patients received low-dose subcutaneous anakinra in addition to non-invasive ventilation and standard treatment; however, anakinra treatment was interrupted after 7 days because of a paucity of effects on serum C-reactive protein and clinical status. At 21 days, treatment with high-dose anakinra was associated with reductions in serum C-reactive protein and progressive improvements in respiratory function in 21 (72%) of 29 patients; five (17%) patients were on mechanical ventilation and three (10%) died. In the standard treatment group, eight (50%) of 16 patients showed respiratory improvement at 21 days; one (6%) patient was on mechanical ventilation and seven (44%) died. At 21 days, survival was 90% in the high-dose anakinra group and 56% in the standard treatment group (p=0·009). Mechanical ventilation-free survival was 72% in the anakinra group versus 50% in the standard treatment group (p=0·15). Bacteraemia occurred in four (14%) of 29 patients receiving high-dose anakinra and two (13%) of 16 patients receiving standard treatment. Discontinuation of anakinra was not followed by inflammatory relapses.
Interpretation
In this retrospective cohort study of patients with COVID-19 and ARDS managed with non-invasive ventilation outside of the ICU, treatment with high-dose anakinra was safe and associated with clinical improvement in 72% of patients. Confirmation of efficacy will require controlled trials.
Funding
None.
Introduction
As of April 29, 2020, the coronavirus disease 2019 (COVID-19) pandemic has affected 3 018 681 people worldwide, causing the death of 207 973.1 Current management of COVID-19 is supportive, and respiratory failure from acute respiratory distress syndrome (ARDS) is the main cause of death.2, 3 Mortality in patients with COVID-19 and ARDS who are admitted to the intensive care unit (ICU) is high: a study of 24 patients reported that 50% had died at 14 days,4 whereas mortality in other reports ranges from 28% to 78%.2, 5, 6, 7, 8 Mortality is increased in patients with pronounced systemic inflammation.2, 3
In areas where the COVID-19 pandemic is overwhelming, the number of patients with COVID-19 and ARDS can exceed the maximum capacity of ICUs.9 As a shortage of ICU beds has emerged, with an unsettling element of overall lethality,9 many patients with COVID-19 and ARDS have received maximum supportive treatment, including non-invasive ventilation in medical wards, while awaiting access to the ICU and further therapeutic approaches. Treatments are needed to effectively reduce mortality and prevent ICU admission in this population.
Research in context.
Evidence before this study
Since the coronavirus disease 2019 (COVID-19) outbreak, evidence has emerged that some patients develop acute lung injury and respiratory insufficiency as a result of an excessive, maladaptive host inflammatory response to severe acute respiratory syndrome coronavirus 2. Published work has delineated a similarity between this subgroup of patients with COVID-19 and those with hyperinflammatory syndromes (eg, resembling the cytokine storm that develops in patients with macrophage activation syndrome or after chimeric antigen receptor T-cell treatment). On this basis, use of cytokine-blocking agents has been proposed for treatment of patients with COVID-19; however, data are scarce for the efficacy and safety of these treatments in this population. In particular, no published study has assessed interleukin-1 (IL-1) blockade with anakinra, despite previous evidence of efficacy and safety of this treatment for patients with hyperinflammatory syndromes.
Added value of this study
Our retrospective cohort study is, as far as we know, the first to describe IL-1 blockade with high-dose intravenous anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation.
Implications of all the available evidence
Our study, together with pre-existing evidence of the efficacy and safety of anakinra in patients with hyperinflammatory syndromes, suggests that this agent deserves consideration and controlled testing for the treatment of COVID-19.
Anakinra is an interleukin (IL)-1 receptor antagonist that blocks activity of the proinflammatory cytokines IL-1α and IL-1β and is used to treat autoinflammatory disorders (eg, adult-onset Still's disease, systemic-onset juvenile idiopathic arthritis, and familial Mediterranean fever) at a daily dose of 100 mg subcutaneously in adult patients.10 A subgroup of patients with COVID-19 show hyperinflammatory symptoms that resemble the cytokine storm after chimeric antigen receptor T-cell treatment or in patients with macrophage activation syndrome, with release of IL-1, IL-6, IL-18, and interferon γ.3, 11 Cytokine-blocking agents, including high-dose anakinra, are effective treatments for these disorders.12, 13, 14 However, compared with other cytokine-blocking agents, anakinra has a remarkable record of safety and a short half-life, which allows prompt discontinuation, and it is therefore suitable for use in critically ill patients.15, 16 Indeed, reanalysis of data from a previous phase 3 randomised controlled trial of anakinra in sepsis showed significant survival benefits in patients with hyperinflammation.17 We aimed to assess clinical outomes at 21 days in consecutive patients with COVID-19, ARDS, and hyperinflammation who received anakinra in addition to non-invasive ventilation and standard treatment outside of the ICU compared with a historical cohort of patients who did not receive anakinra.
