Abstract
Hematopoietic stem cell transplantation is an established treatment for many malignant and non-malignant haematological disorders. In the current case report, we describe the first haploidentical stem cell transplantation, used for the first time in the Romania, the case of 33 year-old young woman diagnosed with Hodgkin’s lymphoma that has underwent a haploSCT after she relapsed from several chemotherapy regiments, as well as after an autologous stem cell transplantation. This success represent a premiere in Romanian clinical hematology, being the first case of a haploSCT in Romania, as well as in South-Eastern Europe.
Keywords: haploidentical stem cell transplantation, Hodgkin’s lymphoma, first case in Romania
Abstract
Transplantul hematopoietic de celule stem reprezinta un tratament standard pentru multe afectiuni hematologice, atat neoplazice cat si benigne. In prezenta lucrare, descriem aplicarea transplantului haploidentic de celule stem hematopoietice pentru prima data in Romania. Este descris cazul unei paciente de 33 de ani diagnosticate cu limfom Hodgkin, ce a beneficiat de un transplant haploidentic dupa ce a recidivat in urma a multiple cure de chimioterapie, dar si in urma unui transplant autolog de celule stem. Acest succes reprezinta o premiera in hematologia clinica romaneasca, fiind primul caz de transplant haploidentic din tara si chiar din sud-estul Europei.
Introduction.
Hematopoietic stem cell transplantation (SCT) is an established treatment for many malignant and non-malignant haematological disorders. More than 30.000 SCTs arecurrently performed each year in the Western world [1,2]. The first SCT was performed more than 50 years ago. Ever since, this therapy has been used for the treatment of various conditions that include congenital or acquired bone marrow failure, as well as hematological malignancies such as leukemias, lymphomas or multiple myelomas [3]. Cure from a hematological malignancy is believed to rely on exploiting the graft-versus-leukemia (GVL) effects by allogeneic immune cells in addition to the effects by conditioning regimen [4,5].
In the current case report, we describe the very same technique used for the first time in the Romania, the case of 33 year-old young woman that has underwent a haploSCT after she relapsed from several chemotherapy regiments, as well as after an autologous stem cell transplantation. This success represent a premiere in Romanian clinical hematology, being the first case of a haploSCT in Romania, as well as in South-Eastern Europe.
Case report.
Diagnosis and previous treatment.
A 27-year old young woman was diagnosed in February 2009 with stage IIIB nodular sclerosing Hodgkin’s lymphoma (HL). Staging revealed a large mediastinal mass of approximately 10 cm in diameter with multiple metastatic lymph nodes localized around the mesenteric artery, the hepatic hilum, as well as the lombo-aortic artery and the common iliac arteries, as seen in Image 1. Following diagnosis, three cycles of chemotherapy with adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) were given , according to the protocols of Canellos et al [6]. As sub-diaphragmatic metastatic lymph nodes and a persistent mediastinal mass were diagnosed on the computer tomography (CT) scan, as seen in Images 2 A and B, a diagnosis of partial remission was concluded. Thus, the therapy was changed to escalated-dose BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone),as according to Diehl et al [7]. After 4 chemotherapy cycles, a complete remission was diagnosed on a positron emission tomography (PET scan).
Image 1.
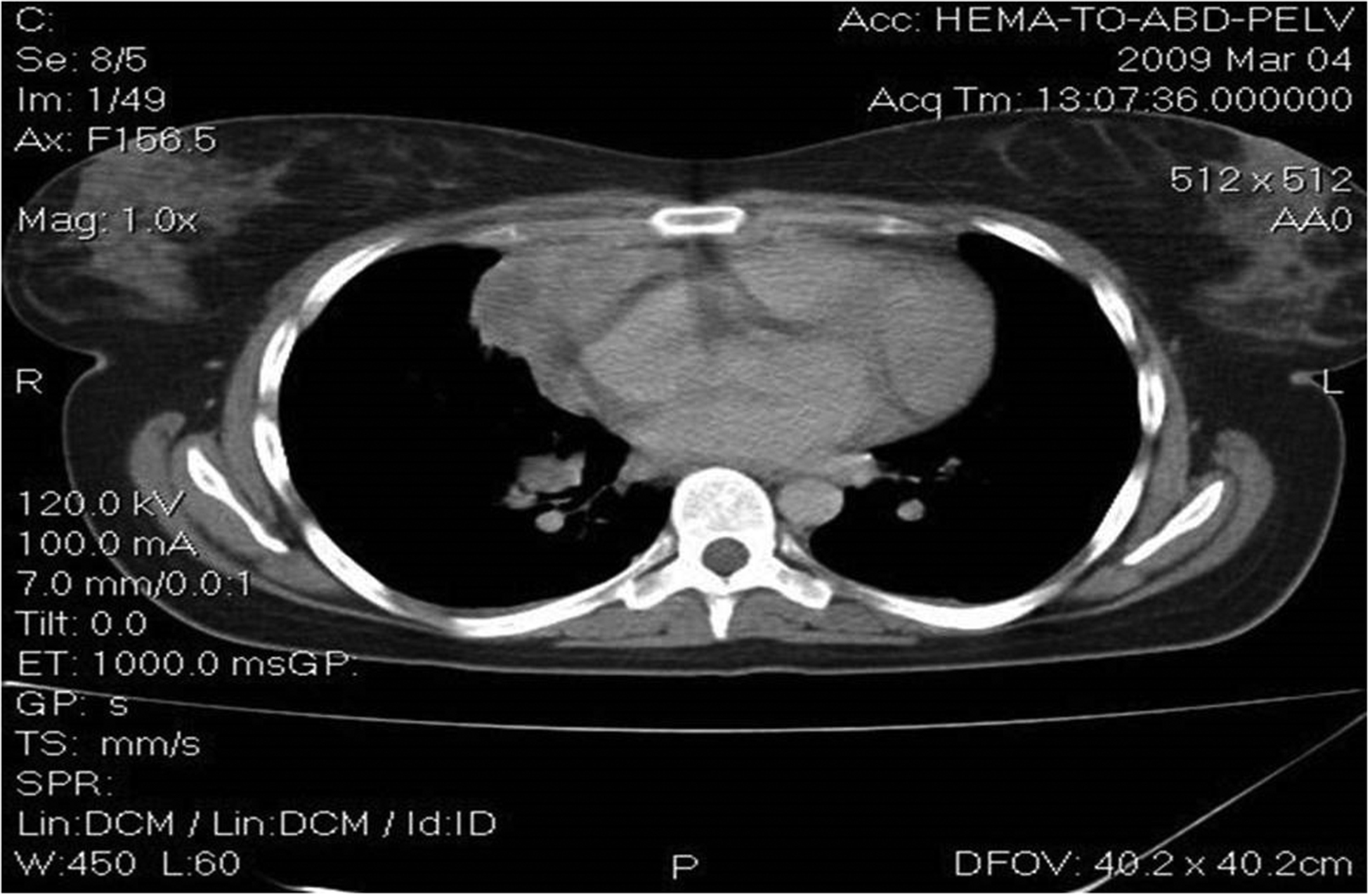
Large mediastinal mass of 10 cm in diameter with multiple metastatic lymph nodes localized around the mesenteric artery, the hepatic hilum, as well as the lombo-aortic artery and the common iliac arteries.
