Abstract
Type 2 diabetes (T2D) is a major comorbidity of COVID-19. However, the impact of blood glucose (BG) control on the degree of required medical interventions and on mortality in patients with COVID-19 and T2D remains uncertain. Thus, we performed a retrospective, multi-centered study of 7,337 cases of COVID-19 in Hubei Province, China, among which 952 had pre-existing T2D. We found that subjects with T2D required more medical interventions and had a significantly higher mortality (7.8% versus 2.7%; adjusted hazard ratio [HR], 1.49) and multiple organ injury than the non-diabetic individuals. Further, we found that well-controlled BG (glycemic variability within 3.9 to 10.0 mmol/L) was associated with markedly lower mortality compared to individuals with poorly controlled BG (upper limit of glycemic variability exceeding 10.0 mmol/L) (adjusted HR, 0.14) during hospitalization. These findings provide clinical evidence correlating improved glycemic control with better outcomes in patients with COVID-19 and pre-existing T2D.
Keywords: COVID-19, SARS-CoV-2, diabetes mellitus, blood glucose control, mortality
Graphical Abstract
Type 2 diabetes (T2D) correlates with a worse outcome for COVID-19. Here, Zhu et al. show that among ∼7,300 cases of COVID-19, T2D is associated with a higher death rate, but diabetics with better controlled blood glucose die at a lower rate than diabetics with poorly controlled blood glucose.
Context and Significance
Although type 2 diabetes (T2D) is a major comorbidity of novel coronavirus disease 2019 (COVID-19), the impact of blood glucose control on the degree of medical interventions required and on all-cause mortality of patients with COVID-19 and pre-existing T2D remains unclear. Here, Zhu et al. report that among ∼7,300 individuals with COVID-19 (among which nearly 1,000 had T2D) in Hubei Province, China, those with T2D had significantly increased medical interventions and mortality risk. But among the patients with T2D, those with well-controlled blood glucose regulation (upper limit ≤ 10 mmol/L) fared much better than those with poorly controlled blood glucose (upper limit > 10 mmol/L). These findings provide clinical evidence correlating more proper blood glucose control with improved outcomes in patients with COVID-19.
Introduction
The novel coronavirus disease 2019 (COVID-19) is caused by infection from the newly emerged, highly contagious coronavirus SARS-CoV-2 (Wu and McGoogan, 2020). SARS-CoV-2 mainly invades the respiratory tract and lungs, leading to a new type of coronavirus pneumonia (Zhu et al., 2020). The severe cases of COVID-19 can rapidly progress to acute respiratory distress syndrome (ARDS), septic shock, and multiple organ dysfunction syndrome (MODS) (Guan et al., 2020). Elderly individuals, along with those with pre-existing conditions, such as hypertension, cancer, cardiovascular diseases, diabetes mellitus, and acute kidney injury, have a demonstrated higher risk for developing more severe cases of COVID-19, as well as suffering a higher risk of mortality (Wang et al., 2020, Zhang et al., 2020b, Zhou et al., 2020). The collision between the two global pandemics of COVID-19 and type 2 diabetes (T2D) has led to the grim reality that T2D is already the second most common comorbidity of COVID-19 (Zhou et al., 2020). However, current evidence implicating T2D in worse COVID-19 prognosis has mostly come from relatively limited-sized cohorts (Deng and Peng, 2020, Zhang et al., 2020a, Zhou et al., 2020). Thus, the clinical features of patients with T2D infected by SARS-CoV-2 remain to be comprehensively clarified in a large-scale analysis, which is needed to more efficiently and precisely manage the treatment of such patients.
It has been well established that patients with diabetes are more susceptible to infections in general and exhibit worse prognosis once infected compared to the non-diabetic population (Kumar Nathella and Babu, 2017, Xu et al., 2019). Such a higher susceptibility has also been previously observed for other coronaviral epidemics. For example, in patients with severe acute respiratory syndrome (SARS), pre-existing T2D was independently associated with poor outcomes. The percentage of known T2D history was significantly higher among patients who succumbed to SARS than who survived (Booth et al., 2003, Yang et al., 2006). Further, epidemiological studies also indicate that T2D was the primary comorbidity associated with severe or lethal MERS-CoV infections (Alqahtani et al., 2018). And with regard to the current COVID-19 pandemic, several recent studies, though with limited participants, have already suggested that T2D is a common comorbidity and constitutes a higher proportion of patients with severe and ICU-admitted cases of COVID-19 than patients with mild symptoms (Deng and Peng, 2020, Wang et al., 2020, Zhang et al., 2020a, Zhou et al., 2020). These associations between diabetes and worse outcome in viral infections are not unexpected as hyperglycemia is detrimental to the control of viremia and inflammation, aggravating morbidity and mortality in a variety of patients (Forbes et al., 2018). However, an overly rigid glucose control may increase the risk of severe hypoglycemia, which can also lead to an increased mortality (Rodriguez-Gutierrez et al., 2019). Consequently, previous clinical trials examining the effects of glucose control on mortality have yielded conflicting results (Forbes et al., 2018, Van den Berghe et al., 2006). For individuals with COVID-19 and pre-existing T2D, a key challenge for clinicians is to improve outcomes in the face of uncertainty regarding the degree of glycemic management that should be maintained and any effects this might have on the benefits and risks of overall treatment. Thus, detailed analyses of data from such patients is needed that links plasma glucose levels with clinic outcomes, including mortality.
In this report, we performed a retrospective longitudinal, multi-centered study from a cohort of 7,337 confirmed COVID-19 cases enrolled among 19 hospitals in Hubei Province, China, focusing on the association between plasma glucose levels and clinic outcomes in COVID-19 patients with T2D. In addition to a significant association between diabetic status and higher mortality rate in patients with COVID-19 and pre-existing T2D versus non-diabetic subjects with COVID-19, our study indicated that well-controlled glycemia was associated with a markedly improved outcome of patients with COVID-19 and pre-existing T2D.
