Abstract
This study describes the pathology and clinical information on 20 placentas whose mother tested positive for the novel Coronovirus (2019-nCoV) cases. Ten of the 20 cases showed some evidence of fetal vascular malperfusion or fetal vascular thrombosis. The significance of these findings is unclear and needs further study.
Keywords: villitis, placenta, pathology, Covid-19, pregnancy, thrombosis
Introduction
With the recent pandemic of novel coronavirus (2019-nCoV), hospitals can expect an influx of Covid-19 positive patients to labor and delivery. Not surprisingly, little is known about placental findings in such cases, with only 1 report of 3 cases in the world literature.1 Findings reported were nonspecific, including variable degrees of increased perivillous fibrin and focal increased syncytial knots. One placenta had massive infarction, and a chorangioma was present in another. Using reverse transcriptase - polymerase chain reaction (RT- PCR), the authors found no evidence of viral nucleic acids in these 3 cases. Recently, a number of Covid-19 positive patients who have delivered newborns have been seen by us. This report catalogs our experience.
Materials and Methods
Placentas received by the Department of Pathology at Weill Cornell Medical Center and consisted of 20 cases. Weill Cornell Institutional Review Board approval was given. Due to the infectious nature of the tissue, fixation for 48 hours was performed prior to dissection. Typical sections were fixed in formalin, processed into paraffin blocks, and stained with usual Hematoxylin and Eosin stain. Clinical information was retrieved from the electronic medical record or surgical pathology accession sheet, which is given in Table 1. Testing for Covid-19 was not performed on placental tissue. However, all mothers and infants were tested via RT-PCR at Weill Cornell Department of Pathology and Laboratory Medicine.
Table 1.
Clinical Information.
| Case | Maternal Age | GA | G | P | Birthweight (g) | Delivery | History |
|---|---|---|---|---|---|---|---|
| 1 | 35 | 39w6d | 6 | 2032 | 3650 | VD | Focal accreta × 2, fever |
| 2 | 30 | 38w0d | 8 | 7017 | 3360 | VD | Fever, GBS+ |
| 3 | 29 | 40w4d | 6 | 5 | 3400 | VD | Nuchal cord |
| 4 | 40 | 39w4d | 3 | 2 | 3720 | CS | PPH, Uterine atony |
| 5 | 26 | 39w2d | 6 | 2 | 3050 | VD | |
| 6 | 40 | 37w0d | 7 | 5 | 2072 | VD | Meconium, SGA |
| 7 | 19 | 38w0d | 1 | 0 | 2390 | VD | Pneumonia, acute hypoxia |
| 8 | 28 | 40w3d | 3820 | VD | Sickle cell trait | ||
| 9 | 37 | 39w0d | 4 | 3 | 2415 | CS | Nuchal cord × 1, ITP, Planned repeat CS, SGA |
| 10 | 26 | 40w1d | 2 | 1 | 3799 | VD | |
| 11 | 40 | 36wod | 2 | 1 | 2680 | CS | Placenta previa, chronic diabetes |
| 12 | 38 | 39w0d | 15 | 10 | VD | Readmitted for hypoxia/shortness of breath at 3d postpartum | |
| 13 | 28 | 40w0d | 2 | 1 | 3800 | VD | HTN |
| 14 | 40 | 33w2d | 1 | 0 | CS | Severe preeclampsia | |
| 15 | 41 | 40w0d | 1 | 0 | 4115 | VD | Group B Strep screen positive |
| 16 | 16 | 32w2d | 3 | 0 | 3314 | VD | Preterm labor |
| 17 | 36 | 35w3d | 10 | 9 | CS | Twins, severe preeclampsia | |
| 18 | 23 | 39w5d | 2 | 1 | 3580 | VD | |
| 19 | 25 | 38w4d | 2 | 1 | 3920 | VD | Group B Strep screen positive |
| 20 | 32 | 37w6d | 3 | 1 | 3160 | VD | Hypothyroidism |
Abbreviations: CS, Cesarean section; d, days; G, gravidity; GA, week of gestation; GBS, Group B Streptococcus carrier status; HTN, hypertension; P, parity; ITP, idiopathic thrombocytopenic purpura; PPH, postpartum hemorrhage; SGA, small for gestational age; VD, vaginal delivery; w, weeks.
Results
All expectant mothers at our institution are tested for Covid-19 even if asymptomatic and all mothers in this study tested positive. Two mothers had a fever on presentation (cases 1 and 2). In case 7, the mother presented with pneumonia and acute hypoxia but was later discharged home. One woman (case 12) was readmitted for hypoxia and shortness of breath 3 days postpartum. No women were admitted to the intensive care unit or intubated. The remaining women were asymptomatic prior to delivery and in the postpartum period. In all cases, the infants had 5-minute Apgars of 8 or 9, were admitted to the well-baby nursery, and discharged home without apparent sequelae. All infants tested negative for Covid-19 by RT-PCR.
