Abstract
Identifying factors that contribute to inter-individual differences in emotional reactivity is central to understanding the basic mechanisms that give rise to adaptive emotion reactivity and to disruptions that may occur in psychopathology. The current study related emotional reactivity in an unselected young adult sample (N = 101) to individual difference factors relevant to emotional functioning and mood pathology, specifically anhedonia, depressed mood, and current affective state. To assess emotional reactivity, participants rated their emotional responses to 100 pictures from the International Affective Picture System. Increased self-reported anhedonia (i.e. reduced hedonic capacity) predicted blunted emotional reactivity to both positive and negative images, relative to neutral images, while elevated depressed mood predicted potentiated emotional reactivity to negative vs. neutral images. Anhedonia also accounted for far greater variance in emotional reactivity than depressed mood. Further, more positive affective state predicted potentiated reactivity to positive versus neutral images while more negative affective state predicted potentiated reactivity to negative versus neutral images beyond effects of anhedonia and depressed mood. The current study identified separable effects of anhedonia, depressed mood, and current affect on emotional reactivity.
Keywords: Emotional reactivity, Anhedonia, Depression, Individual differences, Affect, IAPS
Introduction
Our emotional reactions to our environment are one of the most integral aspects of human experience; they inform and differentiate our experiences, memories, and interactions with the world. Yet, there are substantial differences among individuals in the way and degree to which we experience emotional reactions to the stimuli around us. Understanding the factors that contribute to inter-individual differences is central to understanding the basic mechanisms that give rise to adaptive emotion reactivity (ER) as well as the disruptions in these mechanisms that may occur in psychopathology. Although an oversimplification, studies of ER often focus on positive ER and negative ER as a useful heuristic, referencing an individual’s change in emotional state from baseline affect in response to positive and negative stimuli, respectively (Bylsma et al. 2008). As we discuss below, disruptions in positive and negative ER are among the core deficits observed in mood pathology, such as major depressive disorder (MDD), though the direction of observed effects is often inconsistent, particularly regarding negative ER. Importantly, there are large individual differences in ER in healthy populations as well as across individuals with mood pathology. The goal of the current study was to examine how individual differences in hedonic capacity and depressed mood relate to ER in a normative population, which informs our basic understandings of the role of individual differences in ER and how such relations may help resolve conflicting findings regarding ER in MDD.
The largest meta-analysis to date examining emotion reactivity in MDD provided clear support for a blunting of positive ER, i.e. less positive responses to positive stimuli in depressed groups (Bylsma et al. 2008; Grimm et al. 2009; Kellough et al. 2008; Sloan et al. 1997). This has been described as the “positive attenuation” hypothesis of MDD, i.e. decreased positive ER (Rottenberg et al. 2005). This same meta-analysis also found evidence of less negative responses to negative stimuli in MDD (Bylsma et al. 2008). Together, results from this meta-analysis support the “Emotional Context Insensitivity” (ECI) hypothesis, which postulates that mood pathology is associated with a blunting of both positive and negative ER (Bylsma et al. 2008; Rottenberg et al. 2005). However, several studies have also noted increased ER to negative images in MDD (Grimm et al. 2009; Kellough et al. 2008), termed “negative potentiation,” i.e. increased negative ER. Together, the inconsistencies in the literature regarding ER in MDD have led to several different hypotheses about ER that are somewhat conflicting; specifically, while the positive attenuation and ECI hypotheses both predict blunted ER to positive stimuli, the negative potentiation and ECI hypotheses make opposing predictions about negative ER in depression.
We propose that such mixed findings may be the result of examining depression as unitary construct, thus disregarding the heterogeneity of individual differences present within both healthy populations and groups with mood pathology. Particularly, an MDD diagnosis requires an individual to experience a loss of pleasure (anhedonia) and/or depressed mood, as well as five or more secondary emotional and somatic symptoms for most or all days (American Psychiatric Association 2013). Therefore, people diagnosed with MDD may exhibit either anhedonia, depressed mood, or both, at varying levels of severity. Similarly, non-depressed individuals also vary in the degree to which they experience subclinical depressed mood and anhedonic symptomology. Critically however, the literature has yet to disentangle the unique relations between these constructs and ER in either healthy or depressed individuals.
