Abstract
Objectives
The aim of this study was to evaluate if de novo hepatic lipid synthesis contributes to fatty acid overload in the liver of cats with feline hepatic lipidosis (FHL).
Methods
Lipogenic gene expression of peroxisome proliferator-activated receptor-alpha (PPAR-α), peroxisome proliferator-activated receptor-gamma (PPAR-γ), fatty acid synthase (FASN) and sterol regulatory element-binding factor (SREBF1) were evaluated using quantitative RT-PCR in liver tissue of six cats with FHL and compared with the liver tissue of eight healthy cats.
Results
In liver tissue, PPAR-α, PPAR-γ and FASN mRNA expression levels were not significantly different (P >0.12, P >0.89 and P >0.5, respectively) in the FHL group compared with the control group. SREBF1 gene expression was downregulated around 10-fold in the FHL group vs the control group (P = 0.039).
Conclusions and relevance
The downregulation of SREBF1 in the liver tissue of cats with FHL does not support the hypothesis that de novo lipogenesis in the liver is an important pathway of fatty acid accumulation in FHL.
Keywords: Feline hepatic lipidosis, de novo lipogenesis, SREBP-1c, qRT-PCR
Introduction
Feline hepatic lipidosis (FHL) is considered the most common hepatobiliary disease in cats and is characterised by the accumulation of excessive triglycerides (TGs) in more than 80% of hepatocytes, resulting in liver dysfunction and cholestasis.1–4 The pathophysiology of FHL is complex and the recent literature has highlighted how FHL results from multiple alterations in the pathways involved in the hepatic uptake of fatty acids (FAs), lipoproteins and glucose, TG degradation and FA oxidation, and lipoprotein secretion in the form of very-low-density lipoproteins.1,3–5 De novo TG synthesis in the liver could cause some of the hepatic FA load and may further exacerbate liver fat accumulation.4–6
De novo lipogenesis (DNL) is a complex and highly regulated metabolic pathway.7,8 In normal conditions DNL converts excess carbohydrate into FAs, which are then esterified to storage triacylglycerols. The main product of DNL is palmitic acid (C16:0). Palmitic acid can be desaturated by stearoyl-CoA desaturase 1 (SCD-1) to produce palmitoleic acid, or it can be elongated to yield stearic acid (C18:0) and other shorter FAs.8–10
In healthy humans, DNL is active in both liver and adipose tissue, whereas in healthy cats the adipose tissue is primarily involved and the liver plays a secondary role. Furthermore, while glucose is the precursor for DNL in humans, in cats the substrate to form the FA palmitate is acetate.3,11–13 DNL is considered to be a minor contributor to lipid formation and lipid homeostasis in healthy individuals.14–17
However, in humans, hepatic DNL has been demonstrated to contribute to liver steatosis in different metabolic diseases that cause deregulation of the lipogenic pathways, such as obesity, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD).18–22
The adipogenic transformation of hepatocytes, with the expression of gene profiles characteristic of healthy adipose tissue, contributes to liver overload with excess free FAs (FFAs).8,23,24 DNL has been demonstrated to contribute up to 26% of FFAs that accumulate in the liver of patients with NAFLD.8,10,25
The role of the molecular mediators of lipogenesis in NAFLD has recently been reviewed. 26
Sterol regulatory element-binding protein 1c (SREBP-1c) has been defined as the pivotal controller of lipid biosynthesis and main regulator of the expression of several genes involved in lipogenesis.27–30 In patients with NAFLD and obese patients, elevated de novo hepatic FA synthesis and secondary TG accumulation in the liver has been reported to be secondary to increased mRNA expression of hepatic SREBP-1c. Increasing SREBP-1c by inducing lipogenic gene expression, such as those for fatty acid synthase (FASN) and peroxisome proliferator-activated receptor gamma (PPAR-γ), and suppressing the mRNA expression of peroxisome proliferator-activated receptor alpha (PPAR-α), favours hepatic lipogenesis over FA oxidation and leads to steatosis.28–33 FASN is involved in conversion of malonyl-CoA into 16-carbon saturated FA palmitate. 26 PPAR-γ is required for normal adipocyte differentiation and is expressed at very low levels in the liver of healthy individuals. Overexpression of PPAR-γ in the liver leads to hepatic steatosis, also by enhancing the expression of other adipogenic genes in the liver.32,34–36
