Abstract
Objective:
To generate preliminary data about the subgingival microbiome of pregnant, African American women to calculate power for a future larger study and to explore associations among the microbiome, periodontal inflammation, and preterm birth.
Design:
Comparative descriptive pilot study design.
Setting:
Urban area in southeastern United States.
Participants:
Thirty-four African American women in the third trimester of pregnancy.
Methods:
Based on visual assessment, participants were placed in two groups: healthy gingiva and gingivitis. Saliva samples were analyzed for interleukin-1 beta (IL-1β), matrix metalloproteinase-8 (MMP-8), and C-reactive protein (CRP). DNA was extracted from subgingival plaque samples, and amplicons of the fourth hypervariable region were sequenced.
Results:
We found no differences in overall microbiome diversity between the healthy gingiva (n=22) and the gingivitis (n=12) groups, although significant differences were found among the bacterial taxa present. The gingivitis group had higher levels of salivary IL-1β and MMP-8, while CRP was not different between groups. Overall microbiome diversity was positively associated with CRP level. We found no significant relationships among the subgingival microbiome, periodontal inflammation, and preterm birth.
Conclusion:
Gingivitis in pregnancy did not appear to shift overall composition or diversity of the subgingival microbiome, although differences in several bacterial taxa suggest that inflamed gingiva in pregnant women are associated with a disruption in the stability of the subgingival microbiome. A correlation between the abundance of bacteria and CRP also suggests an association between the microbiome and systemic inflammation. These findings provide support for future research about how the oral microbiome and progression of periodontal disease in pregnant women link to adverse pregnancy outcomes.
Keywords: pregnancy, gingivitis, premature birth, microbiota, oral health, inflammation, gestational age, biomarkers
Precis
Gingivitis in pregnancy is associated with perturbations in the composition of the subgingival microbiome; increased abundance of bacteria may trigger an acute-phase, systemic, inflammatory response.
Oral health is integral to overall good health and well-being (Petersen, 2009). Pregnant women are particularly susceptible to alterations in oral health because of a variety of factors, including hormonal shifts that contribute to inflammation and decreased immunocompetence (Steinberg, Hilton, Iida, & Samelson, 2013); changes in dietary habits, such as frequent snacking or increased consumption of carbohydrate rich and decay-promoting foods; stomach acids from nausea and vomiting that contribute to the breakdown of tooth enamel; and decreased likelihood of seeking dental care during pregnancy (Detman, Cottrell, & Denis-Luque, 2010). Furthermore, assessment and promotion of oral health is often overlooked by prenatal health care providers (George et al., 2012).
In the United States, it is estimated that almost half of all adults older than the age of 30 have periodontitis (Eke et al., 2015). Gingivitis, the most common oral disease in pregnancy, has a prevalence of 50% to 70% (Anil et al., 2015). Symptoms of gingivitis in pregnancy typically emerge during the first trimester (Giglio, Lanni, Laskin, & Giglio, 2009) and present as red, swollen gingival margins and bleeding that occurs with the slightest provocation (Han, 2011) Gingivitis, which represents the milder form of periodontal disease, is an inflammatory condition that affects the soft and hard structures that support the teeth; it is a direct, immune-inflammatory response to the microorganisms that inhabit the biofilm (plaque) at the interface between the surface of the tooth and the gingiva (Lang, Schätzle, & Löe, 2009). Underlying this condition is a complex interaction between these microorganisms and the host defense system. In response to microbial pathogens, host mediated destruction of periodontal tissue is caused by hyperactivity of leukocytes and the generation of cytokines, eicosanoids, and matrix metalloproteinases (AlMoharib et al., 2014). These biomarkers of the inflammatory process can be found in gingival crevicular fluid and whole saliva (AlRowis et al., 2014). For some, progressive gingivitis leads to periodontitis, which is a polymicrobial inflammatory disease that involves the irreversible destruction of supportive soft tissue and bone and ultimately leads to tooth loss (Lang et al., 2009). The incidence of periodontitis in pregnant women ranges between 5% and 20% (Polyzos et al., 2010).
Periodontal Disease and Preterm Birth
In 1996, Offenbacher reported that women with periodontal disease had a seven-fold increase in the risk of preterm birth. Since then, results from several other studies have demonstrated an association between periodontal disease and preterm birth. For example, after controlling for other known risk factors of preterm birth, moderate to strong associations were found between periodontal disease and/or inflammation and spontaneous preterm birth (Guimarães, Silva-Mato, Miranda Cota, Siqueira, & Costa, 2010; Khader et al., 2009; Macedo et al., 2014; Stadelmann et al., 2014). The evidence, however, is not clear (Hunter & Yount, 2011). In critical reviews of the literature, authors cautioned that the association should be considered in light of a high degree of variability in study populations, recruitment, and assessment (Agueda, Echeverría, & Manau, 2008; Ide & Papapanou, 2013). Furthermore, the treatment of periodontal disease during pregnancy has yielded mixed outcomes (Jeffcoat et al., 2011; Macones et al., 2010; Polyzos et al., 2010). These inconsistencies suggest a gap in knowledge regarding the underlying mechanisms of the observed association between periodontal disease and preterm birth.
Within-Race Study of the Microbiome
African American women are at increased risk for preterm birth and periodontal disease compared to non-Hispanic white women (Martin, Hamilton, Osterman, Driscoll, & Drake, 2018). Socioeconomic disparities related to prenatal oral hygiene practices and dental service utilization during pregnancy also exist (Boggess et al., 2010; Eke, Dye, Wei, Thornton-Evans, & Genco, 2012) and are major, public health concerns. Several structural and behavioral factors may be at play, including limited health care resources (Lee, Milgrom, Huebner, & Conrad, 2010), lack of understanding about the importance of prenatal, preventive, oral health practices (Marchi, Fisher-Owens, Weintraub, Yu, & Braveman, 2010), and poor adherence to recommendations from dentists and maternal health care providers regarding oral health care (Lee et al., 2010; George et al., 2012). In order to investigate the high rate of preterm birth in African American women and prevent confounding that may result from comparison across racial groups, studies that focus on risk within a racial subgroup are needed.
