Highlights
-
•
These guidelines provide orientation to perform clinical neurophysiological studies during COVID-19 pandemic.
-
•
Guide to perform protective procedures for healthcare personal doing clinical neurophysiological studies.
-
•
Offer a protocol for the disinfection of equipment and supplies in clinical neurophysiological studies.
Keywords: Hygiene, EEG, EMG, Neurophysiological test, COVID-19, Coronavirus
Abbreviations: CDC, Center for Disease Control, United States; CN, Clinical Neurophysiology; CNS, Central Nervous System; COVID-19, coronavirus disease 2019; CPAP, continuous positive airway pressure; EEG, electroencephalography; EMG, electromyography; EP, evoked potential; HP, healthcare professional; IONM, intraoperative neurophysiological monitoring; NCS, nerve conduction study; PPE, personal protective equipment; PSG, polysomnography; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; US, ultrasound; WHO, World Health Organization
Abstract
On 31st December 2019, China notified the World Health Organization of an outbreak of atypical pneumonia from patients at a local seafood market in Wuhan, Hubei, China, responsible for a new coronavirus called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that caused COVID-19 disease, which spread rapidly around the world. WHO declared a state of pandemic (11th March, 2020), which has caused more than 1 million infected and more than 110,000 deaths; it was observed that up to 29% of those infected were health care personnel.
The main route of transmission of SARS-CoV2 is through respiratory secretions and direct contact with contaminated surfaces and material. The pandemic induced an international saturation of health care services and a rupture in the supply chain of protective equipment for healthcare personnel, which poses a high occupational risk to all. Based on the different healthcare systems, human resources, infrastructure and medical emergencies that will warrant the conduct of clinical neurophysiology studies and the lack of a guide for the management of the situation, it was decided by an expert task force of the Latin American Chapter of the International Federation of Clinical Neurophysiology to carry out these guidelines for the protection of patient and healthcare professionals conducting clinical neurophysiological studies.
1. Introduction
On December 31th, 2019, the Wuhan Municipal Health Commission notified the World Health Organization (WHO) of an outbreak of 27 cases of pneumonia of unknown cause in Wuhan City, Hubei Province, People's Republic of China (Wang et al., 2020a). The results of the research suggested the diagnosis of viral pneumonia, and the genetic sequencing on January 9th, 2020 discovered a new strain of coronavirus (Gorbalenya et al., 2020). On February 11th, 2020, WHO called the disease COVID-19, short for “coronavirus disease 2019” and the International Committee on Virus Taxonomy called the virus “severe acute respiratory syndrome coronavirus 2” SARS-CoV-2 (Gorbalenya et al., 2020, World Health Organization, 2020e).
COVID-19 is characterized by fever, dry cough, dyspnea, general discomfort and chest pain (Guan et al., 2020). The mortality rate is higher in people over 60 years, immunosuppressed or previously affected by other conditions such as obesity, diabetes, respiratory, cardiovascular or cancer diseases (Yi et al., 2020). The overall COVID-19 mortality rate ranges from 2.7 to 3.4% (East and Surveillance, 2020, Verity et al., 2020), while the reproduction number (the expected number of new cases of an infection caused by an infected individual) of COVID-19 (R0) is 2.68, suggesting a high level of contagiosity (East and Surveillance, 2020, Sorbello et al., 2020).
In America, the first case of COVID-19 was reported in Washington, D.C., USA, on January 15th, 2020, through the Centers for Disease Control (CDC). WHO declared the status of pandemic by COVID-19 on March 11th, 2020 (World Health Organization, 2020c). Until April 12th, 2020, more than one million people have been infected and more than 105,952 deaths have been reported (World Health Organization, 2020a).
In America, United States has become the center of the pandemic with more than half million of cases and over 20,000 deaths, however, other Latin-American countries also have a high number of confirmed cases (World Health Organization, 2020a). Cases continue to increase due to the rapidly evolving situation, which is a challenge for health care, especially for severe COVID-19 patients and uninfected severely ill patients (World Health Organization, 2020b).
The main route of transmission of SARS-CoV2 has been shown to be through respiratory secretions and direct contact with contaminated surfaces and material. The possibility of the transmission of the virus from asymptomatic carriers through the same routes after a close contact with non-infected people was also documented (Verity et al., 2020, Zhou et al., 2020). Healthcare professionals (HP) are exposed to a close contact with possible asymptomatic or symptomatic carriers from which transmission is possible (Li et al., 2020a). Currently, the extent of those affected by this virus in the HP in Latin America is unknown, however, there are multiple reports of a high incidence (Ng et al., 2020, Sorbello et al., 2020). On February 11th, 2020, China reported 3019 healthcare workers with COVID-19, corresponding to a proportion of 7.06% of cases in China with at least 6 deaths in that group (Ran et al., 2020). In Italy, the International Nursing Council reported on March 19th, 2020 2609 infected health workers (Nurses, 2020). International media reported that Spain's Ministry of Health reported that 14% of COVID-19 cases reported were infected HP (Minder and Peltier, 2020). This situation predisposes to the collapse of health systems, reduction of healthcare personnel and even the death of healthcare workers and their families (Minder and Peltier, 2020).
2. Justification
The advent of the SARS-CoV-2 pandemic and the saturation of health services internationally pose a high occupational risk to all healthcare-related staff (Centers for Disease Control and Prevention, 2020d). WHO encourages countries to prepare hospitals and health facilities, protect their health staff, and decide what kind of social distancing methods should be implemented and for how long, among other actions (World Health Organization, 2019). In addition, an increasing number of COVID-19 patients with manifestations of central and peripherical nervous system condition have been reported (Li et al., 2020b, Baig et al., 2020, Mao et al., 2020, Pleasure et al., 2020).