Table 2 shows a summary of the pathologic diagnoses.2 Diagnoses were made and lesions graded as per the Amsterdam criteria.2 Interestingly, in these first 20 cases, the most common lesion was fetal vascular malperfusion which was seen in 9 cases (45%). In most cases, this was the presence of intramural fibrin deposition in 1 or 2 foci (cases 2, 12, and 13), 2 cases showed only foci of villous stromal-vascular karyorrhexis (cases 3 and 10), while the remaining cases (1, 4, 5, and 7) showed multiple lesions. A few cases showed intramural nonocclusive thrombi which were very recent. In all cases, the fetal vascular malperfusion was low grade (Figures 1 to 3). Other miscellaneous findings included meconium macrophages (6 cases), lesions of maternal vascular malperfusion (5 cases), and focal increase in perivillous fibrin deposition. One case (7), in which the patient had pneumonia and acute hypoxia, showed evidence of ascending infection with acute chorioamnionitis and acute funisitis. Four cases showed chronic villitis (8, 13, 17, and 18), which was high grade in 2 cases and was associated with obliterative vasculopathy in 1 case (case 8).
Table 2.
Pathology.
| Case | Histology of FVM | Other Findings |
|---|---|---|
| 1 | Thrombosis, intramural fibrin deposition | Focal increase in fibrin, intervillous thrombus, focal chorangiosis, furcate insertion of umbilical cord |
| 2 | Intramural fibrin deposition | Meconium |
| 3 | Villous stromal-vascular karyorrhexis | Meconium |
| 4 | Thrombosis, avascular villi, intramural fibrin deposition | Meconium |
| 5 | Thrombosis, intramural fibrin deposition | |
| 6 | None | Meconium, maternal vascular malperfusion (infarction, accelerated villous maturity, intraplacental hematoma) |
| 7 | Intramural fibrin deposition, avascular villi, villous stromal-vascular karyorrhexis | Decidual vasculopathy, acute chorioamnionitis and funisitis, meconium |
| 8 | None | High-grade chronic villitis with associated avascular villi (obliterative vasculopathy) |
| 9 | None | Maternal vascular malperfusion (accelerated villous maturity) |
| 10 | Villous stromal-vascular karyorrhexis | |
| 11 | None | |
| 12 | Intramural fibrin deposition | Meconium, early acute funisitis |
| 13 | Intramural fibrin deposition | Basal chronic villitis |
| 14 | None | Maternal vascular malperfusion (accelerated villous maturity) |
| 15 | None | Old retromembranous hematoma, meconium |
| 16 | None | Maternal vascular malperfusion (accelerated villous maturity, multiple chorionic cysts) |
| 17 | None | Twin 1 – Villous infarctTwin 2 – High-grade chronic villitis |
| 18 | None | Low-grade chronic villitis |
| 19 | None | |
| 20 | None | Hypercoiled umbilical cord, marginal insertion of umbilical cord |
Abbreviation: FVM, fetal vascular malperfusion.
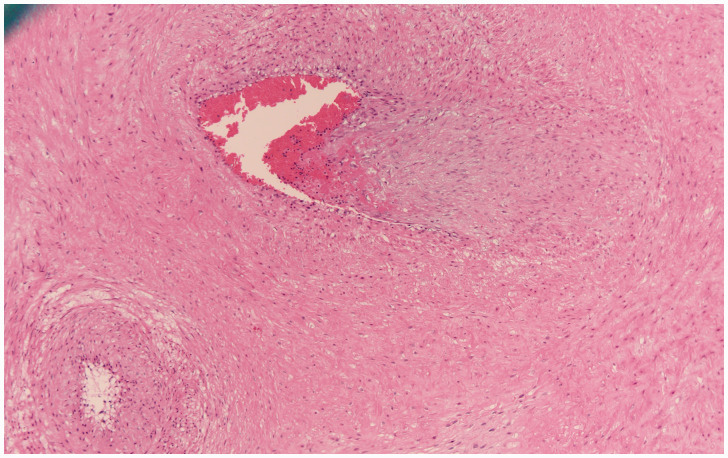
Figure 1.
Section of a stem vessel in the placenta showing fetal vascular malperfusion, specifically intramural fibrin deposition where fibrin is deposited in the intima of the vessel. This was the most common type of thrombotic lesion in these placentas. H&E original magnification 200×.
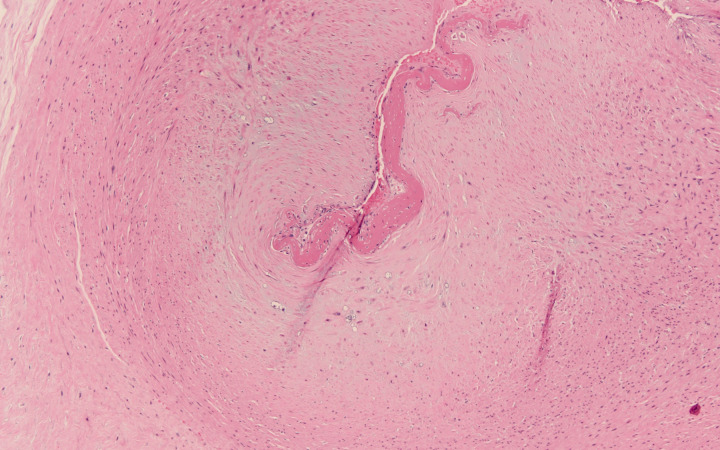
Figure 2.
