Abstract
The World Health Organization declared COVID-19 a global pandemic in March 2020. A major challenge in this worldwide pandemic has been efficient and effective large-scale testing for the disease. In this communication, we discuss lessons learned in the set up and function of a locally organized drive-through testing facility.
In December of 2019, a cluster of cases of respiratory tract infections caused by a novel coronavirus was reported in Wuhan, the heart of Hubei province of China.1 Subsequently, several clusters of COVID-19 outbreaks were reported in various countries around the world, leading the World Health Organization to declare the escalating situation as a global pandemic.2 One of the biggest challenges in a pandemic is the availability of widespread community testing to identify cases and inform effective isolation strategies and prompt contact tracing. In that endeavor, Mayo Clinic, Rochester, at the onset of the pandemic in the United States, set up an efficient drive-through specimen-collection site, inspired, in part, by efficient and safe drive-through testing strategies in South Korea.3 At our center, discussions on best steps to manage the pandemic started very early in the outbreak.4
Efficient, effective, and coordinated large-scale testing has been one of the major challenges of the response to the pandemic. In Rochester, Minnesota, the Mayo Clinic microbiology laboratory developed in-house capability to test for SARS-CoV-2, the causative agent of COVID-19, via an in-house laboratory-developed real-time polymerase chain reaction (RT-PCR) assay. To optimize use of this resource, Mayo Clinic implemented a drive-through specimen-collection site for patients meeting prespecified criteria.
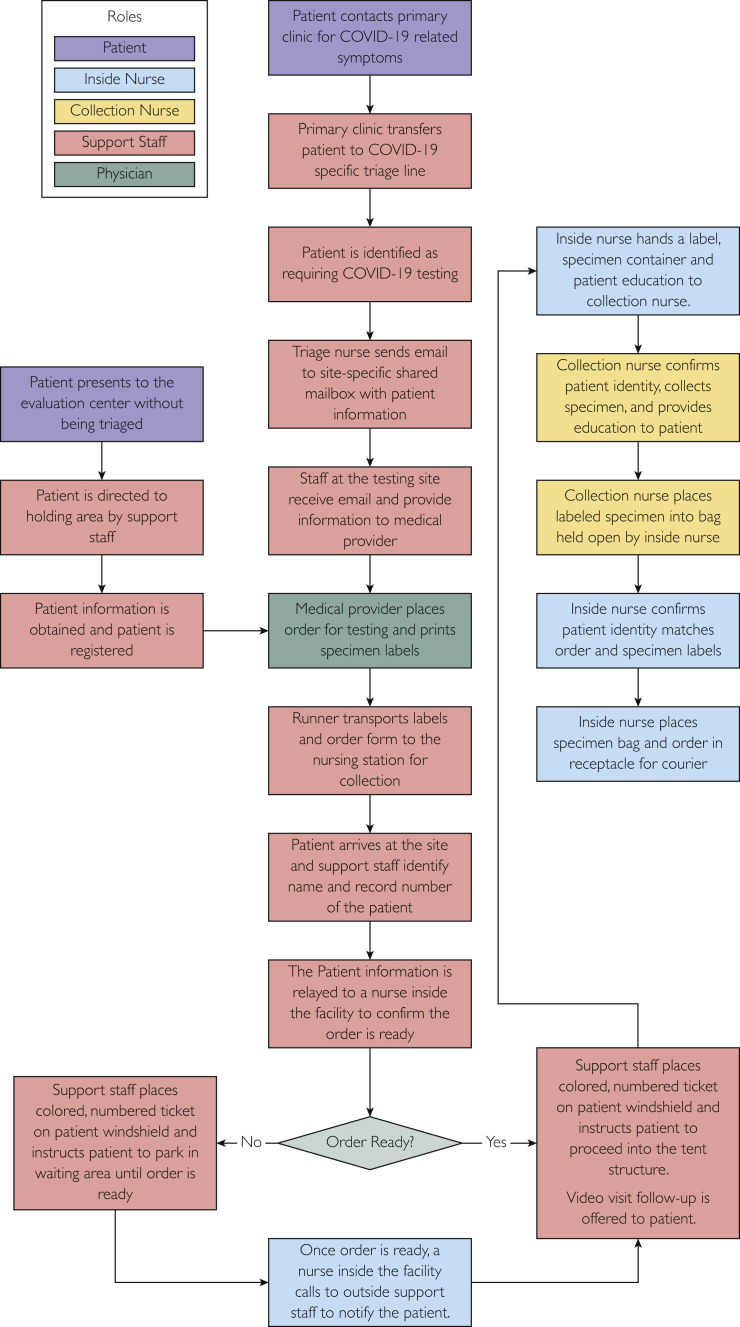
Following the diagnosis of the first case of SARS-CoV-2 infection (COVID-19) on March 3, 2020, in Minnesota,5 a multidisciplinary team of infectious disease, general internal medicine, and hospital administration experts at our institution established a drive-through specimen- collection site on March 10, 2020. At the onset of the response, Mayo Clinic adopted a liberal testing strategy to identify cases rapidly and initiate early and appropriate isolation measures. Patients on our health network were asked to call their local providers if they had symptoms consistent with SARS-CoV-2 infection (COVID-19). These included fever with or without cough or shortness of breath. On a daily basis, we made iterative changes to our screening algorithm as more information regarding SARS-CoV-2 infection (COVID-19) epidemiology and disease risk factors emerged. These patients were then directed to a nurse triage phone line, currently staffed by 60 to 70 nurses. Triaged patients were assessed based on symptoms, risk for severe disease, and potential for community exposure (health care workers, residents of