Methods
Study design and patients
We conducted a retrospective cohort study at the San Raffaele Hospital, a tertiary health-care centre in Milan, Italy, which was designated as a COVID-19 hub by Italian health authorities. We included patients (aged ≥18 years) with COVID-19, moderate-to-severe ARDS, and hyperinflammation. COVID-19 was diagnosed by quantitative RT-PCR and either chest radiography or CT. Moderate or severe ARDS was defined as acute-onset respiratory failure with bilateral infiltrates on chest radiography or CT, hypoxaemia (ratio of the partial pressure of oxygen in arterial blood to the fractional concentration of oxygen in inspired air [PaO2:FiO2] ≤200 mm Hg with a positive end-expiratory pressure [PEEP] of at least 5 cm H2O), and no evidence of left atrial hypertension (to rule out cardiogenic oedema; appendix p 2).18 Hyperinflammation was defined as an increase in either the serum inflammation marker C-reactive protein (≥100 mg/L) or ferritin (≥900 ng/mL), or both. We excluded patients who were already admitted to the ICU for mechanical ventilation, those with evidence of bacterial infection (appendix p 2), individuals with concomitant administration of other anti-inflammatory agents or glucocorticoids, and people who had been enrolled concomitantly in clinical trials.
On obtaining written individual patient's consent, we recorded clinical data daily from all consecutive patients admitted to hospital for COVID-19, using a dedicated electronic case report form, according to an institutional protocol (ethics committee approval no 34/int/2020).19 All patients also gave written informed consent for off-label use of anakinra.
The comparison group in our study consisted of patients admitted to hospital before initiation of this study who retrospectively fulfilled eligibility criteria for anakinra treatment. Specifically, these patients had COVID-19, ARDS, and hyperinflammation; received the same institutional standard treatment and continuous positive airway pressure (CPAP) outside of the ICU; did not receive anti-inflammatory agents or glucocorticoids; and were not enrolled in other clinical trials.
Procedures
All patients in the study received respiratory support by non-invasive ventilation with CPAP (PEEP of 10 cm H2O), 200 mg hydroxychloroquine twice a day orally, and 400 mg lopinavir with 100 mg ritonavir twice a day orally, as per standard treatment administered at our institution at the time. Patients did not receive glucocorticoids or other anti-inflammatory agents. Detailed information on institutional treatment protocols is available in the appendix (pp 3–4). No management changes were introduced during the timeframe of this study.
Anakinra (Swedish Orphan Biovitrum, Stockholm, Sweden) was one of the immediately available anti-inflammatory therapeutic options at the time of the COVID-19 outbreak. Low-dose anakinra was administered off-label subcutaneously at a dose of 100 mg twice daily. High-dose anakinra was administered off-label intravenously at a dose of 10 mg/kg per day (5 mg/kg twice daily, infused over 1 h). The duration of treatment was protracted until sustained clinical benefit, defined as a 75% reduction in serum C-reactive protein and sustained respiratory improvement (PaO2:FiO2 >200 mm Hg) for at least 2 days, or until death, bacteraemia (appendix p 2), or side-effects arose (eg, increase in liver aminotransferase enzymes above three-fold the upper normal limit). In patients achieving sustained clinical benefit, discontinuation of high-dose anakinra was followed by daily low-dose subcutaneous administration (100 mg twice daily) for 3 days, which was deemed useful to avoid possible inflammatory relapses. In case of side-effects, anakinra was interrupted without previous dose reduction (eg, to allow standard management of infection without concomitant risk of immunosuppression).