Link between Periodontal Disease and Preterm Birth
One theory regarding the underlying link between periodontal disease and preterm birth relates to an immune-inflammatory mechanism. Healthy pregnancy appears to have unique inflammatory phases that correspond to implantation of the conceptus and fetal development (Mor, Aldo, & Alvero, 2017). These inflammatory phases can be disrupted by external stimuli such as infections (Mor et al., 2017) that place the fetus and the pregnancy at risk (Han, 2011). Although vaginal infections that occur during pregnancy are thought to be the most common source of systemic inflammation (Romero, Dey, & Fisher, 2014), periodontal infections that initiate systemic inflammation or infectious agents that actually translocate through the bloodstream to the uterus may likewise increase risk during pregnancy (Madianos, Bobetsis, & Offenbacher, 2013). During the period between successful implantation and onset of labor, the woman, placenta, and fetus are symbiotic, and the predominant environment is shifted toward an anti-inflammatory state (Mor et al., 2017) mediated by anti-inflammatory cytokines such as interleukin- (IL) 4, IL-5, IL-10, IL-13, and granulocyte-macrophage stimulating factor. This shift is thought to be critical to prevent the rejection of the fetal allograft (Challis et al., 2009). Dysregulation of this anti-inflammatory state would be represented by a shift toward the pro-inflammatory cytokines represented by IL-1, IL-2, IL-6, IL-12, IL-15, IL-18, interferon-γ, and tumor-necrosis factor-α (Challis et al., 2009). An elevation in pro-inflammatory cytokines caused by periodontal disease could then potentially play an important role in this dysregulation of the normal immunological state during pregnancy (Han, 2011). Supporting this theory are studies that demonstrate that pregnant women with periodontal disease have elevated levels of plasma C-reactive protein (CRP), which indicates elevated, systemic inflammation (Sharma, Ramesh, & Thomas, 2009). Evidence also suggests that periodontal disease is associated with an increased level of pro-inflammatory cytokines in the peripheral circulation (Andrukhov et al., 2011).
The Subgingival Microbiome
Despite the suspected connection between periodontal disease and preterm birth, the current knowledge about the subgingival bacterial environment that is most closely related to periodontal disease in pregnant women is incomplete. As a result, our ability to understand its relationship to inflammation and to assess or treat early signs of periodontal infection/inflammation as a potentially modifiable risk factor for preterm birth is limited. Several pathogens are thought to play roles in periodontal infection and inflammation in non-pregnant individuals, including Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia (Lamont, Koo, & Hajishengallis, 2018) and Prevotella intermedia (Lima et al., 2015). Evidence implicates these organisms in periodontal disease in pregnancy but is inconsistent (Adriaens, Alessandri, Spörri, Lang, & Persson, 2009; Carrillo-de-Albornoz, Figuero, Herrera, & Bascones-Martínez, 2010; Novak et al., 2008; Vettore, Leao, Leal, Feres, & Sheiham, 2008). This may be because older methodologies in which only specific periopathogens were targeted were not powerful enough to identify the hundreds of other bacterial species in the oral cavity (Dewhirst et al., 2010).
More recent research findings about the oral microbiome based on next generation sequencing in non-pregnant populations suggest that our understanding of infection/inflammation must move from a focus on individual pathogens to the ecological context of a particular microbial community (Abusleme et al., 2013; Duran-Pinedo et al., 2014; Griffen et al., 2012). Also, a state of periodontal inflammation may represent a shift in the overall ecological balance of microbial flora rather than the appearance of individual pathogens. Given this, it is critical to investigate the subgingival microbiome of pregnant women to identify associations among the microbiome, inflammation, and preterm birth. This is particularly the case for African American women who are at increased risk for periodontal disease and preterm birth (Eke et al., 2015; Martin et al., 2018). Therefore, the primary objective of this pilot study was to generate preliminary data about the characterization of the subgingival microbiome of pregnant, African American women that could be used to calculate power for a larger study. The secondary objective was to explore associations among the maternal subgingival microbiome, inflammatory indicators of periodontal disease, and preterm birth.
Methods
Design
We used a comparative descriptive design for this pilot study and obtained approval from the Emory Institutional Review Board before the commencement of study activities.
Setting and Sample
We enrolled 34 pregnant, African American women from two prenatal clinics located in Atlanta, GA, between December 2016 and February 2017. Participants were recruited from an ongoing, larger investigation of the associations among a woman’s oral, vaginal, and gut microbiomes during pregnancy and preterm birth (Corwin et al., 2017). Inclusion criteria for the larger, parent study included self-identification as African American, 18 to 40 years of age, ability to speak and read English, singleton gestation, no chronic medical problems, and no prescription medications. Recruitment of women to participate in this pilot study occurred during their third trimesters of pregnancy when participants in the parent study were asked if they would also be willing to allow inspection of the gingival tissue and collection of saliva and subgingival microbiome samples. To be included, women had to have a minimum of 20 natural teeth and no professional dental cleaning in the past 3 months.
Results from two recent studies conducted in non-pregnant populations (Abusleme et al., 2013; Griffen et al., 2012) indicated that effect sizes for differences in the Shannon and Chao indices (used to measure the richness and evenness of microbial communities) range from large to very large (.82 – 1.4). Power analysis estimates from Gpower3.1 software (Faul, Erdfelder, Buchner, & Lang, 2009) indicated that 40 total participants (20 per group) were required to detect large effect sizes with 80% power. Given the one-year time constraint of the funding mechanism, and pilot nature of this study, we determined that a study with 30 total participants (15/group) would produce sufficiently large effect sizes with 70% power for foundational data to support the design of future adequately powered subgingival microbiome studies.
Procedures
Three teeth (#25, 28, 30) from the right lower quadrant of each participant’s mouth were assessed for visual signs of gingival inflammation using the Modified Gingival Index (MGI) (Lobene, Weatherford, Ross, Lamm, & Menaker, 1985) by a single, trained examiner. MGI scores for the three teeth were averaged, and participants with mean MGI scores less than or equal to 1 were placed in the healthy group; those with scores greater than 1 were assigned to the gingivitis group. Saliva and subgingival plaque samples were then collected from participants in both groups and were visually assessed using the sterile paperpoint method (Zhou & Li, 2015). Supragingival plaque was first removed with sterile gauze and each tooth site held dry using cotton rolls while one sterile paperpoint was inserted into the pocket of each tooth for 20 seconds. The three paperpoints were pooled and immediately placed in 750uL of MoBio buffer contained in sterile MoBio bead tubes (MoBio laboratories, Inc., Carlsbad, CA). Saliva and plaque samples were placed on ice and transported for storage at −80°C until ready for analysis.
DNA isolation and 16S rRNA gene library preparation and sequencing.
Specimens were sent to Microbiome Insights (Vancouver, BC, Canada), leaders in the field of microbiome research, for extraction and sequencing. Pooled paper points were homogenized, and DNA was isolated using the MoBio PowerMag Soil DNA Isolation Kit. The highly conserved 16S rRNA gene, which is widely used to characterize taxonomic diversity in microbial communities, was polymerase chain reaction-amplified with dual-barcoded primers targeting the fourth hypervariable region as per the protocol of Kozich, Westcott, Baxter, Highlander, and Schloss (2013). Normalized library concentrations of 1–2 ng/μl (as per the specifications of the Sequal Prep normalization kit) were used. Amplicons were sequenced with an Illumina MiSeq using the 250-base pair paired-end kit (v.2). Sequences were denoised, taxonomically classified using a built-in RDP (Ribosomal Database Project) Classifier with the Greengenes (version 13_8_99) reference database, and clustered into 97%-similarity operational taxonomic units (OTUs) with the Mothur software package (v. 1.39.5; Schloss et al. 2009), following the recommended procedure. The default Mothur Needleman alignment was used to join reads. Ambiguous base pairs in joined reads were filtered out and a maximum length of 275 base pairs was specified.