Results and Discussion
Clinical Characteristics of Patients with COVID-19 and Pre-existing T2D upon Admission
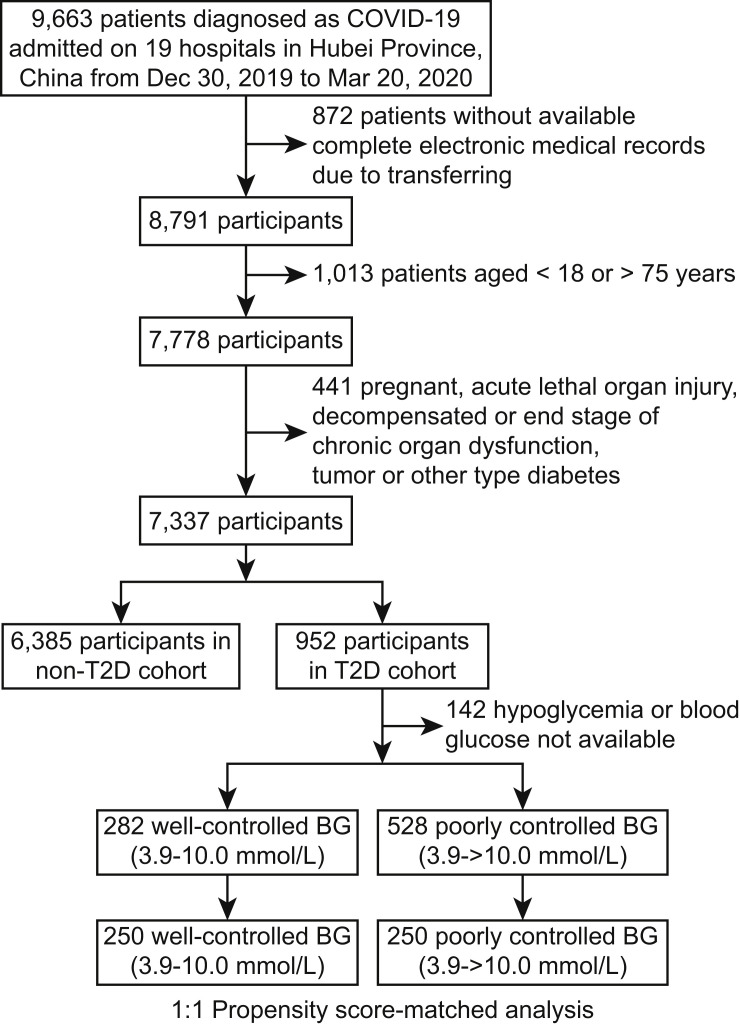
Clinic characteristics were collected from a total of 7,337 participants out of 9,663 confirmed cases of COVID-19, including 952 subjects with pre-existing T2D (n = 510 male, 53.6%) and 6,385 non-diabetic cases (n = 2,967 male, 46.5%) (Figure 1 ). Of the initial 9,663 cases enrolled, 2,326 patients with COVID-19 were excluded from the study, including 1,013 patients younger than 18 or older than 75 years old, 872 patients without complete medical records, 13 patients with acute myocardial infarction, 5 patients with acute coronary syndrome, 8 patients with acute pulmonary embolism, 10 patients with acute stroke, 11 patients with acute severe pancreatitis, 9 patients with cirrhosis, 37 patients with above stage 3 chronic renal insufficiency, 7 patients with severe congestive heart failure, 104 patients with pregnancy, 227 patients with malignancy, 7 patients with type 1 diabetes, 2 patients with a history of gestational diabetes, and 1 patient with drug-induced diabetes. From the final cohort of 7,337 COVID-19 patients analyzed, the prevalence of T2D was 13.0%, which was similar to the nationwide prevalence of T2D in China (about 10.9%) (Wang et al., 2017). The median ages were 62 (55–68) and 53 (40–63) in the diabetic and non-diabetic groups, respectively (Table S1). The median body mass index (BMI) in patients with or without T2D was 24.7 (22.0–26.4) and 23.4 (21.0–26.0), respectively. The median duration from the first symptom to admission was 10 days (6–19) for both groups. The major symptoms for both groups were fever (71.8%), cough (63.5%), fatigue (32.3%), and dyspnea (16.1%), similar to the general population of patients (Chen et al., 2020, Guan et al., 2020, Huang et al., 2020, Wang et al., 2020). Patients with T2D reported significantly higher incidences of fatigue (38.0% versus 31.4%) and dyspnea (20.5% versus 15.4%) compared to the non-diabetic group. Pre-existing hypertension (53.4% versus 19.7%), coronary heart disease (CHD; 13.7% versus 3.7%), cerebrovascular disease (5.6% versus 1.5%), and chronic kidney disease (4.9% versus 1.3%) had higher frequencies in the T2D group compared to the non-diabetic group. Chest CT scans indicated that the incidence of bilateral lung lesion was higher (88.1% versus 80.4%) in the diabetic group compared to non-diabetic patients.
Figure 1.
Study Inclusion Criteria
A schematic overview illustrating participant enrollment in the cohort study and the various exclusion and inclusion criteria among the initial case group. Briefly, a total of 9,663 patients with COVID-19 were included. After various exclusion criteria, 2,326 patients were removed from the study. Of the remaining 7,337 patients, data from 6,385 patients without diabetes (non-T2D) were placed in one group, while 952 individuals with type 2 diabetes (T2D) were placed in a second group. Of the 952 cases with T2D, 142 cases were further excluded due to hypoglycemia or lack of BG readings. Of the remaining 810 cases of T2D, 282 were considered to have well-controlled BG, while 528 had poorly controlled BG. And of these two T2D groups, 250 of each were used for propensity score-matched analysis.
While heart rate and respiratory rates did not show differences between the diabetic and the non-diabetic groups, systolic blood pressure was modestly higher in the diabetic group (130 mmHg [120–142] versus 126 mmHg [120–136]). Lab findings showed that blood glucose (BG) level was much higher in the diabetic group compared to the non-diabetic group, as expected (8.3 mmol/L [6.2–12.4] versus 5.2 mmol/L [4.7–6.1]), with higher levels of HbA1c (7.9% [6.8%–9.5%] versus 6.1% [5.7%–6.6%]). Patients with T2D had a significantly higher incidence of lymphopenia (44.5% versus 32.6%), and higher ratio of elevation of leukocyte (11.3% versus 6.6%) and neutrophil (17.2% versus 9.9%) counts in peripheral blood, relative to the non-diabetic individuals. At the same time, elevated serum markers, indicating inflammation (C-reactive protein, CRP [57.0% versus 42.4%] and procalcitonin [33.3% versus 20.3%]), decreased kidney function (creatinine [12.0% versus 5.0%]), and increased coagulation status (D-dimer [50.5% versus 33.3%]), were found more frequently in the T2D group than in the non-diabetic group. Furthermore, SpO2 lower than 95% occurred more frequently in the diabetic group versus the non-diabetic group (18.8% versus 13.2%) on admission.
Patients with COVID-19 and Pre-existing T2D Require More Intensive In-Hospital Treatment
The patients with pre-existing T2D received significantly more intensive integrated treatments to manage their symptoms of COVID-19 than the non-diabetic subjects. The former group registered a higher need for antibiotics (61.3% versus 56.9%), antifungal drugs (2.5% versus 1.2%), systemic corticosteroids (29.4% versus 22.8%), immunoglobin (23.0% versus 17.7%), anti-hypertensive drug (45.1% versus 21.1%), and even vasoactive drugs (7.7% versus 2.2%). Oxygen inhalation (76.9% versus 61.2%), noninvasive ventilation (10.2% versus 3.9%), and invasive ventilation (3.6% versus 0.7%) were also applied significantly more frequently to the individuals with T2D compared to the patients without T2D (Table S2).
T2D Is Correlated with a Higher Risk of All-Cause Mortality and Detrimental Comorbidities in Patients with COVID-19
During the 28-day follow-up period, we performed a retrospective longitudinal analysis on various parameters starting from the time of admission to the hospital for each patient in the study. We noticed that, despite having received more aggressive treatment against COVID-19 and the comorbidities, the diabetic group had greater incidences of decreased lymphocyte counts and increased neutrophil counts, as well as higher levels of serum interleukin-6 (IL-6), CRP, and lactic dehydrogenase (LDH), accompanied by higher BG levels, compared to the non-diabetic group. The BG level was also significantly associated with comorbid hypertension, CHD, the incidences of decreased lymphocyte count, and elevated neutrophil count and the levels of serum CRP and creatinine in the entire cohort (Figure S1).
During the 28-day follow-up period starting from admission, the in-hospital death rate was significantly higher in patients with pre-existing T2D relative to the non-diabetic individuals (7.8% versus 2.7%, p < 0.001) (Table S3). The crude HR of the 28-day all-cause mortality in the diabetic group versus non-diabetic individuals was 2.90 (95% CI, 2.21–3.81; p < 0.001) (Table S4). After adjusting for age, gender, and hospital site on admission, the HR of the all-cause mortality between these two groups was 1.70 (95% CI, 1.29–2.24; p < 0.001) (Table S4). We further adjusted for the severity of COVID-19 and found that the HR of the all-cause mortality between these two groups was 1.49 (95% CI, 1.13–1.96; p = 0.005) (Figure S2; Table S4). We here did not adjust for comorbidities closely related to T2D, including hypertension, CHD, cerebrovascular disease, and chronic kidney disease, as these diseases often co-exist with T2D.