long-term care facilities). The nurse team then sent an e-mail with the patient's details, and a provider at the specimen-collection site would order SARS-CoV-2 (COVID-19) testing. At the command center (a structure adjacent to the specimen collection site, housing our team), we had a team of approximately 5 primary care providers—including internal medicine, family medicine, and pediatrics—working closely with 3 infectious disease physicians (Figure 1 ). The function of this team was to order tests for patients, assist triage nurses with appropriateness of testing, and conduct video or phone visits for patients calling in with respiratory symptoms. In addition, this close collaborative effort allowed real-time observation of the appropriateness of the screening algorithm, permitting serial refinement to optimize the test strategy. To support the providers, there was an administrative team present on site, assisting with communication with the nursing line, tracking testing volumes, coordinating information technology (IT) resources, and managing schedules.
Figure 1.
Workflow.
At the drive-through specimen-collection site, 4 covered automobile lanes were set up adjacent to an existing Mayo Clinic building located in the Northwest part of Rochester, Minnesota. Leading up to the tents, were 3 lanes, with clear signs directing patients to our specimen-collection site if they had been prescreened by the nurse triage line. Patients who had not received prescreening, or who did not have medical record numbers in our health system, were directed to additional booths dedicated for registration. Campus security and Rochester city police helped coordinate the flow of cars through an approximate 50-meter area leading up to the collection tents (Figure 2 A). Once patients made their way to the tent in their cars, they were instructed to cough with their windows rolled up (Figure 2B). Following this instruction, the windows were rolled down, and a nurse in full personal protective equipment (PPE) obtained a nasopharyngeal swab.
Figure 2.
(A,B) Tent. (C) Warming station.
Nursing was critical to the success of this endeavor. The nursing team was composed of 20 to 24 staff members, including 1 supervisor working 11-hour days, 7 days a week. Warming stations were located about 10 meters from the collection tents to keep staff safe in March temperatures of 20 °F to 30 °F when the collection center opened (Figure 2C). Once a car pulled up, a nurse, wearing PPE—gown, gloves, face mask, and eye shield—would walk up to the car and collect specimens. Initially, nursing staff was collecting nasopharyngeal and oropharyngeal swabs for the SARS-CoV-2 (COVID-19) polymerase chain reaction (PCR), but this was simplified to collection of a single nasopharyngeal swab after Centers for Disease Control guidance. To preserve PPE, nursing staff would only change gloves between patients.