Potential for contamination was addressed by co-sequencing DNA amplified from specimens and from four each of template-free controls and extraction kit reagents processed the same way as the specimens. Two positive controls consisting of cloned SUP05 DNA were also included (number of copies = 2*10^6). Operational taxonomic units were considered putative contaminants and were removed if their mean abundance in controls reached or exceeded 25% of their mean abundance in specimens. An average of 12,382 quality-filtered reads were generated per sample. The resulting dataset had 4,248 unique OTUs.
Salivary immune marker assays.
Saliva samples were used for immunoassay analysis to determine the concentrations of Interleukin-1β (IL-1 β), matrix metalloproteinase (MMP-8), and C-reactive protein (CRP). Enzyme-linked immunosorbent assays for IL-1β, CRP (Salimetrics, Carlsbad, CA), and MMP-8 (Fisher Scientific, Hampton, NH), and appropriate, compatible software programs were used to generate the raw data reports. All study samples were assayed following the manufacturer’s guidelines.
Measures
The Modified Gingival Index (MGI) is based on visual inspection, eliminates the need for probing or pressure, and therefore does not disrupt the subgingival plaque (Lobene et al., 1985). The scale of the index makes early detection and subtle changes in gingival inflammation detectable (Lobene et al., 1985). This instrument has demonstrated interrater reliability for early visual changes in gingivitis, with an average interrater correlation coefficient of .81 (Lobene et al., 1985). The MGI also has a positive correlation (r = .88, p = .0094) with dental plaque (Sosa-Jurado et al., 2014) and satisfies the criteria for a gingival index specified in guidelines from the Council on Scientific Affairs (Council on Dental Therapeutics, 1986) and has been generally accepted for use in clinical trials (Ata-Ali et al., 2015; He, Qu, Chang, & Wang, 2018; Kinane et al., 2015).
Clinical and Demographic Data
Additional data were obtained from the parent study data, including self-reported clinical variables (smoking status, illicit substance use, oral infection, and oral self-care by self-report), demographic variables (age, income, and education) by self-report verified by clinical record, and gestational age at birth by clinical record review. Gestational age at birth was based on last menstrual period (LMP) and/or ultrasound before 14 weeks’ gestation according to standard clinical criteria, with a preterm birth defined as a birth occurring prior to 37 weeks gestation (American College of Obstetricians and Gynecologists, 2014).
Analysis
Clinical and demographic data were compared between groups using independent t-tests and chi-square tests as appropriate. Inflammatory markers were non-normally distributed as determined by the Shapiro-Wilk test; thus, the logarithmic function was used to transform the observations, after which independent t-tests were used to compare the two groups.
Operational taxonomic units, which are clusters of organisms grouped by DNA similarity, were aggregated into each taxonomic rank (phylum, class, order, family, genus, species), and the most abundant were plotted according to relative abundance. Generating diversity scores that could be used to calculate power for a future larger study was a primary objective of this pilot study. Alpha diversity, a measure of species diversity within a particular ecosystem, was estimated with Chao1 and Shannon indices using the Vegan – R package (Oksanen, 2015) on raw OTU abundance tables after filtering out contaminants. Chao1 describes richness or the total number of species present in a community, i.e. the higher the Chao1 score, the greater the number of species present (Lemos, Fulthorpe, Triplett, & Roesch, 2011). The Shannon index provides richness with evenness (Magurran, 2013). Communities numerically dominated by one or a few species exhibit a low Shannon score, whereas communities in which abundance is distributed equally among species will exhibit high evenness. Chao1 calculations were performed with 1000 permutations at a rarefaction depth of 3000 reads. The significance of diversity differences was tested with an analysis of variance (ANOVA). To estimate beta diversity across samples, OTUs that occurred in fewer than 10% of the samples with a count of less than 3 were excluded, then Bray-Curtis indices were computed.
We visualized beta diversity, which describes differences across samples, using non-metric multidimensional ordination. Variation in community structure was assessed with permutational multivariate ANOVA with the gingivitis group as the main fixed factor and using 4,999 permutations for significance testing. We used general linear models using the DESeq2 package (Love, Huber, & Anders, 2014) to determine the statistical significance of differences in abundance of individual OTUs between groups with p-adjustment using the Benjamin Hochberg method (Love et al., 2014). We investigated associations among the alpha-diversity scores, transformed levels of biochemical indicators of periodontal disease (interleukin-1β, matrix metalloproteinase, and CRP) and gestational age at birth (in weeks) using Pearson product-moment correlation coefficients.
Results
The mean age of participants was 26.03 ± 5.12 years. Other sociodemographic and oral health characteristics of the participants can be found in Table 1. Of the 34 participants enrolled, 22 had healthy gingiva and 12 had visual signs of gingivitis as indicated by mean MGI scores greater than 1. We found no significant differences between groups in age, income level, education level, smoking, other drug use, reported mouth/gum infection, or oral self-care habits.
Table 1.