The handful of studies investigating anhedonia in ER suggest that elevated anhedonia (i.e., reduced hedonic capacity) in healthy controls and those with schizophrenia/schizoaffective disorder relates to blunted positive and negative ER, i.e. decreased emotional response to all stimuli (Berenbaum and Oltmanns 1992; Burbridge and Barch 2007; Dowd and Barch 2010). This blunting of both negative and positive ER observed in individuals with elevated anhedonia is consistent with the ECI hypothesis of depression. While there are no studies, to our knowledge, relating depressed mood specifically to both positive and negative ER, a recent study in healthy children found that elevated depressed/negative mood related to enhanced behavioral responsiveness to negative feedback, consistent with the negative potentiation hypothesis of depression (Luking et al. 2015). Together, these studies suggest that severity of specific types of symptoms (anhedonia and depressed mood) observed in healthy populations may relate to different theories of ER in MDD that might otherwise conflict.
The goal of the current study was to examine whether individual differences in hedonic capacity and depressed mood differentially predict positive and negative ER in an unselected sample. We hypothesized that elevated anhedonia (i.e. reduced hedonic capacity) would be associated with an overall blunting of ER, i.e. less positive responses to positive stimuli and less negative responses to negative stimuli. Furthermore, we hypothesized that increased depressed mood would be associated with a general increase in negative emotional processing, resulting in less positive responses to positive stimuli and more negative responses to negative stimuli. Support for these two hypotheses would provide valuable information about the factors influencing normative individual differences in emotional reactivity and would have further implications for the study of mood pathology. Specifically, support for these hypotheses would suggest that the positive attenuation and ECI hypotheses are likely characterizing the relations between anhedonia and ER, while negative potentiation is a better characterization of the effects of depressed mood. Finally, as an exploratory follow-up, we examined whether a participant’s current affective state would further influence individual differences in ER over and above effects of potentially more trait-level factors, like hedonic capacity and depressed mood.
Methods
Participants
One hundred and twenty-eight people from Washington University in St. Louis and the surrounding St. Louis area enrolled in the present study. Of those enrolled, 19 did not arrive for their session or failed to complete the full protocol. Participants taking psychoactive medication (N = 6), as well as participants who did not meet our age inclusion criterion of 18 to 30 years (N = 2) were excluded. The remaining 101 participants (65 female) were included in the present analyses. These individuals identified as White (N = 33), Asian (N = 46), African-American (N = 11), non-White Hispanic (N = 1), or multiracial (N = 9), and one participant chose not report their ethnicity.
Design and materials
Using an online scheduling system, Experimetrix, participants were recruited and enrolled in a behavioral study. Participants completed consent as well as several questionnaires online either before or at the beginning of the in-person session. Participants completed additional questionnaires on paper during the in-person session. During the session, participants completed several tasks assessing reward and emotional processing, including the Emotional Picture Rating Task (EPRT), which was focus of the current study. Participants were paid $10 per hour of participation, in addition to $5 for completing the online questionnaires and any money won during tasks with monetary incentives. No money could be won during the EPRT. The institutional review board at Washington University in St. Louis approved all study procedures.
Individual difference measures
Participants completed forms to assess depressive symptoms, Beck depression inventory (BDI, Beck et al. 1996), and hedonic capacity, Snaith-Hamilton Pleasure Scale (SHPS, Snaith et al. 1995), Fawcett-Clark Pleasure Scale (FCPS, Fawcett et al. 1983), and Behavioral Inhibition System /Behavioral Approach System Scales (BIS, Carver and White 1994). In addition, participants completed forms regarding demographics and medication usage and several other measures not of interest to the current analyses (See Supplement for complete list). Descriptive statistics are provided in Table 1. The demographics form, FCPS, SHPS, and BIS/BAS were completed online either before or at the start of the in-person session. The BDI, Mood Rating Questionnaires (described below), and other measures were completed on paper during the session.
Table 1.
