Abstract
The coronavirus disease 2019 pandemic is wreaking havoc on society, especially health-care systems, including disrupting bariatric and metabolic surgery. The current limitations on accessibility to non-urgent care undermine postoperative monitoring of patients who have undergone such operations. Furthermore, like most elective surgery, new bariatric and metabolic procedures are being postponed worldwide during the pandemic. When the outbreak abates, a backlog of people seeking these operations will exist. Hence, surgical candidates face prolonged delays of beneficial treatment. Because of the progressive nature of obesity and diabetes, delaying surgery increases risks for morbidity and mortality, thus requiring strategies to mitigate harm. The risk of harm, however, varies among patients, depending on the type and severity of their comorbidities. A triaging strategy is therefore needed. The traditional weight-centric patient-selection criteria do not favour cases based on actual clinical needs. In this Personal View, experts from the Diabetes Surgery Summit consensus conference series provide guidance for the management of patients while surgery is delayed and for postoperative surveillance. We also offer a strategy to prioritise bariatric and metabolic surgery candidates on the basis of the diseases that are most likely to be ameliorated postoperatively. Although our system will be particularly germane in the immediate future, it also provides a framework for long-term clinically meaningful prioritisation.
Introduction
Bariatric surgery has been used for decades to treat patients with severe obesity. In 2016, global guidelines established through the Diabetes Surgery Summit (DSS), an international consensus conference series, formally recognised gastrointestinal surgery as a standard therapy for type 2 diabetes; this practice is known as metabolic surgery.1 During the coronavirus disease 2019 (COVID-19) outbreak, under unprecedented pressure to free up inpatient capacity, and because of intraoperative risks for viral contagion among patients and staff, hospitals worldwide have been obliged to postpone most elective operations, including bariatric and metabolic surgery. Increased hazards of severe COVID-19 complications in patients with obesity, type 2 diabetes, or both,2, 3, 4, 5 further support the rationale for a pause in elective surgery during the peak of the pandemic.
The return to normal services will be gradual, with surgeons competing for reduced capacity to address a backlog of elective procedures. Hence, access to bariatric and metabolic surgery will continue to be constrained. Given the uncertainty regarding the effects and duration of the COVID-19 outbreak, combined with the progressive nature of obesity, diabetes, and related conditions, delaying bariatric and metabolic surgery could increase the risks for morbidity and mortality in surgical candidates. The risk of harm, however, is variable among individuals, depending on the type and severity of disease and their indications for bariatric and metabolic surgery. The traditional, weight-centric criteria for patient selection in bariatric surgery, which are still commonly used today, do not reflect severity of disease,6 and they therefore cannot be used to prioritise treatment based on actual clinical needs. Furthermore, physical distancing policies and continued lockdowns might limit adherence to lifestyle interventions, worsening metabolic deterioration among candidates for bariatric and metabolic surgery. Additionally, reduced access to non-urgent care during the COVID-19 pandemic might impede postoperative monitoring for potential surgical and nutritional complications.
A clear and urgent need therefore exists for strategies to mitigate harm to patients during and after the COVID-19 pandemic. These approaches should include non-surgical interventions to optimise metabolic and weight control in patients awaiting surgery, telemedicine protocols for postoperative surveillance, and use of appropriate criteria to triage surgical candidates during a foreseeable period of reduced capacity for elective surgery. To address these issues, the DSS1 organisers directed a group of international experts to assess the effect of the COVID-19 pandemic on candidates for surgical treatment of obesity and type 2 diabetes. Our specific aim was to develop criteria to help prioritise bariatric and metabolic surgery for when elective surgery is resumed and beyond.
Elective surgery: definitions and prioritisation
Surgery ameliorates a wide range of conditions and diseases, both acute and chronic. Emergency surgery is required when acute problems pose immediate threat to life, organs, or limbs, and must be done without delay. Elective surgery refers to operations that can be planned and scheduled in advance. These procedures, however, are not optional, because they can have important, life-changing implications. When access to elective surgery is reduced, doctors should prioritise patients with the greatest need or with a greater risk of harm from delayed treatment. In some health-care systems, elective surgery is categorised into urgent, semi-urgent, or non-urgent.7, 8 Urgent elective surgery is required within 30 days for conditions that might deteriorate quickly. Semi-urgent conditions are those that, although not likely to deteriorate quickly, could reasonably cause severe pain or dysfunction or further harm if delayed beyond 3 months. Non-urgent elective surgery is planned for conditions that are unlikely to cause substantial discomfort, dysfunction, or harm if treated within 1 year.
Although some complications from bariatric and metabolic operations can require emergency surgical treatment (eg, haemorrhage, leak, or intestinal obstruction), most bariatric and metabolic procedures represent genuine elective surgery. To date, however, no consensus exists for criteria to identify urgent, semi-urgent, or non-urgent indications in bariatric and metabolic surgery on the basis of the type and severity of patients' conditions.
Delaying elective surgery during the peak of the COVID-19 pandemic
There are many reasons why most bariatric and metabolic operations should be suspended during the most intense phase of the COVID-19 pandemic, including infection risks among patients and staff, factors inherent to the operations, and increased hazards of severe COVID-19 complications among patients with obesity or type 2 diabetes.
Procedure-related risks
Laparoscopic surgery involves aerosol-generating techniques such as carbon dioxide, pneumoperitoneum, electrocautery, and ultrasonic shearing. These techniques could easily increase the risk of viral contagion for staff,9, 10 including with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Upper gastrointestinal endoscopy (another aerosol-producing procedure) is also commonly done before bariatric and metabolic surgery. Patients undergoing major surgery are at risk of life-threatening inflammatory complications such as infection (including from viruses), the systemic inflammatory response syndrome, and sepsis.11
Although there is no conclusive evidence that laparoscopy or upper endoscopy can promote COVID-19 transmission, postponing elective metabolic and bariatric interventions during the acute phase of the COVID-19 outbreak seems sensible, except for urgent revisional surgery or emergency endoscopic interventions for complications (eg, haemorrhage, stoma stenosis, or leaks).