Changes in clinical outcomes were evaluated for 21 days, or until discharge from hospital, ICU admission, or death, whichever came first. Outcomes included survival, mechanical ventilation-free survival, and changes in PaO2:FiO2, C-reactive protein (assessed until day 14), and clinical status (assessed at baseline [day 0] and day 21).
Clinical status was assessed using a seven-point ordinal scale, recommended by WHO and used in previous studies of patients admitted to hospital with COVID-19.20 For the assessment of ARDS at the time of anakinra initiation, PaO2:FiO2 was calculated with arterial blood gas while the patient was on CPAP (PEEP of 10 cm H2O). For the following days, derived PaO2:FiO2 was calculated with the formula SaO2:FiO2 = 64 + 0·84 × (PaO2:FiO2), which is used for monitoring patients with ARDS (SaO2 is the oxygen saturation).21 ARDS was defined as moderate if PaO2:FiO2 was 100–200 mm Hg and as severe if PaO2:FiO2 was less than 100 mm Hg, according to standard definitions.22 Further details are available in the appendix (pp 2–4).
Statistical analysis
Data were analysed with SPSS version 24.0 (Chicago, IL, USA) and Prism (GraphPad, San Diego, CA, USA). We report categorical variables as number (%) and continuous variables as median (IQR). Survival analysis was done with the Kaplan-Meier approach; comparison between survival curves was done with the log-rank test. Statistical significance was defined as a p value less than 0·05.
This study is part of the COVID-19 Biobank study, which is registered with ClinicalTrials.gov, NCT04318366.
Role of the funding source
No funding was received for this study. GiaDL, CC, ED-T, AT, NF, AR, PR-Q, FC, and GL had full access to raw data. GC and LD had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Between March 10 and March 17, 2020, before initiation of our study, 16 patients were admitted to hospital with COVID-19, ARDS, and hyperinflammation and were managed with non-invasive ventilation (CPAP) outside of the ICU. These patients retrospectively fulfilled eligibility criteria for anakinra treatment but received standard therapy only and were regarded as the comparison group for this study. Baseline characteristics of these patients are shown in table 1 . During this same period, seven additional patients with COVID-19, ARDS, and hyperinflammation were managed with CPAP outside of the ICU and received low-dose subcutaneous anakinra (100 mg twice daily). Their characteristics at baseline are also shown in table 1.
Table 1.
Demographic, clinical, and laboratory characteristics of patients
| Standard treatment (n=16) | Low-dose anakinra (n=7) | High-dose anakinra (n=29) | |
|---|---|---|---|
| Demographics | |||
| Male sex | 14 (88%) | 5 (71%) | 24 (83%) |
| Female sex | 2 (12%) | 2 (29%) | 5 (17%) |
| Age, years | 70 (64–78) | 68 (51–73) | 62 (55–71) |
| Comorbidities | |||
| Tobacco smoking | 2 (13%) | 1 (14%) | 3 (10%) |
| Arterial hypertension | 8 (50%) | 3 (43%) | 15 (52%) |
| Coronary artery disease | 2 (13%) | 1 (14%) | 3 (10%) |
| Diabetes | 3 (19%) | 2 (29%) | 6 (21%) |
| COPD | 2 (13%) | 1 (14%) | 1 (3%) |
| Chronic kidney disease | 3 (19%) | 1 (14%) | 2 (7%) |
| Inflammation markers | |||
| Axillary temperature, °C | 38·0 (37·2–38·5) | 38·3 (38·0–38·5) | 37·8 (37·2–38·5) |
| C-reactive protein, mg/L (normal range <6) | 188 (130–246) | 139 (109–172) | 164 (105–227) |
| Ferritin, ng/mL (normal range 30–400) | 2218 (1389–2980) | 1037 (952–1591) | 1237 (941–3025) |
| Lactate dehydrogenase, U/L (normal range 125–220) | 459 (373–532) | 495 (310–585) | 458 (356–578) |
| Aspartate aminotransferase, U/L (normal range 5–34) | 61 (39–114) | 53 (35–84) | 54 (40–94) |
| Alanine aminotransferase, U/L (normal range 6–59) | 51 (23–73) | 34 (30–57) | 42 (26–61) |
| Respiratory function | |||
| Treated with non-invasive ventilation | 16 (100%) | 7 (100%) | 29 (100%) |
| Duration of non-invasive ventilation, h per day | 9 (4–12) | 8 (6–14) | 12 (12–12) |
| PaO2:FiO2, mm Hg | 96 (73–128) | 107 (100–151) | 77 (68–86) |
| PaO2:FiO2 100–200 mm Hg (moderate ARDS) | 7 (44%) | 6 (86%) | 4 (14%) |
| PaO2:FiO2 <100 mm Hg (severe ARDS) | 9 (56%) | 1 (14%) | 25 (86%) |
Data are n (%) or median (IQR). COPD=chronic obstructive pulmonary disease. PaO2=partial pressure of oxygen in arterial blood. FiO2=fractional concentration of oxygen in inspired air. ARDS=acute respiratory distress syndrome.