The physiological significance of DNL in NAFLD has been recently reviewed by Hodson and Frayn. 27 They argued that the most important role of DNL might not be its quantitative contribution to hepatic FA overload, but its part in the suppression of FA oxidation. 27 Malonyl-CoA, a key a metabolic intermediate in de novo FA biosynthesis in lipogenic tissues, is, in fact, a potent inhibitor of the mitochondrial outer membrane enzyme carnitine-palmitoyl transferase 1 (CPT-1). 37 CPT-1 is responsible for the transport of fatty acyl-CoA into the mitochondrion, a fundamental step in FA oxidation. 38 The increased expression of SREBP-1c in NAFLD suppresses PPAR-α, further inhibiting CPT-1 and FA beta-oxidation. 31
The role of DNL in the development of hepatic lipidosis has been evaluated in the American mink (Neovison vison), an obligated carnivore often used as an experimental animal model for NAFLD.12,13 These animals share physiological similarities with the domestic cat and they can easily develop hepatic lipidosis after a short period of anorexia.4,5,13,39 In a recent study evaluating mRNA expression of lipogenic genes in the liver of mink with hepatic lipidosis, Rouvinen-Watt et al confirmed that liver steatosis is partially secondary to the activation of the hepatic DNL. 13
The concept that hepatic DNL could contribute to liver FA overload in cats with hepatic lipidosis was, for the first time, introduced by Hall et al in their study on the lipid composition of hepatic and adipose tissue in cats with FHL. 40 The FA composition of visceral adipose tissue and of the liver of cats with HL revealed greater percentages of palmitate and monounsaturated FAs (MUFAs) than in healthy controls. The increased hepatic concentrations of palmitate in cats with HL vs healthy cats supported the hypothesis that some de novo synthesis of FAs occurs both in the liver and adipose tissue of cats with hepatic lipidosis. 40 Furthermore, in FHL, acetate, like other ketone bodies, is increased as a result of a more complex catabolic state that includes increased insulin resistance and decreased tolerance to glucose.5,11,41 The increased acetate in the liver of cats with FHL constitutes a potential excess substrate for hepatic DNL, contributing and worsening hepatic FA overload.
To date, the concept of adipogenic transformation of hepatocytes and its contribution to hepatic FA accumulation has not been adequately evaluated in cats.
The aim of the present study was to evaluate adipogenic transformation of hepatocytes and de novo lipid synthesis by determination of lipogenic gene expression of PPAR-α, PPAR-γ, FASN and SREBF1 (equivalent in function to SREBP-1c) using quantitative RT-PCR (qRT-PCR) in the liver tissue of cats with FHL.
Materials and methods
This study was approved by the Committee for the Ethical Care of Animals of Utrecht University. Written, informed consent was obtained from all cat owners prior to study enrolment of the cats.
Animals
Cats admitted from January 2014 to January 2015 to the Department of Clinical Science of Companion Animals (DCSCA) of the Faculty of Veterinary Medicine, Utrecht University diagnosed with HL based on clinical signs (anorexia, vomiting and icterus), ultrasonography findings (diffuse echodensity of the liver) and cytological evaluation of fine-needle aspirates of the liver consistent with FHL were considered for the study. Only cats that were euthanased owing to deterioration of their clinical condition, despite intense treatment and/or because of financial constraints, were enrolled in the study.
In cats with FHL, wedge liver biopsies were immediately collected post mortem via laparotomy. The biopsies were rinsed in normal saline (NaCl 0.9%), rapidly frozen in liquid nitrogen and then stored at −80°C. These frozen biopsy specimens were used to perform qRT-PCR.