Depending on the type of nervous system condition or secondary neurological complication, the assistance of a clinical neurophysiologist may be required. Patients who already have neurological diagnoses requiring studies by the clinical neurophysiology (CN) service during this pandemic should also be taken into consideration. For these two main reasons it is necessary to be prepared and act according to the needs of each patient. Since HP is at the forefront of defense in this pandemic, with SARS-CoV2 infection rates up to 29% (Sun and Guan, 2020), this poses additional risk for intrahospital virus dissemination (Sorbello et al., 2020). Based on the different healthcare systems, human resources, infrastructure and medical emergencies in demand of CN studies, and the lack of a guide for the management of this situation, it was decided to carry out these guidelines.
3. Objective
To establish guidelines to provide protection of HP conducting CN studies in outpatient or inpatient settings during the COVID-19 pandemic. In addition, to establish a protocol for the disinfection of equipment and supplies required in the CN studies.
4. Methodology
We reviewed the state of the art of scientific articles and sources of official health organizations in English and Spanish of policies to protect HP with an emphasis on COVID-19 and developed recommendations adapted to the practice of CN. These practical guidelines were developed with the participation of experts in CN from Latin America under the coordination of The Latin American Chapter of the International Federation of Clinical Neurophysiology of the International Federation of Clinical Neurophysiology (LAC – IFCN).
5. Definitions
Coronavirus: Coronavirus are an extensive family of RNA viruses that can cause disease in both animals and humans. In humans, they cause respiratory infections that can range from the common cold to more serious diseases that can lead to death (World Health Organization, 2019).
COVID-19: is an acute respiratory disease caused by SARS-CoV-2 (World Health Organization, 2020a).
COVID-19 patient confirmed: A person with laboratory confirmation of COVID-19 infection, regardless of clinical signs and symptoms (World Health Organization, 2020a).
Suspected case of COVID-19: A person who has some of the following conditions: A) an acute respiratory disease (fever and at least one sign/symptom of respiratory disease; e.g. cough, dyspnea), and a travel or residency history at a location reporting community transmission of COVID-19 for 14 days prior to the onset of symptoms; B) any acute respiratory disease that has been in contact with a probable or confirmed COVID-19 case in the last 14 days prior to the onset of symptoms; or C) a severe acute respiratory disease (fever and at least one sign/symptom of respiratory disease, e.g. cough, shortness of breath; and requiring hospitalization) in the absence of an alternative diagnosis that fully explains the clinical presentation (World Health Organization, 2020a).
Probable case of COVID-19: A person who meets the following conditions: (A) a suspected case for whom the laboratory tests for the COVID-19 virus are inconclusive; or (B) a suspected case for whom the tests could not be carried out for any reason (World Health Organization, 2020a).
6. General recommendations for clinical neurophysiological studies
The following recommendations should be harmonized with current scientific knowledge, institutional, local, national and international medical policies in force. These guidelines address the most common diagnostic procedures for the practice of CN; however, other procedures may follow similar guidelines. The context in which these recommendations are implemented should be adapted to the different phases of the pandemic and health systems, which may vary rapidly in the affected regions.
6.1. Recommendations for neurophysiology staff with risk factors for COVID-19
Pregnant women and patients over 60 years of age with comorbidities such as obesity, diabetes mellitus, systemic high blood pressure, cardiovascular disease, chronic lung disease or immunosuppression states have an increased risk of contracting the disease and dying (Wang et al., 2020b, Yi et al., 2020). For this reason, CN staff with the above risk factors should avoid conducting CN studies as much as possible and take extreme precautions when there is no other option.
6.2. Recommendations in case of psychiatric signs in CN staff induced by the COVID-19 pandemic
The pandemic and excess information from mass media including the preventive measures taken by governments has generated in a large part of the population anxiety, panic, fear, insomnia and depressive symptoms in the face of the likelihood of acquiring infection and fatal outcome if acquired (Galea et al., 2020, Lai et al., 2020). For this reason it is advisable that there is the possibility of access to psychological and psychiatric support, for example, through an easily accessible telephone line where initial support can be provided to the HP, who has an increased risk of developing these symptoms (Lai et al., 2020).
7. Considerations of clinical neurophysiological studies in any circumstance whether ambulatory, private offices or hospitals
Due to the high risk of infection to patients and HP in the face of exposure, it is recommended to carefully evaluate each and every case to decide which, when and why CN tests are non-urgent elective tests and, therefore, could be postponed, including electroencephalograms (EEG), video-EEG, polysomnography (PSG), use of continuous positive airway pressure (CPAP) devices, evoked potentials (EP), neuromuscular ultrasound (US), electromyography (EMG), nerve conduction studies (NCS) and other CN tests (American Clinical Neurophysiology Society, 2020a).
In the case of a demand for a CN study, the risk/benefit of conducting an ambulatory study should be evaluated. Performance of the study should be preferred through hospitalization if required by the emergency of the medical condition, and the result of the examination should impact decision-making in the treatment. An example is non-convulsive status epilepticus in children or adults (Fogang et al., 2017, Sánchez Fernández et al., 2017). If an outpatient study is needed in a private or public clinic, the guidelines for the protection of the HP and the prevention of transmission through the guidelines for disinfection of the facilities, equipment and material referred to in sections 13, 14 and 15 below shall be followed.
Studies at the patient's home should follow the WHO's recommendations for the care of patients with suspected or confirmed COVID-19, for example in PSG studies with monitors 2 and 3 (American Academy of Sleep Medicine, 2020).
8. Preventive COVID-19 risk assessment process in the department of clinical neurophysiology prior to patient admission
It is useful when evaluating the request for a CN study to call the patient or family relatives and perform clinical and epidemiological screening. The CN lab secretary or assistant should do the following survey, which is very easy to apply (Liang, 2020).
-
1.
Does the patient have respiratory symptoms and/or fever, no matter whether mild?
-
2.