Between March 17 and March 27, 2020, 29 patients with COVID-19 and ARDS received high-dose intravenous anakinra in addition to CPAP and standard treatment outside of the ICU. The median age of this population was 62 years (IQR 55–71); 24 (83%) patients were male (table 1). Before initiation of anakinra treatment, median C-reactive protein was 164 mg/L (IQR 105–227) and median ferritin was 1237 ng/mL (941–3025). All patients were on CPAP, with a median PaO2:FiO2 of 77 (68–86) mm Hg. Notably, 25 (86%) patients had severe ARDS (PaO2:FiO2 ≤100 mm Hg). No patients from the comparison group or the low-dose anakinra group transitioned into the high-dose anakinra group.
Seven patients received low-dose subcutaneous anakinra (100 mg twice daily). At 7 days, this treatment was neither associated with reductions in serum C-reactive protein (appendix p 5) nor with improvements in clinical status (table 2 ). Although no safety concerns emerged, a paucity of meaningful clinical or anti-inflammatory effects led to early termination of this preliminary experience after 7 days: low-dose anakinra was discontinued; two patients went on to receive anti-inflammatory agents other than anakinra.
Table 2.
Assessment of clinical status in patients receiving low-dose anakinra (n=7)
| Day 0 | Day 7 | ||
|---|---|---|---|
| Discharged from hospital with resumption of normal activities (1) | 0 | 0 | |
| Discharged from hospital but unable to resume normal activities (2) | 0 | 0 | |
| Hospitalised, not requiring supplemental oxygen (3) | 0 | 0 | |
| Hospitalised, requiring supplemental oxygen (4) | 0 | 1 (14%) | |
| Hospitalised, requiring non-invasive mechanical ventilation, high-flow supplemental oxygen, or both (5) | 7 (100%) | 4 (57%) | |
| PaO2:FiO2 >200 mm Hg | 0 | 0 | |
| PaO2:FiO2 100–200 mm Hg (moderate ARDS) | 6 | 2 | |
| PaO2:FiO2 <100 mm Hg (severe ARDS) | 1 | 2 | |
| Hospitalised, requiring invasive mechanical ventilation (6) | 0 | 2 (29%) | |
| Death (7) | 0 | 0 | |
Data are n (%). Clinical status was graded on a 7-point scale at the time of treatment initiation (day 0) and after 7 days. PaO2=partial pressure of oxygen in arterial blood. FiO2=fractional concentration of oxygen in inspired air. ARDS=acute respiratory distress syndrome.
High-dose intravenous anakinra (5 mg/kg twice daily) was assessed based on the hypothesis that increased dosing might be needed to suppress COVID-19-related hyperinflammation. 29 patients received high-dose anakinra, with a median duration of treatment of 9 days (IQR 7–11). Clinical outcomes of these patients are shown in table 3 and are compared with those of the 16 patients who received standard treatment only. After 21 days of follow-up, 21 (72%) patients treated with high-dose anakinra had improved respiratory function. Specifically, 13 (45%) patients were discharged from hospital, three (10%) were no longer in need of supplemental oxygen, three (10%) were receiving low-flow supplemental oxygen, and two (7%) were weaning from CPAP and no longer had ARDS. Of the eight patients who did not have improved respiratory function, five (17%) were on mechanical ventilation and three (10%) died. In the standard treatment group, respiratory improvements were seen in eight (50%) patients, of whom seven (44%) were discharged from hospital and one (6%) was still hospitalised and receiving low-flow supplemental oxygen. Of the eight patients who did not show respiratory improvement, one (6%) was on mechanical ventilation and seven (44%) died.