Aged-matched liver biopsies of eight healthy cats were used as controls. These liver biopsies were retrieved from surplus material from a study on the effect of administration of vitamin A on skeletal and hepatic tissue as part of the university’s 3R-policy. 42 Good health in these cats was based on a normal physical examination and haematological, biochemical and coagulation parameters within the reference intervals. In this study, percutaneous ultrasound-guided liver biopsies at the beginning and end of the study were taken with a 16 G, 20 cm long biopsy needle (Pro-Mag Biopsy Needle; Argon Medical Devices) under general anaesthesia. The liver biopsies were snap frozen in liquid nitrogen and then stored at −80°C. Only liver biopsies taken at the beginning of the study before supplementation of vitamin A was initiated were used for evaluation of lipogenesis gene expression.
RNA isolation, reverse transcription and quantitative RT-PCR
Total RNA was isolated from liver tissue using a RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. An on-column DNase-I (QIAFEB, RNase-free DNase kit) treatment was included to avoid contamination with residual genomic DNA. RNA concentrations and quality were measured spectrophotometrically using the Nanodrop ND-1000 (Isogen Life Science). RNA integrity was checked on a Bioanalyzer 2100 (Agilent Technologies). The RNA integrity number of all samples exceeded seven. Per sample, at least 3 µg of RNA was used for further processing. cDNA was synthesised from all RNA samples with the iScriptTM cDNA Synthesis Kit (BioRad), which contained both oligo-dT and random hexamer primers to ensure that the cDNA levels were reliably representative of the mRNA levels. Around 600 ng of RNA was incubated with iScript reaction mix, iScript reverse transcriptase and nuclease-free water at 42°C for 30 mins in a 60 μl reaction volume.
As required under MIQE-precise expression stability of the reference genes was evaluated and a combination of ribosomal protein S5 (RPS5), β-glucuronidase (GUSB) and tyrosine 3-monooxygenase/tryptophan 5-momooxygenase activation protein zeta isoform (YWHAZ) was optimal. 43 Primer sequences for specific sequence-confirmed amplicons and optimal annealing temperature are described in Table 1. The qRT-PCRs were performed in duplicate using the BioRad detection system. Amplifications were carried out in a volume of 25 μl containing 12.5 μl 2 × SYBR green supermix (BioRad), 0.4 µM forward and reverse primer, and 1 μl cDNA and Milli-Q water. Cycling conditions were as follows: denaturation at 95°C for 3 mins, followed by 45 cycles of denaturation (95°C for 10 s) and amplicon-specific annealing temperature for 30 s. A melt curve analysis was performed for every reaction to verify amplicon specificity. IQ5 Real-Time PCR detection system software (BioRad) was used for data analysis. A no template control was also run in duplicate with each plate as a negative control. Expression levels were calculated taken the PCR efficiency into account; the PCR efficiency was always between 96% and 106%.
Table 1.
Primers for FASN, PPAR-γ, SREBF1 and PPAR-α
| Gene | Primer 5′ → 3′ | Annealing temperature (ºC) | Amplicon size (bp) |
|---|---|---|---|
| FASN | F: GAAATCGGCAAATTCGACCT | 65 | 115 |
| R: CTGTTCCCACCTTCATCCA | |||
| PPAR-γ | F: TGTGACCTTAACTGTCGTATCC | 66–67 | 134 |
| R: CTTCTCTTTCTCCGCCTGTG | |||
| SREBF1 | F: CGTTTCTTCGTGGATGGG R: ACAAATTCAGTGCTGCTC |
63 | 140 |
| PPAR-α | F: GACAAATGTGACCGTAGCTG | 60 | 109 |
| R: AAACGAATTGCGTTATGGGA | |||
| RPS5 | F: CAGGTCTTGGTGAATGCG | 58 | 129 |
| R: CCAGATGGCCTGATTCAC | |||
| GUSB | F: TGACATCACCATCAGCACCAGC | 67 | 114 |
| R: GCCTTCCTCATCCAGAAGACGC | |||
| YWHAZ | F:GAAGAGTCCTACAAAGACAGCACGC | 65 | 115 |
| R: AATTTTCCCCTCCTTCTCCTGC |
Data are reported as median (range). The Mann–Whitney U-test was used to determine significance for qRT-PCR results between groups, with statistical significance being set at P <0.05.