Images 2A and 2B.
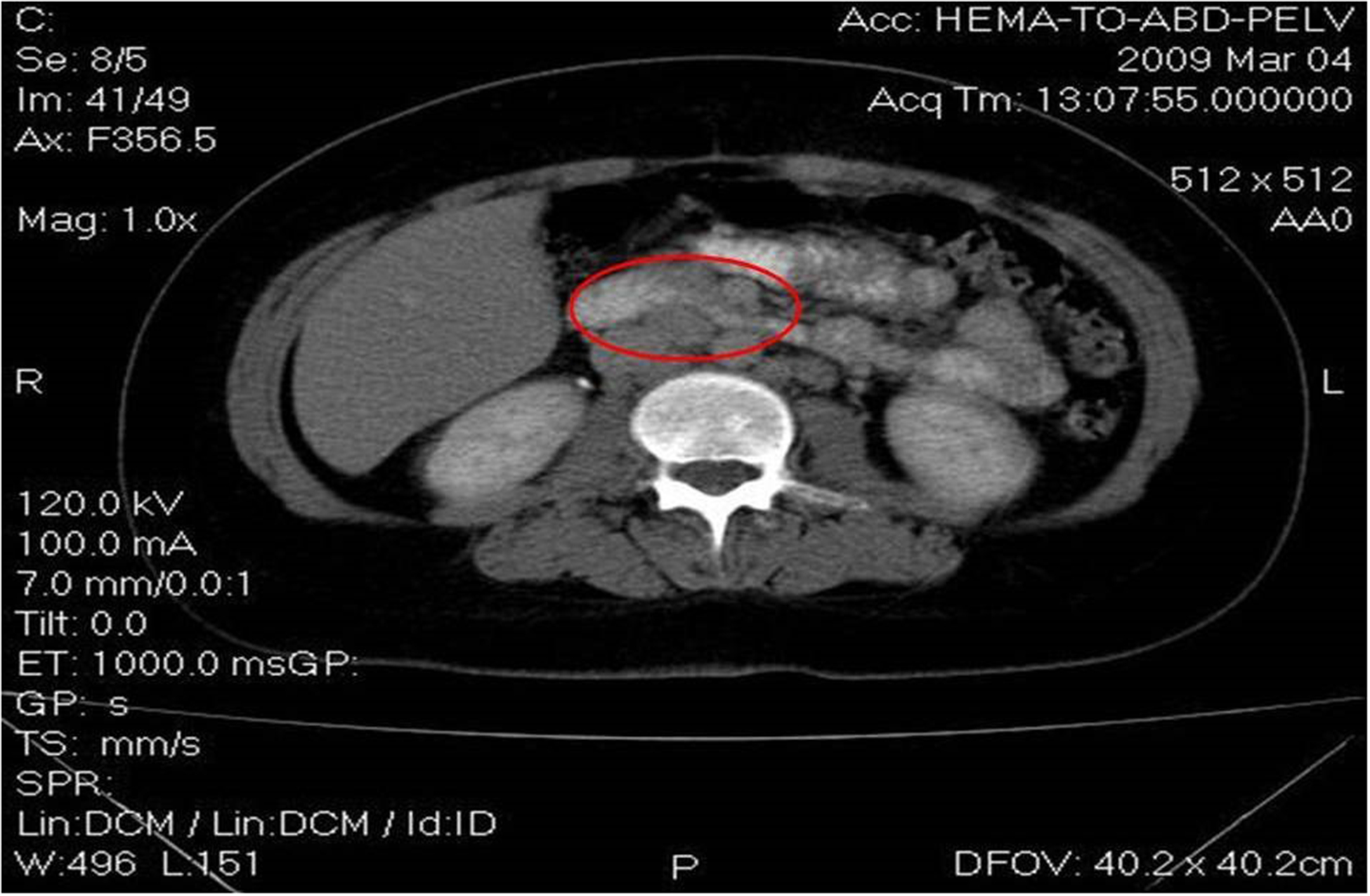
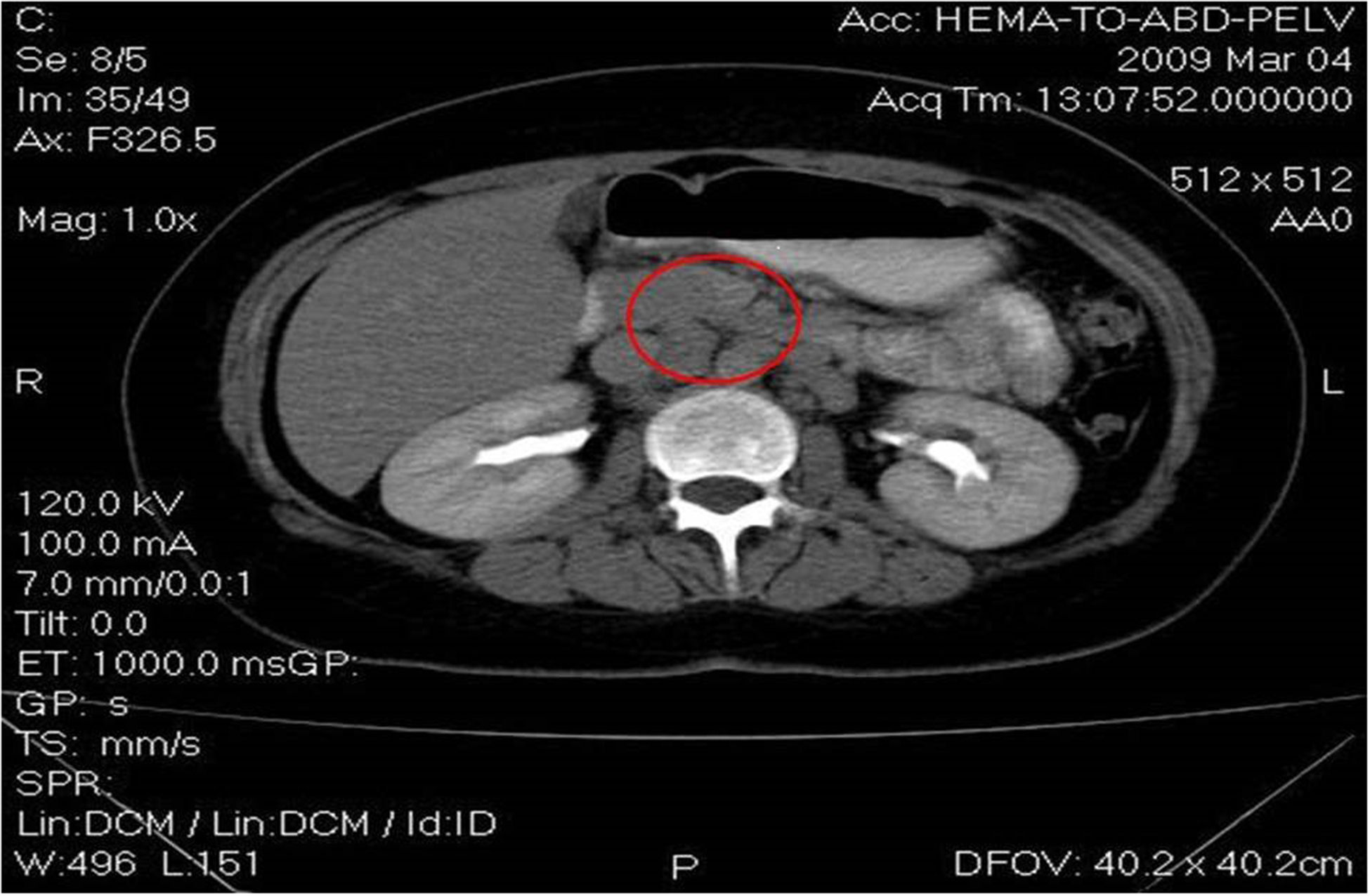
Computer tomography shows sub-diaphragmatic metastatic lymph nodes and a persistent mediastinal mass.
One year later, a relapse was treated with 3 cycles of salvage therapy with ifosfamide, gemcitabine and vinorelbine (IGEV), according to the study of Santoro et al [8]. After IGEV chemotherapy, the patient refused an autologous stem cell transplantation. A PET Scan showed a complete remission, followed by yet another relapse one year later (Image 3), treated with 3 cycles of dexamethasone, high dose cytarabine and cisplatin (DHAP) [9]. The 3 cycles of DHAP were followed by an autologous stem cell transplantation, with BEAM conditioning