Has the patient travelled in countries where COVID-19 cases have been detected in the last 14 days?
-
3.
Has the patient been in contact with people who have been traveling or reside in countries with proven cases of COVID-19 in the last 14 days?
-
4.
Has the patient been to places where 2 or more people with respiratory symptoms and/or fever have been detected in the last 14 days (home, workplace, school)?
-
5.
Has the patient been in contact with healthy people who have been in contact with patients with proven COVID-19?
-
6.
Has the patient been in contact with people with a proven or suspected COVID-19 infection in the last 14 days?
Patients can be classified as: risk 1, if all answers are negative; risk 2, if any single of the answers to questions 1–5 is “yes”; risk 3: if two or more answers to questions 1–5 are “yes”; risk 4 (maximum risk) if the answer to question 6 is “yes”.
In the event that all responses are negative, a “risk 1” is assigned since the patient may still be asymptomatic infected and then could spread the disease. Depending on the risk, neurophysiology service personnel should take the necessary precautions in accordance with institutional and governmental standards (Liang, 2020).
9. Considerations of clinical neurophysiology studies in hospitalized patients
Medical condition scenarios that demand for CN studies are multiple and depend on multiple factors including the type of hospital, for example, if a general hospital or a specialized neurological hospital, pediatric or adult or mixed, etc.
It is known that human coronaviruses can be spread from the respiratory tract to the central nervous system (CNS) through trans-neuronal and hematogenic pathways, resulting in encephalitis and neurological diseases (Verity et al., 2020, Zhou et al., 2020). SARS-CoV2 was identified in the cerebrospinal fluid (CSF) in a patient confirmed with COVID-19 in China (Sun and Guan, 2020). The authors proposed that invasion into the CNS, peripheral nervous system and muscle is responsible for some neurological symptoms or syndromes (Li et al., 2020c), including as the most common entities musculoskeletal injury (19.3%), impaired consciousness (14.8%) and acute cerebral vascular disease (5.7%) (Desforges et al., 2014). However, it has been reported that hyposmia, anosmia and dysgeusia may occur up to 11% of patients prior to the symptoms of nasal congestion (Lechien et al., 2020).
In general, all requests for CN studies should be reviewed by the physician performing or interpreting the studies to assess the benefit/risk ratio and appropriate time for conducting studies in patients with COVID-19, suspected COVID-19 or patients without COVID-19 infection. A driving plan for staff and patients should be in place, considering the following events:
-
1.
Before the patient's arrival
-
2.
When the patient reaches the clinic or department or attends the intra-hospital area
-
3.
During staff visit to the department, or during the execution of the exam at the patient's bed.
-
4.
When the patient is removed from the emergency room area or returned outside the COVID-19 care area (Centers for Disease Control and Prevention, 2020c).
9.1. EEG
-
1.
EEG studies (with or without 20-min scalp surface video-EEG) should be conducted following the protection and infection prevention protocol. It is recommended that the physician reviews (remotely if possible) the recording before removing the electrodes and equipment to avoid repositioning in case a continuous EEG (cEEG) is required (with or without video-EEG).
-
2.
If relevant, cEEG monitoring can be initiated and interpreted remotely.
-
3.
The number of HP directly involved with the CN study should be minimized and the same staff should be the one that makes the connection and removal of the equipment in case of routine EEG studies.
-
4.All elective admissions for hospitalized cEEG monitoring should be cancelled. The following are alternatives for diagnosis if they are feasible by public health policies.
-
1.Home video with electronic devices.
-
2.Outpatient EEG. If the HP went to the patient’s home, the WHO recommendations should be followed (World Health Organization, 2020a).
-
1.
-
5.
In the case of EEG/Video EEG Unit (VEEG) monitoring, the flow of patients and staff should be limited and the availability of beds for potential patients with COVID-19 should be optimized. Many institutions have decided to cancel the VEEG examinations completely.
-
6.
For patients who show up to the emergency service with frequent events, admission for non-elective EEG video monitoring for a quick diagnosis should be considered and subsequent visits avoided if possible, but risk versus benefit should always be balanced. Maneuvers should be avoided that involve airway handling. Also, requesting the patient to hyperventilate should be avoided.
9.2. EMG/NCS
-
1.
It is generally recommended to perform EMG/NCS 2 weeks after clinical onset of any neurological disease (Rubin, 2012), except for repetitive nerve stimulation and/or single-fiber EMG tests on patients with suspected neuromuscular disease in critical condition (Oh et al., 2019).
-
2.
CN staff should take special attention to the cases of EMG of facial, laryngeal and pharyngeal muscles, oral cavity, diaphragmatic and intercostal muscles due to the increased risk of aerosolization, contamination and medical complications.
-
3.
EMG/NCS studies have not been reported to provide data to contribute to the prognosis of recovery of critically ill COVID-19 patients with mechanical ventilation.
-
4.
If NCS are required in critically ill patients for diagnostic purposes, an abbreviated protocol with examination of fewer nerves than usual should be preferred (Moss et al., 2014).
9.3. Polysomnography
-
1.
All elective procedures shall be cancelled.
-
2.
If necessary, PSG of confirmed or suspected COVID-19 patients should be performed with appropriate use of PPE (Table 1 ) and following appropriate hygiene policy.
-
3.
Divided night studies should be limited where the risk of using CPAP increases the risk of airbrushing and contamination (Federación Latinoamericana de Sociedades del Sueño, 2020).
-
4.
Remote interpretation of the study and home studies are recommended.
Table 1.