Sociodemographic and Oral Health Characteristics of Participants (N = 34)
| Characteristic | Frequency (%) |
|---|---|
| Education | |
| < High School Completion | 4 (11.8) |
| ≥ High School Completion | 27 (79.4) |
| Missing | 3 (8.8) |
| Income | |
| < 100% Federal Poverty Level | 17 (50) |
| ≥ 100% Federal Poverty Level | 14 (41.2) |
| Missing | 3 (8.8) |
| History of mouth/gum infection | |
| Yes | 5 (14.7) |
| No | 26 (76.5) |
| Missing | 3 (8.8) |
| Brushed teeth in the last 2 days | |
| Yes | 27 (79.4) |
| No | 1 (2.9) |
| Missing | 6 (17.6) |
| Flossed in the last month | |
| Yes | 11 (32.4) |
| No | 20 (58.8) |
| Missing | 3 (8.8) |
| Visited the dentist in the last month | |
| Yes | 3 (8.8) |
| No | 28 (82.4) |
| Missing | 3 (8.8) |
| Smoked cigarettes in the last month | |
| Yes | 2 (5.8) |
| No | 32 (24.1) |
| Missing | 1 (2.9) |
| Other drug use | |
| Yes | 1 (2.9) |
| No | 33 (97.1) |
Characterization of the Oral Microbiome in Periodontal Health and Disease
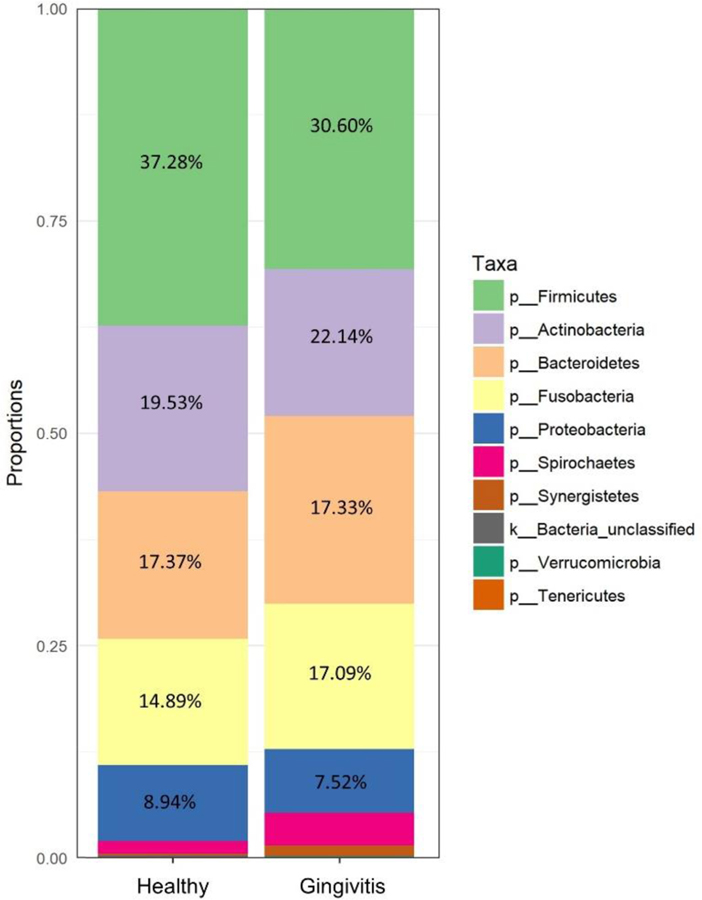
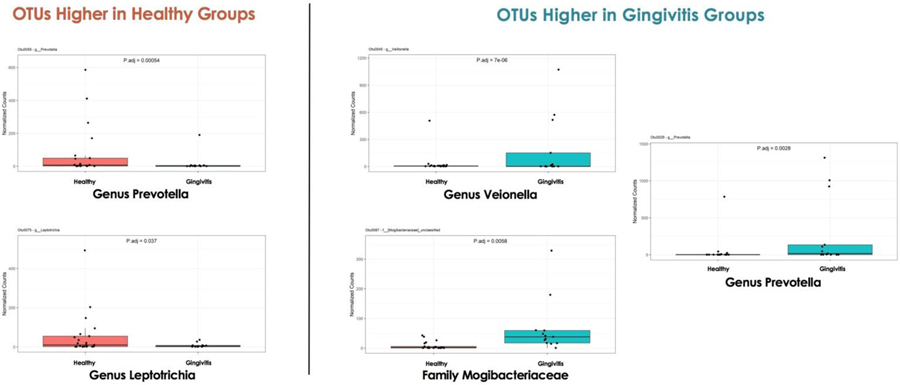
As illustrated in Figure 1, comparison of the two groups at the level of the phylum, revealed a similar, overall microbiome composition between groups. However, significant differences existed in several OTUs that were classified at the genus and family levels. OTUs that belonged to the genera Prevotella and Leptotrichia were more prevalent in healthy women, whereas OTUs that belonged to the family Mogibacteriaceae and genera Veionella and Prevotella were more prevalent in the gingivitis group (p < .01, see Figure 2).
Figure 1.
Taxonomic composition of the most abundant phyla in the healthy and gingivitis groups. Other represents unclassified and lower abundance taxa.
Figure 2.
Individual operational taxonomic unit (OTU) differences between healthy and gingivitis groups.
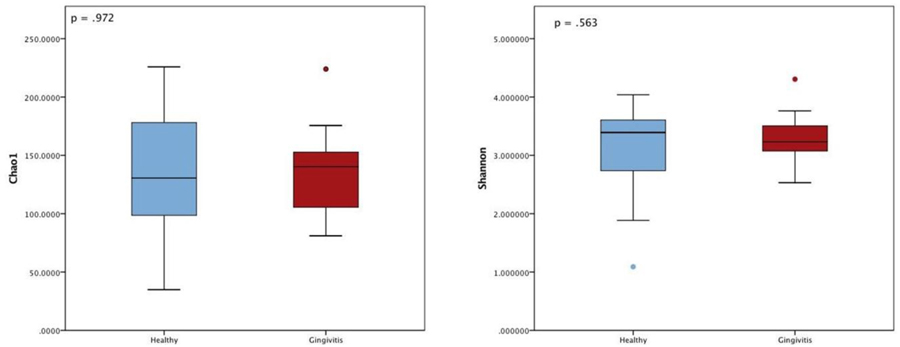
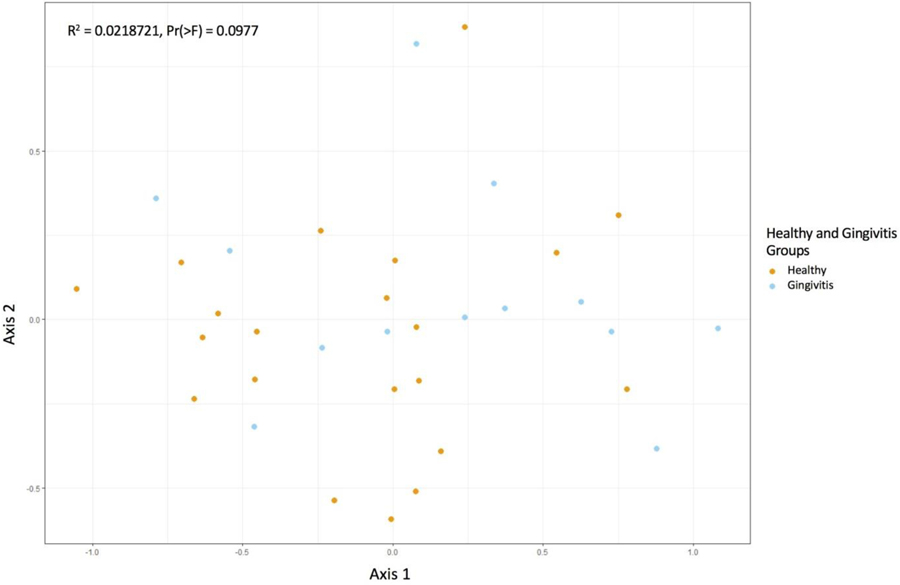
Beyond taxonomic description of the subgingival microbiome, our primary objective was to generate diversity scores that would be used to calculate power for a future larger study. We found no significant differences in measures of alpha-diversity between groups in the Chao1 or Shannon index, meaning that the two groups were similar in terms of the abundance and evenness of OTUs within each sample (p > .05, see Figure 3). Similarly, a visualization of beta diversity using non-metric multidimensional ordination and permutational multivariate ANOVA using distance matrices confirms that the groups were similar (Pr(>F) = 0.0977), see Figure 4).
Figure 3.