chemotherapy (high-dose carmustine, etoposide, cytarabine and melphalan), as described by the same German Hodgkin Lymphoma Study Group [10]. Another PET Scan, done 100 days after the autologous stem cell transplantation (autoSCT) showed an increased metabolic activity in various sub-diaphragmatic lymph nodes, as well as in the left iliac crest, as seen in Image 4. Salvage radiotherapy with 30 Gy was later on done on the left hemi-pelvis [11], but 6 months later another positive PET Scan showed active disease in the sub-diaphragmatic lymph nodes, confirmed later on by new bone lesions in the ischium, pubis and vertebrae (Images 5 A to C).
Image 3.
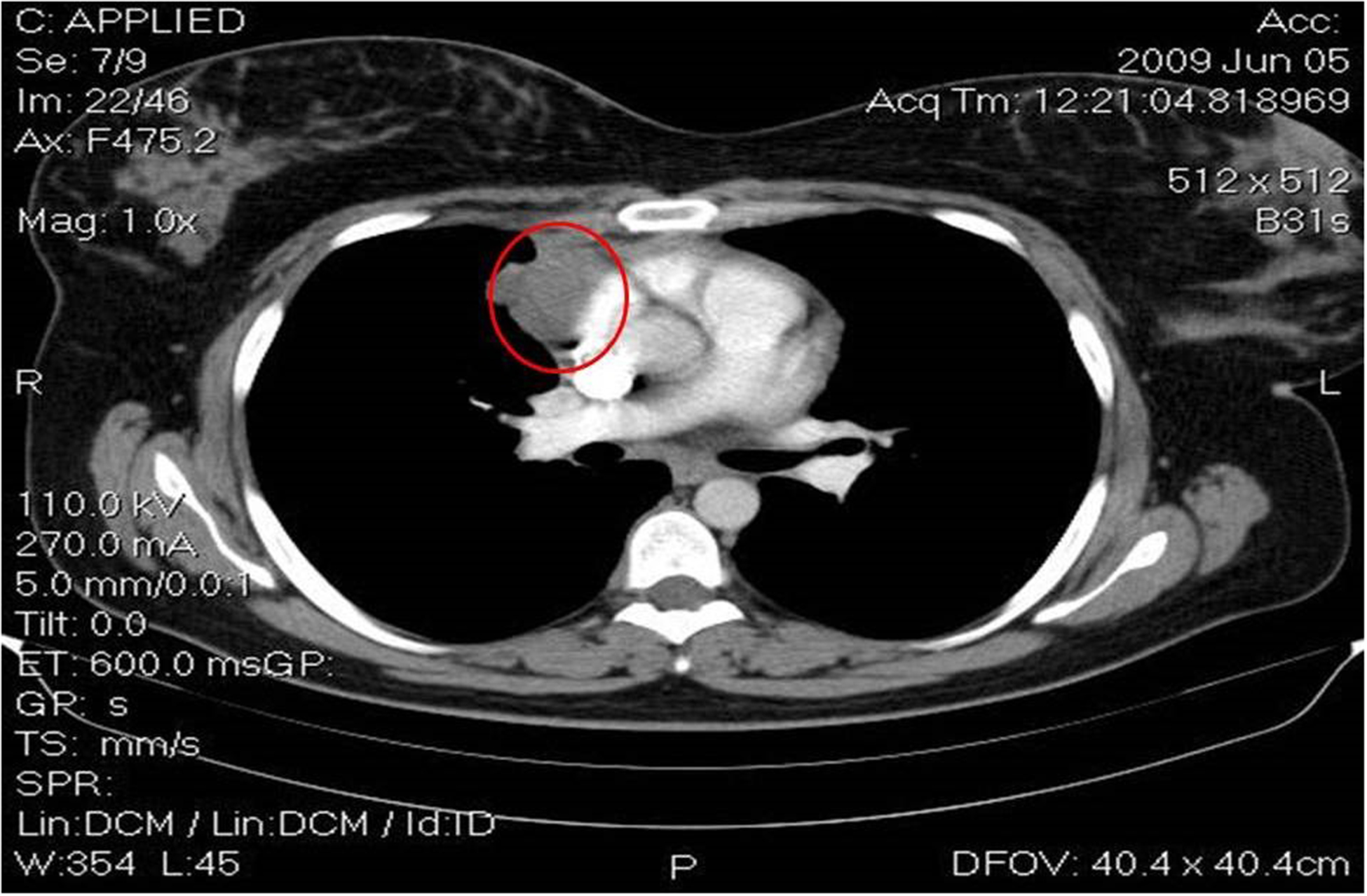
Positron emission tomography showing lymphoma relapse.