Despite the potential for a higher risk of contagion, the laparoscopic approach in bariatric and metabolic surgery is associated with substantial benefits compared with traditional open surgery, especially in patients with severe obesity. These benefits include lower rates of mortality and complications (including pulmonary and procedural), and shorter hospital stays.12, 13 For these reasons, laparoscopic access should remain the preferred approach over open techniques when elective bariatric and metabolic surgery resumes. Appropriate personal protective equipment should be used, however, given the increased risk of SARS-CoV-2 infection for staff.
Risks of severe COVID-19 complications in obesity and type 2 diabetes
Obesity increases the risk of complications from viral respiratory infections. During the 2009 influenza H1N1 pandemic in California, 91% of people who died had obesity, and higher BMI was associated with mortality.14 In patients admitted to intensive care for SARS-CoV-2, class 2–3 obesity (BMI >35 kg/m2) is an independent risk factor for disease severity.5 Similarly, patients with diabetes have augmented risk for severe COVID-19 and mortality.2, 3, 4, 5
Several mechanisms have been suggested to increase the risk of complications from viral infections in obesity and type 2 diabetes, including low-grade chronic inflammation with overproduction of proinflammatory cytokines, reduced natural killer cell number and activity, and impaired antigen-stimulation responses.15, 16, 17 Another factor that might have a role in the relationship between obesity, diabetes, and increased risk for complications is that SARS-CoV-2 enters host cells by binding to the angiotensin-converting-enzyme 2 (ACE2) receptor. ACE2 transforms angiotensin 2 to angiotensin,14, 15, 16, 17, 18, 19, 20 thereby reducing vasoconstriction, sodium retention, inflammation, and metabolic degeneration.21 Chronic hyperglycaemia downregulates ACE2 expression,22 and further reduction of ACE2 during COVID-19 infection could contribute to hyperinflammation and respiratory failure in patients with type 2 diabetes.23 People with obesity are also prone to hypoventilation syndrome, cardiovascular disease,24 heart failure,25 and other conditions that could increase the risk of COVID-19 mortality.
When elective bariatric and metabolic surgery resumes, the pandemic will be contained, but SARS-CoV-2 will probably still circulate in the population. Given the risks of severe complications from COVID-19 in patients with obesity and type 2 diabetes, we recommend that COVID-19 screening should be mandatory preoperatively for patients considering bariatric and metabolic surgery.
Risk of disease progression from delayed operations
Class 2–3 obesity and type 2 diabetes, the most common indications for bariatric and metabolic surgery, are associated with reduced quality of life and increased morbidity and mortality. Their ability to cause life-threatening complications, however, varies depending on the severity or stage of disease and the burden of comorbidities. The degree of harm from delaying metabolic and bariatric surgery depends on each patient's condition, the surgical efficacy at different stages of disease, and the availability and effectiveness of non-surgical therapies to control disease progression while awaiting surgery. Understanding the prognostic factors of morbidity and mortality in obesity and type 2 diabetes can help to define criteria for surgical prioritisation.
Prognosis and prognostic factors of type 2 diabetes
Diabetes is a major cause of morbidity and death, including from cardiovascular, renal, neurological, and retinal complications. Approximately two-thirds of people with diabetes die of cardiovascular disease, with a relative risk 1·8–2·6 times greater than in people without diabetes.26 The biological progression of type 2 diabetes, characterised by declining β-cell function and continuing insulin resistance, is manifested clinically by deteriorations in multiple parameters, including HbA1c, fasting, and postprandial glucose levels. The UK prospective diabetes study27 reported significant associations between hyperglycaemia and development of diabetes complications or death, and a 21% risk reduction for any diabetes-related endpoint with each 1% absolute HbA1c reduction.
Factors beyond hyperglycaemia can also influence type 2 diabetes prognosis. In the TRIAD study,28, 29 predictors of all-cause mortality at 4 years and 8 years of study follow-up included older age, male sex, non-Hispanic white race, lower education and income, longer duration of diabetes, lower BMI, hypertension, macrovascular disease, retinopathy, nephropathy, and neuropathy. Among the specific predictors of cardiovascular mortality were also treatment with insulin (with or without oral medication), higher LDL cholesterol, history of nephropathy, transient ischaemic attack, stroke, angina, myocardial infarction, coronary artery and peripheral vascular disease, and use of antihypertensive or cholesterol-lowering medications.
Factors predicting obesity-related morbidity and mortality
Obesity increases the risks of many other illnesses, including diabetes, hypertension, dyslipidaemia, liver disease, coronary artery and cerebrovascular disease, many cancers, cholelithiasis, infertility, psychosocial dysfunction, osteoarthritis, chronic kidney disease, and now also COVID-19. Together, these complications powerfully reduce quality of life and exacerbate obesity-associated mortality. Even before COVID-19, obesity reduced life expectancy by 5–20 years.30 Notably, higher all-cause mortality is associated with obesity class 2 (BMI 35–39·9 kg/m2) and 3 (BMI ≥40 kg/m2), corresponding to candidates for bariatric surgery, but not with class 1 obesity (BMI 30–34·9 kg/m2).31
Obesity hypoventilation syndrome and obesity-associated heart failure substantially increase mortality. Obesity hypoventilation syndrome represents the combination of obesity and chronic daytime hypercapnia.32, 33 The prevalence of obesity hypoventilation syndrome is highest among patients with a BMI of more than 50 kg/m2.34 Mortality from untreated obesity hypoventilation syndrome can be as high as 24% at 1·5–2 years after diagnosis.35 Obesity heart failure is associated with increased mortality, and for each 5-unit increase in BMI, heart failure-related mortality increases by 1·4 times.36
Since BMI alone does not reflect obesity-related mortality and morbidity, staging systems such as the King's Obesity Criteria37 and Edmonton Obesity Staging System (EOSS)38 have been developed to assess individual patients' risk on the basis of evidence of subclinical, established, or end-stage comorbidities.39 Retrospective application of EOSS to data from the National Health and Nutrition Examination Survey showed that patients in stages 2–4 of EOSS have increased all-cause mortality compared with stages 0 or 1. This finding supports the idea that the presence, type, and severity of obesity-related complications, in addition to BMI,39 should inform decision making about the prioritisation of treatment, especially surgery.
Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease is characterised by excess hepatic fat. Its more aggressive form, non-alcoholic steatohepatitis, includes hepatocyte injury, inflammation, and fibrosis.40, 41, 42 These two conditions affect 20–25% of the western population, with rates rising worldwide.40, 43 66% of patients with obesity and diabetes have non-alcoholic fatty liver disease or non-alcoholic steatohepatitis.44, 45
Non-alcoholic steatohepatitis can lead to cirrhosis (in 15–20% of cases), liver failure, or hepatocellular carcinoma.46 Beyond liver-related mortality, non-alcoholic steatohepatitis can substantially increase microvascular and macrovascular complications, and cardiovascular mortality in patients with obesity and type 2 diabetes.40, 41, 42, 43, 47, 48 Non-randomised trials suggest that Roux-en-Y gastric bypass resolves the histological features of non-alcoholic steatohepatitis in up to 80% of patients.49, 50
Effects of bariatric and metabolic surgery
Randomised clinical trials and observational studies show that in patients with all classes of obesity, bariatric and metabolic surgery promotes greater long-term weight loss than the best available non-surgical interventions, regardless of the operation chosen.47, 51, 52, 53 Multiple observational studies also indicate that bariatric and metabolic surgery lowers long-term risk of all-cause mortality compared with matched non-surgical patients.54, 55, 56, 57, 58, 59 Data from eight observational studies involving a total of 635 642 patients suggest that bariatric and metabolic surgery is associated with a reduced risk of all types of cancer (odds ratio [OR]=0·72; 95% CI 0·59–0·87) and obesity-associated cancer (OR=0·55; 95% CI 0·31–0·96).60, 61, 62 Without exception, each of the 29 all-cause mortality studies published to date shows that patients who have bariatric and metabolic surgery live longer than matched non-surgical controls.54, 55, 56, 57, 58, 59, 63, 64
Concerning type 2 diabetes, at least 12 randomised controlled trials comparing bariatric and metabolic surgery with conventional diabetes therapies (ie, lifestyle plus medication) in patients with type 2 diabetes show that surgery is superior for control of hyperglycaemia, reduction of cardiovascular and overall mortality risk, improvement in quality of life, and reduction in risk of renal complications.1, 65, 66 The safety of bariatric and metabolic surgery compares favourably with that of most elective operations, including hysterectomy, cholecystectomy, and knee replacement. Surgical treatments for diabetes are highly cost-effective, with the cost per quality-adjusted life-year ranging between US$3200 and $13 000.1, 65, 67
Based on this evidence, DSS guidelines, which have been formally endorsed by 56 worldwide medical or scientific organisations and recognised by payers worldwide, recommend the consideration of bariatric and metabolic surgery for appropriate candidates (including those with only class 1 obesity), who do not achieve adequate glycaemic control with medical therapy.1
Health and economic costs of delaying bariatric and metabolic surgery
The delay of bariatric and metabolic surgery that is occurring due to COVID-19 will augment the burden of disease among surgical candidates. This increase will particularly affect patients with type 2 diabetes, given that metabolic surgery causes remission of hyperglycaemia in most cases.65 The likelihood of hyperglycaemia remission, however, depends upon how soon an operation is done during the natural history of diabetes. Algorithms designed to predict surgical remission (eg, DiaRem-2, AD-DiaRem, DiaBetter, and ABCD)68, 69, 70, 71 consistently show that longstanding disease is one of the most powerful indicators of failure to achieve this benefit.72 Remission rates drop off notably after 10 years of diabetes. Moreover, the SOS study73 reported substantially lower type 2 diabetes remission among patients with only 4 years of known disease than in those with 2 years of known disease. Thus, delaying metabolic surgery reduces the chances of diabetes remission.
Delayed metabolic surgery might cause even greater harm to patients with type 2 diabetes who are at higher risk of microvascular and macrovascular complications and mortality, especially when medications and lifestyle interventions are not achieving adequate metabolic control. Patients without diabetes but with severe respiratory (obesity hypoventilation syndrome), cardiac, or renal complications of obesity, and individuals for whom weight reduction is crucial to advancing time-sensitive and life-saving treatments (eg, organ transplants) also have greater risks of harm from delaying bariatric and metabolic surgery.
Patients with surgically remediable metabolic diseases, especially diabetes, incur more health-care costs per day than do those without these conditions. All studies that compared costs for 1–5 years between surgical and non-surgical patients found that pharmacy expenses decrease substantially after bariatric and metabolic surgery compared with matched non-surgical patients,74, 75, 76, 77, 78 primarily due to lower diabetes medication costs.69 Hence, metabolic surgery decreases daily health-care costs, especially for patients requiring multidrug therapy. The longer surgery is delayed for these patients, the less cost-saving it becomes.
Management of surgical candidates and postoperative follow-up in times of COVID-19
Various non-surgical options can be used to mitigate the harm from delaying bariatric and metabolic surgery and to manage patients who have had surgery (panel 1 ). Regarding the need to optimise glycaemic control in patients with type 2 diabetes, especially those with advanced microvascular or macrovascular complications,79 we considered available evidence of pharmacological strategies that promote weight loss, such as glucagon-like peptide-1 receptor agonists (GLP-1RA) or sodium/glucose cotransporter 2 (SGLT-2) inhibitors, or both.80 GLP-1RAs reduce HbA1c by about 1%81 while promoting clinically relevant weight loss.82 SGLT-2 inhibitors, however, might be contraindicated with COVID-19, because of concerns about potential subclinical vascular congestion and risk of acute metabolic decompensation associated with these drugs.83
Panel 1. Diabetes Surgery Summit recommendations for managing bariatric and metabolic surgical candidates and postoperative patients during the coronavirus disease 2019 pandemic.