Section of a chorionic plate vessel showing fetal vascular malperfusion, also with deposition of fibrin in the intimal of the vessel extending into the lumen. H&E original magnification 100×.
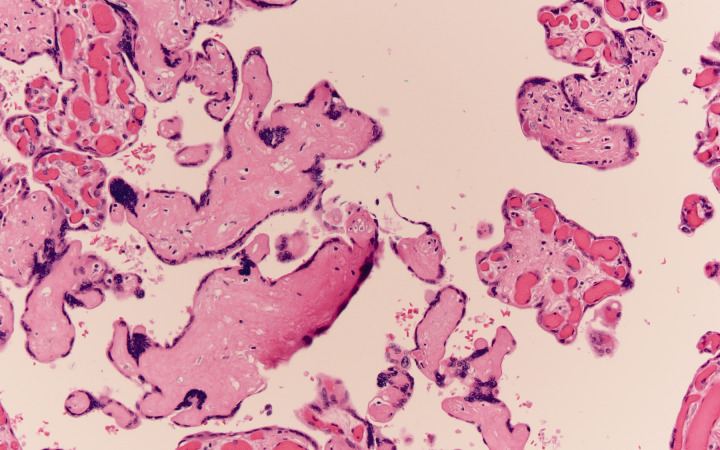
Figure 3.
Section of chorionic villi which are avascular. This is another lesion of fetal vascular malperfusion which develops due to thrombosis upstream from the chorionic villi leaving to loss of fetal circulation downstream from the thrombosis. Loss of circulation ultimately leads to loss of fetal vessels with preservation of surface trophoblast. Here, the villi are avascular and the stroma is hyalinized. H&E original magnification 400×.
Discussion
Very little is currently known about the effects of Covid-19 on the human placenta and neonate. The mouse hepatitis virus, a coronavirus often used as a study model, has been shown to infect the placenta and affect the fetus.3 Human SARS has been vertically transmitted and in some cases showed fetal thrombotic vasculopathy (fetal vascular malperfusion).4 In humans, early evidence did not demonstrate vertical transmission of Covid-19 in small cohorts of patients.5,6 However, demonstration of IgM antibodies to Covid-19 in a single neonate, who also had elevated cytokines suggests that vertical transmission is possible, even if uncommon.7
Covid-19 infection has been associated with hypercoagulability,8 with development of ischemic changes including gangrene of fingers and toes, with evidence of d-dimer elevation, and, in some patients, with disseminated intravascular coagulopathy in one series.9 Whether the fetal vascular malperfusion in some of the cases described in this study is related to hypercoagulability associated with Covid-19 and whether villitis of unknown etiology is related to an antiviral immune response need further study.
This is a brief report of initial findings seen in placentas of Covid-19 positive mothers. While one of our cases had fetal vascular malperfusion findings potentially related to a furcate cord insertion, in 8 cases there was no gross umbilical cord abnormality known to be associated with fetal vascular malperfusion. This suggests that maternal Covid-19 infection might be associated with propensity for thrombosis in the fetal circulation. This, in turn, may have significant clinical implications for the mother and infant. On the other hand, as the lesions were low grade, more than half did not have thrombotic lesions and the infants tested negative; hence, these findings may be unrelated. Further studies with additional cases are necessary to determine the reproducibility and significance of these initial findings.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Chen S, Huang B, Luo DJ, et al. [Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases]. Zhonghua Bing Li Xue Za Zhi Published online March 1, 2020. doi:10.3760/cma.j.cn112151-20200225-00138 [DOI] [PubMed]
- 2.Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group consensus statement. Arch Pathol Lab Med. 2016; 140(7):698–713. [DOI] [PubMed] [Google Scholar]
- 3.Barthold SW, Beck DS, Smith AL. Mouse hepatitis virus and host determinants of vertical transmission and maternally-derived passive immunity in mice. Arch Virol. 1988: 100:171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng WF, Wong SF, Lam A, et al. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology. 2006; 38:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Go J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020; 395:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karim-Zarchi M, Neamatzadeh H, Dastgheib SA, et al. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr Pathol Published online April 2, 2020. doi:10.1080/15513815.2020.1747120 [DOI] [PMC free article] [PubMed]
- 7.Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. Published online March 26, 2020. doi:10.1001/jama.2020.4621 [DOI] [PMC free article] [PubMed]
- 8.Li T, Lu H, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020; 9(1):687–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Cao W, Xiao M, et al. [Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia]. Zhonghua Xue Ye Xue Za Zhi. Published online March 28, 2020. doi:10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PMC free article] [PubMed]