Table 3.
Assessment of clinical status in patients receiving standard treatment (n=16) and high-dose anakinra (n=29)
|
Day 0 |
Day 21 |
||||
|---|---|---|---|---|---|
| Standard treatment | High-dose anakinra | Standard treatment | High-dose anakinra | ||
| Discharged from hospital with resumption of normal activities (1) | 0 | 0 | 7 (44%) | 13 (45%) | |
| Discharged from hospital but unable to resume normal activities (2) | 0 | 0 | 0 | 0 | |
| Hospitalised, not requiring supplemental oxygen (3) | 0 | 0 | 0 | 3 (10%) | |
| Hospitalised, requiring supplemental oxygen (4) | 0 | 0 | 1 (6%) | 3 (10%) | |
| Hospitalised, requiring non-invasive mechanical ventilation, high-flow supplemental oxygen, or both (5) | 16 (100%) | 29 (100%) | 0 | 2 (7%) | |
| PaO2:FiO2 >200 mm Hg | 0 | 0 | 0 | 2 | |
| PaO2:FiO2 100–200 mm Hg (moderate ARDS) | 7 | 4 | 0 | 0 | |
| PaO2:FiO2 <100 mm Hg (severe ARDS) | 9 | 25 | 0 | 0 | |
| Hospitalised, requiring invasive mechanical ventilation (6) | 0 | 0 | 1 (6%) | 5 (17%) | |
| Death (7) | 0 | 0 | 7 (44%) | 3 (10%) | |
Data are n (%). Clinical status was graded on a 7-point scale at the time of treatment initiation (day 0) and after 21 days. PaO2=partial pressure of oxygen in arterial blood. FiO2=fractional concentration of oxygen in inspired air. ARDS=acute respiratory distress syndrome.
Compared with standard treatment, high-dose anakinra was associated with a higher survival rate at 21 days, with cumulative survival of 90% in the anakinra group versus 56% in the standard treatment group (p=0·009; figure 1A ). Mechanical ventilation-free survival did not differ between treatment groups, with cumulative mechanical ventilation-free survival of 72% in the anakinra group versus 50% in the standard treatment group (p=0·15; figure 1B). Baseline clinical and laboratory features associated with death and mechanical ventilation in patients treated with high-dose anakinra are shown in the appendix (p 5).
Figure 1.
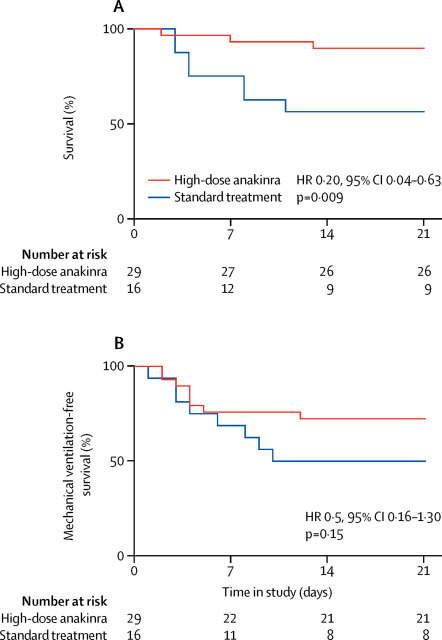
Survival and mechanical ventilation-free survival at 21 days
Plots show survival (A) and mechanical ventilation-free survival (B) at 21 days of patients with COVID-19, ARDS, and hyperinflammation managed outside the intensive care unit with CPAP and high-dose anakinra (n=29) or receiving CPAP and standard treatment only (n=16). For mechanical ventilation-free survival (B), death and mechanical ventilation were considered equivalent to treatment failure. COVID-19=coronavirus disease 2019. ARDS=acute respiratory distress syndrome. CPAP=continuous positive airway pressure. HR=hazard ratio.
Daily changes in serum C-reactive protein and PaO2:FiO2 for every patient were assessed at baseline and for the following 14 days (figure 2 ). Treatment with high-dose anakinra was associated with prompt reductions in serum C-reactive protein (figure 2A) and with progressive improvements in PaO2:FiO2 (figure 2B). Conversely, most patients receiving standard treatment had persistent or recurrent increases in C-reactive protein (figure 2C) and little improvement in PaO2:FiO2 (figure 2D).
