Abstract
Up to one-third of COVID-19 patients admitted to intensive care develop an acute cardiomyopathy, which may represent myocarditis or stress cardiomyopathy. Further, while mortality in older patients with COVID-19 appears related to multi-organ failure complicating acute respiratory distress syndrome (ARDS), the cause of death in younger patients may be related to acute heart failure. Cardiac involvement needs to be considered early on in critically ill COVID-19 patients, and even after the acute respiratory phase is passing. This Statement presents a screening algorithm to better identify COVID-19 patients at risk for severe heart failure and circulatory collapse, while balancing the need to protect health care workers and preserve personal protective equipment (PPE). The significance of serum troponin levels and the role of telemetry and targeted transthoracic echocardiography (TTE) in patient investigation and management are addressed, as are fundamental considerations in the management of acute heart failure in COVID-19 patients.
Keywords: COVID-19, Acute cardiomyopathy, Myocarditis, High sensitivity troponin, Targeted transthoracic echocardiogram, Screening algorithm
Introduction
There is a need to screen for acute heart failure in critically ill COVID-19 patients. Up to one-third of COVID-19 patients who are admitted to the intensive care unit (ICU), albeit a select population, develop an acute cardiomyopathy [1], which could represent myocarditis or stress cardiomyopathy. Fulminant myocarditis has been identified as a cause of death in younger patients with Middle East Respiratory Syndrome (MERS) [2], and a similar trend may follow with COVID-19, even though younger age groups comprise only a small proportion of the overall COVID-19 deaths.
An elevated and/or increasing serum troponin level may serve as an indicator of patients developing COVID-19-related cardiomyopathy [3]. In these patients, an elevated troponin can indicate inflammatory myocarditis rather than myocardial infarction (either type I or type II). Other causes could be pulmonary emboli or right ventricular dysfunction. Retrospective studies from China show that over 50% of patients who died from COVID-19 had evidence of heart failure, whilst nearly 60% had elevated biochemical markers consistent with acute cardiac injury [4].
In two separate cohort studies from Wuhan [3,5], the proportion of COVID-19 patients with evidence of cardiac injury — as defined by elevated serum levels of high sensitivity troponin (hsTn) with or without other biochemical markers — ranged from 20 to 28%. In both studies, patients with elevated hsTn were older and more likely to have a range of cardiovascular comorbidities, including hypertension, diabetes, coronary artery disease and prior heart failure. In both studies, an elevated hsTn was associated with a marked increase in mortality: 50-60% in those with an elevated hsTn compared with a mortality rate of 5-10% for those who had a normal hsTn during hospitalisation. In addition, COVID-19 patients with elevated hsTn during admission experienced higher rates of: acute respiratory distress syndrome (ARDS) necessitating higher rates of non-invasive and invasive ventilation; acute renal failure requiring renal replacement therapy; and, heart failure. A separate but related issue is that tachyarrhythmias (subtypes not known) occurred in 44% of COVID-19 patients who were hospitalised [6].
Whereas the mortality of the majority of older COVID-19 patients appears to be related to the development of multi-organ failure complicating ARDS [[7], [8], [9]], the cause of death in younger COVID-19 patients may be related to myocarditis [10]. This can develop when the pneumonitis itself is not clinically severe [11]. If it is a fulminant myocarditis, then where possible, these patients should be supported through this phase with mechanical support. The question remains as to what is the primary driver of this myocarditis – the virus, or the host inflammatory response? The latter would seem more likely, particularly in younger patients, in keeping with an early and then delayed peak of a ‘cytokine storm’ [12].
Screening Algorithm
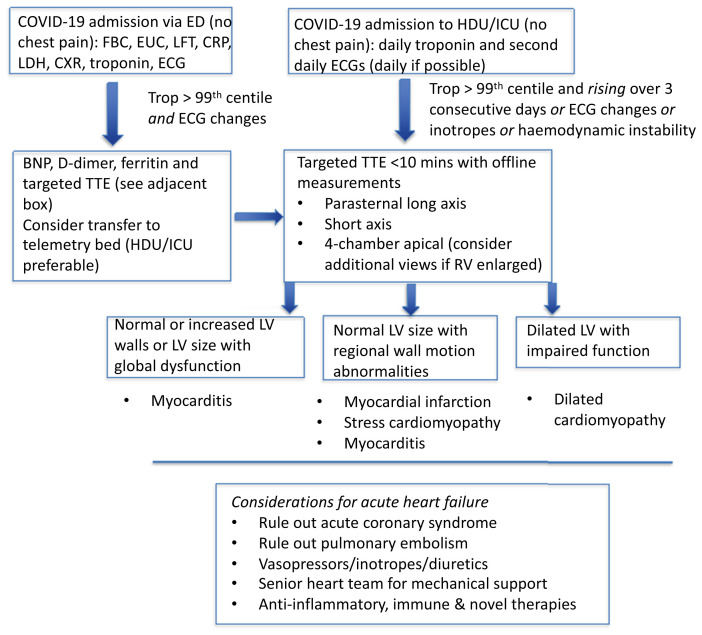
We suggest the following algorithm (Figure 1 ), to better identify COVID-19 patients at risk for severe heart failure and circulatory collapse, whilst balancing the need to protect health care workers from virus exposure and to preserve personal protective equipment (PPE):
-
1.