Furthermore, the individuals with T2D had a greater occurrence of ARDS (16.9% versus 7.2%), acute heart injury (7.3% versus 3.0%), acute kidney injury (3.9% versus 0.8%), septic shock (3.8% versus 1.0%), and disseminated intravascular coagulation (DIC) (0.5% versus 0.2%) than the non-diabetic group (Table S3). Mixed-effect Cox analysis indicated T2D was significantly correlated with the occurrence of ARDS, acute kidney injury, and septic shock with respective adjusted HRs of 1.44 (95% CI, 1.20–1.73), 3.01 (95% CI, 1.94–4.68), and 1.95 (95% CI, 1.18–3.20), after adjusting for age, gender, and severity of COVID-19 among the patients (Table S4). Our current study was based on the largest diabetic COVID-19 cohort so far analyzed, and the results were unequivocal to implicate diabetes mellitus in higher risk of death and other detrimental outcomes of COVID-19. Notably, care must be taken in interpreting the significant difference in outcomes between diabetic and non-diabetic patients with COVID-19, since there were notable differences in the covariate distributions between the two groups.
The pathophysiological mechanisms underlying the impact of T2D on COVID-19 progression remain to be fully investigated. In patients with diabetes, pulmonary dysfunction involving lung volume, pulmonary diffusing capacity, control of ventilation, bronchomotor tone, and neuroadrenergic bronchial innervation have been reported (Fuso et al., 2019), which may account for the propensity of poor outcomes in patients with COVID-19 and T2D. At the same time, a dysregulated immune response caused by T2D is likely also responsible for the increased disease severity of COVID-19 in patients with T2D as a higher ratio of lymphopenia and increased levels of neutrophils, serum CRP, and IL-6 were observed in the patients with COVID-19 and pre-existing T2D in our study. These findings dovetail with immune dysregulation observed in other coronavirus infection-triggered pneumonia (Kulcsar et al., 2019). In an experimental model of MERS, diabetic mice had lower numbers of inflammatory monocytes and macrophages and CD4+ T cells, which was accompanied by lower levels of Ccl2 and Cxcl10 expression (Kulcsar et al., 2019). Furthermore, T2D is associated with activation of the renin-angiotensin system in different tissues (Candido et al., 2002). Considering SARS-CoV-2 utilizes angiotensin-converting enzyme 2 (ACE2) to bind and gain entry to infected cells (Hoffmann et al., 2020, Lu et al., 2020) and reduces the expression of ACE2 (Kuba et al., 2005), overactivation of the renin-angiotensin system may also contribute to the increased adverse risk in patients with COVID-19 and diabetes. In this respect, application of renin-angiotensin system inhibitors may have therapeutic effect in patients with COVID-19 and pre-existing T2D.
Differential Glucose Control Is Associated with Different Outcomes in Patients with COVID-19 and Pre-existing T2D
Among the cohort with COVID-19 and T2D, there were 282 individuals with well-controlled BG (136 males, 48.2%) and 528 individuals with poorly controlled BG (298 males, 56.4%). The median BG level was much lower in the well-controlled BG group than the poorly controlled BG group (6.4 mmol/L [5.2–7.5] versus 10.9 mmol/L [7.6–14.3]) (Figure S3), and the levels of HbA1C in these two groups were 7.3% (6.6%–8.2%) and 8.1% (7.2%–10.1%), respectively. The patients from the well-controlled BG group also had significantly lower incidences of lymphopenia (30.5% versus 49.6%), lower rates of increased counts of leukocyte (6.3% versus 12.2%) and neutrophil (10.7% versus 19.4%), and elevated serum CRP (47.5% versus 59.5%) and procalcitonin (24.2% versus 35.0%). The same pattern was observed for elevated aspartate transaminase (AST) (11.3% versus 20.4%) and D-dimer (37.6% versus 55.4%) (Table 1 ). Notably, fewer individuals from the well-controlled group had SpO2 lower than 95% compared to the poorly controlled group (12.6% versus 22.7%). But other parameters between the two groups were not significantly different. In particular, these two groups had a median age of 62 (55–67) and 63 (56–68), respectively (Table 1). The difference of the median BMI between patients with well-controlled BG or poorly controlled BG was modest (25.0 [23.9–26.4] versus 23.2 [21.0–24.9], respectively).
Table 1.
Characteristics of Patients in the Well-Controlled and Poorly Controlled BG Groups Before and After Propensity Score Matching
| Parameters | Unmatched |
Matched (1:1) |
||||
|---|---|---|---|---|---|---|
| Well Controlled (n = 282) | Poorly Controlled (n = 528) | SD | Well Controlled (n = 250) | Poorly Controlled (n = 250) | SD | |
| Clinical Characteristics on Admission | ||||||
| Age, median (IQR) | 62 (55–67) | 63 (56–68) | −0.094 | 62 (55–67) | 63 (54–68) | 0.008 |
| Male gender, n (%) | 136 (48.2%) | 298 (56.4%) | −0.165 | 126 (50.4%) | 126 (50.4%) | 0.000 |