Descriptive statistics for measures of interest
| Min | Max | Mean | S.D. | Alpha (α) | |
|---|---|---|---|---|---|
| Age in years | 18 | 28 | 19.76 | 1.80 | – |
| Years of education | 12 | 19 | 13.93 | 1.59 | – |
| Behavioral activation reward subscale total | 12 | 20 | 17.66 | 1.66 | 0.56 |
| Snaith-Hamilton pleasure scale total | 32 | 56 | 47.64 | 5.09 | 0.93 |
| Fawcett-Clark pleasure scale totala | 56 | 152 | 111.66 | 16.67 | 0.85 |
| Anhedonia composite scale total | −4.66 | 9.27 | 0.03 | 2.55 | 0.80 |
| Beck depressive inventory II totalb | 0 | 24 | 6.92 | 6.31 | 0.87 |
| Depressed mood subscalec total | 0 | 12 | 2.51 | 3.16 | 0.85 |
| Positive affective state total | 1 | 6.5 | 3.11 | 1.20 | 0.82 |
| Negative affective state total | 1 | 4.75 | 1.86 | 0.91 | 0.50 |
Excludes questions pertaining to intimate relationships or children
Item 9 was not administered
Consists of items 2, 3, 5–8, and 14 of the BDI
Anhedonia composite scale
The literature does not support only one questionnaire or a “standard” questionnaire for assessing individual differences in anhedonia in relation to emotional reactivity. Thus we collected several measures of hedonic capacity/reward responsiveness. We first aimed to evaluate relations between these potential measures of interest by examining correlations between scores on the SHPS (M = 47.62, SD = 5.09), FCPS (M = 111.66, SD = 16.67), and BAS reward responsiveness subscale (BASr; M = 17.66, SD = 1.66). While assessing hedonic capacity and reward responsiveness in different ways, the SHPS, FCPS, and BASr all showed strong inter-correlations (all |r|s ≥ 0.53, all ps <0.001). Thus, the SHPS, FCPS, and BASr scores were z-scored, summed, and inverted to create a composite individual difference measure, ‘Anhedonia’ (Cronbach’s α=0.81). Higher values on this composite indicate increased anhedonia (i.e., reduced hedonic capacity).
Note that, as the mean age of our sample was 19 years old, questions on the FCPS related to intimacy or having children (i.e., items 3, 6, 8, 9, 15, and 32) were often left unanswered as they were likely less relatable to our sample. Thus, these items were excluded from FCPS mean scores. The Cronbach’s alpha of the FCPS improved greatly when these items were removed (α= 0.72 to α=0.93).
Depressed mood scale
We assessed individual differences in depressed mood with a subscale of the BDI identified by Beck and colleagues to measure negative attitudes and emotionality (Steer et al. 1999). Though others have used the same or a similar subscale, there is no consistent term or name used for this scale in the literature. For example, the items used in this scale have been referred to as Cognitive (vs Non-cognitive; Kumar et al. 2002), Cognitive-Affective (vs Somatic-Vegetative; Dozois et al. 1998), Cognitive (vs Somatic-Affective; Steer et al. 1999), Negative Attitude (vs Performance Difficulty vs. Somatic Elements; Osman et al. 1997) or simply unnamed. Thus, for simplicity, we refer to the subscale as a “depressed mood scale” given that the included items describe a group of experiences that include depression related cognitions and negative evaluations about one’s self. This subscale included items assessing pessimism, guilt, negativity, and worthlessness (items 2, 3, 5–9, & 14; note: item 9, ‘suicidality,’ was not collected), but did not include items assessing somatic or anhedonic symptoms. Thus, we used this subscale to assess individual differences in depressed mood symptoms dissociable from self-reports of anhedonia. Particularly, depressed mood scores did not significantly relate to Anhedonia scores in this sample (r(99) = 0.17, p = .09; Fig. 1).