No significant difference in Chao1 and Shannon alpha diversity between groups indicates the two groups were similar in terms of the abundance and evenness of operational taxonomic units within each sample.
Figure 4.
This non-metric multidimensional ordination plot reflects the beta diversity between healthy and gingivitis groups. Each point represents one microbiome sample. The orange dots reflect healthy group samples and the blue dots represent gingivitis group samples. Points that cluster together reflect similarity. The lack of clustering indicates that the healthy and gingivitis communities are not very different from one another. We confirmed this statistically with the R-squared value, which showed that the percentage of variation between groups was not significant.
Associations among Oral Microbiome, Biochemical Indicators of Periodontal Disease, and Preterm Birth
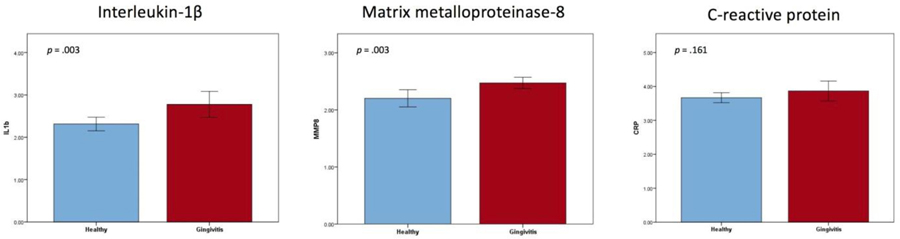
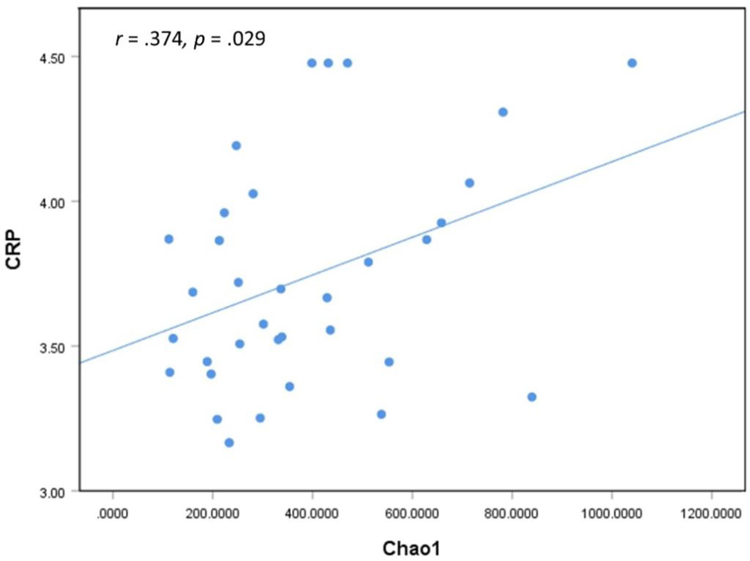
Our results indicated that women in the gingivitis group had significantly higher levels of salivary interleukin-1β (IL-1β) and matrix metalloproteinase (MMP-8); salivary CRP levels did not significantly differ (see Figure 5). We found moderate to strong positive correlations between all three salivary inflammatory markers (IL-1β, MMP-8, CRP; see Table 2). A weak positive association was found between alpha-diversity (Chao1) and CRP (r = .374, p = .029; see Figure 6). We found no relationship between gestational age at birth and any of the inflammatory markers or alpha-diversity.
Figure 5.
Salivary interleukin −1β levels and matrix metalloproteinase levels were significantly higher in the gingivitis group, whereas salivary CRP levels did not significantly differ between groups.
Table 2.
Correlations between Three Inflammatory Markers (N = 34)
| Inflammatory Marker | 1 | 2 | 3 |
|---|---|---|---|
| 1. Interleukin-1 beta | -- | .743** | .603** |
| 2. Matrix metalloproteinase-8 | .743** | -- | .409* |
| 3. C-reactive protein | .603** | .409* | -- |
p < 0.05
p < 0.01
Figure 6.
A weak, positive correlation between Chao1 (a measure of alpha-diversity) and C-reactive protein (CRP) suggests that increased richness or abundance of microorganisms in the subgingival space triggers an acute-phase response and elevates CRP levels.
Discussion
Despite the prevalence of gingivitis in pregnancy and its observed associations with preterm birth and other adverse conditions, little is known about the subgingival bacterial environment of pregnant women. In order to develop nursing assessment and interventions that can mitigate this risk, it is critical to understand the microbiome of pregnancy gingivitis and its relationship with local inflammation and adverse birth outcomes. The aim of this study, therefore, was to characterize the subgingival microbiome of a small group of pregnant African American women in order to generate preliminary data that would help us to calculate power for a larger study. Additionally, we aimed to explore associations between the maternal subgingival microbiome, inflammatory indicators of periodontal disease, and preterm birth.
The top six phyla represented in the subgingival space of both participant groups were Firmicutes, Actinobacteria, Bacteroidetes, Fusobactera, Proteobacteria, and Spirochaetes. In a previous study of non-pregnant populations, researchers used 16S rRNA sequencing and reported that up to 96% of the bacteria in our mouths belong to one of these six phyla (Dewhirst et al., 2010). Broadly speaking, pregnancy or gingivitis during pregnancy does not appear to shift the taxonomic composition of the subgingival space.
Despite this overall similarity, differences in individual OTUs existed between groups (see Figure 2). The five OTUs that differentiated participants with healthy gingiva from those with signs of gingivitis were all characterized as taxa that are commonly found in the oral cavity and contain species that are known periodontal pathogens or opportunistic pathogens. For example, the genus Prevotella is the largest genus in the phylum Bacteroidetes (Dewhirst et al., 2010) and contains species such as Prevotella intermedia that are known periodontal pathogens (Cobb et al., 2017). Leptotrichia and Veionella contain species that are typically found in the oral cavity and are known to act as opportunistic pathogens (Eribe & Olsen, 2017) or to stimulate the growth of other pathogenic organisms (Eribe & Olsen, 2017; Knapp et al., 2017). Although our ability to draw species level conclusions was limited by our choice to sequence the fourth hypervariable region of the 16S rRNA gene, our results confirmed findings from contemporary microbiome studies that indicated that diseased gingiva in pregnant women appeared to result from perturbations within the ecology of normally existing oral flora rather than singular pathogens (Costalonga & Herzberg, 2014).
These perturbations can lead to shifts in the diversity of microbial communities that affect the balance of commensal organisms to pathogenic organisms and are associated with periodontal disease in non-pregnant populations (Cobb et al., 2017). Microbial community diversity can be described two ways. Alpha diversity describes how many OTUs are present in a community and how evenly they are distributed; beta diversity describes the diversity between two different environments. Chao1 and Shannon are two common indices used to describe diversity within a bacterial community as previously described. Based on findings in non-pregnant populations, alpha diversity should be higher in individuals with periodontal disease (Abusleme et al., 2013; Griffen et al., 2012) since gingival bleeding creates a nutrient source, and deepened periodontal pockets create anaerobic niches. Both provide ideal conditions for the proliferation of more organisms and a successively more diverse bacterial community. Despite the differences in specific OTUs, we found no significant difference in alpha or beta diversity between the two participant groups, which suggests that these individual OTU differences were not large enough to affect overall community diversity. This is likely attributable to the limited range of disease in this cohort since the gingival status of most women indicated only mild inflammation.