Recommendations of rational and appropriate use of Personal Protective Equipment (PPE) in health personnel and patients during clinical neurophysiology studies. Adaptation of the WHO recommendations (World Health Organization, 2020c).
|
Area |
Staff or Patient |
Activity |
Personal Protection Equipment |
|---|---|---|---|
| Health Care Environment | |||
| In-hospital Environment | |||
| Patient's Room | Healthcare Professionals including technicians. | Providing care direct to patients with COVID-19. | Medical mask, Apron or Gown, Gloves, Goggles or Full-face screen |
| Procedures in patients with COVID-19 generating aerosols | Mask N95 or FFP2 standard or similar. Gloves, Goggles or Full-face screen. Apron or Gown | ||
| Cleaners | Providing care direct to patients with COVID-19. | Medical mask, Apron or Gown, Hard work Gloves, Goggles or Full-face shield, Work covered shoes | |
| Other areas for patient transit. (e.g. corridors). | All the staff, including HP | Any activity that doesn't involve direct contact with COVID-19 patients | No PPE required. |
| Administrative Areas | All the staff, including HP | Administrative Tasks that doesn't involve direct contact with COVID-19 patients | No PPE required. |
| Outpatient Environment | |||
| Consultation Room | HP and technologists. | Physical examination of patients with respiratory symptoms | Medical mask, Apron or Gown, Gloves, Goggles or Full-face shield |
| HP and technologists. | Physical examination of patients with respiratory symptoms | PPE according to standard precautions and risk assessment | |
| Patients with respiratory symptoms | NA | Provide him a medical mask if tolerates it | |
| Patients with respiratory symptoms | NA | Provide him a medical mask ** | |
| Cleaners | After and between appointments with patients with respiratory symptoms | Medical mask, Apron or Gown, Hard work Gloves, Goggles or Full-face shield, Work covered shoes | |
| Waiting room | Patients with respiratory symptoms | None | Provide him a medical mask if tolerates it. Immediately transfer the patient to an isolated room or area apart from other patients; if not feasible, ensure at least 1 m distance from other patients. |
| Patients without respiratory symptoms | Provide him a medical mask ** | ||
| Administrative areas | All staff including HP | Administrative Activities | No PPE required. |
| House | Patients with respiratory symptoms | None | Keeping the distance of at least 1 meter. Provide medical mask if tolerates it, except when sleeping. |
| Caregivers | Provide direct attention or assistance to patients with COVID-19 at home. | Medical Mask, Apron or Gown, Gloves, Goggles or Full-face screen | |
| Entrance Administrative areas | All the staff. | None. | No PPE required. |
Note: HP-Healthcare Professionals.
9.4. Neuromuscular ultrasound
-
1.
All elective procedures shall be cancelled.
-
2.
Diaphragmatic neuromuscular US studies have not been reported to contribute to the prognosis of critical COVID-19 patients with mechanical ventilation. However, a previous systematic review suggested that neuromuscular US may be useful for detecting diaphragmatic dysfunction in critically ill patients, predicting the success or failure of extubating, and monitoring the ventilatory function (Zambon et al., 2017).
-
3.
If necessary to perform neuromuscular US, it should be done at the patient's bedside, with equipment and transducer dedicated to caring for patients with confirmed and suspected COVID-19 (Abu-Rustum et al., 2020).
9.5. Intraoperative Neurophysiological Monitoring (IONM)
-
1.
Several centers recommend rescheduling all elective surgeries, and only trauma, selected cancer and urgent neurosurgical procedures should be performed (Burke et al., 2020, Sorbello et al., 2020).
-
2.
Communication between neurophysiological, surgical and anesthesia teams is critical in all peri-operative periods in all cases (Skinner et al., 2017).
-
3.
IONM staff need to avoid stay in the operative room during the intubation process or to be at least 2 meters away from the patient during this activity, and during the IONM (Canelli et al., 2020).
-
4.
CN staff should take special attention to the cases of EMG of facial, laryngeal and pharyngeal muscles, oral cavity and intercostal muscles due to the increased risk of aerosolization or contamination, as well as medical complications. The role of the glass box used during endotracheal intubation “aerosol box” for the placement of EMG electrodes has not been evaluated, although it has been proposed as an additional protective measure attached to PPE in patients with COVID-19 (Canelli et al., 2020).
-
5.
Remote IONM could reduce the exposure of the physician interpreting the study (Galloway, 2013).
9.6. Evoked potentials
-
•
All elective diagnostic procedures should be rescheduled. If an urgent EP is needed the recommendations in section 11 should be followed.
9.7. Transcranial magnetic stimulation
-
•
All elective diagnostic procedures should be rescheduled.