Non-surgical options to mitigate harm from delaying surgery
-
•
Glycaemic control should be optimised in patients awaiting metabolic surgery for type 2 diabetes, especially for those with advanced microvascular or macrovascular complications; this is desirable to prepare for surgery and also in case of severe acute respiratory syndrome coronavirus 2 infection
-
•
In patients who do not achieve glycaemic targets with lifestyle modifications and metformin, the addition of a glucagon-like peptide-1 receptor agonist (GLP-1RA) or sodium/glucose cotransporter 2 (SGLT-2) inhibitor, or both, can advance the combined goals of improving metabolic control and causing weight loss or limiting weight gain; use of SGLT-2 inhibitors, however, is not recommended in the case of acute coronavirus disease 2019 (COVID-19) infection because of concerns about potential subclinical vascular congestion and risk of acute metabolic decompensation associated with these drugs
-
•
For patients with multiple weight-responsive comorbidities who face prolonged waiting times for surgery, dietary or pharmacological interventions for weight control might become necessary
-
•
Diets with higher protein content and lower glycaemic index can be effective and should be considered
-
•
Among patients already taking weight-loss medications, efforts should be made to continue the drug(s) until surgery is scheduled, since rapid weight regain is predictable when they are discontinued
-
•
In countries where weight-loss medications (eg, phentermine, orlistat, GLP-1RAs, naltrexone–bupropion, and phentermine–topiramate) are accessible, clinicians could consider their use when weight loss or weight maintenance is important, such as for patients with multiple weight-responsive comorbidities
Management of patients who have had surgery
-
•
Telemedicine strategies that are supervised by specialist bariatric and metabolic surgery providers should be used
-
•
In people with persistent or recurrent type 2 diabetes after surgery, weight-reducing diabetes medications (eg, GLP-1RAs) should be considered; weight maintenance should also be encouraged in patients with type 2 diabetes remission to mitigate risk of disease recurrence
-
•
There is insufficient evidence to justify deviations from current evidence-based recommendations for postoperative nutritional care in patients who have had bariatric and metabolic surgery
-
•
To minimise risk of nutrition-related complications, providers should engage with patients at the same intervals as in current guidelines
-
•
Clinical signs (eg, weight, visual changes, rash, weakness, oedema or anasarca, and neuropsychiatric signs), and symptoms (eg, nausea, tingling, bowel-habit changes, and fatigue) of nutritional deficiency must be assessed during virtual clinic sessions
-
•
Routine laboratory tests (eg, albumin, thiamine, B12, vitamin A, vitamin D, iron, and calcium) should not be deferred but obtained at standard intervals, particularly for patients who had operations with greater risk of nutrient malabsorption, such as long-limb diversionary procedures
-
•
Urgent face-to-face meetings and laboratory tests are mandated when symptoms suggest severe biochemical deficiencies or surgical complications (eg, intestinal obstruction or acute cholecystitis)
Preparation for surgery and surgical technique
-
•
Misconceptions and stigma about obesity and bariatric and metabolic surgery might further penalise candidates for surgical treatment of obesity and diabetes in times of limited resources; clinicians, policy makers, and hospital managers should recognise the seriousness of the diseases that require metabolic and bariatric surgery and ensure that these operations are not further delayed
-
•
Given the risks of severe complications from COVID-19 in patients with obesity and type 2 diabetes, COVID-19 screening should be mandatory preoperatively for patients considering bariatric and metabolic surgery
-
•
Despite the potential higher risk of contagion for staff, the risk and benefit of a laparoscopic approach remain favourable for patients and should be preferred over the use of open techniques
-
•
Appropriate personal protective equipment should be used as recommended by professional bodies and public health agencies to minimise risk for staff and operators
We also considered available data regarding the efficacy of dietary or pharmacological interventions for weight loss,84, 85, 86, 87, 88 or both, as a strategy to achieve weight loss or weight maintenance in patients with multiple weight-responsive comorbidities who face prolonged waiting times for bariatric and metabolic surgery. Regarding strategies to maximise surgical outcomes in patients who have already had surgery, our recommendations are based on results from studies investigating the efficacy of pharmacological approaches in people with persistent or recurrent type 2 diabetes after surgery. Among these individuals, a recent study89 showed that the GLP-1RA liraglutide can reduce HbA1c by 1·2%, with up to 5% additional weight loss. We reviewed existing evidence-based recommendations for postoperative nutritional care79 to define safe and pragmatic methods of virtual consultation by telemedicine (panel 1).
Priorities in resuming elective bariatric and metabolic procedures
Even before the COVID-19 pandemic, metabolic and bariatric surgery was underused for many reasons, including misconceptions and stigma about obesity and bariatric surgery.90 Such barriers might further penalise candidates for this surgery in times of limited resources. Given the seriousness of the diseases that require metabolic and bariatric surgery, clinicians, hospital managers, and policy makers should ensure that these operations are not further delayed because of the widespread misconception that they are a last resort.90
Eventually, the COVID-19 crisis will abate, and elective operations will resume, leaving an enormous backlog of patients who would benefit from bariatric and metabolic surgery. How should we prioritise whom to serve first with limited resources? At a broad level, the answer is simple. If patients are well enough to be safe surgical candidates, preference should be afforded to those with the greatest risk of morbidity and mortality from their disease, if it is probable that this risk can be reduced by surgery. This logic would apply, for instance, to many surgical candidates with poorly controlled type 2 diabetes or substantial metabolic, respiratory, or cardiovascular disease.
Traditional BMI-centric criteria for patient selection, however, tend to skew access to bariatric and metabolic surgery in the opposite direction. Despite strong evidence that surgery achieves its greatest health benefits among patients with type 2 diabetes, a minority of those who have such operations have preoperative type 2 diabetes or cardiometabolic disease.91 Furthermore, in many publicly funded health-care systems (eg, UK National Health Service), candidates for bariatric and metabolic surgery are currently placed on a single elective surgery waiting list, regardless of their indication. Priority is established largely on a first-come first-served basis, rather than on clinical need. This approach is comparable to putting all colorectal surgery candidates on the same waiting list with similar priority, regardless of whether their diagnosis is cancer or benign neoplasia. A strong need therefore exists for clinically sound criteria to help prioritise access to surgery in times of pandemics with limited resources. These criteria can also inform future waiting list management and decision making about the structure of surgical services.