| Female gender, n (%) | 146 (51.8%) | 230 (43.6%) | 0.165 | 124 (49.6%) | 124 (49.6%) | 0.000 |
| Heart rate, median (IQR), bpm | 84.0 (77.0–95.0) | 85.0 (76.3–97.0) | −0.103 | 84.0 (76.5–93.5) | 83.0 (76.0–96.0) | −0.048 |
| Respiratory rate, median (IQR), bpm | 20.0 (18.0–20.0) | 20.0 (19.0–21.0) | −0.180 | 20.0 (18.0–20.0) | 20.0 (19.0–21.0) | 0.008 |
| SBP, median (IQR), mmHg | 130.0 (120.0–142.0) | 130.0 (120.0–142.0) | 0.073 | 130.0 (120.0–142.0) | 130.0 (120.0–142.0) | 0.085 |
| DBP, median (IQR), mmHg | 80.0 (73.0–89.0) | 80.0 (72.0–86.0) | 0.074 | 80.0 (73.0–86.5) | 80.0 (72.0–86.0) | 0.025 |
| Symptom onset to admission, median (IQR), day | 13.0 (7.0–23.0) | 10.0 (6.0–17.0) | 0.261 | 12.0 (7.0–20.0) | 10.0 (6.0–18.8) | 0.177 |
| Fever, n (%) | 182 (64.5%) | 381 (72.2%) | −0.164 | 166 (66.4%) | 171 (68.4%) | −0.043 |
| Cough, n (%) | 169 (59.9%) | 350 (66.3%) | −0.132 | 155 (62.0%) | 153 (61.2%) | 0.016 |
| Fatigue, n (%) | 90 (31.9%) | 218 (41.3%) | −0.196 | 87 (34.8%) | 90 (36.0%) | −0.025 |
| Dyspnea, n (%) | 48 (17.0%) | 117 (22.2%) | −0.130 | 44 (17.6%) | 39 (15.6%) | 0.054 |
| Comorbidities on Admission | ||||||
| Hypertension, n (%) | 156 (55.3%) | 282 (53.4%) | 0.038 | 136 (54.4%) | 135 (54.0%) | 0.008 |
| Coronary heart disease, n (%) | 42 (14.9%) | 68 (12.9%) | 0.058 | 39 (15.6%) | 32 (12.8%) | 0.080 |
| Chronic liver disease, n (%) | 5 (1.8%) | 10 (1.9%) | −0.009 | 4 (1.6%) | 4 (1.6%) | 0.000 |
| Cerebrovascular diseases, n (%) | 18 (6.4%) | 27 (5.1%) | 0.055 | 18 (7.2%) | 15 (6.0%) | 0.048 |
| Chronic renal diseases, n (%) | 17 (6.0%) | 17 (3.2%) | 0.134 | 13 (5.2%) | 9 (3.6%) | 0.078 |
| COPD, n (%) | 4 (1.4%) | 8 (1.5%) | −0.008 | 4 (1.6%) | 3 (1.2%) | 0.034 |
| Chest CT on Admission | ||||||
| Unilateral lesion, n/N (%) | 25/266 (9.4%) | 22/468 (4.7%) | 0.184 | 19/239 (8.0%) | 18/212 (8.5%) | −0.020 |
| Bilateral lesions, n/N (%) | 230/266 (86.5%) | 425/468 (90.8%) | −0.137 | 210/239 (87.9%) | 184/212 (86.8%) | 0.032 |
| Laboratory Examination on Admission | ||||||
| Leukocyte count > 9.5, 10ˆ9/L, n/N (%) | 17/272 (6.3%) | 61/500 (12.2%) | −0.207 | 15/242 (6.2%) | 18/231 (7.8%) | −0.063 |
| Neutrophil count > 6.3, 10ˆ9/L, n/N (%) | 29/272 (10.7%) | 97/500 (19.4%) | −0.246 | 28/242 (11.6%) | 24/231 (10.4%) | 0.038 |
| Lymphocyte count < 1.1, 10ˆ9/L, n/N (%) | 83/272 (30.5%) | 248/500 (49.6%) | −0.397 | 81/242 (33.5%) | 85/231 (36.8%) | −0.070 |
| C-reactive protein increase > ULNa, n/N (%) | 103/217 (47.5%) | 209/351 (59.5%) | −0.244 | 97/195 (49.7%) | 78/166 (47.0%) | 0.055 |
| Procalcitonin level increase > ULNa, n/N (%) | 51/211 (24.2%) | 143/409 (35.0%) | −0.238 | 48/188 (25.5%) | 40/180 (22.2%) | 0.078 |
| ALT increase > 40 U/L, n/N (%) | 31/266 (11.7%) | 80/476 (16.8%) | −0.148 | 27/235 (11.5%) | 22/219 (10.1%) | 0.047 |
| AST increase > 40 U/L, n/N (%) | 30/266 (11.3%) | 97/476 (20.4%) | −0.251 | 30/235 (12.8%) | 25/219 (11.4%) | 0.041 |
| Creatinine > ULNa, n/N (%) | 20/267 (7.5%) | 68/498 (13.7%) | −0.201 | 20/237 (8.4%) | 15/231 (6.5%) | 0.074 |
| D-dimer > ULNa, n/N (%) | 88/234 (37.6%) | 252/455 (55.4%) | −0.362 | 83/208 (39.9%) | 91/206 (44.2%) | −0.087 |
| K+ < 3.5 mmol/L, n/N (%) | 38/266 (14.3%) | 73/496 (14.7%) | −0.012 | 36/235 (15.3%) | 32/228 (14.0%) | 0.036 |
| LDL-c, mmol/L, median (IQR) | 2.5 (1.9–3.0) | 2.4 (1.9–2.9) | 0.043 | 2.4 (1.9–3.0) | 2.4 (1.9–2.9) | −0.020 |
| SpO2, <95%, n/N (%) | 26/206 (12.6%) | 94/414 (22.7%) | −0.267 | 26/182 (14.3%) | 27/189 (14.3%) | 0.000 |
| Blood glucose, mmol/L, median (IQR) | 6.4 (5.2–7.5) | 10.9 (7.6–14.3) | −1.312 | 6.4 (5.2–7.4) | 10.6 (7.4–13.7) | −1.329 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; COPD, chronic obstructive pulmonary disease; ALT, alanine transaminase; AST, aspartate transaminase; LDL-C, low-density lipoprotein cholesterol; IQR, interquartile range; SD, standardized difference.
Upper limit of normal (ULN) was defined according to criteria in each hospital
Other clinical manifestations, including chest CT imaging and the incidences of major comorbidities, were similar between the two groups on admission. Even so, the patients with COVID-19 and T2D with well-controlled BG acquired significantly less integrated treatments than those with poorly controlled BG. There was a significantly lower frequency of pharmacological therapy in the well-controlled BG group versus the poorly controlled group, including the usage of antivirals (62.8% versus 71.2%), antibiotics (53.2% versus 66.5%), antifungal drugs (0.4% versus 2.8%), systemic corticosteroids (20.2% versus 34.9%), immunoglobin (15.3% versus 26.5%), and vasoactive drugs (2.5% versus 8.9%). The incidences of oxygen inhalation (70.2% versus 83.5%), noninvasive ventilation (4.6% versus 11.9%), invasive ventilation (0.0% versus 4.2%), and extracorporeal membrane oxygenation (0.0% versus 0.8%) were lower in the well-controlled group than in the poorly controlled group (Table 2 ).
Table 2.