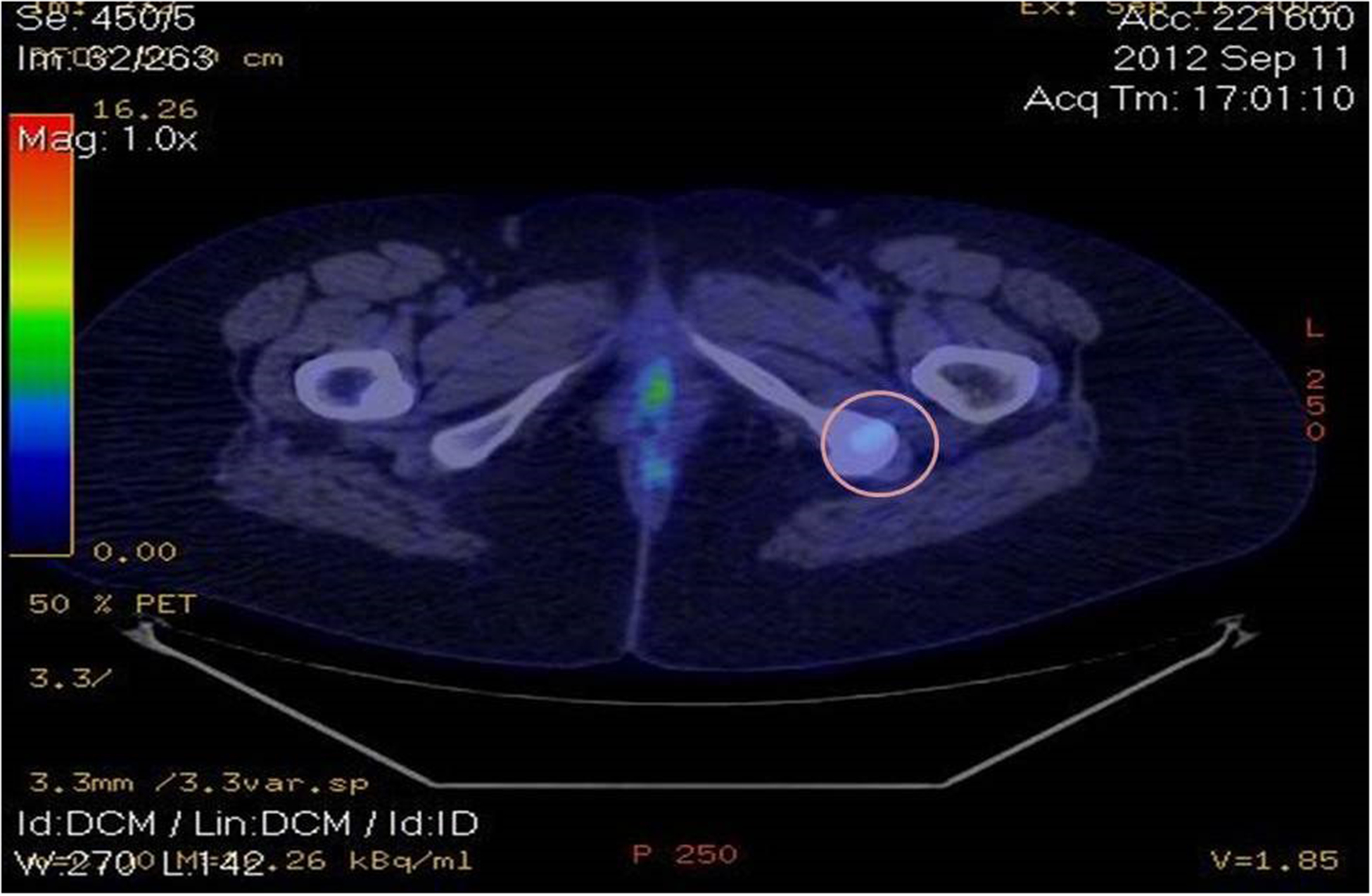
Image 4.