Figure 2.
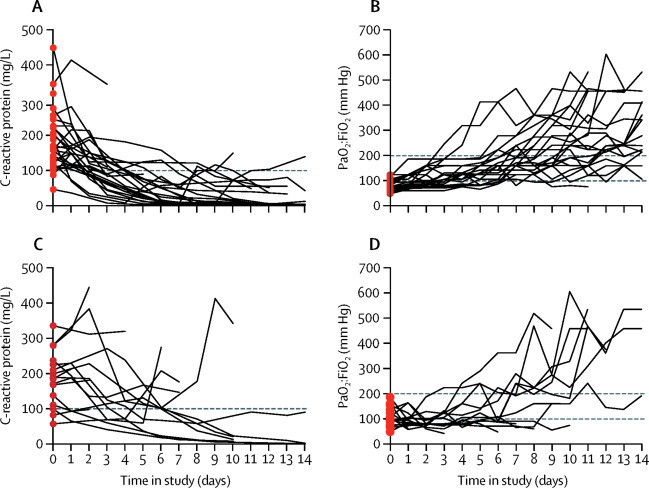
Daily changes in C-reactive protein and PaO2:FiO2
Before and after graphs show daily measurements of C-reactive protein (A) and (C) and PaO2:FiO2 (B) and (D) obtained for patients (red dots) receiving high-dose anakinra (upper panels) and standard treatment (lower panels) for 14 days, until discharge from hospital, or until death or mechanical ventilation, whichever came first. Horizontal black dotted line in (A) and (C) marks the 100 mg/L threshold, which was used to define hyperinflammation. Horizontal black dotted lines in (B) and (D) indicate thresholds for moderate ARDS (PaO2:FiO2 100–200 mm Hg) and severe ARDS (PaO2:FiO2 <100 mm Hg). PaO2=partial pressure of oxygen in arterial blood. FiO2=fractional concentration of oxygen in inspired air. ARDS=acute respiratory distress syndrome.
High-dose anakinra was well tolerated in all patients. However, treatment was discontinued for adverse events in seven (24%) patients after a median treatment duration of 9 days (IQR 8–10). Specifically, four (14%) patients had bacteraemia, with isolation of Staphylococcus epidermidis, whereas three (10%) had increases in serum liver enzymes. In these patients, anakinra was interrupted without previous dose reduction, and no rebound worsening in inflammation or respiratory function was seen. Of note, retrospective assessment showed that bacteraemia and increases in liver enzymes also occurred in two (13%) and five (31%) patients receiving standard treatment, respectively.
Three patients who did not improve while on high-dose anakinra underwent chest CT, which showed thromboembolism in the arterial pulmonary circulation. Since thromboembolism was attributed to COVID-19 intrinsic pathogenic events, including maladaptive inflammation, anakinra was not discontinued in these patients. Thromboembolism in pulmonary arteries was also identified in two patients from the standard treatment group.
Causes of death in patients receiving high-dose anakinra were pulmonary thromboembolism, respiratory insufficiency, and multiorgan failure (n=1 for each). Causes of death in the standard treatment group were respiratory insufficiency (n=3), multiorgan failure (n=3), and pulmonary thromboembolism (n=1).
Discussion
Administration of high-dose intravenous anakinra dampened systemic inflammation and was associated with progressive improvement in respiratory function in patients with COVID-19, moderate-to-severe ARDS, and hyperinflammation, who were managed outside of the ICU in a setting overwhelmed by the COVID-19 pandemic and with a shortage of ICU resources. This effect allowed us to postpone or avoid intubation in most patients. Treatment was well tolerated, and discontinuation was not followed by relapses in systemic inflammation or respiratory dysfunction. High-dose intravenous anakinra showed incremental effects over subcutaneous administration on concentrations of C-reactive protein.