Fig. 1.
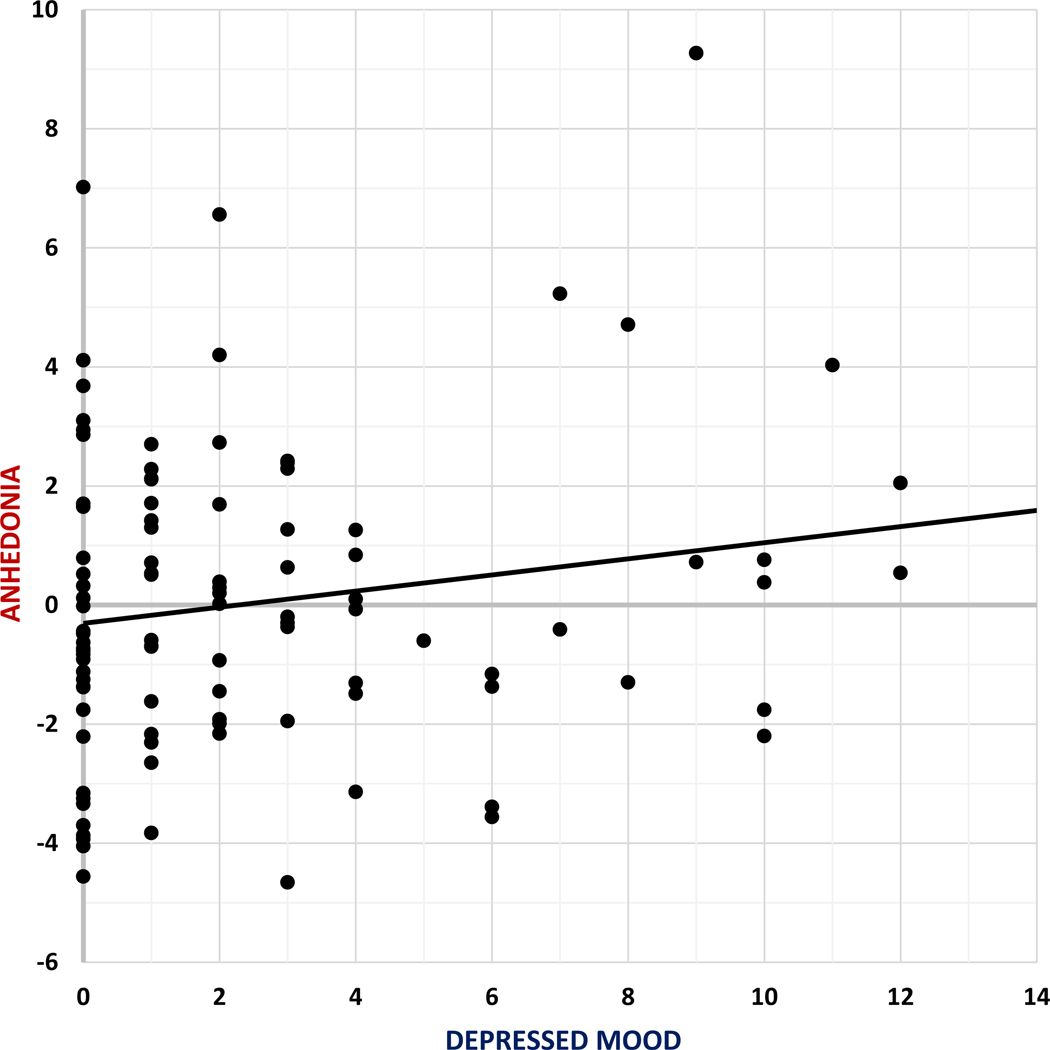
Spread of scores from Anhedonia composite score and depressed mood subscale
Current affective state
The BDI, FCPS, SHPS, and BASr ask individuals to rate their affect over the past several days to two weeks or to rate their expected affect in hypothetical situations. In order to test how an individual’s current affective state could influence ER beyond these more trait-level measures of Anhedonia and depressed mood, Mood Rating Questionnaires (MRQs) were administered throughout the in-person session. MRQs assessed how happy, excited, upset, and stressed participants felt at a given moment using a 7-point Likert scale for each emotion. Ratings collected directly before the EPRT were used in the current analyses. Happy and excited mood ratings were significantly correlated (r(99) = 0.71, p <.001), as were stressed and upset ratings (r(99) = 0.42, p<.001), and happy and excited ratings were not significantly related to stressed or upset ratings (all ps > 0.05). Thus, happy and excited ratings were averaged to form a ‘Positive Affective State’ composite variable while the stressed and upset ratings were averaged to create a ‘Negative Affective State’ composite variable, where higher scores indicated more positive or more negative current affect, respectively.
Calculating emotional reactivity (ER)
Emotional reactivity (ER) is defined in the literature as the change from baseline affect in emotional response to positive or negative stimuli (Bylsma et al. 2008). In our calculation of ER, ratings of neutral images were used as baseline affect, since the ratings are subject to both individual differences and specific emotional cues. Thus positive ER was calculated by subtracting neutral image ratings from positive image ratings (positive-neutral; M = 0.89, SD = 0.32) and negative ER was calculated by subtracting neutral image ratings from negative image ratings (negative-neutral; M=−1.20, SD = 0.43). For positive ER, a larger positive difference score indicates a greater increase in positive ratings to the positive compared to neutral pictures. For negative ER, a larger negative difference scores indicates a greater increase in negative ratings to the negative compared to neutral pictures.
Procedure
Emotional picture rating task (EPRT)
During the Emotional Picture Rating Task, participants rated their emotional response to 100 pictures from the International Affective Pictures System (IAPS, Lang et al. 2008, see Supplementary Table 4 for a complete list of images used). Pictures were chosen to create three valence categories, negative, neutral, and positive images, based on the original IAPS ratings assessed on the 9-point Self-Assessment Manikin (SAM, Bradley and Lang 1994). We selected 40 negative pictures with valence ratings between 1.00 and 4.00 (M = 2.76, SD = 0.64), 20 neutral pictures with valence ratings between 4.00 and 6.00 (M = 4.87, SD = 0.53), and 40 positive pictures with valence ratings between 6.00 and 9.00 (M = 7.30, SD = 0.58).