10. General protective procedures for healthcare personnel
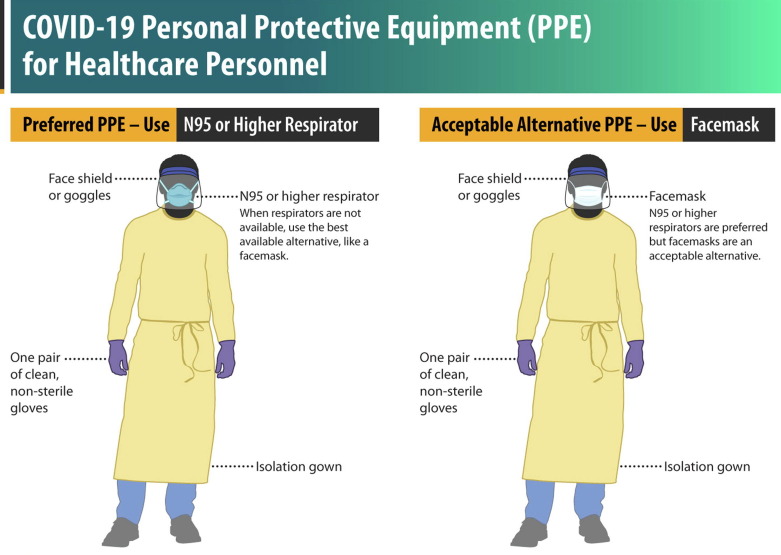
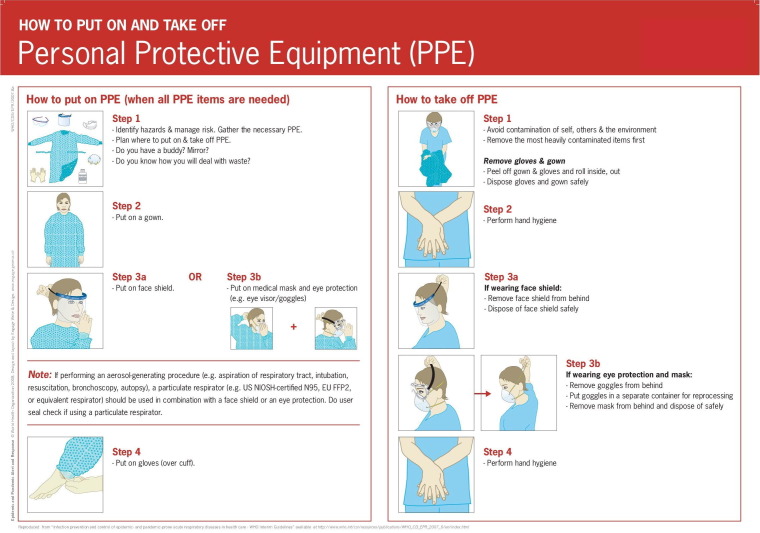
The HP should follow the policies to protect themselves and prevent intrahospital transmission. Precautions include rational use and appropriate selection of PPE (Fig. 1, Fig. 2 and Table 1); training on how to put it on, remove it and discard it (World Health Organization, 2020d). In addition, care should be taken that the work area is in proper hygienic conditions (World Health Organization, 2020c).
Fig. 1.
COVID-19 Personal Protective Equipment (PPE) for healthcare personnel. Center for Disease Control, USA. https://www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html.
Fig. 2.
How to put on and take off Personal Protective Equipment (PPE), World Health Organization. https://www.who.int/csr/resources/publications/putontakeoffPPE/en/.
It is recommended to use physical barriers to reduce exposure to patients with COVID-19 or suspect cases, such as glass or plastic windows. This includes administrative areas where patients will first show up, such as triage areas or the registration desk at the CN department (World Health Organization, 2020b).
HP access to COVID-19 patient rooms should be restricted if HP is not involved in direct care or will not perform a CN study. HP should consider grouping activities to minimize the number of times to enter a room and plan what activities will need to be done at the bed side (World Health Organization, 2020b).
Some considerations about the PPE are as follows:
Apron or Gown must be resistant to liquids and must be placed before entering the contaminated environment. After the study, the dressing apron (or gown) must be removed, inside the room and deposited in the corresponding container.
Masks: They may be surgical or medical being flat or folded. The use of only medical or surgical masks is insufficient to provide the appropriate level of protection (World Health Organization, 2020b). Respirators (e.g. N95, FFP2 or equivalent standard) are useful when caring for multiple patients who have the same diagnosis and should be used without withdrawal, and evidence indicates that respirators can maintain their protection even when used for extended periods of time. However, using a respirator for more than 4 hours can cause discomfort and should be avoided (World Health Organization, 2020b). Surgical masks have a lifespan of 3 hours. The N95 respirator can be reused, however, the maximum possible number of safe reuses is unknown, so it is advisable to review the recommendations of the manufacturer, the respiratory protection program of the hospital and the infection control department (Centers for Disease Control and Prevention, 2020a). The CDC has recommended that the N95 must be discarded when it has been used in aerosol-generating procedures, when contaminated with blood, nasal or respiratory secretions, or other fluids from the patient (Centers for Disease Control and Prevention, 2020a). The N95 mask lasts 15 days, it must always be stored on paper to avoid moisture, and it is advisable to review the manufacturer's recommendations (Centers for Disease Control and Prevention, 2020a).
Goggles and full-face screens or shields should properly cover the eyes and protect them from splashes or aerosols.
Gloves made of latex, or nitrile in case of allergy, are recommended.
11. Protective procedures for the CN technologist
It is important to the CN technologist to know the policies to contain COVID-19 infection. As a first instance, technical staff should have theoretical knowledge of the infectious agent (COVID-19). In this way, they will be aware of the infection mechanism of the virus and will be able to recognize the means or routes of contagion. The CN technologist should also follow the health institution's hygiene recommendations.
Before starting the diagnostic study, the CN technologist should:
-
1.
Obtain the responsible physician’s approval for the CN study.
-
2.
Obtain as much information as possible about the patient's condition including COVID-19 status.
-
3.
Verify equipment, material and supplies necessary to perform the examination.
-
4.
Determine the appropriate PPE level, see Table 1 and Fig. 1, Fig. 2.
-
5.
Remove all disposable items that are not needed from the neurodiagnostic equipment.
-
6.
Pick up hair, remove jewelry and makeup, watches and unnecessary personal items. If lenses are required, attach them to the face.
-
7.
Clean all surfaces of the neuro-diagnostic equipment.
-
8.
It is advisable to cover the equipment with a clear plastic liner, taking care not to block the computer fans. It is important also to cover the cables especially those that may have contact with the ground.
-
9.
Wash hands with the appropriate technique (minimum 20 seconds).
-
10.
Wear the appropriate PPE equipment (Table 1) Fig. 2 (Liang, 2020).
At the site of the diagnostic study
-
1.
Follow the policies of the area where the study will be carried out.
-
2.
Check that the patient also follows the recommended protective policies to reduce the risk of contagion.
-
3.
Conduct the study following international guidelines.
-
4.
If a routine study requires conversion to a prolonged one, inform the CN medical staff to avoid relocations and decrease the exposure time.
-
5.
