1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome (SARS) which first appeared in Wuhan, China, December 2019, and has since spread globally [1]. To date, official figures released by World Health Organization (WHO), indicate over 3 million cases worldwide with over 200,000 deaths. However, the number of newly diagnosed patients has started to decline, suggesting that the rate of transmission is beginning to be controlled by countries [2]. Although most patients have mild symptoms and good prognosis after infection, some develop severe symptoms and die due to multiple organ failure [[3], [4], [5]]. Based on the published literature and clinical observations, researchers and clinicians around the world have postulated about the pathogenesis of this viral infection in humans. We know that the virus has the capacity to cross mucous membranes (especially nasal and larynx mucosa), and in severe cases leads to multiple systemic manifestations and pneumonia requiring mechanical ventilation [6].
Interestingly, COVID-19 disease prognosis is strongly correlated to clinical characteristics of patients, with high risk associated to those with a concomitance of cardiovascular risk factors, primarily obesity, hypertension, and diabetes mellitus [4,7]. In the context of COVID-19-associated cardiovascular manifestations, more recent studies report that the disease is commonly complicated with coagulopathy linked to disseminated intravascular coagulation (DIC) and/or thrombotic and thromboembolic disease [4,8,9]. For these reasons, many patients with severe COVID-19 meet the Third International Consensus Definitions for Sepsis (SEPSIS-3) [10].
Moreover, we must consider that the development of thrombotic and thromboembolic disease could be a direct consequence of the systemic inflammatory process related to interleukin (IL)-6 and IL-17A up-regulation [[11], [12], [13], [14]]. This clinical scenario has prompted the use of intravenous immunoglobulin (IVIG) and low molecular weight heparin (LMWH) anticoagulant therapy as early as possible, particularly when circulating T and B cells numbers decrease, and inflammatory cytokines and D-Dimer (a non-specific parameter of thrombi formation) increase abnormally [[15], [16], [17]]. While IVIG has shown efficacy in the treatment of patients with influenza and SARS, more clinical data is required, for both IVIG and LMWH, to confirm significant efficacy in COVID-19 patients [[18], [19], [20]].
2. IL-17A and COVID-19-related thrombotic and vascular mechanisms
Viral infection and subsequent systemic and/or local inflammation is a common cause of DIC [[21], [22], [23]] due to increased synthesis of cytokines such as as tumor necrosis factor-α (TNF-α), IL-1β, IL-6, IL-17A and IL-18 [24].
IL-17A (commonly known as IL-17) is the most studied member of the IL-17 cytokine family. It is produced by T-helper (Th)-17 lymphocytes, and by innate cellular components [25,26]. This “unique” pro-inflammatory cytokine, highly produced and modulated in patients with chronic inflammatory-based diseases, also plays a role in the cardiovascular system, more specifically, it is involved in cardiovascular complications associated with autoimmune and inflammatory-based diseases [27].
Indeed, in the attempt to find a link between inflammatory markers and endothelial dysfunction, Marder et al., [28] demonstrated that elevated IL-17A levels strongly correlated with vascular dysfunction in subjects affected by rheumatoid arthritis. Furthermore, it has been shown that, human umbilical vein endothelial cells (HUVECs), treated with IL-17A, synergistically with TNF-α, induces tissue factor (TF) expression and modulates thrombomodulin [29] and thrombosis formation [30,31].
In addition to IL-17A role on the vascular endothelium, data, from our research group and others has also highlighted a role for this cytokine in platelet biology. We previously reported IL-17A ability to increase, in both mouse and human, platelet activation [32] and to modulate, in vivo, arterial thrombus formation [33] through the extracellular signal-regulated kinase-2 (ERK-2) signaling pathway [34]. Moreover, a study from Ding et al., [35] investigated the role of IL-17A in mouse and human deep vein thrombosis (DVT) formation, and found that this cytokine promotes DVT pathogenesis by enhancing platelet activation/aggregation, neutrophil infiltration, and endothelial cell (EC) activation. Collectively, these data suggest that the use of an anti-IL-17A neutralising monoclonal antibody may be useful for DVT-related syndromes.