During the EPRT, participants first viewed a picture for two seconds and then were asked to rate the valence and arousal level of their emotional response on the abbreviated 5-point SAM. Ratings were obtained using the number pad on a keyboard and were self-paced with the image remaining on the screen throughout the ratings. To assess valence, participants were told to select “the graphic that best describes how the picture makes you feel.” A rating of ‘1’ indicated negative valence and a rating of ‘5’ indicated positive valence. Participants were then told to select “the graphic that best describes how stimulated you are by the image or how intense the image makes you feel.” A rating of ‘1’ indicated low arousal and a rating of ‘5’ indicated high arousal. Arousal ratings were not examined in the current analyses. The order of the pictures was pseudorandomized so that no more than two pictures of the same valence type were shown in a row. Participants had the option to skip images they did not wish to view/rate (only nine participants ever used this option).
Data analysis
Individual difference measures analyses
Descriptive statistics for the relevant questionnaires are presented in Table 1. Pearson’s correlations were used to describe the relations between individual difference questionnaire measures (Table 2).
Table 2.
Intercorrelations between measures of interest
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| (1) Age in years | |||||||||
| (2) Years of education | 0.82*** | ||||||||
| (3) Behavioral activation reward subscale total | − 0.14 | − 0.13 | |||||||
| (4) Snaith-Hamilton pleasure scale Total | − 0.13 | − 0.12 | 0.52*** | ||||||
| (5) Fawcett-Clark pleasure scale Totala | − 0.25* | − 0.24* | 0.60*** | 0.60*** | |||||
| (6) Anhedonia composite scale Total | 0.20* | 0.19* | − 0.84*** | − 0.84*** | − 0.87*** | ||||
| (7) Beck depressive inventory II Totalb | 0.00 | − 0.02 | − 0.07 | − 0.22* | − 0.07 | 0.14 | |||
| (8) Depressed mood subscalec Total | 0.01 | − 0.02 | − 0.08 | − 0.27** | − 0.07 | − 0.17 | − 0.87*** | ||
| (9) Positive affective state total | 0.06 | 0.13 | 0.14 | 0.15 | 0.21* | − 0.19* | − 0.17 | − 0.14 | |
| (10) Negative affective state total | − 0.15 | − 0.06 | 0.00 | − 0.09 | 0.01 | 0.03 | 0.37*** | 0.30** | 0.02 |
p<.001
p<.01
p<.05
Excludes questions pertaining to intimate relationships or children
Item 9 was not administered
Consists of items 2, 3, 5–8, and 14 of the BDI
Emotional reactivity and individual difference analyses
We conducted Pearson’s correlations to evaluate the relations between emotional reactivity and individual difference measures, specifically Anhedonia, depressed mood, and positive and negative affective states.
Emotional picture rating task and normed data analyses
To compare image ratings from our sample to those of the original IAPS ratings, we calculated the mean valence rating across all participants for each image and correlated those values with the normed valence ratings. Further, three paired t tests were run to assess whether the mean ratings of each valence type (Neutral, Negative, Positive) were significantly different from one another.
Emotional picture rating task ratings and individual differences analyses
EPRT valence ratings and individual differences
As higher valence ratings of positive images reflect higher positive ER and higher valence ratings of negative images reflect lower negative ER, our first hypothesis predicts an interaction between ER valence and Anhedonia, such that elevated anhedonia is associated with blunted positive ER (less positive ratings of positive images) and blunted negative ER (less negative ratings of negative images). Our second hypothesis predicts a main effect of depressed mood such that elevated depressed mood is associated with blunted positive ER (less positive ratings of positive images) and potentiated negative ER (more negative ratings of negative images).
To test these hypotheses, we first conducted a general linear model (GLM) with repeated measures to assess relationships between Image Valence and individual differences in Anhedonia and depressed mood. Image Valence served as the within-subject repeated measure (3 levels: negative, neutral, and positive). Anhedonia and depressed mood were included as continuous predictors. Greenhouse-Geisser corrections are reported for analyses that violated Mauchly’s test of sphericity.