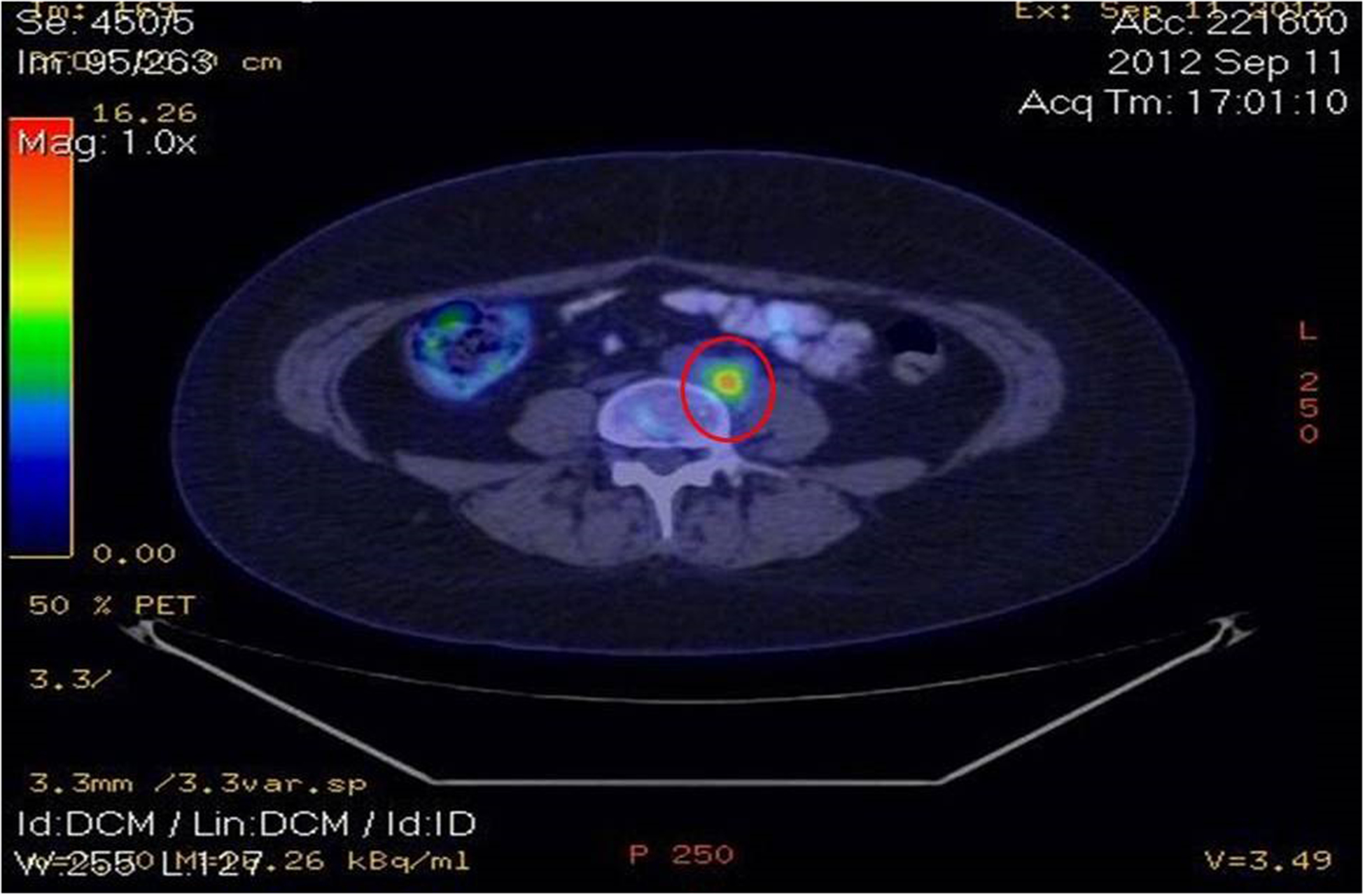
Positron emission tomography showing an increased metabolic activity in various sub-diaphragmatic lymph nodes, as well as in the left iliac crest.
Images 5A, 5B and 5c.
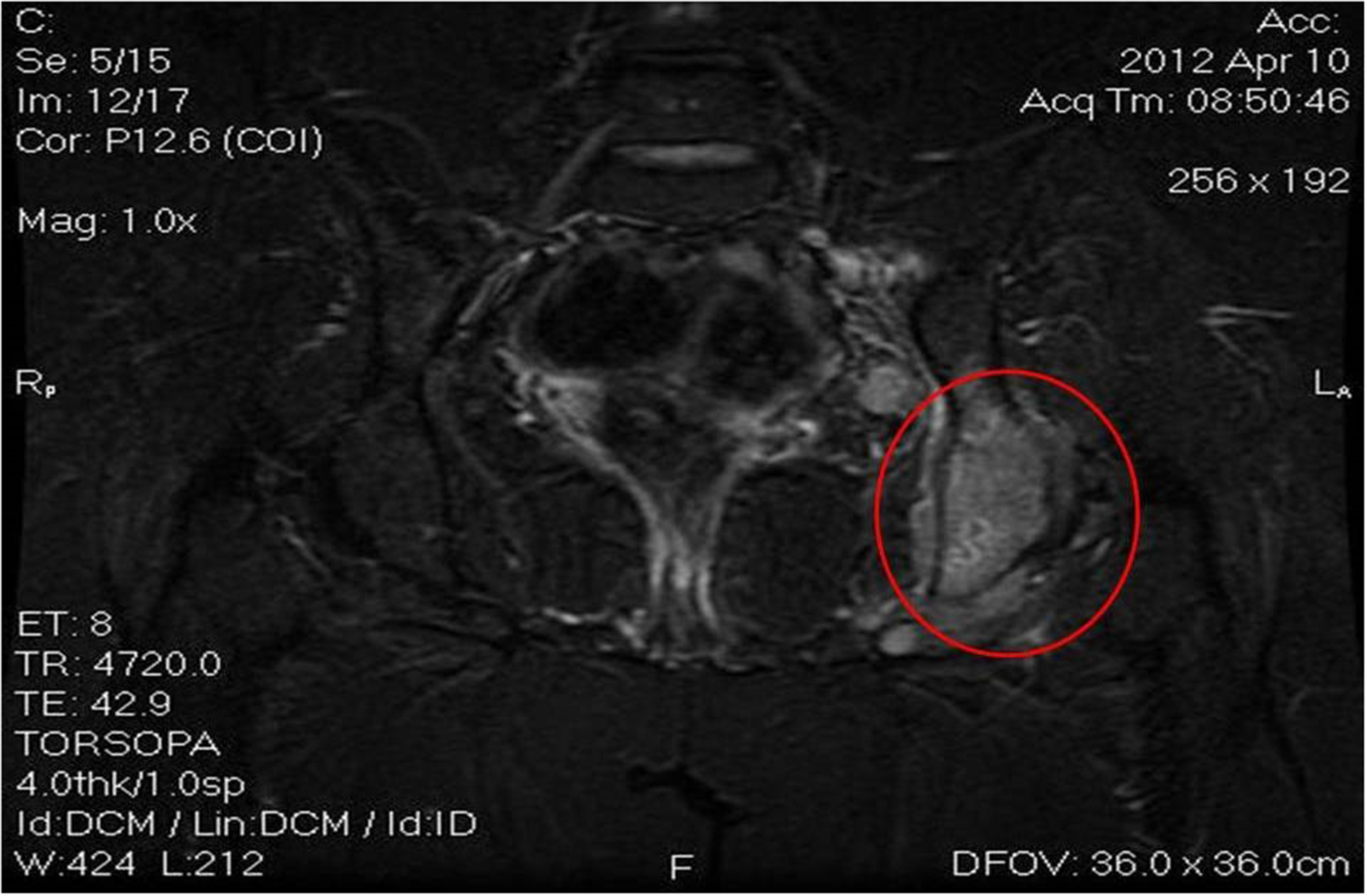
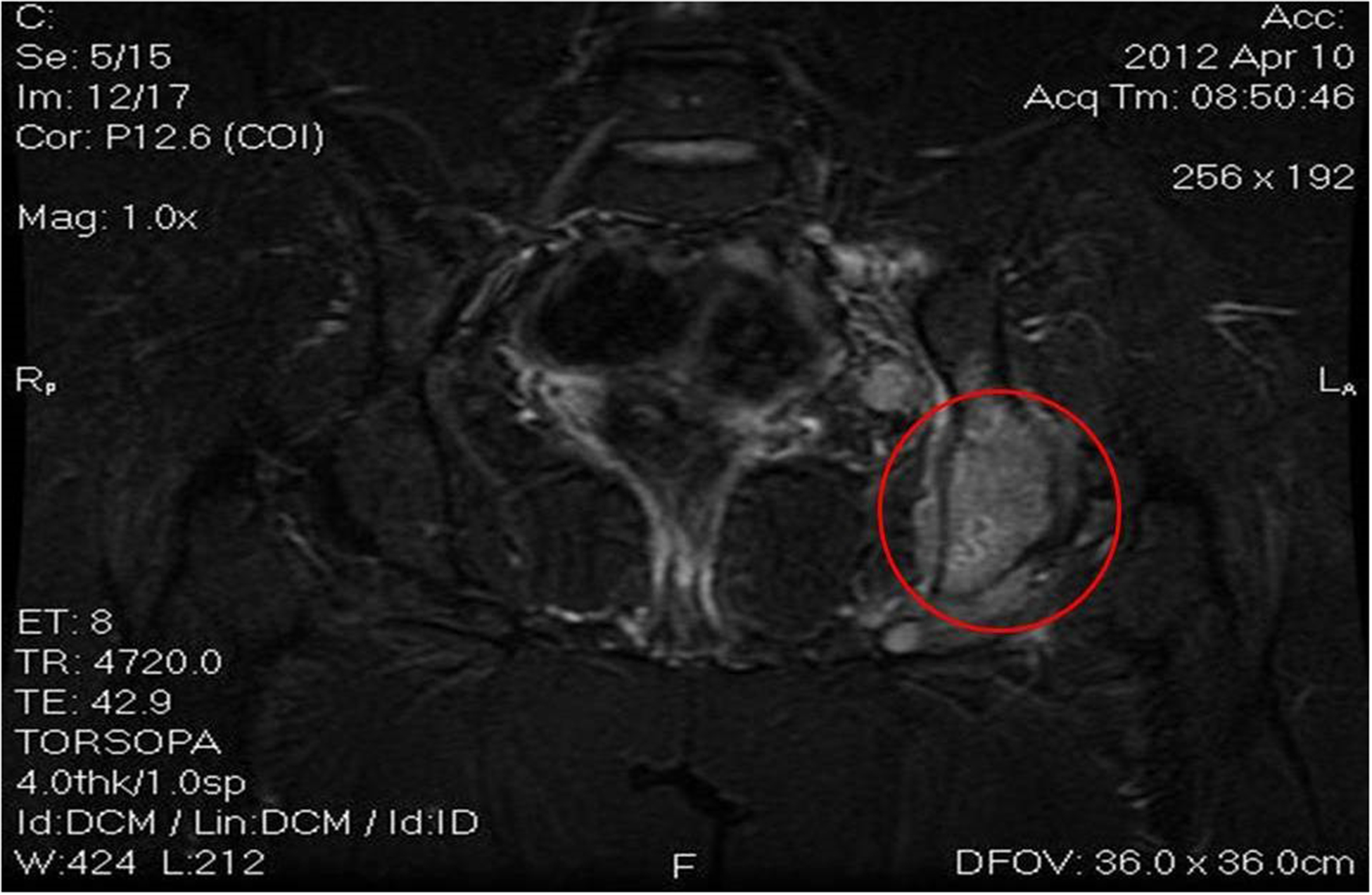
Active disease in the sub-diaphragmatic lymph nodes, confirmed later on by new bone lesions in the ischium, pubis and vertebrae
The progressive disease required another transplant, this time a matched unrelated donor (MUD) allogeneic stem cell transplantation (alloSCT) and 2 cycles of salvage chemotherapy with gemcitabine and oxaliplatin was the only therapeutic option. Brentuximab Vedotin was not used instead of gemcitabine and oxaliplatin because this drugs was not found in Romania at the time. The CT Scan confirmed again the progressive disease before 9/10 HLA typing match was found in the International Bone Marrow Registry. Three cycles of chlorambucil, vinblastine, procarbazine and prednisolone (ChIVPP) were administered [12], but just before the transplant the donor was diagnosed with leukopenia and thrombocytopenia. Thus, the procedure was postponed.
Four months later, a PET Scan showed new bone lesions in the left iliac crest and in the vertebral bodies of L4 and L5, for which intensity modulated radiotherapy treatment (IMRT) with 35 Gy was done. At this point, it was obvious that the only curative treatment was a haploidentical stem cell transplantation.
Transplantation.