Results
Animals
The FHL group consisted of six client-owned cats (four male castrated, one male intact and one female spayed) that met the inclusion criteria for the study. Three cats were European Shorthairs, two were Maine Coons, one was a Norwegian Forest Cat and one a Turkish Angora. Median age was 4 years and 4 months (range 2–8.5 years) and median body weight was 4 kg (range 3–6.7 kg). Anorexia, lethargy and icterus were the major problems for which 5/6 cats were referred to the DCSCA. One cat was admitted for dyspnoea secondary to chronic traumatic diaphragmatic rupture and developed HL while in hospital.
The control group consisted of six castrated males and two intact males cats. They all were European Shorthair cats. Median age was 4 years and 3 months (range 1.5–7 years) and median body weight was 4.6 kg (range 3–6.3 kg).
Lipogenic gene expression
In liver tissue, PPAR-α, PPAR-γ and FASN mRNA expression levels were not significantly different (P >0.12, P >0.89 and P >0.5, respectively) in the FHL group compared with the control group (Figure 1). SREBF1 gene expression was downregulated around 10-fold in the FHL group vs the control group (P = 0.039) (Figure 1).
Figure 1.
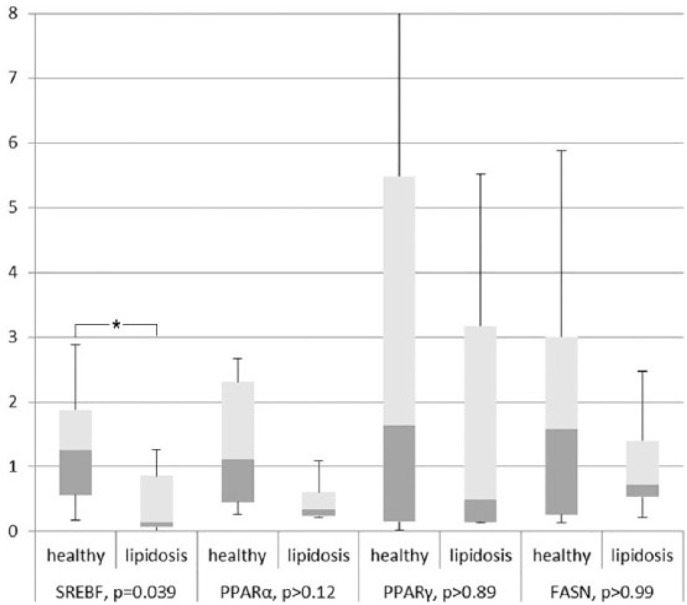
Presented are median, first quartile (box) and range (whiskers). Expression per gene is related to the average of the healthy control group, which is set at ‘1’. Expression corrected for three reference genes RPS5, GUSB and YWHAZ. *Only the expression of SREBF1 is significantly downregulated in feline hepatic lipidosis samples compared with heathy controls
Discussion
In humans, in situations of metabolic dysregulation, DNL has been suggested to be abnormally increased and contributes to the pathogenesis of liver steatosis in diseases such as obesity and NAFLD.10,20–22 The increased DNL is characterised by the presence of the FA palmitate, the primary product of de novo FA synthesis, and the MUFA palmitoleic acid, synthesised from palmitate by the action of the enzyme SCD-1.9,10
In cats with FHL, a higher concentration of palmitate and MUFAs were found both in the visceral adipose tissue and liver, when compared with healthy cats. 40 This finding was suggestive of an increased DNL in visceral adipose tissue and most likely also in the liver as part of the pathophysiological mechanism of liver steatosis, contributing and worsening FA liver overload. 40
In this study the decrease in liver SREBF1 gene expression and no expression change in other DNL-related enzymes suggest that DNL does not occur in the liver of cats with FHL. This is in contrast with what has been reported in humans with NAFLD and in other obligated carnivores.13,18–21 Furthermore, expression of PPAR-α was similar to its expression in healthy cats. This suggests that CPT-1a expression and the following degree of β-oxidation in the liver of cats with hepatic lipidosis is not affected by a downregulation of PPAR-α.