Another potential consideration is the race of the participants. Recently, researchers have begun to elucidate substantial divergences in the microbiome between individuals from different races and ethnicities (Gupta, Paul, & Dutta, 2017). For example, Mason and colleagues (2013), found that 33 of 77 genera in the oral cavity significantly differed in abundance between non-Hispanic Black, non-Hispanic White, Chinese, and Hispanic individuals in the United States and that it was possible to identify an individual’s ethnicity from subgingival microbial signatures. Although the microbiome profile of the African American women in our sample does not clearly distinguish itself as unique, subtle variations in taxonomic abundance may have affected the diversity scores and contributed to the lack of difference between groups.
Interleukin-1β and MMP-8 are known salivary biomarkers of periodontal disease (AlMoharib et al., 2014). Because periodontal disease represents an inflammatory process accompanied by destruction of connective tissue, circulating molecules such as IL-1β and MMP-8 are integral to these processes and can be detected in the saliva (Sexton et al., 2011). Interleukin-1β was shown to function in concert with other cytokines to regulate the localized inflammatory response in the periodontium (AlRowis et al., 2014). Matrix metalloproteinase-8 is a leading enzyme in the destruction of tissue in individuals with periodontal disease (AlRowis et al., 2014). As expected, participants in the gingivitis group had significantly higher salivary levels of IL-1β and MMP-8 than participants with healthy gingiva.
In our study, CRP was not different between groups. C-reactive protein is produced by the liver and is a systemic marker released during the acute phase of an inflammatory response (AlMoharib et al., 2014). C-reactive protein reaches the saliva via gingival crevicular fluid or salivary glands (AlMoharib et al., 2014) and is elevated in the saliva of individuals with chronic or progressive periodontal disease (Miller et al., 2010; Shojaee et al., 2013). Plasma CRP has also been shown to be elevated in pregnant women with periodontal disease (Sharma et al., 2009). In our study, however, it is conceivable that gingivitis, a milder form of periodontal disease exhibited by most participants in the gingivitis group, may not have triggered enough of an acute-phase response to elevate systemic levels of CRP (Jain, Gautam, & Naseem, 2011).
Despite this, our analysis provides one clue to suggest an association between the subgingival microbiome and systemic inflammation. The correlation between CRP and Chao1 diversity suggests that increased richness or abundance of microorganisms in the subgingival space triggers an acute-phase response and elevates CRP levels. This important finding suggests an association between the subgingival microbiome in pregnancy and systemic inflammation, which may ultimately affect pregnancy health. Although these results are preliminary, they strongly indicate the need for further investigation of the microbiome as an explanatory mechanism that links periodontal disease in pregnancy with adverse birth outcomes, such as preterm birth.
Our study findings do not have a direct application to nursing practice or the improvement of maternal newborn health outcomes; however, our study lays the groundwork for future, more in depth research investigating the maternal oral microbiome and its relationship to maternal newborn health. Future, microbiome research studies are important for researchers who seek to understand the biobehavioral underpinnings of maternal newborn health and for clinicians who directly assess, educate, and care for pregnant women. A deeper understanding of the function and diversity of microbes in the mouth will inform both preconception and prenatal education regarding the importance of oral hygiene behaviors and oral healthcare. Future research may also be instrumental in the development of new tools and diagnostics shaping prenatal oral health assessment, education, and intervention guidelines that promote a healthy mouth and good birth outcomes.
Limitations
Limitations of this study include its cross-sectional design and limited range of periodontal disease severity; longitudinal investigation of the oral microbiome in pregnancy with a larger cohort with a more diverse range of disease is needed. Furthermore, bias may have been introduced since the investigator performed the visual assessment, the group assignment, and the collection of specimens. In spite of these limitations, the results of this pilot study provide new information about the subgingival microbiome of pregnant women with healthy and diseased gingiva.
Conclusion
Because of the effects of hormonal changes in pregnant women, the likelihood of a hyperreactive inflammatory response in pregnancy is heightened (Steinberg et al., 2013). Gingivitis in pregnancy is an early form of periodontal disease that has been associated with preterm birth. Our findings provide an initial description of the microbial environment of the subgingival space of African American women during pregnancy and the relationships among the subgingival microbiome, inflammatory markers of periodontal disease, and gestational age at birth. Information gained from this study provides preliminary support for future research investigating the progression of periodontal disease in pregnant women and helps explain how this disease might be linked to adverse pregnancy outcomes.
Footnotes
Disclosure
The authors report no conflict of interest or relevant financial relationships.
Contributor Information
Irene Yang, Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA.
Anna K. Knight, Genetics and Molecular Biology Program, Graduate Division of Biological and Biomedical Sciences, Emory University, Atlanta, GA.
Anne L. Dunlop, Nell Hodgson Woodruff School of Nursing, Department of Family & Preventive Medicine, School of Medicine, Emory University, Atlanta, GA.
Elizabeth J. Corwin, Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA.