Principles of prioritisation for bariatric and metabolic surgery
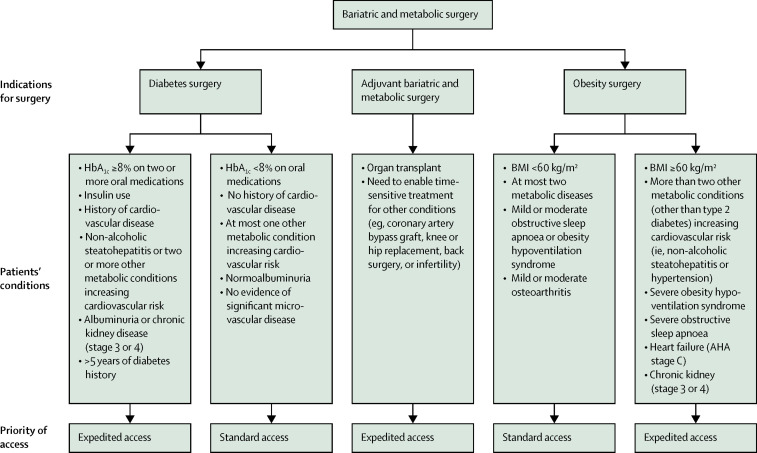
The prioritisation of any elective operation should seek to facilitate access according to clinical need, maximise equity of access, and minimise the harm from delayed access. We have adapted previous categorisations of elective surgery7 to define an objective prioritisation system reflecting these principles for bariatric and metabolic operations (panel 2 ; figure ).
Panel 2. Categories of access to bariatric and metabolic surgery.
Urgent access: surgery within 30 days
Patient's condition is associated with one of the following:
-
•
Conditions with potential to deteriorate quickly
-
•
Severe symptoms or dysfunction
-
•
Examples include severe dysphagia or vomiting from anastomotic stenosis, symptomatic internal hernia, severe nutritional deficiencies, or acute band-related complications
Expedited access: surgery within 90 days
Patient's conditions are not likely to deteriorate quickly but are associated with one of the following:
-
•
Substantial risk of morbidity or mortality
-
•
Reasonable risk of harm or reduced efficacy of treatment if surgery is delayed beyond 90 days
-
•
Complex medical regimens or insulin requirement
-
•
Weight loss, metabolic improvement, or both, are required to allow other time-sensitive treatments (eg, organ transplants or orthopaedic surgery)
Standard access: surgery after 90 days
-
•
Patient's conditions are unlikely to deteriorate within 6 months
-
•
Only mild dysfunction or symptoms
-
•
Delaying surgical treatment beyond 90 days is unlikely to significantly reduce effectiveness of surgery
Figure.
Examples of conditions that warrant expedited access to bariatric and metabolic surgery
AHA=American Heart Association.
Given the factors contributing to morbidity and mortality in obesity and type 2 diabetes, surgical prioritisation should be based on disease-specific considerations. For patients with type 2 diabetes, we suggest that surgery be prioritised for patients at increased risk of morbidity and mortality. This risk would be indicated by poor glycaemic control despite maximal medical therapy, use of insulin, previous cardiovascular disease, albuminuria and chronic kidney disease, non-alcoholic steatohepatitis, or multiple cardiometabolic comorbidities.28 Insulin use is a meaningful prioritisation criterion because it correlates with increased cardiovascular mortality28 and reduced quality of life.92 Moreover, metabolic surgery reduces or abolishes the need for insulin in most patients.1, 47, 52 To mitigate the risk of substantially reducing treatment efficacy, we suggest prioritising surgery in patients with more than 5 years of diabetes. This suggestion is based on evidence that individuals with shorter diabetes duration have greater chances of achieving disease remission,92 whereas type 2 diabetes duration of 8–10 years remits far less often postoperatively.1, 65, 93
The severity of obesity-associated symptoms (eg, mobility issues or joint pain as a consequence of extremely high BMI, regardless of comorbidities) must also be considered when establishing priorities. Equally important is the effect of obesity-related conditions that increase morbidity and mortality (eg, obesity hypoventilation syndrome, chronic kidney disease, or severe obstructive sleep apnoea).38 The availability of non-surgical options that slow disease progression (ie, pharmacological diabetes treatments achieving adequate glycaemic control) reduces need for prioritisation. Expedited access to surgery should also be considered when bariatric and metabolic operations are used as adjuvant therapy to enable other time-sensitive treatments that are made unfeasible or unsafe by excess weight, poor metabolic control, or both (figure).
Many candidates for bariatric and metabolic surgery are at high risk of morbidity and mortality from comorbid conditions. For these patients, access to surgical treatment should be prioritised on the basis of disease-focused clinical needs, rather than primarily on BMI, to mitigate harm from delaying surgery. This approach is especially needed in periods in which access to surgery is reduced, as in the current COVID-19 pandemic. Societal crises often spur developments that provide benefits long after the storm passes. Disease-oriented, medically meaningful strategies to triage patients seeking metabolic surgery after the COVID-19 crisis should help prioritise patients in more urgent need, both now and long into the future.
Search strategy and selection criteria
We did a rapid narrative literature review for this Personal View. For references about the effect of viral infections including coronavirus disease 2019 (COVID-19) on diabetes, obesity, and laparoscopic surgery, we searched PubMed for articles in English published between Jan 1, 2002, and April 10, 2020. We used combinations of terms such as “SARS”, “H1N1”, “coronavirus”, “COVID-19”, “SARS-CoV-2”, “diabetes”, “obesity”, “BMI” “laparoscopy”, “endoscopy”, “severe acute respiratory syndrome”, “acute respiratory distress syndrome”, and “co-morbidities”.
We also reviewed recent guidelines from professional organisations and public health agencies about elective surgery and the COVID-19 pandemic. For evidence about the benefits of bariatric and metabolic surgery, the predicting factors of morbidity and mortality from type 2 diabetes, obesity, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis, and the classification of elective surgery, we reviewed recently published systematic reviews and consensus statements by major scientific societies and relevant individual articles cited in these documents.