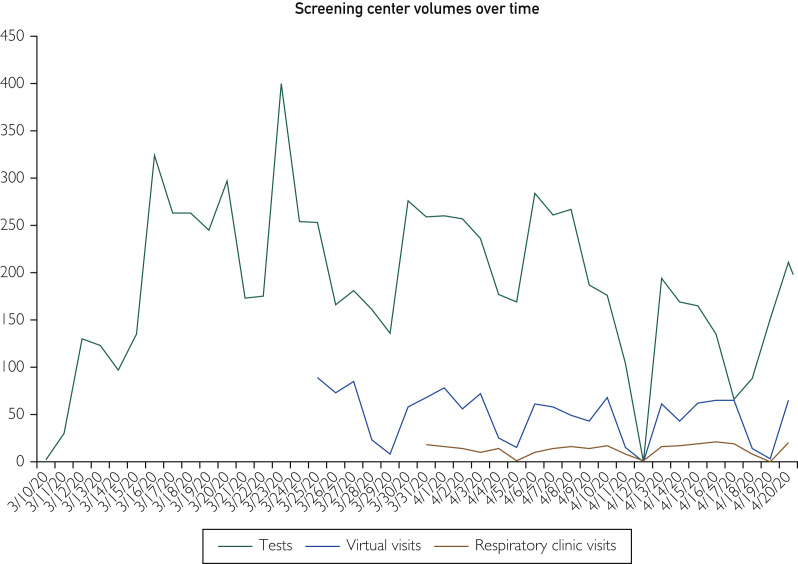
Collected samples were stored in laboratory-designated storage refrigerators. Refrigerated samples were transported twice a day to our local laboratory for testing. The specimen-collection site averaged approximately 200 swabs per day but was able to manage as many as 400 swabs on its busiest days (Figure 3 ). Owing to inclement weather, the specimen collection site remained closed for 1 day.
Figure 3.
Screening center volumes over time.
Discussion
Key components of the Mayo Clinic’s COVID-19 pandemic response included rapid planning, execution, ongoing evaluation, and adaptation of new workflow and processes. A critical part of planning and execution includes widespread community testing of symptomatic patients for SARS-CoV-2 (COVID-19).6 This is considered to be of critical importance in the ongoing efforts to flatten the epidemic curve. As an organization, our focus was to institute these measures early in the pandemic to minimize impact on the local population, its economy, and its health care workforce.
An initial part of the strategic plan for a highly functional center included availability of resources to ensure smooth functioning. Our goal was to have minimal to no wait time for patients calling our nurse triage line. For this purpose, we set up a dedicated nurse triage line phone number that could be accessed by providers in our health network to facilitate needed testing for symptomatic patients. The average wait for a patient to talk to a nurse on the triage line was less than 10 seconds.
The combined presence of administrators, IT, nurses, occupational health, logistics specialists, and physicians in a single center facilitated rapid trouble shooting and dynamic changes to improve the process. For example, IT assisted by establishing dedicated telephone lines, computers, and specialized specimen label printers. The command center administrative support staff established a communication pathway with the Hospital Incident Command System (HICS) to secure needed resources. Necessary infrastructure was put in place to begin testing within 24 hours. Having an institutional command system with authority to make real-time decisions and reallocate resources was essential for the establishment of the specimen-collection site.
Another key part of our specimen-collection site was establishment of notification pathways for providing test results to patients. The operation team established a clear communication plan for patients to explain the results and develop a follow-up plan. The operation team set up an internal message in basket pool in our electronic medical record (EMR) Epic, to which all test results—positive or negative—would be forwarded automatically. We would be notified of positive test results both via our EMR and our laboratory colleagues. While their results were pending, all patients were offered virtual visits—by either phone or video—to assess their symptoms and offer any appropriate treatment for their acute complaint (Figure 3). Algorithms were created for the management of a positive or negative test result once available (generally within 24 hours). Negative test results were sent by a central team to the patient via patient online services or by telephone. Positive SARS-CoV-2 (COVID-19) test results were communicated by physicians to patients by phone. The notifying physician would also assess the severity of the patient’s ongoing symptoms to determine the need for further medical care.