In-Hospital Management of Patients with COVID-19 in the Well-Controlled or Poorly Controlled BG Group
| Management | Total (N = 810) | Well Controlled (n = 282) | Poorly Controlled (n = 528) | p Valueb |
|---|---|---|---|---|
| Traditional Chinese medicine (%) | 650 (80.3%) | 235 (83.3%) | 415 (78.6%) | 0.129 |
| Antiviral drug, n (%) | 553 (68.3%) | 177 (62.8%) | 376 (71.2%) | 0.017 |
| Antibiotics drug, n (%) | 501 (61.9%) | 150 (53.2%) | 351 (66.5%) | <0.001 |
| Systemic corticosteroids, n (%) | 241 (29.8%) | 57 (20.2%) | 184 (34.9%) | <0.001 |
| Immunoglobin, n (%) | 183 (22.6%) | 43 (15.3%) | 140 (26.5%) | <0.001 |
| Anti-hypertensive drug, n (%) | 380 (46.9%) | 128 (45.4%) | 252 (47.7%) | 0.575 |
| Lipid-lowering drug, n (%) | 126 (15.6%) | 40 (14.2%) | 86 (16.3%) | 0.493 |
| Vasoactive drug, n (%) | 54 (6.7%) | 7 (2.5%) | 47 (8.9%) | 0.001 |
| Antifungal medications, n (%) | 16 (2.0%) | 1 (0.4%) | 15 (2.8%) | 0.031 |
| Metformin, n (%) | 278 (34.3%) | 76 (27.0%) | 202 (38.3%) | 0.002 |
| Sulfonylurea, n (%) | 106 (13.1%) | 22 (7.8%) | 84 (15.9%) | 0.002 |
| DPP-4 inhibitor, n (%) | 55 (6.8%) | 11 (3.9%) | 44 (8.3%) | 0.025 |
| Insulin, n (%) | 328 (40.5%) | 40 (14.2%) | 288 (54.6%) | <0.001 |
| Alpha-glucosidase inhibitor, n (%) | 337 (41.6%) | 90 (31.9%) | 247 (46.8%) | <0.001 |
| Trizaolidinedione, n (%) | 9 (1.1%) | 2 (0.7%) | 7 (1.3%) | 0.508 |
| Meglitide | 35 (4.3%) | 7 (2.5%) | 28 (5.3%) | 0.089 |
| Oxygen inhalation, n (%) | 639 (78.9%) | 198 (70.2%) | 441 (83.5%) | <0.001 |
| Noninvasive ventilation, n (%)a | 76 (9.4%) | 13 (4.6%) | 63 (11.9%) | 0.001 |
| Invasive ventilation, n (%)a | 22 (2.7%) | 0 (0.0%) | 22 (4.2%) | 0.001 |
| Renal replacement therapy, n (%) | 15 (1.9%) | 5 (1.8%) | 10 (1.9%) | 1.000 |
| Extracorporeal membrane oxygenation, n (%)a | 4 (0.5%) | 0 (0.0%) | 4 (0.8%) | 0.304 |
Noninvasive ventilation, invasive ventilation, and extracorporeal membrane oxygenation are mutually exclusive
p values were calculated by Fisher's exact test or χ2 test
Well-Controlled Blood Glucose Is Correlated with Reduced Risk of All-Cause Mortality and Detrimental Complications in Patients with COVID-19 and Pre-existing T2D
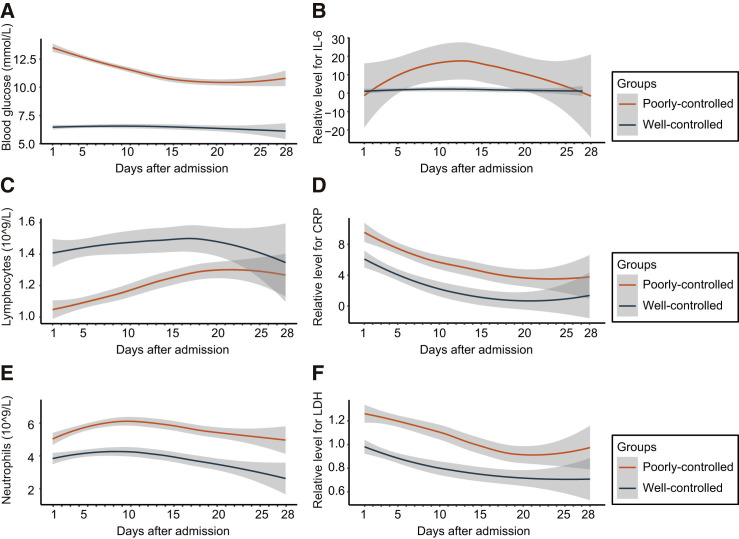
Correlated with persistent lower BG levels, the well-controlled group had higher lymphocyte counts, lower neutrophil counts, and lower serum levels of IL-6, CRP, and LDH throughout the 28-day observation period starting from the time of hospital admission (Figure 2 ). We further noticed that the in-hospital death rate was significantly lower (1.1% versus 11.0%) in the well-controlled group relative to the poorly controlled group (Table S5). The crude HR for the 28-day all-cause mortality between the two groups was 0.09 (95% CI, 0.03–0.30; p < 0.001) (Table S6). After adjusting for age, gender, the severity of COVID-19, comorbidities, and site effect, the HR of the all-cause mortality in the well-controlled BG group versus the poorly controlled BG group was 0.13 (95% CI, 0.04–0.44; p < 0.001) (Table S6). The E-value for the point estimate was 14.87 with upper limit of CI at 3.97. Further, relative to the poorly controlled BG control, the patients from the well-controlled BG group developed less frequent occurrences of ARDS (7.1% versus 21.4%), acute heart injury (1.4% versus 9.9%), acute kidney injury (0.7% versus 3.8%), septic shock (0.0% versus 4.7%), and DIC (0.0% versus 0.6%) (Table S5). After adjusting for age, gender, the severity of COVID-19, site effect, and comorbidities, the respective HRs of ARDS and acute heart injury were 0.41 (95% CI, 0.25–0.66, p < 0.001) and 0.21 (95% CI, 0.07–0.59, p = 0.003) between the well-controlled BG group and poorly controlled BG group (Table S6).
Figure 2.
Dynamics of BG, Lymphocytes, Neutrophils, IL-6, CRP, and LDH in Well-Controlled and Poorly Controlled BG Groups during Hospitalization
Dynamic trajectories of blood glucose (A), lymphocytes (C), and neutrophils (E), and relative levels for IL-6 (B), CRP (D), and LDH (F) during the 28-day follow-up duration, with 95% confidence interval represented by shaded regions, in patients with poorly controlled BG (orange) or patients with well-controlled BG (blue). The BG represents the averaged median BG of patients on the day tested.
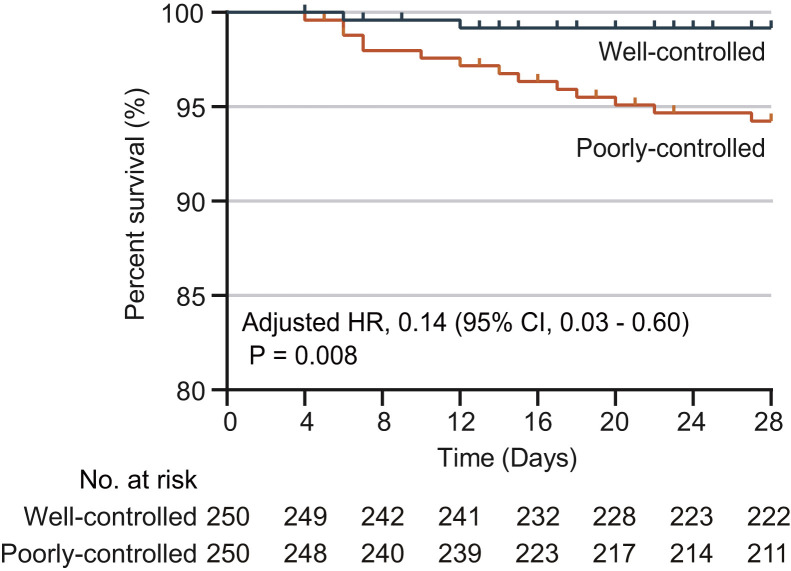
A propensity score-matched (PSM) analysis was further performed to avoid confounding variables that could have interfered with the association between BG levels and all-cause mortality. Due to the lack of reliable indicators for the severity of T2D, we specifically included T2D-related comorbidities for matching since comorbidities are closely related to the severity of T2D. These comorbidities included hypertension, cardiovascular disease, cerebrovascular disease, chronic liver disease, and chronic kidney injury. We were able to match 250 patients from the well-controlled BG group to 250 patients from the poorly controlled BG group at a ratio of 1:1, and these 500 individuals were included in the PSM analysis (Figure 1). By applying mixed-effect Cox model using the hospital site as a random effect and adjusting imbalanced durations from symptom onset to admission, the results consistently and significantly demonstrated a lower risk of all-cause mortality in the patients from the well-controlled BG group (adjusted HR, 0.14; 95% CI, 0.03–0.60; p = 0.008) compared to those from the poorly controlled BG group (Figure 3 ; Tables 3 and S7), although stronger integrated treatment was applied to the poorly controlled BG group (Table S8). The robustness of the association between glycemic variability and mortality was further assessed with additional sensitivity analyses by using different matching variables. The results in these sensitivity analyses were similar to the above analysis with HRs of 0.17 (95% CI, 0.05–0.61; p = 0.006) in the first sensitivity analysis and of 0.16 (95% CI, 0.05–0.58; p = 0.005) in the second analysis (Table S9). In the PSM analysis, the respective adjusted HRs of ARDS, acute heart injury, and acute kidney injury were 0.47 (95% CI, 0.27–0.83, p = 0.009), 0.24 (95% CI, 0.08–0.71, p = 0.010), and 0.12 (95% CI, 0.01–0.96, p = 0.046) between the well-controlled BG group and poorly controlled BG group (Table 3).