3. Discussion
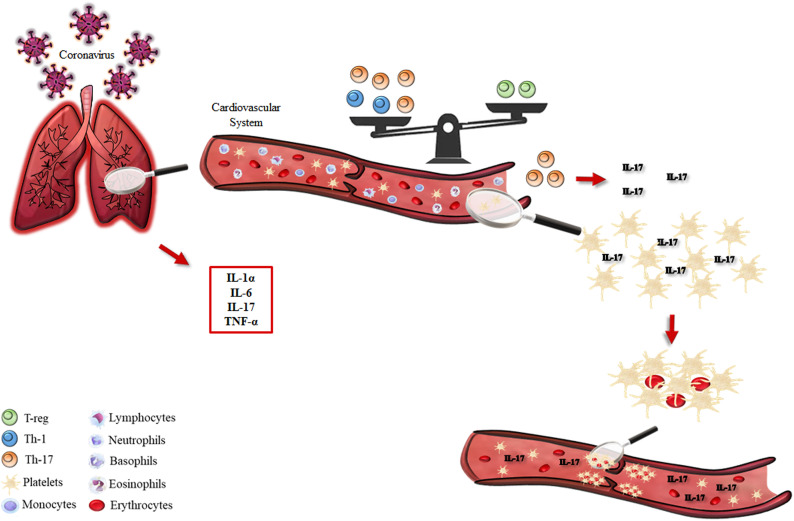
Taking all these various pieces of evidence (albeit minimal), we would like to hypothesize that in COVID-19 patients, IL-17A could potentially promote a pro-thrombotic state in the vascular system. Indeed, the increased level of this cytokine in COVID-19 infection would not be, per se, a stimulus for thrombogenesis but, most likely, support platelet aggregate formation at sites of vascular injury (Fig. 1 ). Based on these assumptions, it would be fascinating to characterize IL-17 levels in bronchoalveolar lavage fluid (BALF) and plasma/serum samples of mild- and severe-infected COVID-19 patients, and potentially go on to test the efficacy of antibodies targeting IL-17A (alone or in a sequential therapy with anti-IL-6 agents) for the treatment of thrombotic, as well respiratory and systemic manifestations of severe COVID-19. These could be useful not only for new therapeutic strategies but also for improving our understanding of the etiopathogenesis and genetic susceptibility of COVID-19 infection.
Fig. 1.
Schematic representation of inflammatory pathways (left part) involved in the COVID-19-related respiratory syndrome. The inflammatory scenario induced by COVID-19 has cardiovascular implications (right part, top panel) in terms of Th-1/Th-17/T-reg balance that favors the production of IL-17A. The overproduction of this cytokine (right part, bottom panel) amplifies platelet hyper-reactivity and thrombus formation.
Author contributions
FR, AAM, GMC and AS drafted the manuscript. FC, RS, NM, AJI and FM wrote and revised the manuscript. All Authors gave final approval to the publication.
Declaration of Competing Interest
This article has been conducted and written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was in part supported by MIUR (PRIN 2017; 2017A95NCJ/2017A95NCJ_002, “Stolen molecules - Stealing natural products from the depot and reselling them as new drug candidates”). AJI is supported by Birmingham Fellowship and AAM by a Saudi Government/KKU scholarship.
Contributor Information
Asif Jilani Iqbal, Email: A.J.Iqbal@bham.ac.uk.
Francesco Maione, Email: francesco.maione@unina.it.