For the haploSCT, the donor was the mother of the patient, a 57-year old woman with 8/10 human leukocyte antigen (HLA) compatibility and a common haplotype with the patient. The donor had a AII Rh positive blood type, positive cytomegalovirus (CMV) IgG, negative HBS antigen, negative HBS antigen, negative anti-HCV antibodies and negative human immunodeficiency virus (HIV) infection, and negative Anti-HLA antibodies. The patient had a AII Rh positive blood type, positive CMV IgG,negative HBS antigen, negative HBS antigen, negative anti-HCV antibodies and negative HIV infection. Conditioning chemotherapy included melphalan 140 mg/m2 at day −8 and fludarabine 40 mg/m2at days −5 to −3. The patient rested at days −2 and −1 and then the donor stem cell infusion was done at day 0 without any incidents and with a TNC of 3.4 × 108 cells.
Post-transplant immunosuppression consisted of 50 mg/kg/day of cyclophosphamide at days +3 and +4, tacrolimus 2g/day starting with day +5 and 15 mg/kg/dose mycophenolate mofetil (MMF) p.o. TID to Day +100.5mcg/kg filgrastim was also administered starting with day +5. Aplasia was diagnosed starting with day −1, with 980/mm3 white blood cells (WBC), 9.1 g/dl hemoglobin (Hb) value and 11800/mm3 platelets (PLT). Complications included a grade III mucositis, and fever. Supportive care was done with meronem 1 every 8 hours and colistin every 8 hours, for 9 days. At day +15, aplasia was overcomed, with 8.6 g/dl Hb and 19000/mm3 PLT. 16 days post-haploSCT, a 100% donor chimerism was observed, confirming the success of the transplant.
Discussions.