Figure 3.
Survival Curves of Patients with Well-Controlled BG or Poorly Controlled BG in the PSM Model
Kaplan-Meier Curves for cumulative probability of COVID-19 mortality during the 28-day follow-up duration in the well-controlled BG (blue) or poorly controlled BG (orange) cohort among 500 patients with T2D in the PSM model. The blips on the curve indicate censoring of cases during 28 days of follow-up.
Table 3.
Hazard Ratios for Outcomes in Well-Controlled and Poorly Controlled BG Cohorts under Cox Adjusted Model and Propensity Score-Matching Model
| Well-Controlled versus Poorly Controlled | Unmatched |
Matchedb |
||||
|---|---|---|---|---|---|---|
| Crude |
Adjusteda |
Adjustedc |
||||
| HR (95% CI) | p Valued | HR (95% CI) | p Valued | HR (95% CI) | p Valued | |
| All-cause mortality | 0.09 (0.03,0.30) | <0.001 | 0.13 (0.04,0.44) | <0.001 | 0.14 (0.03,0.60) | 0.008 |
| Septic shock | – | – | – | – | – | – |
| ARDS | 0.31 (0.19,0.50) | <0.001 | 0.41 (0.25,0.66) | <0.001 | 0.47 (0.27,0.83) | 0.009 |
| DIC | – | – | – | – | – | – |
| Acute kidney injury | 0.19 (0.04,0.80) | 0.024 | 0.22 (0.05,1.03) | 0.055 | 0.12 (0.01,0.96) | 0.046 |
| Acute heart injury | 0.14 (0.05,0.39) | <0.001 | 0.21 (0.07,0.59) | 0.003 | 0.24 (0.08,0.71) | 0.010 |
HR, hazard ratio; CI, confidence interval.
In mixed-effect Cox model, adjusted variables for comparing BG well-controlled and BG poorly controlled cohorts included age, gender, indicators of the severity of COVID-19, and comorbidities (hypertension, coronary heart disease, cerebrovascular diseases, chronic liver diseases, and chronic renal diseases)
In the propensity score-matched model, age, gender, hospital sites, indicators of the severity of COVID-19, comorbidities (hypertension, coronary heart disease, cerebral vascular disease, chronic liver disease, and chronic renal diseases), and incidence of increased creatinine were matched
Mixed-effect Cox model using the hospital site as a random effect and adjusting imbalanced durations from symptom onset to admission
p values were calculated based on Cox proportional hazard model
Glycemic variability has been shown to be an important indicator and a possible risk predictor for death and other complications in individuals with T2D (Forbes et al., 2018). The impact of hyperglycemia on the pathogenesis of viral-induced respiratory diseases remains unclear. Elevated BG level has been reported to increase the glucose concentration in airway epithelial secretion (Philips et al., 2003), which may disrupt the defensive capacity of airway epithelia. On the other hand, too rigid glucose control increases the risk of severe hypoglycemia, which can also result in an increased mortality (Rodriguez-Gutierrez et al., 2019). In this study, we found that compared to individuals with well-controlled BG, poor glycemic control in patients with COVID-19 and pre-existing T2D was associated with worse outcome, involving increased need for medical interventions, multi-organ injuries, and higher mortalities. In contrast to such patients, in those with glycemic variability between 3.9 and 10.0 mmol/L there was a significant association with reduced medical interventions, major organ injuries, and all-cause mortality. The findings here provide direct evidence supporting the recent suggestions for clinical management of T2D during COVID-19 (Bornstein et al., 2020).
Conclusions
In conclusion, T2D is an important risk factor for COVID-19 progression and adverse endpoints, and well-controlled BG, maintaining glycemic variability within 3.9 to 10.0 mmol/L, is associated with a significant reduction in the composite adverse outcomes and death. These findings provide critical insights into the clinical characteristics of patients with COVID-19 and pre-existing T2D and the possible avenues to improving their disease outcomes.
Limitations of Study
Due to the retrospective nature of the study and the unprecedented scale of the COVID-19 pandemic, this study has several limitations. First, all data were obtained from patient cohorts admitted in the 19 hospitals in Hubei Province, China. Therefore, the effect of BG control may be different among patients with COVID-19 and pre-existing T2D in the outpatient setting or in ethnically or geographically diverse populations. Second, we were not able to retrieve the pre-hospital status of T2D from the current cohort due to the urgent circumstance of the COVID-19 pandemic. The status of pre-hospital T2D could be significantly associated with numerous clinical parameters, which are known independent risk factors for the poor outcomes of COVID-19, including cardiovascular abnormalities and immunological dysfunction. While we performed PSM analysis to support the independent association of T2D with COVID-19 pathology, the influence from these cofounders cannot be fully excluded. Also, given this lack of pre-hospital data, it was not possible for us to access if BG levels changed as result of COVID-19 progression and/or severity. Third, the number of the patients with T2D and well-controlled BG in this study was modest and might not be powered sufficiently to reflect the overall complexity of the general population. Therefore, large-scale prospective cohort studies will be required in ethnically and geographically diverse cohorts to better understand the association and importance of BG control in the disease progression of COVID-19. Fourth, given the retrospective nature of the study, it was not possible for us to determine if active management of BG levels to a more normal range could ameliorate COVID-19 severity or adverse outcomes. Finally, individuals with type 1 diabetes were excluded from our analysis as there were too few of them in the initial cohort, but it is possible that blood glucose control may also affect their outcomes during COVID-19.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and Algorithms | ||
| R-3.6.3 | R Foundation for Statistical Computing | https://www.r-project.org/ |
| Graphpad Prism 8 | Graphpad | https://www.graphpad.com/ |
| SPSS statistics 23.0 | IBM Corporation | http://www.spss.com.hk/software/statistics/ |
| Adobe illustrator CC 2019 | Adobe company | https://www.adobe.com/cn |
| Coxme-2.2.16 | Therneau and Pankratz, 2003 | https://cran.r-project.org/web/packages/coxme/index.html |
| MatchIt-3.0.2 | Ho et al., 2007 | https://cran.r-project.org/web/packages/MatchIt/ |
| Matching-4.9-7 | Sekhon, 2011 | https://cran.r-project.org/web/packages/Matching/ |
| Tableone-0.11.1 | Kazuki Yoshida | https://github.com/kaz-yos/tableone |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to the Lead Contact, Hongliang Li (lihl@whu.edu.cn).
Materials Availability
The study did not generate any new reagents or materials.
Data and Code Availability
Data related to the findings of this study will be available from the corresponding author upon reasonable request. The research team will provide an email address for communication once the data are approved to be shared with others. The proposal with detailed aims, statistical plan, and other information/materials may be required to guarantee the rationality of requirement and the security of the data. The patient-level data, but without names and other identifiers, will be shared after review and approval of the submitted proposal and any related requested materials.
Method Details
Study Design and Participants
This was a multi-centered, retrospective cohort study and the study protocol was approved by the Institution Ethic Committee of Renmin Hospital of Wuhan University and Zhongnan Hospital of Wuhan University. The study design was also individually approved by each collaborating hospital or their institutional ethics boards. Given the urgency of the COVID-19 pandemic, the informed consent forms were waived by the ethics boards of the hospitals.