Using labelled FAs, Donnelly et al concluded that 59% of TGs in the livers of patients with NAFLD were from increased lipolysis in the adipose tissue and increased FA flux to the liver, 26% were from hepatic DNL and 15% were from the diet. 25 Hepatic DNL contributes significantly to FA accumulation in the liver of subject with NAFLD.
The development of hepatic lipidosis has been investigated in the American mink, as a model of NAFLD.12,13 In the American mink, food deprivation for 3 days was adequate to induce hepatic lipidosis, with liver fat percentage increasing twice in the fasted group vs the control group. The liver steatosis in the initial days of fasting was secondary to the flux of FAs from extensive peripheral lipolysis from the visceral adipose tissue.12,13 In the following days, the percentage of liver lipid accumulation increased dramatically to the end of the 7-day fasting period. Significant increases were observed in the levels of mRNA encoding for acetyl-CoA carboxylase 1 (ACC-1) and FASN after 5–7 days of fasting compared with non-fasted mink. It was concluded that hepatic DNL could have further exacerbated liver fat accumulation in fasted minks and that 5–7 days of fasting appeared to be the critical time period for this to develop. Although SREBP-1c was not measured in this study, the higher expression of FASN and ACC-1, and the higher hepatic palmitate and MUFA concentrations, suggested that the gene expression of the key enzymes for DNL was increased. Feeding the minks for 28 days after the end of the fasting period allowed the relative expression of ACC-1 and FASN to return to pre-fasting levels. 13
In a model of a diet-induced overweight state in cats, lipogenic gene expression (SREBP-1c, FASN and ATP citrate lyase [ACLY]) in lipid sensitive tissue, that is liver, subcutaneous and abdominal adipose tissue, has been evaluated and compared with lean animals. 46 As already demonstrated in humans, a positive energy imbalance due to overnutrition and obesity can activate SREBP-1c and other secondary lipogenic gene expressions, enhancing lipogenesis in adipose and liver tissues contributing to liver steatosis.18,19,21 In the overweight cats, mRNA expression of SREBP-1c and ATP citrate lyase were markedly decreased in liver tissue, whereas FASN expression remained similar to that of control cats. Abdominal SREBP-1c mRNA expression was markedly lower in abdominal subcutaneous and higher in omental adipose tissues of overweight cats than in control cats. The conclusion of this study was that in an overweight cat model DNL does not occur in the liver, but it occurs in the visceral adipose tissue. 46 This supports the finding of Hall et al, 40 that the origin of the enhanced hepatic TGs in cats with FHL is the visceral adipose tissue and not the liver.
Our study has some limitations. Although the collection process of healthy liver tissue (ie, needle biopsies) and of FHL livers (ie, post-mortem wedge material) were different, there are no indications that the biopsy technique itself could have caused differences in relative mRNA expression levels.
In order to validate the qRT-PCR data, the reduced mRNA level needs to be confirmed by quantitative measurement at the protein level. This confirmation step is currently hampered by the very low hepatic expression of SREBF1 together with the lack of validated antibodies for SREBF1 in feline tissues. Interestingly, the downregulation of SREBF1, as observed here in vivo, is similar to the downregulation observed in three-dimensional feline liver organoids cultured in vitro under FA overload, lending credence to the robustness of the qRT-PCR measurements. 44
Conclusions
The downregulation of SREBF1 in the liver tissue of cats with FHL does not support the hypothesis that DNL in the liver is an important pathway of FA accumulation in FHL. As cats with hepatic lipidosis have an increased concentration of palmitate and MUFAs vs healthy cats in their liver and visceral adipose tissue, DNL in FHL most likely occurs in the abdominal adipose tissue. Subsequently, the de novo synthetised MUFAs are transported from the adipose tissue to the liver, where they contribute to fat overload in FHL.
Footnotes
Accepted: 27 May 2019
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: LCP received a grant from The Netherlands Organization for Health Research and Development (NWO Zon/MW number 16004121) for liver progenitor research.