A subgroup of patients with COVID-19 develops ARDS after a maladaptive, detrimental host inflammatory response to severe acute respiratory syndrome coronavirus 2, which is mirrored systemically by pronounced increases in serum C-reactive protein and ferritin.2, 3, 11 The network of mediators orchestrating inflammatory responses to tissue damage includes IL-1α and IL-1β: specifically, IL-1α is released by dying epithelial and endothelial cells, whereas IL-1β is produced by infiltrating monocytes, macrophages, and neutrophils.23 The main endogenous regulatory mechanism preventing excessive IL-1-mediated inflammation is the IL-1 receptor antagonist.10 Indeed, in a study of ARDS assessing serial bronchoalveolar lavage fluids for cytokine content, IL-1β concentrations were shown to reach peak levels at the time of disease onset; however, a slower increase in IL-1 receptor antagonist eventually follows, which provides a physiological mechanism for dampening the excessive inflammatory response in the lung.24
Anakinra is a recombinant IL-1 receptor antagonist, used to treat autoinflammatory disorders. In adult patients, this drug is usually administered subcutaneously at a standard dose of 100 mg/day;10 however, high-dose intravenous anakinra is used off-label for the treatment of hyperinflammatory conditions, such as macrophage activation syndrome.13 In view of the short half-life of anakinra (3 h), intravenous drug is typically administered every 6 h.25 In this study, high-dose anakinra was administered at a dose of 5 mg/kg twice a day in slow intravenous infusions, a choice aimed at maximising use in a high-intensity situation.
Targetable cytokines that play a part in the pathogenesis of lung inflammation in COVID-19 also include IL-6, tumour necrosis factor, and interferon γ.22 Hence, different cytokine-blocking monoclonal antibodies, or inhibitors of the JAK–STAT signalling pathway (eg, baricitinib or ruxolitinib) also represent attractive therapeutic options.26 However, a remarkable safety profile makes anakinra especially suitable for use in critically ill patients.10 First, the short half-life of anakinra allows prompt discontinuation and clearance from the circulation. Moreover, opportunistic infections in patients treated with anakinra are rare,27, 28 and it is routine practice when caring for children with autoinflammatory diseases to not discontinue anakinra during infections.29 We postulate that severe and difficult-to-treat infections will be a concern for patients with COVID-19 receiving other cytokine-blocking agents, such as anti-IL-6 monoclonal antibodies, which have a half-life of 2–3 weeks.30
In this study, comparable rates of bacteraemia and increases in liver enzymes were recorded in patients receiving anakinra or standard treatment only. In patients receiving anakinra, these side-effects occurred after protracted treatment and after improvements in clinical status. Since our study population was especially frail and had multiple risk factors (ie, prolonged hospitalisation with non-invasive ventilation and central intravenous catheters), we deemed it prudent to discontinue anakinra in patients with infection. Discontinuation of anakinra was not associated with relapses in inflammation or worsening of clinical status.
In view of the urgent need for an effective treatment for COVID-19, patients worldwide have received several off-label or compassionate-use treatments, including remdesivir,31 immune-modulating compounds,32 and convalescent plasma,33 all in uncontrolled settings. Our study arose from a specific need: medical management of patients with COVID-19, hyperinflammation, and ARDS outside of the ICU, to prevent death or escalation of intensity of care. The severity of our patient population was indeed notable: besides substantial increases in serum concentrations of C-reactive protein and ferritin (eligibility criteria for this study), poor prognostic factors included raised serum concentrations of lactate dehydrogenase, respiratory failure, older age, and comorbid conditions.5, 6 Anakinra is already approved by the US Food and Drug Administration and the European Medicines Agency, is accessible and available, and could represent a safe and controllable off-label treatment to dampen detrimental inflammation in patients with COVID-19. Of note, previous studies indicated that IL-1 inhibition might also limit endothelial dysfunction,34 which has a role in the development of coagulopathy in COVID-19.35
Our study has clear limitations. The retrospective nature and the relatively small size of the cohorts (particularly the historical comparator group) limit interpretation of results and preclude definitive conclusions. A more extended follow-up is also needed to assess long-term outcomes of treated patients. Nevertheless, a sound rationale for use, objective improvements in respiratory and inflammatory variables, and comparisons with published studies assessing different treatments suggest that high-dose intravenous anakinra could have clinical benefit in COVID-19.
The uncontrolled nature of our study mandates caution in interpretation of findings, and validation is absolutely required in a controlled setting. A randomised phase 2 clinical trial of intravenous anakinra in COVID-19 is ongoing (NCT04324021). Compared with our study, that trial is assessing lower doses (400 mg/day, approximately half the dose of 10 mg/kg per day in our study) and is not enrolling patients with ARDS. Controlled evidence is awaited, as IL-1 blockade with high-dose intravenous anakinra deserves consideration among anti-inflammatory treatments for COVID-19.