In case the study is prolonged (e.g. cEEG or video-EEG) the number of technologists entering the area during the shift should be limited. Ensure that there is a distance greater than 2 m from the monitoring patient and use physical barriers if possible.
-
6.
Consider using clear plastic bags to cover CN equipment.
-
7.
Avoid maneuvers that promote airway handling. Avoid request of hyperventilation or use of CPAP (CDC, 2020).
After the diagnostic study
-
1.
Dispose the non-reusable CN (e.g. electrodes) material.
-
2.
Plan the cleaning procedure for equipment and reusable material. See Section 13.
-
3.
Remove the PPE equipment (Fig. 2) following the recommendations of its dispensing or reuse in the area where you conducted the study, it can be a separate room, but avoid walking in other areas.
-
4.
Wash hands with appropriate technique.
-
5.
Proceed to the disinfection process of diagnostic equipment and electrodes with a new appropriate PPE equipment. Table 1.
-
6.
Remove the PPE. Fig. 2.
-
7.
Perform hand hygiene.
12. Protective procedures for the clinical neurophysiologist
Clinical neurophysiologists shall coordinate the staff under their supervision and follow the principles of professional responsibility at the institutional, regional and national level of the practice of CN following international, ethical, safety and hygiene principles.
Before the study
-
1.
The physician should review and approve the indication of the studies based on risk/benefit assessment.
-
2.
Promote communication between the patient and the diagnostic center and between other health personnel.
-
3.
Rationally select and use the PPE.
During and after the CN studies, the clinical neurophysiologist should follow the same principles as the technical CN staff, described in Section 11.
13. Hygiene policy for work areas, medical equipment and supplies
SARS-CoV2 is an RNA virus that is wrapped with a fragile external lipid membrane. In general, wrapped viruses are less stable in the environment and are more susceptible to oxidizers such as chlorine. Persistence of coronaviruses on surfaces shows great variability, ranging from about 2 hours to 9 days. Survival time depends on different factors such as surface type, temperature, relative humidity and the specific strain of the virus (World Health Organization, 2020d). The SARS-CoV2 could be eliminated within 1 minute using common disinfectants such as 70% ethanol or sodium hypochlorite. COVID-19 has not yet been detected in drinking water supplies and, based on current evidence, the risk of infection from water supplies is low (World Health Organization, 2020d).
According to the U.S. CDC, sterilization is the process that destroys or eliminates all microscopic life forms and is performed in hospitals or clinics using physical or chemical methods, e.g. dry heat, ethylene oxide gas, steam under pressure, hydrogen peroxide plasma and liquid chemicals. Disinfection is the process that eliminates most or all microorganisms, except bacterial spores on inanimate objects, these are eliminated in health facilities by using chemical liquids or wet pasteurization. Cleaning is the removal of visible dirt (e.g. organic and inorganic material) from objects and surfaces and is usually done manually or mechanically using water with detergents or enzymatic products (Centers for Disease Control and Prevention, 2016).
Manufacturer policies, hygiene and hazardous waste management policies of national institutions and regulations on the use or reuse of diagnostic inputs and equipment are recommended. E.g., use of EEG electrode sets or disposable EMG needles.
14. Environmental disinfection, operating room and areas of clinical neurophysiology study
Evidence-based strategies for attenuation of environmental contamination involve a combination of deep cleaning with surface disinfectants and ultraviolet (UV) light. UV radiation has proven to reduce intra-hospital bacterial and viral contamination of both surfaces and air (Fraise et al., 2012).
In general, cleaning and disinfection measures should be implemented on any surface that had contact with the patient. Hospital grade cleaning agents are recommended (Table 2 ) and it is suggested that the bathrooms be cleaned at least twice a day and when needed. Dirty surfaces should first be cleaned with a detergent and then applied to the hospital grade disinfectant, in accordance with the manufacturer's recommendations for volume and contact time. After contact time has elapsed, the disinfectant is rinsed with clean water. SARS-CoV2 will be inactivated after 5 minutes of contact with household laundry disinfectants (World Health Organization, 2020d).
Table 2.
Survey of medical grade disinfectants. Severe acute respiratory syndrome coronavirus 2 is sensible to all this medical grade disinfectants (Acosta-Gnass and Stempliuk, 2008), considering that it is a RNA virus with an external lipid membrane.
| Compound | Concentration | Level of Disinfection | B | VL | VH | M | H | E | Mechanism of Action |
|---|---|---|---|---|---|---|---|---|---|
| Chlorine | 100 ppm | Intermediate/low | + | + | + | + | + | IE, DP, IAN | |
| Iodine | 30–50 ppm | Intermediate | + | + | + | + | + | − | RP |
| − | − | ||||||||
| Hydrogen Peroxide | 3–25% | Intermediate | + | + | − | + | + | − | ROH |
| Alcohols | 60–95% | Intermediate | + | + | − | + | + | − | DP |
| Phenols | 0.4–5% | Intermediate /low | + | + | + | − | + | − | IE |
| − | − | ||||||||
| Quaternary Ammonias | 0.4–1.6% | Low | + | + | − | − | + | − | IE, DP |
| − | |||||||||
| Peracetic Acid | 0.001–0.2% | Alto | + | + | + | + | + | + | Oxidant |
| Chlorhexidine | 0.05% | Low | + | + | + | − | + | − | Cytoplasmic |
| − | |||||||||
| Glutaraldehyde | 2% | Chemical Sterilizing | + | + | + | + | + | + | Alkylation of DNA, RNA |
Note: B-bacteria, VL-lipophilic viruses, VH-hydrophilic viruses, M-mycobacteria, H-fungal, E- spores, IE-enzymatic inactivation, DP-denaturation of proteins, IAN-inactivation of nucleic acids.
Cleaning staff should use appropriate PPE. See Table 1 (World Health Organization, 2020d). Health risks associated with the use of germicides in hospital spaces range from irritation of mucous membranes to death (Chataigner, et al., 1991, Hess et al., 1991).