Finding a suitable donor is the main challenge for an allogeneic stem cell transplantation (allo-SCT). For up to 50% of all potential transplant patients, an HLA-matched sibling donor (MSD) or a HLA-matched unrelated donor (MUD) can’t be identified in time and the patient often relapses and dies from the hematological malignancy. On the other hand, a partially HLA-matched, also known as a HLA-haploidentical first degree relative is a minor obstacle for the transplant hematologist as these potential HLA-haploidentical donors include the biological parents or children. Each of these potential donors have at least a 50% chance of sharing one HLA haplotype. The HLA haplotype is defined as the case when both the patient and the donor have exactly the same HLA alleles on chromosome 6. The diagnosis is possible through high resolution typing for HLA-A, -B, -C, -DRB1 and -DQB1. A half-matched transplant was initially considered impossible to be implemented in the clinic because the T lymphocytes that respond to the allogeneic HLA molecules were responsible for the very high incidence of graft versus host disease (GVHD) and graft rejection, as reported by Powles et al [13]. The patients enrolled in this study died from pulmonary edema, fever, fluid retention or kidney failure. This syndrome is known as engraftment syndrome, a diagnosis confirmed in another study by Beatty et al [14].
For patients lacking a HLA-matched donor, the big question is whether haploSCT are a better or a worse therapeutic option in comparison with the double unrelated donor umbilical cord blood transplantation (dUCB). The Blood and Marrow Transplant Clinical Trials Network (BMT-CTN) have recently published the results of two parallel phase 2 trials of reduced intensity conditioning and either HLA-haploSCT with high dose post-transplant cyclophosphamide or dUCB transplantation [15]. The one year probability of overall survival (OS) or progression-free survival (PFS) were 54% and 46% for dUCB in comparison with 62% and 48% for haploSCT. The day 56 cumulative incidence of neutrophil recovery was 94% for dUCB and 96% for haploSCT, whereas the day incidence of GVHD was 40% for dUCB in comparison with 32% for haploSCT [16–18]. The quality of life after transplantation, apart from all the statistical data regarding survival also suggest haploSCT as the best treatment available for leukemia or lymphoma patients that relapse aster standard chemotherapy or after an autologous SCT and for which a matched donor cannot be found.
Transplantation of haploidentical stem cells is becoming a well-established approach in lymphoma patients, which makes a potential donor available for almost all patients. CD34+ positive selection was the most common procedure for graft manipulation in the past years, whereas T and B cell depletion is a promising new method. GVHD could herewith be effectively reduced and primary engraftment was reported in 83–100% of patients after transplantation of high stem cell doses. For patients with lymphomas in remission, disease-free survival at 3 years ranged between 22 and 48%. Treatment-related mortality, mainly because of viral infections, was improved by the use of reduced-intensity conditioning (which helped to speed up T cell recovery) and by close monitoring of viral loads and prophylactic/preemptive therapy. The role of donor-derived Ag-specific T cells against viral and fungal antigens is currently under investigation. Patients with active disease at the time of transplantation had a poor outcome and several attempts to improve these results are currently evaluated, such as co-infusion of natural killer cells, co-transplantation of mesenchymal stem cells, use of new drugs and post-transplant cyclophosphamide [19]. Outcomes in haploidentical stem cell transplantations have improved and outcomes are comparable to matched transplants, both for hematological malignancies, as well as for other conditions [20]. In a seminal paper, Bashey et al have compared in Atlanta retrospectively 271 patients that have underwent an allogeneic stem cell transplant, out of which 100 had received a MSD, 101 a MUD a 53 a haploidentical transplant. GVHD prophylaxis consisted of post-transplantation cyclophosphamide, tacrolimus and MMF for the haploSCT cases, while the matched transplantations had received conventional treatment for GVHD. For MSD, MUD and haploSCT cases, the 2-year cumulative incidence of NRM was 13%, 16% and respectively 7%, while relapse was similar (34% vs 34% vs 33%). The 2-year disease-free survival was 53%, 52% and 60% [21]. The transplant unit from MD Anderson Cancer Center in the United States have analyzed the outcomes of 227 patients treated with fludarabine 120–160 mg/m2, melphalan 100–140 mg/m2 and thiotepa 5–10 mg/kg, for the haploidentical cases. 87 underwent a MSD, 108 a MUD and 18 of them a haploSCT transplant. For the matched donors, GVHD prophylaxis was done with tacrolimus and mini-methotrexate with thymoglobulin in MUDs, whereas the haploSCT cases had received PTCy, tacrolimus and MMF. MSD, MUDs and haploSCT cases had similar grade II-IV acute GVHD (24 %, 19 %, and 26 %) for the ones in remission, whereas severe acute GVHD was reported to be of 4 %, 4 % and respectively 0 %. PFS at 3 years was concluded to be similar (57 %, 45 %, and 41 %) but a significant trend towards a better 3-year progression-free survival was reported in those with a matched sibling donor [22]. Thus, almost identical results are between haploSCT transplants and the matched ones, for both lymphoid and myeloid malignancies, mostly due to various conditioning chemotherapy regimens. HaploSCT are even more important taking into consideration that in Romania, transplant centers don’t have access to a public cord blood bank and thus, a HLA-half-matched donor may be the life-saving solution for patients diagnosed with various hematologic malignancies.
As haploSCT are emerging as the best treatments available, in the current case report we emphasize the need to continue this trend in stem cell transplantation and offer patients diagnosed with various hematological malignancies in Romania as well as in Eastern Europe the possibility of a haploSCT, as we have already cured our first case.
Conclusion.
A conditioning chemotherapy regimen based on post-transplant cyclophosphamide is a reduced intensity conditioning regimen. This regimen is used mostly for patients diagnosed with aggressive lymphoproliferative diseases. Hodgkin’s lymphomas and aggressive lymphomas are usually cured after the first line of chemotherapy, but around half of them relapse or have a progressive disease, usually cured by high dose chemotherapy and autologous stem cell transplantation. Still, a large number of patients need further therapy and classic myeloablative regimens for allogeneic stem cell transplantation have been associated with disappointing results due to a high non-relapse mortality, as well as due to the lack of a HLA-compatible donor for most cases. Thus, one solution was the use of a haploidentical T-cell repleted stem cell transplantation, as is the case of the present patient. Without a haploidentical stem cell transplantation, the prognosis would have been dismal, but by using the protocol described in the current paper the patient is disease-free one year after the transplant.
Contributor Information
Alina Tanase, Department of Stem Cell Transplantation, Fundeni Clinical Institute, Bucharest, Romania.