In addition to testing and virtual visits, the center also had capabilities to do in-person visits in a respiratory clinic. These visits were reserved for patients with respiratory symptoms, who had tested negative for SARS-CoV-2 (COVID-19), but who needed in-person visits from providers for further assessment and treatment guidance (Figure 3).
Funding and staffing for the specimen-collection site was provided entirely by Mayo Clinic. Allocated staff members were reassigned from their routine duties. Using this approach, we were able to increase testing workflow and numbers significantly (Figure 3).
One of the operational challenges the team encountered initially was with patients who arrived our center without previously being triaged and following the workflow outlined here. To help with specimen collection of unregistered patients, we created a new workflow and set up logistical support to generate medical record numbers. Traffic control directed unregistered patients to different parking areas so the registration could be completed by phone without patients having to leave their vehicles and without delaying other patients. This strategy also kept patients in their vehicles, minimizing potential exposures of staff that might have occurred when waiting in a typical clinical waiting area. Registration staff was present in the parking lot to assist with communication with the team inside the clinic. These staff members were also wearing appropriate PPE.
A second challenge was dealing with a backlog of cars, which could potentially hamper the traffic flow around the specimen collection site. We sought assistance of both campus security personnel and city police. With their logistical support, we were able to increase throughput to a daily average of 200 tests without difficulty or long queues.
Another challenge was to create a third workflow for employees who needed specimen collection. We involved the occupational health team early and implemented parallel pathways for employee screening, notification, and return to work. Occupational health staff was present on site initially to help with the creation of appropriate messaging for staff in collaboration with the infectious disease and primary care providers. This real-time collaboration allowed the teams to respond quickly to a rapidly changing environment while ensuring consistent communications to all members of the specimen-collection site.
Early in the establishment of the specimen-collection site, a need of on-the-ground infectious disease expertise and support was identified. For this purpose, we had 1 infectious disease consultant and 2 to 3 infectious disease fellows help with troubleshooting and logistics at our command center. The role of the consultant was to communicate with the infection prevention and control team at our main campus, with creation of protocols and algorithms for real-time challenges. These activities included generation of telephone scripts for the nurse triage line, algorithms for stratification of patients calling into the center, protocols for testing of and isolation of health care workers, and notification of patients with positive or negative test results. The role of the fellows at the command center was answering questions from the general medicine staff, speaking with nurse triage to decide testing for cases that needed infectious disease clinical judgment, ordering tests for the patients, and helping with matters requiring infectious disease expertise in general.
Conclusion
A major logistical challenge in the ongoing pandemic, worldwide, is adopting a fast, efficient, safe, and cost-effective way of testing a large proportion of the community. This has been successfully accomplished in countries such as South Korea and Singapore that have then managed to flatten the curve of their disease burden through aggressive testing and isolation of cases.7 , 8 We adapted strategies developed elsewhere to create a rapidly adaptable, high-efficiency, and multidisciplinary team capable of quickly responding to the dynamic changes present in the current pandemic. Similar models should be adopted nationwide for flattening the curve during the COVID-19 pandemic.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Novel coronavirus (COVID-19). World Health Organization website. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Published 2020.
- 3.Kwon K.T., Ko J.H., Shin H., Sung M., Kim J.Y. Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. J Korean Med Sci. 2020;35(11):e123. doi: 10.3346/jkms.2020.35.e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah A., Kashyap R., Tosh P., Sampathkumar P., O’Horo J.C. Guide to understanding the 2019 novel coronavirus. Mayo Clin Proc. 2020;95(4):646–652. doi: 10.1016/j.mayocp.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minnesota Department of Health Health officials confirm first case of novel coronavirus in Minnesota. https://www.health.state.mn.us/news/pressrel/2020/covid19030620.html
- 6.World Health Organization Critical preparedness, readiness and response actions for COVID-19. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/critical-preparedness-readiness-and-response-actions-for-covid-19
- 7.Shim E., Tariq A., Wongyeong C., Lee Y., Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pung R., Chiew C.J., Young B.E. on behalf of the Singapore 2019 Novel Coronavirus Outbreak Research Team. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395(10229):1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]