The study subjects included patients with COVID-19 diagnosed between December 30th, 2019 and March 20th, 2020. COVID-19 was diagnosed based on chest computed tomography (CT) manifestations and/or reverse transcription-polymerase chain reaction (RT-PCR) following the criteria of the New Coronavirus Pneumonia Prevention and Control Program (5th edition) published by the National Health Commission of China and WHO interim guidance (National Health Commission of China, 2020, World Health Organization, 2020, Zhang et al., 2020b). A total of 9,663 patients with COVID-19 were initially screened for the study. Data from individuals, however, were excluded if the subjects were younger than 18 or older than 75 or had incomplete medical records (e.g., transfer to any other hospital), acute lethal organ injury (e.g., acute myocardial infarction, acute coronary syndrome, acute pulmonary embolism, or acute stroke), decompensated or end stage of chronic organ dysfunction (e.g., decompensated cirrhosis, decompensated chronic renal insufficiency, severe congestive heart failure), pregnancy, type 1 diabetes, getational diabetes, or malignancy. For further study, the remaining cohort (n = 7,337) was categorized into diabetic (n = 952) and non-diabetic (n = 6,385) groups, according to the clinical diagnosis and/or medical history on admission (Figure 1).
Data Collection
The medical records of patients were analyzed by an integrated research team, including physicians, data scientists and statisticians. After deidentification process by removing the personal information (e.g., name and ID) of the participants and designating using a coding system, the basic information, epidemiological records, clinical manifestations, laboratory findings, radiographic characteristics from CT, treatments and outcomes during hospitalization were recorded. Major clinical symptoms (i.e., fever, cough, fatigue, dyspnea and comorbidities) were collected. The laboratory findings included routine blood test, fasting blood glucose (BG) and 2 h postprandial BG (2 hPG), C-reactive protein (CRP), procalcitonin, D-dimer, and serum indicators for liver injury, kidney injury and heart dysfunction. An experienced physician team reviewed, interpreted and double-checked all data to guarantee the accuracy.
Definition
The date of disease onset was defined as the day when the first symptom of COVID-19 was noticed. Severe cases were defined according to whether the patients had indication of respiratory rate > 30 breaths/min, or SpO2 ≤ 93% on room air, or PaO2/FiO2 ≤ 300 mmHg. T2D status was designated based on the patient’s medical history and guideline for the prevention and control of T2D in China (2017) (Chinese Diabetes Society, 2018). The timing and frequency of obtaining the fasting and 2-h postprandial BG (2 hPG) varied between individuals, depending on the necessity related to the severity of the comorbid T2D. Severe T2D status accordingly obtained more frequent BG surveillances. Glycemic variability was defined as the range between the lowest fasting BG (FBG) and 2 hPG level during the observation period. Well-controlled BG was defined when glycemic variability ranged from 3.9 to 10.0 mmol/L since the normal range of fasting BG is 3.9 - 6.1 mmol/L and lower than 10 mmol/L is the targeting level of 2 hPG in DM management according to the guideline for the prevention and control of T2D in China (2017) (Chinese Diabetes Society, 2018). Poorly-controlled BG was defined when the lowest fasting BG was above or equal 3.9 mmol/L and the highest 2 hPG level exceeded 10.0 mmol/L during the observation window.
Hypertension was diagnosed when systolic blood pressure is equal or above to 140 mm Hg and/or diastolic blood pressure is equal or above 90 mm Hg. ARDS and septic shock were defined following WHO interim guideline for “clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected.” Cardiac injury was defined when the serum level of cardiac injury biomarkers (cardiac troponin I [cTNI], cardiac troponin T [cTNT], or high sensitivity cardiac troponin I [hs-cTNI]] were higher than the upper limit of normal (ULN). Acute liver injury was defined when an acutely increased level of serum alanine aminotransferase (ALT) and serum alkaline phosphatase (ALP) of upper limit of normal (ULN) was observed (Marrone et al., 2017). Acute kidney injury was indicated by the value of serum creatinine level when it reached or exceeded 26.5 μmmol/L within 48 h (Kellum et al., 2012). Disseminated intravascular coagulation (DIC) was diagnosed according to the criteria published by the International Society on Thrombosis and Hemostasis (ISTH) (Gando et al., 2013). The primary endpoint of the study was 28-day all-cause death in COVID-19 patients. The secondary endpoints were occurrence of ARDS, septic shock, acute cardiac injury, acute kidney injury, or DIC.
Propensity Score-Matched Analysis
The variables potentially confounding the association between BG and the outcomes of COVID-19 were addressed using the propensity score-matching (PSM) method (Waljee et al., 2013). PSM requires a complete set of variables for every patient, randomly missing values of 10 selected parameters from noninvasive tests were therefore imputed. The non-parameter imputation method missForest was applied and the estimation of the imputation error was 4.08%. The bootstrapped cross validation was further applied and repeated 10 times for evaluating imputation performance on the training data after 10% more parameters were randomly introduced. The missForest method yielded 4.11% (IQR 1.91%–5.44%) differences between the datasets before and after parameters were introduced. The algorithm of the PSM is as follows:
Logistic(P) = Ln[P/(1-P)] = β0 + β1X1 + β2X2 + …+ βkXk p = e(β0 + β1X1 + β2X2 + …+ βkXk)/1+e(β0 + β1X1 + β2X2 + …+ βkXk). The P in this formula is the predicted probability of glucose control procedure, and the beta value before each variable is the regression coefficient of that variable.
The variables potentially confounding the association between BG and the outcomes of COVID-19 were addressed using the PSM method. When evaluating the association of glucose control level and outcomes, the PSM cohorts were identified by balancing age, gender, fever, cough, dyspnea, CT-diagnosed lung lesions, SpO2, breath frequency, incidence of increased leukocyte count, ALT, AST, D-dimer, creatinine, CRP, procalcitonin and decreased of lymphocyte count, as well as comorbidities (hypertension, coronary artery disease, cerebrovascular disease, chronic liver diseases and chronic renal disease). The propensity score, a predicted probability of glucose change contributed by the above variables, were estimated based on multivariable logistic regression model. The matching ratio was at 1:1 for well-controlled BG versus poorly-controlled BG during the entire hospitalization. Exact matching with a caliper size of 0.05 was applied for all matching pairs according to the propensity scores. Evaluation of the balance between covariates was conducted by estimating standardized differences before and after matching. Only those with small absolute value less than 0.1 were considered qualified balancing.
Sensitivity Analysis
The robustness of the association between BG level and all-cause mortality was assessed by analyzing E-value in the Mixed-effect Cox proportional hazards model to address unmeasured confounders using the methodology of VanderWeele and Ding (Haneuse et al., 2019, Mathur et al., 2018, VanderWeele and Ding, 2017). Two sensitivity analyses were performed to evaluate the robustness of propensity score-matched cohort analyses, among all pairs.
Quantification and Statistical Analysis
All statistical analysis was performed using R-3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS Statistics (version 23.0, IBM, Armonk, NY, USA). Data with continuous variables were presented as median and interquartile range (IQR), and data with categorical variables were presented as frequency rates and percentage (%). Comparison between 2 groups was analyzed using Student’s t tests (normally distributed) or Mann-Whitney U test (nonnormally distributed) for continuous variables. Comparison of categorical variables was analyzed by Fisher’s exact test or χ2 test. Generalized linear model (GLM) was performed to evaluate correlation between the median of blood glucose and factors related to viral infection or glycemic control in patients with diabetes. The risk for composite endpoints and corresponding hazard ratio (HR) were analyzed using Cox proportional hazard model and mixed-effect Cox model. The cumulative rates of death were plotted by applying Kaplan-Meier method. A difference with a two-side α less than 0.05 was considered statistically significant.