Members of the expert panel were selected on the basis of their previous participation in the Diabetes Surgery Summit series and their relevant expertise. Additional experts were also invited to join the group and provide complementary expertise or ensure global representation, or both. A subgroup of the expert panel did a first appraisal of the evidence and draft recommendations, and they generated the first draft of the report, synthetising the literature review in response to each specific query. The entire expert group then engaged in online discussion to further appraise the evidence and refine the final consensus recommendations.
Contributors
FR conceived the idea for this initiative. FR, RVC, GM, CWR, JIM, DEA, JV, and DEC reviewed relevant medical literature and prepared the first draft of this report. GA, SAA, RLB, SB, GC, SDP, JBD, RHE, DH, BMM, APan, APat, FP, PRS, and PZZ provided additional input in the appraisal of evidence and in manuscript preparation. All co-authors participated in the development of the recommendations and reviewed and approved this report.
Declaration of interests
FR is on advisory boards for GI Dynamics, Keyron, and Novo Nordisk, has received consulting fees from Ethicon Endosurgery and Medtronic, and has received research grants from Ethicon Endosurgery and Medtronic. CWR reports receiving research grants from Science Foundation of Ireland, Health Research Board, and Irish Research Council, personal advisory board fees from Novo Nordisk and GI Dynamics, honoraria for lectures and advisory work for Eli Lilly, Johnson and Johnson, Sanofi Aventis, AstraZeneca, Janssen, Bristol-Myers Squibb, Boehringer-Ingelheim, AnaBio, and Keyron. JIM has received honoraria for lectures and programme development from Abbott Nutrition. DEA reports receiving grants from the US National Institutes of Health and Patient-Centered Outcomes Research Institute, and travel expenses from World Congress for Interventional Therapy for Diabetes and from International Federation of Surgery for Obesity Latin American Chapter. SAA reports receiving advisory member fees from Medtronic, Novo Nordisk, Abbott, and Roche via her employer, King's College London. RLB is a principal investigator for clinical trials funded by Novo Nordisk and Fractyl (all funds go directly to her institution, University College London), and has consultancy agreements with Novo Nordisk, Pfizer, ViiV, and International Medical Press. JBD reports consultancy with Bariatric Advantage, iNova, and Reshape, is on advisory boards for Novo Nordisk and Nestlé Health Science, and receives research support from Australia's National Health and Medical Research Council. PRS is a board member and advisory panel member for GI Dynamics, has consulted for Ethicon, Medtronic, WL Gore, Global Academy for Medical Education, and BD Surgical, and has received research support from Ethicon, the US National Institutes of Health, Medtronic, and Pacira. All other authors declare no competing interests.
References
- 1.Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861–877. doi: 10.2337/dc16-0236. [DOI] [PubMed] [Google Scholar]
- 2.Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020 doi: 10.1001/jama.2020.4812. published online March 24. [DOI] [PubMed] [Google Scholar]
- 3.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. published online March 23. [DOI] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. published online Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22831. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livingston EH. Inadequacy of BMI as an indicator for bariatric surgery. JAMA. 2012;307:88–89. doi: 10.1001/jama.2011.1950. [DOI] [PubMed] [Google Scholar]
- 7.Bureau of Health Information Elective surgery hospital quarterly: performance of NSW public hospitals April to June 2013. 2013. http://www.bhi.nsw.gov.au/__data/assets/pdf_file/0003/196446/HQ13_ES_Apr-Jun2013.pdf
- 8.Government of Western Australia Department of Health Elective Surgery Patient Information. August, 2014. http://www.health.wa.gov.au/electivesurgery/docs/Elective_Surgery_Patient_Information_ENGLISH.pdf
- 9.Kwak HD, Kim S-H, Seo YS, Song K-J. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857–863. doi: 10.1136/oemed-2016-103724. [DOI] [PubMed] [Google Scholar]
- 10.Wisniewski PM, Warhol MJ, Rando RF, Sedlacek TV, Kemp JE, Fisher JC. Studies on the transmission of viral disease via the CO2 laser plume and ejecta. J Reprod Med. 1990;35:1117–1123. [PubMed] [Google Scholar]
- 11.Alazawi W, Pirmadjid N, Lahiri R, Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. 2016;264:73–80. doi: 10.1097/SLA.0000000000001691. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen NT, Hinojosa M, Fayad C, Varela E, Wilson SE. Use and outcomes of laparoscopic versus open gastric bypass at academic medical centers. J Am Coll Surg. 2007;205:248–255. doi: 10.1016/j.jamcollsurg.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Weller WE, Rosati C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann Surg. 2008;248:10–15. doi: 10.1097/SLA.0b013e31816d953a. [DOI] [PubMed] [Google Scholar]
- 14.Louie JK, Acosta M, Samuel MC, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1) Clin Infect Dis. 2011;52:301–312. doi: 10.1093/cid/ciq152. [DOI] [PubMed] [Google Scholar]
- 15.Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 16.Torres-Castro I, Arroyo-Camarena ÚD, Martínez-Reyes CP, et al. Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose. Immunol Lett. 2016;176:81–89. doi: 10.1016/j.imlet.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Cheng C-I, Chen P-H, Lin Y-C, Kao Y-H. High glucose activates Raw264.7 macrophages through RhoA kinase-mediated signaling pathway. Cell Signal. 2015;27:283–292. doi: 10.1016/j.cellsig.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16:112–123. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol. 2019;19:282–290. doi: 10.1038/s41577-019-0139-2. [DOI] [PubMed] [Google Scholar]
- 20.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol. 2009;302:193–202. doi: 10.1016/j.mce.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masa JF, Pépin J-L, Borel J-C, Mokhlesi B, Murphy PB, Sánchez-Quiroga MÁ. Obesity hypoventilation syndrome. Eur Respir Rev. 2019;28 doi: 10.1183/16000617.0097-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aune D, Sen A, Norat T, et al. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose–response meta-analysis of prospective studies. Circulation. 2016;133:639–649. doi: 10.1161/CIRCULATIONAHA.115.016801. [DOI] [PubMed] [Google Scholar]