References
- Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, … Diaz PI (2013). The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME Journal, 7(5), 1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaens LM, Alessandri R, Spörri S, Lang NP, & Persson GR (2009). Does pregnancy have an impact on the subgingival microbiota? Journal of Periodontology, 80(1), 72–81. [DOI] [PubMed] [Google Scholar]
- Agueda A, Echeverría A, & Manau C (2008). Association between periodontitis in pregnancy and preterm or low birth weight: Review of the literature. Medicina Oral Patología Oral Y Cirugía Bucal, 13(9), E609–615. [PubMed] [Google Scholar]
- AlMoharib HS, AlMubarak A, AlRowis R, Geevarghese A, Preethanath R, & Anil S (2014). Oral fluid based biomarkers in periodontal disease: Part 1. Saliva. Journal of International Oral Health, 6(4), 95–103. [PMC free article] [PubMed] [Google Scholar]
- AlRowis R, AlMoharib HS, AlMubarak A, Bhaskardoss J, Preethanath R, & Anil S (2014). Oral fluid based biomarkers in periodontal disease–Part 2. Gingival crevicular fluid. Journal of International Oral Health: JIOH, 6(5), 126–135. [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. (2014). Method for estimating due date. Committee opinion No. 611. Obstetrics and Gynecology, 124, 863–866. [DOI] [PubMed] [Google Scholar]
- Andrukhov O, Ulm C, Reischl H, Nguyen PQ, Matejka M, & Rausch-Fan X (2011). Serum cytokine levels in periodontitis patients in relation to the bacterial load. Journal of Periodontology, 82, 885–892. [DOI] [PubMed] [Google Scholar]
- Anil S, Alrowis RM, Chalisserry EP, Chalissery VP, AlMoharib HS, & Al-Sulaimani AF (2015). Oral Health and Adverse Pregnancy Outcomes. In Virdi M (Ed.), Emerging trends in oral health sciences and dentistry. Retrieved from https://www.intechopen.com/books/emerging-trends-in-oral-health-sciences-and-dentistry/oral-health-and-adverse-pregnancy-outcomes
- Ata-Ali J, Flichy-Fernández AJ, Alegre-Domingo T, Ata-Ali F, Palacio J, & Peñarrocha-Diago M (2015). Clinical, microbiological, and immunological aspects of healthy versus peri-implantitis tissue in full arch reconstruction patients: A prospective cross-sectional study. BMC Oral Health, 15, 43 https://bmcoralhealth.biomedcentral.com/track/pdf/10.1186/s12903-015-0031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess KA, Urlaub DM, Massey KE, Moos M-K, Matheson MB, & Lorenz C (2010). Oral hygiene practices and dental service utilization among pregnant women. The Journal of the American Dental Association, 141(5), 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-de-Albornoz A, Figuero E, Herrera D, & Bascones-Martínez A (2010). Gingival changes during pregnancy: II. Influence of hormonal variations on the subgingival biofilm. Journal of Clinical Periodontology, 37(3), 230–240. [DOI] [PubMed] [Google Scholar]
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, & Petraglia F (2009). Inflammation and pregnancy. Reproductive Sciences, 16(2), 206–215. [DOI] [PubMed] [Google Scholar]
- Cobb CM, Kelly PJ, Williams KB, Babbar S, Angolkar M, & Derman RJ (2017). The oral microbiome and adverse pregnancy outcomes. International Journal of Women’s Health, 9, 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, Hogue CJ, Pearce B, Hill CC, Read TD, Mulle J, & Dunlop AL (2017). Protocol for the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Cohort Study. BMC pregnancy and childbirth, 17(1), 161 https://bmcpregnancychildbirth.biomedcentral.com/track/pdf/10.1186/s12884-017-1357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costalonga M, & Herzberg MC (2014). The oral microbiome and the immunobiology of periodontal disease and caries. Immunology Letters, 162(2), 22–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council on Dental Therapeutics. (1986). Guidelines for acceptance of chemotherapeutic products for the control of supra gingival dental plaque and gingivitis. Journal of the American Dental Association, 112, 529–532. [DOI] [PubMed] [Google Scholar]
- Detman LA, Cottrell BH, & Denis-Luque MF (2010). Exploring dental care misconceptions and barriers in pregnancy. Birth, 37(4), 318–324. [DOI] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu W-H, … Wade WG (2010). The human oral microbiome. Journal of Bacteriology, 192(19), 5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Pinedo AE, Chen T, Teles R, Starr JR, Wang X, Krishnan K, & Frias-Lopez J (2014). Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. The ISME Journal, 1659–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke P, Dye B, Wei L, Thornton-Evans G, & Genco R (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. Journal of Dental Research, 91(10), 914–920. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, … Genco RJ (2015). Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. Journal of Periodontology, 86(5), 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eribe ER, & Olsen I (2017). Leptotrichia species in human infections II. Journal of Oral Microbiology, 9(1), 1368848 https://www.tandfonline.com/doi/full/10.1080/20002297.2017.1368848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang A-G (2009). Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. [DOI] [PubMed] [Google Scholar]
- George A, Shamim S, Johnson M, Dahlen H, Ajwani S, Bhole S, & Yeo AE (2012). How do dental and prenatal care practitioners perceive dental care during pregnancy? Current evidence and implications. Birth, 39, 238–247. [DOI] [PubMed] [Google Scholar]
- Giglio JA, Lanni SM, Laskin DM, & Giglio NW (2009). Oral health care for the pregnant patient. Journal of the Canadian Dental Association, 75, 43–48. [PubMed] [Google Scholar]
- Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, … Leys EJ (2012). Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME Journal, 6(6), 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães AN, Silva-Mato A, Miranda Cota LO, Siqueira FM, & Costa FO (2010). Maternal periodontal disease and preterm or extreme preterm birth: an ordinal logistic regression analysis. Journal of Periodontology, 81(3), 350–358. [DOI] [PubMed] [Google Scholar]
- Gupta VK, Paul S, & Dutta C (2017). Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Frontiers in Microbiology, 8, 1162 https://www.frontiersin.org/articles/10.3389/fmicb.2017.01162/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y (2011). Oral health and adverse pregnancy outcomes–what’s next? Journal of Dental Research, 90(3), 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T, Qu L, Chang J, & Wang J (2018). Gingivitis models-relevant approaches to assess oral hygiene products. Journal of Clinical Dentistry, 29, 45–51. [PubMed] [Google Scholar]
- Holt SC, & Ebersole JL (2005). Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000, 38(1), 72–122. [DOI] [PubMed] [Google Scholar]
- Hunter LP, & Yount SM (2011). Oral health and oral health care practices among low-income pregnant women. Journal of Midwifery & Women’s Health, 56(2), 103–109. [DOI] [PubMed] [Google Scholar]
- Ide M, & Papapanou PN (2013). Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes–systematic review. Journal of Periodontology, 84, S181–S194. [DOI] [PubMed] [Google Scholar]
- Jain S, Gautam V, & Naseem S (2011). Acute-phase proteins: As diagnostic tool. Journal of Pharmacy and Bioallied Sciences, 3(1), 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffcoat M, Parry S, Sammel M, Clothier B, Catlin A, & Macones G (2011). Periodontal infection and preterm birth: Successful periodontal therapy reduces the risk of preterm birth. BJOG: An International Journal of Obstetrics & Gynaecology, 118(2), 250–256. [DOI] [PubMed] [Google Scholar]