Individual differences predicting emotional reactivity
In cases where there was a significant effect of a continuous predictor, post-hoc hierarchical linear regressions were conducted to assess effects on ER magnitude between valences. Specifically, we examined post-hoc regressions predicting positive ER and negative ER to investigate significant interactions between ER valence and continuous predictors. Step 1 of the regressions included background demographic variables, i.e. dichotomous variables for sex (Female > Male) and ethnicity (Asian vs. not, White vs. not). Anhedonia and depressed mood as predictors of positive ER and negative ER were added in step 2. Finally, to test whether current mood predicted valence ratings over and above trait-level individual differences, Positive Affective State and Negative Affective State were included in step 3.
We also present regression results predicting valence ratings of positive, negative, and neutral images as independent conditions in the supplement (Supplementary Tables 2–4). They serve as post-hoc tests to further clarify the source of relations with the above ER results. We used a Bonferonni correction for multiple comparisons to correct for testing of our two main hypotheses (0.05/2), i.e. Anhedonia and depressed mood were considered significant predictors when they reached p < .025. Greenhouse-Geisser corrections are reported for analyses that violated Mauchly’s test of sphericity.
EPRT reaction time and individual differences
As depressive symptoms have been linked to slower reaction time (RT, Gollan et al. 2008; White et al. 1997) and greater time viewing an image could lead to differences in valence ratings, we conducted a final control analysis to examine rating RT. Particularly, a GLM with repeated measures was used to assess whether median RT for image ratings differed based on Image Valence and individual difference factors. Image Valence served as the within-subject repeated measure (3 levels; positive, neutral, and negative). Anhedonia, depressed mood, Positive Affective State and Negative Affective were included as continuous predictors, while Sex and Ethnicity (Asian vs. Not Asian, White vs. Not White) were included as between-subject predictors. This analysis was used to understand the relationship between RT and predictors of interest in the main GLM analyses’. Bonferonni correction for multiple comparisons was again used to correct for testing of our two predictors of interest (0.05/2), i.e. Anhedonia and depressed mood were considered significant predictors when they reached p < .025. Greenhouse-Geisser corrections are reported for analyses that violated Mauchly’s test of sphericity.
Results
Individual difference measures
Table 1 presents descriptive statistics for the self-report measures of interest, while intercorrelations between the measures of interest can be found in Table 2. In summary, Anhedonia was significantly negatively correlated with Positive Affective State (r(99)=−0.20, p = .04), but was not significantly correlated with Negative Affective State (r(99) = 0.03, p = . 80). Depressed mood was positively correlated with Negative Affective State (r(99) = 0.30, p < .003), but was not significantly correlated to Positive Affective State (r(99)=−0.14, p = .17). Finally, Positive Affective State was not significantly correlated with Negative Affective State, r(99) = 0.02, p = .85).
Emotional reactivity and individual difference correlations
Positive and negative ER were negatively correlated; r(99)=−0.59, p < .001. Further, positive ER was negatively correlated with Anhedonia (r(99)=−0.44, p < .001) and positively correlated with Positive Affective State (r(99) = 0.32, p = .001), but was not significantly correlated with depressed mood (r(99)=−0.10, p = .31) or Negative Affective State (r(99) = 0.10, p = .32). Negative ER was positively correlated with Anhedonia (r(99) = 0.46, p < . 001) and negatively correlated with Negative Affective State (r(99)=−0.21, p = .04), but was not significantly correlated with depressed mood (r(99)=−0.10, p = .31) or Positive Affective State (r(99) = 0.17, p = .09).
EPRT and normed data
The valence ratings of images acquired in the current study were highly consistent with ratings from the original IAPS data (r(98) = 0.97, p < .001). Paired t tests indicated a significant difference between the valence ratings of positive images and negative images (t(100)=−31.45, p < .001), between positive and neutral images (t(100) = 27.63; p < .001), as well as between negative and neutral images (t(100)= −28.49, p < .001).
EPRT and individual differences
EPRT valence ratings and individual differences
As hypothesized, there was a significant interaction between Anhedonia and Image Valence (F(1.20,117.90) = 31.79, p < . 001) indicating that the relation between Anhedonia and magnitude of ER differed depending on ER valence. There was no significant main effect of Anhedonia (F(1,98) = 1.33, p = .25). Also as hypothesized, there was a significant main effect of depressed mood (F(1,98) = 5.10, p = .02) indicating a similar effect of depressed mood across Image Valence ((e.g., more negative ratings of negative and neutral pictures and less positive ratings of positive pictures), but no significant interaction with Image Valence (F(1.20,117.90) = 1.90, p = .17).