- 26.Centres for Disease Control and Prevention National diabetes statistics report 2020: estimates of diabetes and its burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 27.Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEwen LN, Karter AJ, Waitzfelder BE, et al. Predictors of mortality over 8 years in type 2 diabetic patients: Translating Research Into Action for Diabetes (TRIAD) Diabetes Care. 2012;35:1301–1309. doi: 10.2337/dc11-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEwen LN, Kim C, Karter AJ, et al. Risk factors for mortality among patients with diabetes: the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care. 2007;30:1736–1741. doi: 10.2337/dc07-0305. [DOI] [PubMed] [Google Scholar]
- 30.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 31.Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5:161. doi: 10.21037/atm.2017.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudnick EF, Walsh JS, Hampton MC, Mitchell RB. Prevalence and ethnicity of sleep-disordered breathing and obesity in children. Otolaryngol Head Neck Surg. 2007;137:878–882. doi: 10.1016/j.otohns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Kaditis AG, Alexopoulos EI, Hatzi F, et al. Adiposity in relation to age as predictor of severity of sleep apnea in children with snoring. Sleep Breath. 2008;12:25–31. doi: 10.1007/s11325-007-0132-z. [DOI] [PubMed] [Google Scholar]
- 34.Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116:1–7. doi: 10.1016/j.amjmed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Hartemink N, Boshuizen HC, Nagelkerke NJ, Jacobs MA, van Houwelingen HC. Combining risk estimates from observational studies with different exposure cutpoints: a meta-analysis on body mass index and diabetes type 2. Am J Epidemiol. 2006;163:1042–1052. doi: 10.1093/aje/kwj141. [DOI] [PubMed] [Google Scholar]
- 36.Aune D, Sen A, Norat T, et al. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysos of prospective studies. Circulation. 2016;133:639–649. doi: 10.1161/CIRCULATIONAHA.115.016801. [DOI] [PubMed] [Google Scholar]
- 37.Aylwin S, Al-Zaman Y. Emerging concepts in the medical and surgical treatment of obesity. Front Horm Res. 2008;36:229–259. doi: 10.1159/000115368. [DOI] [PubMed] [Google Scholar]
- 38.Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton Obesity Staging System to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ. 2011;183:e1059–e1066. doi: 10.1503/cmaj.110387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuk JL, Ardern CI, Church TS, et al. Edmonton Obesity Staging System: association with weight history and mortality risk. Appl Physiol Nutr Metab. 2011;36:570–576. doi: 10.1139/h11-058. [DOI] [PubMed] [Google Scholar]
- 40.Sattar N, Forrest E, Preiss D. Non-alcoholic fatty liver disease. BMJ. 2014;349 doi: 10.1136/bmj.g4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tai FW, Syn WK, Alazawi W. Practical approach to non-alcoholic fatty liver disease in patients with diabetes. Diabet Med. 2015;32:1121–1133. doi: 10.1111/dme.12725. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care. 2018;41:372–382. doi: 10.2337/dc17-1902. [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A, Zaza G, Byrne CD, et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: a systematic review and meta-analysis. Metabolism. 2018;79:64–76. doi: 10.1016/j.metabol.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32–42. doi: 10.1038/nrgastro.2016.147. [DOI] [PubMed] [Google Scholar]
- 45.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 46.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 47.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Brien R, Johnson E, Haneuse S, et al. Microvascular outcomes in patients with diabetes after bariatric surgery versus usual care: a matched cohort study. Ann Intern Med. 2018;169:300–310. doi: 10.7326/M17-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;1 doi: 10.1002/14651858.CD007340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149:379–388. doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Ikramuddin S, Korner J, Lee WJ, et al. Lifestyle intervention and medical management with vs without Roux-en-Y gastric bypass and control of hemoglobin A1c, LDL cholesterol, and systolic blood pressure at 5 years in the Diabetes Surgery Study. JAMA. 2018;319:266–278. doi: 10.1001/jama.2017.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964–973. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 53.Simonson DC, Halperin F, Foster K, Vernon A, Goldfine AB. Clinical and patient-centered outcomes in obese patients with type 2 diabetes 3 years after randomization to Roux-en-Y gastric bypass surgery versus intensive lifestyle management: the SLIMM-T2D study. Diabetes Care. 2018;41:670–679. doi: 10.2337/dc17-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maciejewski ML, Livingston EH, Smith VA, et al. Survival among high-risk patients after bariatric surgery. JAMA. 2011;305:2419–2426. doi: 10.1001/jama.2011.817. [DOI] [PubMed] [Google Scholar]
- 55.Padwal RS, Klarenbach SW, Wang X, et al. A simple prediction rule for all-cause mortality in a cohort eligible for bariatric surgery. JAMA Surg. 2013;148:1109–1115. doi: 10.1001/jamasurg.2013.3953. [DOI] [PubMed] [Google Scholar]
- 56.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 57.Telem DA, Talamini M, Shroyer AL, et al. Long-term mortality rates (>8-year) improve as compared to the general and obese population following bariatric surgery. Surg Endosc. 2015;29:529–536. doi: 10.1007/s00464-014-3714-4. [DOI] [PubMed] [Google Scholar]
- 58.Perry CD, Hutter MM, Smith DB, Newhouse JP, McNeil BJ. Survival and changes in comorbidities after bariatric surgery. Ann Surg. 2008;247:21–27. doi: 10.1097/SLA.0b013e318142cb4b. [DOI] [PubMed] [Google Scholar]
- 59.Arterburn D, Livingston EH, Schifftner T, Kahwati LC, Henderson WG, Maciejewski ML. Predictors of long-term mortality after bariatric surgery performed in Veterans Affairs medical centers. Arch Surg. 2009;144:914–920. doi: 10.1001/archsurg.2009.134. [DOI] [PubMed] [Google Scholar]