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (2016YFF0101504 to Z.-G.S.), the National Natural Science Foundation of China (81630011 to Haomiao Li, 81970364 to Z.-G.S., 81970070 to X.-J.Z., 81970011 to P.Z., and 81870171 to J.C.), the Major Research Plan of the National Natural Science Foundation of China (91639304 to Haomiao Li), the Hubei Science and Technology Support Project (2019BFC582, 2018BEC473, and 2017BEC001 to Haomiao Li), and Medical Flight Plan of Wuhan University.
Author Contributions
L.Z., Z.-G.S., X. Cheng, and J.-J.Q. designed study, collected and analyzed data, and wrote manuscript. H.W., W.W., P.Z., X.S., Chaozheng Zhang, L.B., D.X., M.-M.C., Y.L., Y. Yan, M.L., W.M., J.Z., L.L., G.C., P.L., B.X., Z.Z., Z.L., J.W., Haomiao Li, X.X., D.W., X.L., G.P., P.Y., J.Y., and Y. Yuan collected and revised clinical, laboratory, and radiological data. F.L. and Haomiao Li performed statistical analysis. J.X., X. Cheng, and M.X. reviewed, interpreted, and checked clinical data. X.-J.Z. and J.C. wrote the manuscript and provided valuable suggestions for study design and data analysis. X.H., J.G., B.-H.Z., and Hongliang Li contributed equally, designed the project, edited manuscript, and supervised the study. All authors have approved the final version of this paper.
Declaration of Interests
The authors declare no competing interests.
Published: April 30, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.cmet.2020.04.021.
Supplemental Information
References
- Alqahtani F.Y., Aleanizy F.S., Ali El Hadi Mohamed R., Alanazi M.S., Mohamed N., Alrasheed M.M., Abanmy N., Alhawassi T. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol. Infect. 2018;147:e35. doi: 10.1017/S0950268818002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., Boehm B., Amiel S., Holt R.I., Skyler J.S. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30152-2. Published online April 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido R., Jandeleit-Dahm K.A., Cao Z., Nesteroff S.P., Burns W.C., Twigg S.M., Dilley R.J., Cooper M.E., Allen T.J. Prevention of accelerated atherosclerosis by angiotensin-converting enzyme inhibition in diabetic apolipoprotein E-deficient mice. Circulation. 2002;106:246–253. doi: 10.1161/01.cir.0000021122.63813.32. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Diabetes Society Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition) Zhongguo Shiyong Neike Zazhi. 2018;38:53. [Google Scholar]
- Deng S.Q., Peng H.J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J. Clin. Med. 2020;9:E575. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes A., Murrells T., Mulnier H., Sinclair A.J. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:476–486. doi: 10.1016/S2213-8587(18)30048-2. [DOI] [PubMed] [Google Scholar]
- Fuso L., Pitocco D., Antonelli-Incalzi R. Diabetic lung, an underrated complication from restrictive functional pattern to pulmonary hypertension. Diabetes Metab. Res. Rev. 2019;35:e3159. doi: 10.1002/dmrr.3159. [DOI] [PubMed] [Google Scholar]
- Gando S., Wada H., Thachil J., Scientific and Standardization Committee on DIC of the International Society on Thrombosis and Haemostasis (ISTH) Differentiating disseminated intravascular coagulation (DIC) with the fibrinolytic phenotype from coagulopathy of trauma and acute coagulopathy of trauma-shock (COT/ACOTS) J. Thromb. Haemost. 2013;11:826–835. doi: 10.1111/jth.12190. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. Published online February 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneuse S., VanderWeele T.J., Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321:602–603. doi: 10.1001/jama.2018.21554. [DOI] [PubMed] [Google Scholar]
- Ho D.E., Imai K., King G., Stuart E.A. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit. Anal. 2007;15:199–236. [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum J.A., Lameire N., Aspelin P., Barsoum R.S., Burdmann E.A., Goldstein S.L., Herzog C.A., Joannidis M., Kribben A., Levey A.S. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012;2:1–138. [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcsar K.A., Coleman C.M., Beck S.E., Frieman M.B. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4:131774. doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Nathella P., Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology. 2017;152:13–24. doi: 10.1111/imm.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone G., Vaccaro F.G., Biolato M., Miele L., Liguori A., Araneo C., Ponziani F.R., Mores N., Gasbarrini A., Grieco A. Drug-induced liver injury 2017: the diagnosis is not easy but always to keep in mind. Eur. Rev. Med. Pharmacol. Sci. 2017;21(Suppl):122–134. [PubMed] [Google Scholar]
- Mathur M.B., Ding P., Riddell C.A., VanderWeele T.J. Web site and R package for computing E-values. Epidemiology. 2018;29:e45–e47. doi: 10.1097/EDE.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of China . National Health Commission of China; 2020. New Coronavirus Pneumonia Prevention and Control Program.http://www.nhc.gov.cn [Google Scholar]
- Philips B.J., Meguer J.X., Redman J., Baker E.H. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003;29:2204–2210. doi: 10.1007/s00134-003-1961-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gutierrez R., Gonzalez-Gonzalez J.G., Zuñiga-Hernandez J.A., McCoy R.G. Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ. 2019;367:l5887. doi: 10.1136/bmj.l5887. [DOI] [PubMed] [Google Scholar]
- Sekhon J.S. Multivariate and propensity score matching software with automated balance optimization: the matching package for R. J. Stat. Softw. 2011;42 doi: 10.18637/jss.v042.i07. [DOI] [Google Scholar]
- Therneau T.M., Pankratz P.M.G.S. Penalized survival models and frailty. J. Comput. Graph. Stat. 2003;12:156–175. [Google Scholar]
- Van den Berghe G., Wilmer A., Hermans G., Meersseman W., Wouters P.J., Milants I., Van Wijngaerden E., Bobbaers H., Bouillon R. Intensive insulin therapy in the medical ICU. N. Engl. J. Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- VanderWeele T.J., Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann. Intern. Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- Waljee A.K., Mukherjee A., Singal A.G., Zhang Y., Warren J., Balis U., Marrero J., Zhu J., Higgins P.D. Comparison of imputation methods for missing laboratory data in medicine. BMJ Open. 2013;3:e002847. doi: 10.1136/bmjopen-2013-002847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Gao P., Zhang M., Huang Z., Zhang D., Deng Q., Li Y., Zhao Z., Qin X., Jin D. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. Published online February 7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance. [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. Published online February 24, 2020. [DOI] [PubMed] [Google Scholar]
- Xu M., Liu P.P., Li H. Innate immune signaling and its role in metabolic and cardiovascular diseases. Physiol. Rev. 2019;99:893–948. doi: 10.1152/physrev.00065.2017. [DOI] [PubMed] [Google Scholar]
- Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y., Sun G.Z., Yang G.R., Zhang X.L., Wang L. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. Published online February 19, 2020. [DOI] [PubMed] [Google Scholar]
- Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J., Liu Y.M., Zhao Y.C., Huang X., Lin L. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.317134. Published online April 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data related to the findings of this study will be available from the corresponding author upon reasonable request. The research team will provide an email address for communication once the data are approved to be shared with others. The proposal with detailed aims, statistical plan, and other information/materials may be required to guarantee the rationality of requirement and the security of the data. The patient-level data, but without names and other identifiers, will be shared after review and approval of the submitted proposal and any related requested materials.