Ethical approval: This work involved the use of client-owned animal(s) only, and followed established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work for the procedure(s) undertaken. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Chiara Valtolina  https://orcid.org/0000-0002-4601-8885
https://orcid.org/0000-0002-4601-8885
References
- 1. Armstrong PJ, Blanchard G. Hepatic lipidosis in cats. Vet Clin North Am Small Anim Pract 2009; 39: 599–616. [DOI] [PubMed] [Google Scholar]
- 2. Center SA, Guida L, Zanelli MJ, et al. Ultrastructural hepatocellular features associated with severe hepatic lipidosis in cats. Am J Vet Res 1993; 54: 724–731. [PubMed] [Google Scholar]
- 3. Center SA. Feline hepatic lipidosis. Vet Clin North Am Small Anim Pract 2005; 35: 225–269. [DOI] [PubMed] [Google Scholar]
- 4. Valtolina C, Favier RP. Feline hepatic lipidosis. Vet Clin North Am Small Anim Pract 2017; 47: 683–702. [DOI] [PubMed] [Google Scholar]
- 5. Verbrugghe A, Bakovic M. Peculiarities of one-carbon metabolism in the strict carnivorous cat and the role in feline hepatic lipidosis. Nutrients 2013; 5: 2811–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang ZG, Robson SC, Yao Z. Lipoprotein metabolism in nonalcoholic fatty liver disease. J Biomed Res 2013; 27: 1–13. DOI: 10.7555/JBR.27.20120077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol 2006; 290: G852–8. DOI: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- 8. Ameer F, Scandiuzzi L, Hasnain S, et al. De novo lipogenesis in health and disease. Metabolism 2014; 63: 895–902. [DOI] [PubMed] [Google Scholar]
- 9. Lee JJ, Lambert JE, Hovhannisyan Y, et al. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am J Clin Nutr 2015; 101: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lambert JE, Ramos-Roman MA, Browning JD, et al. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014; 146: 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aroch I, Shechter-Polak M, Segev G. A retrospective study of serum beta-hydroxybutyric acid in 215 ill cats: clinical signs, laboratory findings and diagnoses. Vet J 2012; 191: 240–245. [DOI] [PubMed] [Google Scholar]
- 12. Rouvinen-Watt K, Mustonen AM, Conway R, et al. Rapid development of fasting-induced hepatic lipidosis in the American mink (Neovison vison): effects of food deprivation and re-alimentation on body fat depots, tissue fatty acid profiles, hematology and endocrinology. Lipids 2010; 45: 111–128. [DOI] [PubMed] [Google Scholar]
- 13. Rouvinen-Watt K, Harris L, Dick M, et al. Role of hepatic de novo lipogenesis in the development of fasting-induced fatty liver in the American mink (Neovison vison). Br J Nutr 2012; 108: 1360–1370. [DOI] [PubMed] [Google Scholar]
- 14. Richard MJ, Holck JT, Beitz DC. Lipogenesis in liver and adipose tissue of the domestic cat (Felis domestica). Comp Biochem Physiol B 1989; 93: 561–564. [DOI] [PubMed] [Google Scholar]
- 15. Campos CF, Duarte MS, Guimaraes SE, et al. Review: animal model and the current understanding of molecule dynamics of adipogenesis. Animal 2016; 10: 927–932. [DOI] [PubMed] [Google Scholar]
- 16. Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr 2005; 135: 2499–2502. [DOI] [PubMed] [Google Scholar]
- 17. Bjorntorp P, Sjostrom L. Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism 1978; 27 Suppl 2: 1853–1865. [DOI] [PubMed] [Google Scholar]
- 18. Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol 2010; 45: 199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Byrne CD. Fatty liver: role of inflammation and fatty acid nutrition. Prostaglandins Leukot Essent Fatty Acids 2010; 82: 265–271. [DOI] [PubMed] [Google Scholar]
- 20. Choudhury J, Sanyal AJ. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liver Dis 2004; 8: 575–594. [DOI] [PubMed] [Google Scholar]
- 21. Diraison F, Dusserre E, Vidal H, et al. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab 2002; 282: E46–E51. [DOI] [PubMed] [Google Scholar]