Individual differences predicting emotional reactivity
With a significant interaction between Anhedonia and Image Valence and a significant main effect of depressed mood, post-hoc hierarchical linear regressions were used to assess the effects of individual differences on positive ER and negative ER (ratings of emotion - neutral images), In the first step of the linear regression assessing positive ER (Table 3) demographic factors were not found to be significant predictors of positive ER. The second step indicated that Anhedonia significantly predicted blunted positive ER, i.e. smaller differences between valence ratings of positive versus neutral images, while depressed mood was not a significant predictor (see Fig. 2 for partial plots). Anhedonia continued to significantly predict blunted positive ER in step 3, while Positive Affective State predicted more positive ratings of positive images as compared to neutral images, above and beyond Anhedonia.
Table 3.
Hierarchal linear regression model predicting positive emotional reactivity
| Predictor/statistic | Step 1 |
Step 2 |
Step 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | β | t | p | b | β | t | p | b | β | t | p | |
| Constant | 0.76 | 6.57 | < 0.001 | 0.84 | 7.63 | < 0.001 | 0.56 | 3.89 | < 0.001 | |||
| Sex (F > M) | 0.08 | 0.11 | 1.10 | 0.27 | 0.03 | 0.05 | 0.54 | 0.59 | 0.05 | 0.08 | 0.83 | 0.41 |
| Asian vs. not | −0.08 | −0.13 | −1.00 | 0.32 | −0.03 | −0.05 | −0.43 | 0.67 | −0.01 | −0.01 | −0.12 | 0.91 |
| White vs. not | −0.03 | −0.04 | −0.32 | 0.75 | −0.01 | −0.02 | −0.13 | 0.90 | 0.00 | 0.00 | 0.02 | 0.99 |
| Anhedonia | −0.05 | −0.42 | −4.34 | <0.001a | −0.05 | −0.37 | −3.93 | <0.001a | ||||
| Depressed mood | 0.00 | −0.03 | −0.33 | 0.74 | 0.00 | −0.04 | −0.40 | 0.69 | ||||
| Positive affective state | 0.06 | 0.24 | 2.60 | 0.01 | ||||||||
| Negative affective state | 0.04 | 0.12 | −1.23 | 0.22 | ||||||||
| Model R2 | 0.03 | 0.20 | 0.26 | |||||||||
| Adjusted R2 | 0.00 | 0.15 | 0.21 | |||||||||
| Model F | 0.86 | 4.62 | 4.76 | |||||||||
| Model p | 0.46 | <0.001 | <0.001 | |||||||||
| Model change p | 0.46 | <0.001 | 0.02 | |||||||||
Note Effects with p < .05 are in bold
Effects of Anhedonia or depressed mood passing Bonferroni correction at p < .025
Fig. 2.
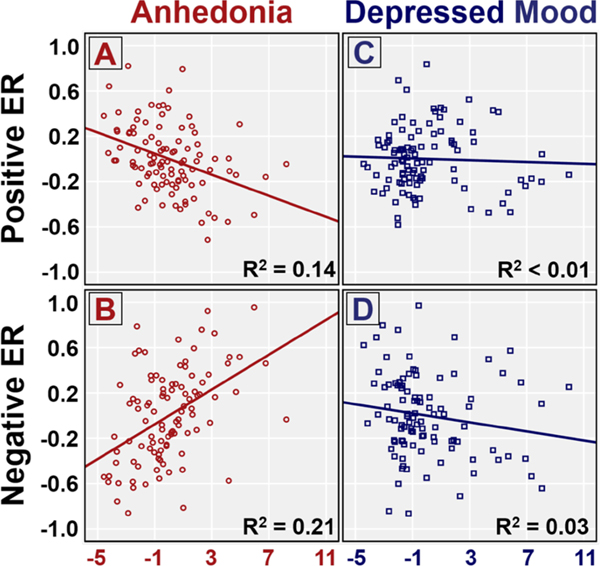
Partial regression plots from step 1 of the differences in valence ratings of positive and neutral images and the differences in valence ratings of negative and neutral image regressions. a less positive (blunted) mean valence ratings of positive images with increased Anhedonia. b less negative (blunted) mean valence ratings of negative images with increased Anhedonia. c no significant change in valence ratings of positive images with increased depressed mood (d) more negative (potentiated) mean valence ratings of negative images with increased depressed mood
Similarly, in the first step of linear regression predicting negative ER (Table 4), demographic factors were not found to be significant predictors of negative ER. In the second step Anhedonia significantly predicted blunted negative ER, i.e. less negative ratings of negative images as compared to neutral images, while depressed mood predicted potentiated negative ER, i.e. greater differences between ratings of negative and neutral images. When adding affective state in step 3, Anhedonia remained a significant predictor, however depressed mood was no longer a significant predictor of negative ER. This may partially be due to the significant correlation between depressed mood and Negative Affective State, which was trend-level predictor of negative ER in this step.