- 60.Wiggins T, Antonowicz SS, Markar SR. Cancer risk following bariatric surgery-systematic review and meta-analysis of national population-based cohort studies. Obes Surg. 2019;29:1031–1039. doi: 10.1007/s11695-018-3501-8. [DOI] [PubMed] [Google Scholar]
- 61.Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95–101. doi: 10.1097/SLA.0000000000002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feigelson HS, Caan B, Weinmann S, et al. Bariatric surgery is associated with reduced risk of breast cancer in both premenopausal and postmenopausal women. Ann Surg. 2019 doi: 10.1097/SLA.0000000000003331. published online April 13. [DOI] [PubMed] [Google Scholar]
- 63.Aminian A, Aleassa EM, Bhatt DL, et al. Bariatric surgery is associated with a lower rate of death after myocardial infarction and stroke: a nationwide study. Diabetes Obes Metab. 2019;21:2058–2067. doi: 10.1111/dom.13765. [DOI] [PubMed] [Google Scholar]
- 64.Aminian ANS. Success (but unfinished) story of metabolic surgery. Diabetes Care (in press). [DOI] [PubMed]
- 65.Cummings DE, Rubino F. Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia. 2018;61:257–264. doi: 10.1007/s00125-017-4513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schauer PR, Mingrone G, Ikramuddin S, Wolfe B. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care. 2016;39:902–911. doi: 10.2337/dc16-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoerger TJ, Zhang P, Segel JE, Kahn HS, Barker LE, Couper S. Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care. 2010;33:1933–1939. doi: 10.2337/dc10-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee WJ, Chong K, Chen SC, et al. Preoperative Prediction of type 2 diabetes remission after gastric bypass surgery: a comparison of DiaRem scores and ABCD scores. Obes Surg. 2016;26:2418–2424. doi: 10.1007/s11695-016-2120-5. [DOI] [PubMed] [Google Scholar]
- 69.Pucci A, Tymoszuk U, Cheung WH, et al. Type 2 diabetes remission 2 years post Roux-en-Y gastric bypass and sleeve gastrectomy: the role of the weight loss and comparison of DiaRem and DiaBetter scores. Diabet Med. 2018;35:360–367. doi: 10.1111/dme.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aron-Wisnewsky J, Sokolovska N, Liu Y, et al. The advanced-DiaRem score improves prediction of diabetes remission 1 year post-Roux-en-Y gastric bypass. Diabetologia. 2017;60:1892–1902. doi: 10.1007/s00125-017-4371-7. [DOI] [PubMed] [Google Scholar]
- 71.Still CD, Wood GC, Benotti P, et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol. 2014;2:38–45. doi: 10.1016/S2213-8587(13)70070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen SC, Wang W, Tam KW, et al. Validating risk prediction models of diabetes remission after sleeve gastrectomy. Obes Surg. 2019;29:221–229. doi: 10.1007/s11695-018-3510-7. [DOI] [PubMed] [Google Scholar]
- 73.Sjöholm K, Pajunen P, Jacobson P, et al. Incidence and remission of type 2 diabetes in relation to degree of obesity at baseline and 2 year weight change: the Swedish Obese Subjects (SOS) study. Diabetologia. 2015;58:1448–1453. doi: 10.1007/s00125-015-3591-y. [DOI] [PubMed] [Google Scholar]
- 74.Neovius M, Narbro K, Keating C, et al. Health care use during 20 years following bariatric surgery. JAMA. 2012;308:1132–1141. doi: 10.1001/2012.jama.11792. [DOI] [PubMed] [Google Scholar]
- 75.Weiner JP, Goodwin SM, Chang HY, et al. Impact of bariatric surgery on health care costs of obese persons: a 6-year follow-up of surgical and comparison cohorts using health plan data. JAMA Surg. 2013;148:555–562. doi: 10.1001/jamasurg.2013.1504. [DOI] [PubMed] [Google Scholar]
- 76.Finkelstein EA, Allaire BT, Globe D, Dixon JB. The business case for bariatric surgery revisited: a non-randomized case-control study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith VA, Olsen MK, Arterburn DE, et al. Long-term expenditures associated with bariatric surgery in VA. JAMA Surg (in press).
- 78.Smith VA, Arterburn DE, Berkowitz TSZ, et al. Association between bariatric surgery and long-term health care expenditures among veterans with severe obesity. JAMA Surg. 2019 doi: 10.1001/jamasurg.2019.3732. published online Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures, 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity (Silver Spring) 2020;28:O1–O58. doi: 10.1002/oby.22719. [DOI] [PubMed] [Google Scholar]
- 80.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98–S110. doi: 10.2337/dc20-S009. [DOI] [PubMed] [Google Scholar]
- 81.Hussein H, Zaccardi F, Dhalwani NN, Davies MJ, Khunti K, Gray LJ. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors (SGLT-2is) and glucagon-like peptide-1 receptor agonists (GLP-1RAs) in patients with type 2 diabetes: a systematic review and network meta-analysis study protocol. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-023206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahrén B, Atkin SL, Charpentier G, et al. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes Obes Metab. 2018;20:2210–2219. doi: 10.1111/dom.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bornstein SR, Rubino F, Khunti K, et al. Consensus recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30152-2. published online April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larsen TM, Dalskov SM, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–2113. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19:110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 88.le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389:1399–1409. doi: 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 89.Miras AD, Pérez-Pevida B, Aldhwayan M, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:549–559. doi: 10.1016/S2213-8587(19)30157-3. [DOI] [PubMed] [Google Scholar]
- 90.Rubino F, Puhl RM, Cummings DE, et al. Joint international consensus statement for ending stigma of obesity. Nat Med. 2020;26:485–497. doi: 10.1038/s41591-020-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rubino F, Shukla A, Pomp A, Moreira M, Ahn SM, Dakin G. Bariatric, metabolic, and diabetes surgery: what's in a name? Ann Surg. 2014;259:117–122. doi: 10.1097/SLA.0b013e3182759656. [DOI] [PubMed] [Google Scholar]
- 92.Jing X, Chen J, Dong Y, et al. Related factors of quality of life of type 2 diabetes patients: a systematic review and meta-analysis. Health Qual Life Outcomes. 2018;16:189. doi: 10.1186/s12955-018-1021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]