- 22. Rector RS, Thyfault JP, Wei Y, et al. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol 2008; 14: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pardina E, Baena-Fustegueras JA, Llamas R, et al. Lipoprotein lipase expression in livers of morbidly obese patients could be responsible for liver steatosis. Obes Surg 2009; 19: 608–616. [DOI] [PubMed] [Google Scholar]
- 24. Westerbacka J, Kolak M, Kiviluoto T, et al. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes 2007; 56: 2759–2765. [DOI] [PubMed] [Google Scholar]
- 25. Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 2004; 114: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hodson L, Frayn KN. Hepatic fatty acid partitioning. Curr Opin Lipidol 2011; 22: 216–224. [DOI] [PubMed] [Google Scholar]
- 28. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002; 109: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horton JD, Goldstein JL, Brown MS. SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb Symp Quant Biol 2002; 67: 491–498. [DOI] [PubMed] [Google Scholar]
- 30. Horton JD. Sterol regulatory element-binding proteins: transcriptional activators of lipid synthesis. Biochem Soc Trans 2002; 30: 1091–1095. [DOI] [PubMed] [Google Scholar]
- 31. Pettinelli P, Del Pozo T, Araya J, et al. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta 2009; 1792: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 32. Pettinelli P, Videla LA. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab 2011; 96: 1424–1430. [DOI] [PubMed] [Google Scholar]
- 33. Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int 2010; 4: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsusue K, Haluzik M, Lambert G, et al. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest 2003; 111: 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu S, Matsusue K, Kashireddy P, et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem 2003; 278: 498–505. [DOI] [PubMed] [Google Scholar]
- 36. Boelsterli UA, Bedoucha M. Toxicological consequences of altered peroxisome proliferator-activated receptor gamma (PPARgamma) expression in the liver: insights from models of obesity and type 2 diabetes. Biochem Pharmacol 2002; 63: 1–10. [DOI] [PubMed] [Google Scholar]
- 37. Saggerson D. Malonyl-CoA, a key signaling molecule in mammalian cells. Annu Rev Nutr 2008; 28: 253–272. [DOI] [PubMed] [Google Scholar]
- 38. Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr 2001; 21: 193–230. [DOI] [PubMed] [Google Scholar]
- 39. Eisert R. Hypercarnivory and the brain: protein requirements of cats reconsidered. J Comp Physiol B 2011; 181: 1–17. [DOI] [PubMed] [Google Scholar]
- 40. Hall JA, Barstad LA, Connor WE. Lipid composition of hepatic and adipose tissues from normal cats and from cats with idiopathic hepatic lipidosis. J Vet Intern Med 1997; 11: 238–242. [DOI] [PubMed] [Google Scholar]
- 41. Chan DL, Freeman LM. Nutrition in critical illness. Vet Clin North Am Small Anim Pract 2006; 36: 1225–1241. [DOI] [PubMed] [Google Scholar]
- 42. Corbee RJ, Tryfonidou MA, Grinwis GC, et al. Skeletal and hepatic changes induced by chronic vitamin A supplementation in cats. Vet J 2014; 202: 503–509. [DOI] [PubMed] [Google Scholar]
- 43. Bustin S, Penning LC. Improving the analysis of quantitative PCR data in veterinary research. Vet J 2012; 191: 279–281. [DOI] [PubMed] [Google Scholar]
- 44. Kruitwagen HS, Oosterhoff LA, Vernooij IGWH, et al. Long-term adult feline liver organoid cultures for disease modeling of hepatic steatosis. Stem Cell Rep 2017; 8: 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Penning LC, Vrieling HE, Brinkhof B, et al. A validation of 10 feline reference genes for gene expression measurements in snap-frozen tissues. Vet Immunol Immunopathol 2007; 120: 212–222. [DOI] [PubMed] [Google Scholar]
- 46. Lee P, Mori A, Takemitsu H, et al. Lipogenic gene expression in abdominal adipose and liver tissues of diet-induced overweight cats. Vet J 2011; 190: e150-3. [DOI] [PubMed] [Google Scholar]