15. Policy for disinfection of the neuro-diagnostic equipment
Neuro-diagnostic equipment
Applies to neuro-diagnostic equipment used for confirmed COVID-19 patients and suspected cases in respiratory isolation (American Clinical Neurophysiology Society, 2020b).
General recommendations:
-
1.
In hospitals, the disinfection should be performed preferably in a separate area close to the area where COVID-19 patients are treated.
-
2.
In outpatient or intrahospital specialized clinics, the procedures should be performed at that site.
Select and wear the appropriate PPE (Table 1) (American Clinical Neurophysiology Society, 2020b). For electronic devices such as cell phones, tablets, touch screens, remote controls, and keyboards, eliminate visible contamination if present.
-
1.
Follow the manufacturer's instructions for all cleaning and disinfection products.
-
2.
If no manufacturer guidance is available, consider using alcohol-based wipes or sprays containing at least 70% alcohol to disinfect touchscreens. Dry surfaces thoroughly to prevent fluid build-up (Centers for Disease Control and Prevention, 2020b).
-
3.
Consider using washable cases for electronic devices.
The use of automated robots with ultraviolet C (UV) light are an alternative for disinfecting neuro-diagnostic devices, diagnostic areas and corridors, however, they are expensive (Ackerman, 2020).
In general, the management of laundry and medical consumable waste should also be performed in accordance with hospital or clinic routine procedures (Raymond Y.W. Chinn, MD, HICPAC Advisor, Sharp Memorial Hospital, San Diego, California) (Centers for Disease Control and Prevention, 2020d).
Author statement
The authors put their efforts to check that the present information is completed and updated, assuming the responsibility for this work. All authors participated in a meaningful way in the preparation of the manuscript.
Declaration of Competing Interest
None of the authors have potential conflicts of interest related to this work.
References
- Abu-Rustum R.S. ISUOG Consensus Statement on organization of routine and specialist obstetric ultrasound services in the context of COVID-19. Ultrasound Obstet Gynecol. 2020 doi: 10.1002/uog.22029. [DOI] [PubMed] [Google Scholar]
- Ackerman E. Autonomous robots are helping kill coronavirus in hospitals – IEEE spectrum; 2020. Available at: https://spectrum.ieee.org/automaton/robotics/medical-robots/autonomous-robots-are-helping-kill-coronavirus-in-hospitals [accessed: 4 April 2020].
- Acosta-Gnass S., Stempliuk V. Manual de esterilización para centros de salud. Organización Panamericana de la Salud. 2008 [Google Scholar]
- American Academy of Sleep Medicine. COVID-19: FAQs for sleep medicine clinicians and sleep facilities | AASM; 2020. Available at: https://aasm.org/covid-19-resources/covid-19-faq [accessed: 4 April 2020].
- American Clinical Neurophysiology Society. COVID-19 resources, April 2, 2020; 2020a. Available at: https://www.acns.org/practice/covid-19-resources [accessed: 2 April 2020].
- American Clinical Neurophysiology Society. PPE and equipment disinfection procedures for patients on respiratory isolation precautions * EEG Staff *; 2020b. Available at: https://www.acns.org/UserFiles/file/UMarylandEEGMachineCleaningProcedures_COVID19_draft.pdf [accessed: 2 April 2020].
- Baig A.M. Evidence of the COVID-19 Virus Targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Burke J.F. Letter: The coronavirus disease 2019 global pandemic: a neurosurgical treatment algorithm. Neurosurgery. 2020 doi: 10.1093/neuros/nyaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canelli R. Barrier enclosure during endotracheal intubation. N Engl J Med. 2020 doi: 10.1056/NEJMc2007589. NEJMc2007589. [DOI] [PubMed] [Google Scholar]
- CDC. Cleaning and disinfection for households interim recommendations for U.S. households with suspected or confirmed coronavirus disease 2019 (COVID-19); 2020.
- Centers for Disease Control and Prevention. Sterilizing practices | disinfection & sterilization guidelines | guidelines library | infection control | CDC; 2016. Available at: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/introduction.html [accessed: 4 April 2020].
- Centers for Disease Control and Prevention. CDC – Recommended guidance for extended use and limited reuse of N95 filtering facepiece respirators in healthcare settings – NIOSH workplace safety and health topic, U.S. Department of Health & Human Services; 2020a. Available at: https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html [accessed: 12 April 2020].
- Centers for Disease Control and Prevention. Infection control: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), vol. 2; 2020b. pp. 2–4. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html [accessed: 3 April 2020].
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. Center for Disease Control and Prevention; 2020c. Available at: https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html [accessed: 22 April 2020].
- Centers for Disease Control and Prevention. Laundry | Background | Environmental Guidelines | Guidelines Library | Infection Control | CDC, Guidelines for Environmental Infection Control in Health-Care Facilities; 2020d. Available at: https://www.cdc.gov/infectioncontrol/guidelines/environmental/index.html [accessed: 4 April 2020].
- Chataigner D et al. ‘Acute accidental poisoning with a hospital disinfectant: 45 cases, 13 deaths. Press Med 1991;20:741–43. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1828591 [accessed: 4 April 2020]. [PubMed]
- Desforges M. Human coronaviruses: Viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East M, Surveillance G. (Home care for patients with COVID-19 presenting with mild symptoms and management of their contacts. March; 2020. p. 17–20. Available at: https://www.who.int/publications-detail/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts [accessed: 3 April 2020].
- Federación Latinoamericana de Sociedades del Sueño. Documento consenso de la Federación Latinoamericana de Sociedades del Sueño – FLASS – Federación Latinoamericana de Sociedades de Sueño; 2020. Available at: https://fedelass.com/documento-consenso-de-la-federacion-latinoamericana-de-sociedades-del-sueno/ [accessed: 4 April 2020].