Patients in the emergency department (ED) who require hospital admission because of COVID-19 should have any prior history of heart failure or left ventricular impairment noted. Patients without chest pain should undergo usual investigations such as serum electrolytes, full blood count, liver function tests and chest X-ray, as well as high sensitivity troponin (hsTn) and an ECG.
Figure 1.
Adult pathway to screen for acute heart failure in COVID-19 patients admitted to hospital.
Abbreviations: ED, emergency department; EUC, electrolytes, urea and creatine; LFT, liver function test; CRP, C-reactive protein; LDH, lactate dehydrogenase; CXR, chest X-ray; ECG, electrocardiograph; HDU, high dependency unit; ICU, intensive care unit; TTE, transthoracic echocardiogram; LV, left ventricular.
Patients with an elevated hsTn (greater than 99th centile for the assay) should be considered for telemetry, preferably in the high dependency or intensive care unit (HDU/ICU). Useful adjunct tests include serum d-dimer, C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, and brain natriuretic peptide (BNP) (or N-terminal pro hormone BNP [NT-proBNP]) although the availability of the latter may vary between institutions.
If there are also ECG changes (see below) then this should trigger a transthoracic echocardiogram (TTE) [13], with limited views (see below). Otherwise, a hsTn should be undertaken daily and an ECG second daily (see caveat below) in the HDU/ICU.
Although the literature for children is limited, we recommend that a child requiring hospitalisation for COVID-19 should have an ECG and serum troponin assay, and if these are found to be abnormal, this should trigger a cardiac assessment which is likely to include a TTE.
-
2.
For patients in the HDU/ICU/telemetry setting, an elevated hsTn (greater than 99th centile for the assay), which continues to rise (in the absence of chest pain) over three consecutive days with or without ECG changes (see below) should trigger a targeted TTE [13] (see below). Haemodynamic instability (e.g. increasing vasopressor requirement), or any inotropic requirement or clinical evidence of heart failure should be independent triggers for a targeted TTE.
Two caveats to note:
-
i)
If telemetry has the capability of interpolated ECG then this can be undertaken on a daily basis as there is no additional exposure to nursing staff. Characteristic ECG changes of significant left ventricular (LV) dysfunction and/or myocarditis include: low QRS voltage consistent with myocardial oedema; occasionally, LV hypertrophy if there is an underlying chronic cardiomyopathy (representative of increased LV mass); ST-segment elevation, either global or in contiguous leads as seen in ST-elevation myocardial infarction (see note below regarding myocardial infarction); ST-segment depression; T wave inversion (may be widespread); QRS widening (including left bundle branch block [LBBB]) and PR-segment depression (pericarditis) [14].
-
ii)
If there is evidence on telemetry of frequent ventricular ectopy and/or non-sustained ventricular tachycardia (VT), then this should trigger a TTE.
-
3.The following points are relevant when interpreting the LV appearance on TTE in the presence of an elevated and rising hsTn:
-
(a)Normal or mildly increased LV size, with preserved or increased wall thickness, with global LV dysfunction (no regional wall motion abnormalities) suggests myocarditis rather than myocardial infarction.
-
(b)Normal or mildly increased LV size with segmental wall motion abnormalities can occur in stress cardiomyopathy and in myocarditis, as well as in myocardial infarction. Consideration of the diagnosis of myocardial infarction and its appropriate treatment should be made on a case-by-case basis. Fulminant myocarditis can display normal LV cavity size with severely impaired systolic function and thickened LV walls representing oedema [14].
-
(c)Significant LV dilatation (e.g. left ventricular end-diastolic diameter [LVEDD] > 6.5cm) with significant LV dysfunction may suggest a pre-existing dilated cardiomyopathy, an underlying predisposition to a dilated cardiomyopathy exacerbated or precipitated by acute viral illness, or the rapid development of a de novo viral cardiomyopathy.