Acknowledgments
Acknowledgments
GC is supported by AIRC under MFAG 2018 - ID. 22136. This study was supported by departmental funds only. We thank our fellow first-line colleagues who, despite working incessantly to contain the COVID-19 pandemic, also found time to provide feedback on this report.
Contributors
GC led the study and wrote the report. MR, DC, CO, BC, CTD, NB, PS, GiuDL, SM, RS, and MT took care of patients and obtained data. GiaDL, CC, ED-T, AT, NF, AR, and PR-Q obtained and analysed data. FC, GL, AZ, and LD analysed data and drafted the report.
Declaration of interests
GC, GiaDL, and LD declare consultation honoraria from SOBI, outside of the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO Coronavirus disease 2019 (COVID-19): situation report—100. April 29, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200429-sitrep-100-covid-19.pdf?sfvrsn=bbfbf3d1_6
- 2.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991. published online March 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatraju PK, Ghassemieh BJ, Nichols M. COVID-19 in critically ill patients in the Seattle region: case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. published online March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. published online Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arentz M, Yim E, Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020 doi: 10.1001/jama.2020.4326. published online March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Zangrillo A, Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. published online April 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalli G, Dinarello CA. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology. 2015;54:2134–2144. doi: 10.1093/rheumatology/kev269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P, McAuley DF, Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12:259–268. doi: 10.1038/nrrheum.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eloseily EM, Weiser P, Crayne CB. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020;72:326–334. doi: 10.1002/art.41103. [DOI] [PubMed] [Google Scholar]
- 15.Cavalli G, Fallanca F, Dinarello CA, Dagna L. Treating pulmonary silicosis by blocking interleukin 1. Am J Respir Crit Care Med. 2015;191:596–598. doi: 10.1164/rccm.201412-2150LE. [DOI] [PubMed] [Google Scholar]
- 16.Cavalli G, Pappalardo F, Mangieri A, Dinarello CA, Dagna L, Tresoldi M. Treating life-threatening myocarditis by blocking interleukin-1. Crit Care Med. 2016;44:e751–e754. doi: 10.1097/CCM.0000000000001654. [DOI] [PubMed] [Google Scholar]
- 17.Shakoory B, Carcillo JA, Chatham WW. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 19.Zangrillo A, Beretta L, Silvani P. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. April 1, 2020. https://ccr.cicm.org.au/supplementary-june-2020/point-of-view [DOI] [PMC free article] [PubMed]
- 20.Cao B, Wang Y, Wen D. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice TW, Wheeler AP, Bernard GR. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 22.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 23.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park WY, Goodman RB, Steinberg KP. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 25.Rajasekaran S, Kruse K, Kovey K. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15:401–408. doi: 10.1097/PCC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 26.Stebbing J, Phelan A, Griffin I. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleischmann RM, Tesser J, Schiff MH. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1006–1012. doi: 10.1136/ard.2005.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavalli G, Dinarello CA. Anakinra therapy for non-cancer inflammatory diseases. Front Pharmacol. 2018;9 doi: 10.3389/fphar.2018.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kullenberg T, Lofqvist M, Leinonen M, Goldbach-Mansky R, Olivecrona H. Long-term safety profile of anakinra in patients with severe cryopyrin-associated periodic syndromes. Rheumatology. 2016;55:1499–1506. doi: 10.1093/rheumatology/kew208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawar A, Desai RJ, Solomon DH. Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: a multidatabase cohort study. Ann Rheum Dis. 2019;78:456–464. doi: 10.1136/annrheumdis-2018-214367. [DOI] [PubMed] [Google Scholar]
- 31.Grein J, Ohmagari N, Shin D. Compassionate use of remdesivir for patients with severe COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. published online April 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020;177 doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen C, Wang Z, Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikonomidis I, Lekakis JP, Nikolaou M. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117:2662–2669. doi: 10.1161/CIRCULATIONAHA.107.731877. [DOI] [PubMed] [Google Scholar]
- 35.Ciceri F, Beretta L, Scandroglio AM. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. April 15, 2020. https://ccr.cicm.org.au/supplementary-june-2020/point-of-view-(1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