Ciprian Tomuleasa, Department of Hematology, Ion Chiricuta Oncology Institute, Cluj Napoca, Romania; Iuliu Hatieganu University of Medicine and Pharmacy, Cluj Napoca, Romania
Alexandra Marculescu, Department of Stem Cell Transplantation, Fundeni Clinical Institute, Bucharest, Romania.
Alexandru Bardas, Department of Stem Cell Transplantation, Fundeni Clinical Institute, Bucharest, Romania.
Anca Colita, Department of Stem Cell Transplantation, Fundeni Clinical Institute, Bucharest, Romania.
Carmen Orban, Department of Stem Cell Transplantation, Fundeni Clinical Institute, Bucharest, Romania.
Stefan Octavian Ciurea, Division of Cancer Medicine, Department of Stem Cell Transplantation, The University of Texas MD Anderson Cancer Center, Houston, TX, United States of America.
References.
- [1].GYURKOCZA B, REZVANI A, STORB RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3(3):285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baron F, STORB R Allogeneic hematopoietic cell transplantation as treatment for hematological malignancies: a review. Springer Semin Immunopathol. 2004;26(1–2):71–94. [DOI] [PubMed] [Google Scholar]
- [3].BARON F, STORB R, LITTLE MT. Hematopoietic cell transplantation: five decades of progress. Arch Med Res. 2003;34(6):528–44. [DOI] [PubMed] [Google Scholar]
- [4].STAAL FJ, BAUM C, COWAN C, DZIERZAK E, HACEIN-BEY-ABINA S, KARLSSON S, et al. Stem cell self-renewal: lessons from bone marrow, gut and iPS toward clinical applications. Leukemia. 2011; 25(7):1095–102. [DOI] [PubMed] [Google Scholar]
- [5].SAULNIER N, DI CAMPLI C, ZOCCO MA, DI GIOCCHINNO G, NOVI M, GASPARRINI A From stem cell to solid organ. Bone marrow, peripheral blood or umbilical cord blood as favorable source? Eur Rev Med Pharmacol Sci. 2005; 9(6):315–24. [PubMed] [Google Scholar]
- [6].CANELLOS GP, ANDERSSON JR, PROPERT KJ, NISSE N, COOPER MR, HENDERSSON ES, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992; 327(21):1478–84. [DOI] [PubMed] [Google Scholar]
- [7].DIEHL V, FRANKLIN J, PFEUNDSCHUCH M, LATHAN B, PAULUS U, HASENCLEVER D, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003; 348(24):2386–95. [DOI] [PubMed] [Google Scholar]
- [8].SANTORO A, MAGAGNOLI M, SPINA M, PINOTTI G, SIRACUSANO L, MICHIELI M, et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin’s lymphoma. Haematologica. 2007; 92(1):35–41. [DOI] [PubMed] [Google Scholar]
- [9].JOSTING A, RUDOLPH C, MAPARA M, GLOSSMANN JP, SIENIAWSKI M, SIEBER M, et al. Cologne high-dose sequential chemotherapy in relapsed and refractory Hodgkin lymphoma: results of a large multicenter study of the German Hodgkin Lymphoma Study Group (GHSG). Ann Oncol. 2005; 16(1):116–23. [DOI] [PubMed] [Google Scholar]
- [10].GLOSSMANN JP, STAAK JO, NOGOVA L, DIEHL V, SCHEID C, KISRO J, et al. Autologous tandem transplantation in patients with primary progressive or relapsed/refractory lymphoma. Ann Hematol. 2005; 84(8):517–25. [DOI] [PubMed] [Google Scholar]
- [11].JOSTING A, NOGOVA L, FRANKLIN J, GLOSSMANN JP, EICH HT, SIEBER M, et al. Salvage radiotherapy in patients with relapsed and refractory Hodgkin’s lymphoma: a retrospective analysis from the German Hodgkin Lymphoma Study Group. J Clin Oncol. 2005; 23(7):1522–9. [DOI] [PubMed] [Google Scholar]
- [12].SELBY P, PATEL P, MILAN S, MELDRUM M, MANSI J, MBIDDE E, et al. ChlVPP combination chemotherapy for Hodgkin’s disease: long-term results. Br J Cancer. 1990; 62(2):279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].POWLES RL, MORGENSTERN GR, KAY HE, MCELWAIN TJ, CLINK HM, DADY PJ. et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983; 1(8325):612–5. [DOI] [PubMed] [Google Scholar]
- [14].BEATTY PG, CLIFT RA, MICKELSON EM, NISPEROS BB, FLOURNOY N, MARTIN PJ, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985; 313(13):765–71. [DOI] [PubMed] [Google Scholar]
- [15].BRUNSTEIN CG, FUCHS EJ, CARTER SL, KARANES C, COSTA LJ, WU J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011; 118(2):282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].AL MALKI MM, HOROWITZ M, HANDGRETINGER R, LEUNG W, ROY DC, HUANG XJ, et al. Proceedings from the Second Haploidentical Stem-cell Transplantation Symposium - Haplo2014, San Francisco, California, December 4, 2014 Biol Blood Marrow Transplant. 2016; S1083–8791(16)00004–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].KANATE AS, MUSSETTI A, KHARFAN-DABAJA MA, AHN KW, DI GILIO A, BEITINJANEH A, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors versus HLA-matched unrelated donors. Blood. 2016; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].BAYRAKTAR UD, MILTON DR, GUINDANI M, RONDON G, CHEN J, AL-ATRASH G, et al. Optimal Threshold and Time of Absolute Lymphocyte Count Assessment for Outcome Prediction after Bone Marrow Transplantation. Biol Blood Marrow Transplant. 2015; S1083–8791(15)00709–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].BRAMMER JE, KHOURI I, GABALLA S, ANDERLINI P, TOMULEASA C, AHMED S, et al. Outcomes of Haploidentical Stem Cell Transplantation for Lymphoma with Melphalan-Based Conditioning. Biol Blood Marrow Transplant. 2015: S1083–8791(15)00685–0. [DOI] [PubMed] [Google Scholar]
- [20].O’DONNEL PV, LUZNIK L, JONES RJ, VOGELSANG GB, LEFFEL MS, PHELPS M, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002; 8(7):377–86. [DOI] [PubMed] [Google Scholar]
- [21].BASHEY A, ZHANG X, SIZEMORE CA, MANION K, BROWN S, HOLLAND HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013; 31(10):1310–6. [DOI] [PubMed] [Google Scholar]
- [22].DI STASI A, MILTON DR, POON LM, HAMDI A, RONDON G, CHEN J, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014; 20(12):1975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