- Fogang Y. Yield of repeated intermittent EEG for seizure detection in critically ill adults. Neurophysiol Clin. 2017;47:5–12. doi: 10.1016/j.neucli.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Fraise A.P., Maillard J.Y., Sattar S.A. Wiley-Blackwell; 2012. Russell, Hugo & Ayliffe’s: Principles and Practice of Disinfection, Preservation and Sterilization, Russell, Hugo & Ayliffe’s: Principles and Practice of Disinfection, Preservation and Sterilization. 10.1002/9781118425831. [Google Scholar]
- Galea S., Merchant R.M., Lurie N. The mental health consequences of COVID-19 and physical distancing. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.1562. [DOI] [PubMed] [Google Scholar]
- Galloway G.M. Intraoperative monitoring: Do you know where your neurophysiologist is? J Clin Neurophysiol. 2013;30:621–622. doi: 10.1097/01.wnp.0000436886.26090.6d. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A.E. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. NEJMoa2002032NEJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JA et al. Epidermal toxicity of disinfectants. Am J Dent 1991;4:51–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1900694 [accessed: 4 April 2020]. [PubMed]
- Lai J. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3:e203976. doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID- 19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, Xuzhou, China. Emerg. Infect. Dis. 2020;26(7) doi: 10.3201/eid2607.200718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y et al. Acute cerebrovascular disease following COVID-19: q single center, retrospective, observational study (3/3/2020); 2020. Available at SSRN: https://ssrn.com/abstract=3550025 or 10.2139/ssrn.3550025. [DOI] [PMC free article] [PubMed]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T. Handbook of COVID-19 prevention and treatment. Handbook of Covid-19, prevention and treatment; 2020. p. 68. Available at: https://www.alnap.org/help-library/handbook-of-covid-19-prevention-and-treatment [accessed: 12 April 2020].
- Mao L. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minder R, Peltier E. Virus knocks thousands of health workers out of action in Europe. The New York Times; 2020. Available at: https://www.nytimes.com/2020/03/24/world/europe/coronavirus-europe-covid-19.html [accessed: 12 April 2020].
- Moss M. Screening for critical illness polyneuromyopathy with single nerve conduction studies. Intensive Care Med. 2014;40:683–690. doi: 10.1007/s00134-014-3251-6. [DOI] [PubMed] [Google Scholar]
- Ng K. COVID-19 and the risk to health care workers: a case report. Ann Intern Med. 2020 doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurses IC. International Council of Nurses 2020. High proportion of healthcare workers with COVID19; 2020.
- Oh S.J. Repetitive nerve stimulation test in myasthenic crisis. Muscle Nerve. 2019;59:544–548. doi: 10.1002/mus.26390. [DOI] [PubMed] [Google Scholar]
- Pleasure S.J., Green A.J., Josephson S.A. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection neurologists move to the frontlines. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1065. [DOI] [PubMed] [Google Scholar]
- Ran L. Risk factors of healthcare workers with corona virus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D.I. Needle electromyography: Basic concepts and patterns of abnormalities. Neurol Clin. 2012;30:429–456. doi: 10.1016/j.ncl.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Sánchez Fernández I. Time to electroencephalography is independently associated with outcome in critically ill neonates and children. Epilepsia. 2017;58:420–428. doi: 10.1111/epi.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner S. Medical error avoidance in intraoperative neurophysiological monitoring: The communication imperative. J Clin Neurophysiol. 2017;34:477–483. doi: 10.1097/WNP.0000000000000419. [DOI] [PubMed] [Google Scholar]
- Sorbello M., El-Boghdadly K., Di Giacinto I., Cataldo R., Esposito C., Falcetta S., Merli G., Cortese G., Corso R.M., Bressan F., Pintaudi S., Greif R., Donati A., Petrini F. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020 doi: 10.1111/anae.15049. [DOI] [PubMed] [Google Scholar]
- Sun T., Guan J. Novel coronavirus and central nervous system. Eur J Neurol. 2020 doi: 10.1111/ene.14227. [DOI] [PubMed] [Google Scholar]
- Verity R. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Advice on the use of masks in the community, during home care and in healthcare settings in the context of the novel coronavirus (2019-nCoV) outbreak. WHO; 2020a. p. 1–2. Available at: https://www.who.int/publications-detail/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak [accessed: 3 April 2020].
- World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19]. Interim guidance. 27 February 2020; 2020b. p. 1–7. Available at: https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf [accessed: 3 April 2020].
- World Health Organization. Situation Report-75 HIGHLIGHTS; 2020c. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200404-sitrep-75-covid-19.pdf?sfvrsn=99251b2b_2 [accessed: 4 April 2020].
- World Health Organization. Water, sanitation , hygiene and waste management for COVID-19, March, 2020d. p. 1–9. doi: 10.1056/NEJMoa2001191.7.
- World Health Organization. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February; 2020e. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 [accessed: 2 April 2020].
- World Health Organization. Coronavirus Disease (Covid-19) outbreak : rights, roles and responsibilities of health workers, including key considerations for occupational safety. World Health Organization (WHO), December; 2019. p. 1–3. Available at: https://www.who.int/publications-detail/coronavirus-disease-(covid-19)-outbreak-rights-roles-and-responsibilities-of-health-workers-including-key-considerations-for-occupational-safety-and-health [accessed: 20 April 2020].
- Yi Y. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon M. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med. 2017;43:29–38. doi: 10.1007/s00134-016-4524-z. [DOI] [PubMed] [Google Scholar]
- Zhou P. Protecting Chinese Healthcare Workers While Combating the 2019 Novel Coronavirus. Infect Control Hosp Epidemiol. 2020:1–4. doi: 10.1017/ice.2020.60. [DOI] [PMC free article] [PubMed] [Google Scholar]