- Khader Y, Al-shishani L, Obeidat B, Khassawneh M, Burgan S, Amarin ZO, … Alkafajei A (2009). Maternal periodontal status and preterm low birth weight delivery: A case–control study. Archives of Gynecology and Obstetrics, 279(2), 165–169. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Zhang P, Benakanakere M, Singleton J, Biesbrock A, Nonnenmacher C, & He T (2015). Experimental gingivitis, bacteremia and systemic biomarkers: a randomized clinical trial. Journal of Periodontal Eesearch, 50, 864–869. [DOI] [PubMed] [Google Scholar]
- Knapp S, Brodal C, Peterson J, Qi F, Kreth J, & Merritt J (2017). Natural competence is common among clinical isolates of Veillonella parvula and is useful for genetic manipulation of this key member of the oral microbiome. Frontiers in Cellular and Infection Microbiology, 7, 139 https://www.frontiersin.org/articles/10.3389/fcimb.2017.00139/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, & Schloss PD (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Applied and Environmental Microbiology, 79(17), 5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Koo H, & Hajishengallis G (2018). The oral microbiota: dynamic communities and host interactions. Nature Reviews: Microbiology, 16, 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang NP, Schätzle MA, & Löe H (2009). Gingivitis as a risk factor in periodontal disease. Journal of Clinical Periodontology, 36, 3–8. [DOI] [PubMed] [Google Scholar]
- Lee RS-Y, Milgrom P, Huebner CE, & Conrad DA (2010). Dentists’ perceptions of barriers to providing dental care to pregnant women. Women’s Health Issues, 20(5), 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos LN, Fulthorpe RR, Triplett EW, & Roesch LF (2011). Rethinking microbial diversity analysis in the high throughput sequencing era. Journal of Microbiological Methods, 86, 42–51. [DOI] [PubMed] [Google Scholar]
- Lima DP, Moimaz S, Garbin C, Sumida DH, Jardim EG Jr, & Okamoto AC (2015). Occurrence of Socransky red complex in pregnant women with and without periodontal disease. Oral Health & Preventive Dentistry, 13(2), 169–176. [DOI] [PubMed] [Google Scholar]
- Lobene R, Weatherford T, Ross N, Lamm R, & Menaker L (1985). A modified gingival index for use in clinical trials. Clinical Preventive Dentistry, 8(1), 3–6. [PubMed] [Google Scholar]
- Love MI, Huber W, & Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology, 15(12), 550 https://genomebiology.biomedcentral.com/track/pdf/10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo J, Ribeiro R, Machado F, Assis N, Alves R, Oliveira A, & Ribeiro L (2014). Periodontal disease and oral health-related behavior as factors associated with preterm birth: a case–control study in south-eastern Brazil. Journal of Periodontal Research, 49(4), 458–464. [DOI] [PubMed] [Google Scholar]
- Macones GA, Parry S, Nelson DB, Strauss JF, Ludmir J, Cohen AW, … Sammel MD (2010). Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: Results from the Periodontal Infections and Prematurity Study (PIPS). American Journal of Obstetrics and Gynecology, 202(2), 147.e1–147.e8. [DOI] [PubMed] [Google Scholar]
- Madianos PN, Bobetsis YA, & Offenbacher S (2013). Adverse pregnancy outcomes (APOs) and periodontal disease: Pathogenic mechanisms. Journal of Clinical Periodontology, 40(s14), S170–S180. [DOI] [PubMed] [Google Scholar]
- Magurran AE (2013). Measuring biological diversity. Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
- Marchi KS, Fisher-Owens SA, Weintraub JA, Yu Z, & Braveman PA (2010). Most pregnant women in California do not receive dental care: Findings from a population-based study. Public Health Reports, 125, 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, & Drake P (2018). Births: Final Data for 2017. National Vital Statistics Reports, 67(8). https://www.cdc.gov/nchs/data/nvsr/nvsr67/nvsr67_08-508.pdf [PubMed] [Google Scholar]
- Mason MR, Nagaraja HN, Camerlengo T, Joshi V, & Kumar PS (2013). Deep sequencing identifies ethnicity-specific bacterial signatures in the oral microbiome. PLoS ONE, 8(10), e77287 https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0077287&type=printable [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, … Jacobson JW (2010). Current developments in salivary diagnostics. Biomarkers in Medicine, 4(1), 171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Aldo P, & Alvero AB (2017). The unique immunological and microbial aspects of pregnancy. Nature Reviews Immunology, 17(8), 469–482. [DOI] [PubMed] [Google Scholar]
- Novak MJ, Novak KF, Hodges JS, Kirakodu S, Govindaswami M, DiAngelis A, … Michalowicz BS (2008). Periodontal bacterial profiles in pregnant women: response to treatment and associations with birth outcomes in the obstetrics and periodontal therapy (OPT) study. Journal of Periodontology, 79(10), 1870–1879. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, … Beck J (1996). Periodontal infection as a possible risk factor for preterm low birth weight. Journal of Periodontology, 67(10), 1103–1113. [DOI] [PubMed] [Google Scholar]
- Oksanen J (2015). Vegan: An introduction to ordination. Retrieved from https://cran.r-project.org/web/packages/vegan/vignettes/intro-vegan.pdf
- Petersen PE (2009). Global policy for improvement of oral health in the 21st century–implications to oral health research of World Health Assembly 2007, World Health Organization. Community Dentistry and Oral Epidemiology, 37(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Polyzos NP, Polyzos IP, Zavos A, Valachis A, Mauri D, Papanikolaou EG, … Messinis IE (2010). Obstetric outcomes after treatment of periodontal disease during pregnancy: Systematic review and meta-analysis. British Medical Journal, 341, c7017 https://www.bmj.com/content/bmj/341/bmj.c7017.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Dey SK, & Fisher SJ (2014). Preterm labor: one syndrome, many causes. Science, 345(6198), 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton WM, Lin Y, Kryscio RJ, Dawson DR, Ebersole JL, & Miller CS (2011). Salivary biomarkers of periodontal disease in response to treatment. Journal of Clinical Periodontology, 38(5), 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Ramesh A, & Thomas B (2009). Evaluation of plasma C-reactive protein levels in pregnant women with and without periodontal disease: A comparative study. Journal of Indian Society of Periodontology, 13, 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaee M, Golpasha MF, Maliji G, Bijani A, Mir SMA, & Kani SNM (2013). C-reactive protein levels in patients with periodontal disease and normal subjects. International Journal of Molecular and Cellular Medicine, 2(3), 151–155. [PMC free article] [PubMed] [Google Scholar]
- Sosa-Jurado F, Hernández-Galindo VL, Meléndez-Mena D, Mendoza-Torres MA, Martínez-Arroniz FJ, Vallejo-Ruiz V, … Santos-López G (2014). Detection of hepatitis C virus RNA in saliva of patients with active infection not associated with periodontal or liver disease severity. BMC Infectious Diseases, 14(1), 72 https://bmcinfectdis.biomedcentral.com/track/pdf/10.1186/1471-2334-14-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann PF, Eick S, Salvi GE, Surbek D, Mohr S, Bürgin W, … Sculean A (2014). Increased periodontal inflammation in women with preterm premature rupture of membranes. Clinical Oral Investigations, 19, 1537–1546. [DOI] [PubMed] [Google Scholar]
- Steinberg BJ, Hilton IV, Iida H, & Samelson R (2013). Oral health and dental care during pregnancy. Dental Clinics, 57, 195–210. [DOI] [PubMed] [Google Scholar]
- Vettore M, Leao A, Leal M. d. C., Feres M, & Sheiham A (2008). The relationship between periodontal disease and preterm low birthweight: clinical and microbiological results. Journal of Periodontal Research, 43(6), 615–626. [DOI] [PubMed] [Google Scholar]
- Zhou X, & Li Y (2015). Atlas of oral microbiology. Amsterdam: Elsevier. [Google Scholar]