References
- 1.Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S. Exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;104761 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Coronavirus disease 2019 (COVID-19) Situation Report – 73. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200402-sitrep-73-covid-19.pdf?sfvrsn=5ae25bc7_4; [Accessed 14 April 2020]
- 3.Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020:12. doi: 10.1038/s41368-020-0075-9. 10.1038%2Fs41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;102538 doi: 10.1016/j.autrev.2020.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivellese F., Prediletto E. ACE2 at the Centre of COVID-19 from paucisymptomatic infections to severe pneumonia. Autoimmun Rev. 2020;102536 doi: 10.1016/j.autrev.2020.102536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395(10229):1054–62. doi: 10.1016/s0140-6736(20)30566-3 Epub 2020 Mar 11. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. Lancet. 2020 Mar 28;395(10229):1038. [DOI] [PMC free article] [PubMed]
- 8.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M. The third international consensus definitions for Sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caso F., Costa L., Ruscitti P., Navarini L., Del Puente A., Giacomelli R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;102537 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y., Yu M., Zhu F., Zhang S., Ding P., Wang M. IL-9 promotes the development of deep venous thrombosis by facilitating platelet function. Thromb Haemost. 2018;118(11):1885–1894. doi: 10.1055/s-0038-1673614. [DOI] [PubMed] [Google Scholar]
- 14.Casillo G.M., Mansour A.A., Raucci F., Saviano A., Mascolo N., Iqbal A.J. Could IL-17 represent a new therapeutic target for the treatment and/or management of COVID-19-related respiratory syndrome? This paper is dedicated to Sofia Maione born during COVID-19 outbreak. Pharmacol Res. 2020;104791 doi: 10.1016/j.phrs.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020 doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alger H.M., Williams Iv J.H., Walchok J.G., Bolles M.M., Fonarow G.C., Rutan C. The role of data registries in the time of COVID-19. Circ Cardiovasc Qual Outcomes. 2020 doi: 10.1161/circoutcomes.120.006766. [DOI] [PubMed] [Google Scholar]
- 17.Atri D., Siddiqi H.K., Lang J., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci. 2020 doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M. Hematological findings and complications of COVID-19. Am J Hematol. 2020 doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samad F., Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122(20):3415–3422. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood 2014; 123(18):2759–67. doi:10.1182%2Fblood-2013-11-462432. [DOI] [PMC free article] [PubMed]
- 23.Jackson S.P., Darbousset R., Schoenwaelder S.M. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133(9):906–918. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 24.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*). Annu. Rev. Immunol. 2009; 27:621–68. doi:10.1146%2Fannurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed]
- 25.D’Acquisto F., Maione F., Pederzoli-Ribeil M. From IL-15 to IL-33: the never-ending list of new players in inflammation. Is it time to forget the humble aspirin and move ahead? Biochem Pharmacol. 2010;79(4):525–534. doi: 10.1016/j.bcp.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Iwakura Y., Ishigame H., Saijo S., Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Maione F. Commentary: IL-17 in chronic inflammation: from discovery to targeting. Front Pharmacol. 2016;7:250. doi: 10.3389/fphar.2016.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marde W, Khalatbari S, Myles JD, Hench R, Yalavarthi S, Lustig S, et al. 17 as a novel predictor of vascular function in rheumatoid arthritis. Ann. Rheum. Dis. 2011; 70(9):1550–5. doi:10.1136%2Fard.2010.148031. [DOI] [PMC free article] [PubMed]
- 29.Hot A., Lenief V., Miossec P. Combination of IL-17 and TNFα induces a pro-inflammatory, pro-coagulant and pro-thrombotic phenotype in human endothelial cells. Ann Rheum Dis. 2012;71(5):768–776. doi: 10.1136/annrheumdis-2011-200468. [DOI] [PubMed] [Google Scholar]
- 30.Bouchnita A., Miossec P., Tosenberger A., Volpert V. Modeling of the effects of IL-17 and TNF-α on endothelial cells and thrombus growth. C R Biol. 2017;340(11−12):456–473. doi: 10.1016/j.crvi.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 31.de Boer O.J., Li X., Teeling P., Mackaay C., Ploegmakers H.J., van der Loos C.M. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb Haemost. 2013;109(2):290–297. doi: 10.1160/th12-06-0425. [DOI] [PubMed] [Google Scholar]
- 32.Maione F., Cicala C., Liverani E., Mascolo N., Perretti M., D’Acquisto F. IL-17A increases ADP-induced platelet aggregation. Biochem Biophys Res Commun. 2011;408(4):658–662. doi: 10.1016/j.bbrc.2011.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maione F., Parisi A., Caiazzo E., Morello S., D’Acquisto F., Mascolo N. Interleukin-17A exacerbates ferric chloride-induced arterial thrombosis in rat carotid artery. Int J Inflam. 2014 doi: 10.1155/2014/247503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S., Yuan J., Yu M., Fan H., Guo Z.Q., Yang R. IL-17A facilitates platelet function through the ERK2 signaling pathway in patients with acute coronary syndrome. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding P., Zhang S., Yu M., Feng Y., Long Q., Yang H. IL-17A promotes the formation of deep vein thrombosis in a mouse model. Int Immunopharmacol. 2018;57:132–138. doi: 10.1016/j.intimp.2018.02.006. [DOI] [PubMed] [Google Scholar]