-
(a)
Therefore, a targeted (less than 10 mins) TTE (so as to minimise operator exposure time) would aim for a minimum of three views:
-
i)
Parasternal long axis;
-
ii)
Short axis (papillary muscle level); and,
-
iii)
Four-chamber apical.
Information gained offline (aside from the assessment of left ventricular systolic function) for each of these views (corresponding to the order above) would include:
-
i)
LVEDD and wall thickness;
-
ii)
Assessment of regional wall motion abnormalities, and for pericardial effusion; and,
-
iii)
LV and RV size.
If the RV is disproportionately large at the time of the study, then additional views/TR Doppler assessment to assess pulmonary pressure should be considered. Further investigation for pulmonary embolism (PE) should be considered on a case-by-case basis given the pro-coagulant state of these patients [4].
It is not only left ventricular systolic function that is important but also the left ventricular morphology and size, which in themselves are prognostic, particularly in these younger patients with acute heart failure. As indicated in the Cardiac Society of Australia and New Zealand (CSANZ) Imaging COVID-19 position statement [13], TTE should be used judiciously to avoid unnecessary staff exposure, and all personal protective precautions be made available.
Managing Acute Heart Failure
Patients who develop acute heart failure should be managed in an ICU environment according to established guidelines, with the use of intravenous diuretics and inotropic/vasopressor support for those who develop hypotension. In patients with progressive cardiogenic shock, who fail to respond to inotropic support, consideration of mechanical circulatory support (e.g. veno-arterial extracorporeal membrane oxygenation [VA ECMO] or Impella [Abiomed, Danvers, MA, USA]) may be appropriate, however, this requires careful evaluation of the overall clinical picture. Given the potential risk posed to health care staff from aerosol generation during high-flow nasal oxygen, non-invasive ventilation or positive pressure ventilation without an adequate seal, institutional and departmental preparation is required for the management of acute respiratory failure [15].
The underlying pathophysiology in these critically ill patients is a likely fulminant myocarditis, so consideration should be given for therapies such as high dose corticosteroids, intravenous immunoglobulin and even selective cytokine blockade that target hyperinflammation [16], although these are not yet proven. Novel markers of inflammation and emerging therapies should also be considered as the evidence comes to hand.
While endomyocardial biopsy is considered the ‘gold standard’ for diagnosis of acute myocarditis, experience with this procedure in COVID-19 patients is extremely limited. Isolated case reports have suggested that the findings on endomyocardial biopsy are non-specific and are non-contributory to patient management. Furthermore, the procedure carries risks for both the patient and proceduralist. Based on these considerations endomyocardial biopsy is not recommended.
We recommend that inpatient cardiac magnetic resonance imaging should also be avoided as the risks of the prolonged scan time and magnet contamination are substantial and immediate management is unlikely to be changed by its use.
Some of these critically ill patients, particularly those with severe respiratory failure, may have RV dysfunction and potentially acute cor pulmonale (similar to acute PE) even without clinical evidence of pulmonary vascular thrombosis. Consequently, they may have an elevated serum troponin level stemming from the right ventricle, which is an aspect that needs to be explored. This may trigger additional modifications to ventilatory management (e.g. positive end-expiratory pressure [PEEP] titration).
Conclusion
The over-riding imperative in these critically unwell patients is that cardiac involvement, including myocarditis and cardiomyopathy, needs to be considered early and even after the acute respiratory phase is passing. Vigilance is required to identify and treat these patients.
Conflicts of Interest
None to declare.
References
- 1.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of Coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1105. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 Mar 25 doi: 10.1001/jamacardio.2020.0950. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 doi: 10.1001/jama.2020.2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., et al. Cardiac involvement in a patient with Coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1096. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 May 16 doi: 10.1093/eurheartj/ehaa190. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wahi S., Thomas L., Stanton T., Taylor A., Mahadevan D., Evan G., et al. CSANZ Imaging Council position statement on echocardiography services during the COVID-19 pandemic. Heart Lung Circ. 2020 doi: 10.1016/j.hlc.2020.04.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kociol R.D., Cooper L.T., Fang J.C., Moslehi J.J., Pang P.S., Sabe M.A., et al. American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Recognition and initial management of fulminant myocarditis: A Scientific Statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 15.Brewster D.J., Chrimes N.C., Do T.B.T., Fraser K., Groombridge C.J., Higgs A., et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust. 2020;212(10):472–481. doi: 10.5694/mja2.50598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta P., McAuley D., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]