Table 4.
Hierarchal linear regression model predicting negative emotional reactivity
| Predictor/statistic | Step 1 |
Step 2 |
Step 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | β | t | p | b | β | t | p | b | β | t | p | |
| Constant | −1.01 | −6.63 | < 0.001 | −1.05 | −7.45 | < 0.001 | −0.80 | −4.28 | < 0.001 | |||
| Sex (F > M) | −0.12 | −0.13 | −1.30 | 0.20 | −0.07 | −0.07 | −0.82 | 0.42 | −0.08 | −0.09 | −1.00 | 0.32 |
| Asian vs. not | 0.06 | 0.07 | −0.55 | 0.58 | −0.03 | −0.03 | −0.27 | 0.79 | −0.06 | −0.07 | −0.62 | 0.54 |
| White vs. not | −0.03 | −0.03 | −0.23 | 0.82 | −0.07 | −0.08 | −0.67 | 0.50 | −0.09 | −0.10 | −0.83 | 0.41 |
| Anhedonia | 0.08 | 0.48 | 5.21 | < 0.001a | 0.08 | 0.46 | 5.00 | < 0.001a | ||||
| Depressed mood | −0.03 | −0.19 | −2.10 | 0.04 | −0.02 | −0.15 | −1.58 | 0.12 | ||||
| Positive affective state | −0.03 | −0.10 | −1.08 | 0.28 | ||||||||
| Negative affective state | −0.08 | −0.18 | −2.00 | 0.05 | ||||||||
| Model R2 | 0.03 | 0.25 | 0.29 | |||||||||
| Adjusted R2 | 0.00 | 0.21 | 0.24 | |||||||||
| Model F | 0.86 | 6.40 | 5.50 | |||||||||
| Model p | 0.46 | <0.001 | <0.001 | |||||||||
| Model change p | 0.46 | <0.001 | 0.07 | |||||||||
Note Effects with p < .05 are in bold
Effects of Anhedonia or depressed mood passing Bonferroni correction at p < .025
Hierarchal regression results predicting positive, negative, and neutral images independently, supported the above post-hoc regressions and are presented in Supplementary Tables 2–4.
EPRT reaction times and individual differences
The GLM with repeated measures results indicated that interactions (F(1.77,161.01) = 2.49, p = .09 and main effects (F(1,91) = 3.79, p = .05) associated with Anhedonia did not pass Bonferroni correction at p < .025. Interactions (F(1.77,161.01) = 3.03, p = .06) and main effects (F(1,91) = 2.08, p = . 15) associated with depressed mood, also did not pass Bonferroni correction at p < .025. Similarly, interactions (F(1.77,161.01) = 0.79, p = .44) and main effects (F(1,91) = 0.32, p = .57) associated with Positive Affective State, as well as, interactions (F(1.77,161.01) = 0.21, p = .78) and main effects (F(1,91) = 0.00, p = .99) associated with Negative Affective State did not pass nominal significance. Finally, Sex, Asian vs. Not, and White vs. Not, also did not pass nominal significance (all ps > 0.05).
Discussion
The goal of the current study was to examine the effects of individual differences in anhedonia/hedonic capacity, depressed mood, and current affective state on emotional reactivity to affective images. We found that increased anhedonia, operationalized as lower hedonic capacity, predicted blunted responses to both positive and negative images, relative to neutral. Increased depressed mood, operationalized as high scores on BDI items assessing pessimism, guilt, negativity, and worthlessness, predicted more overall negative responses to images, (i.e., more negative ratings of negative and neutral and less positive ratings of positive). Greater negative and positive affective states also predicted potentiated responses to negative and positive images vs. neutral, respectively. These results help elucidate the effects of trait and state individual differences on ER. Leveraging this understanding of factors influencing normative ER may help reconcile mixed findings in the MDD ER literature, particularly regarding support for the conflicting negative potentiation and emotional context insensitivity hypotheses.
Consistent with the positive attenuation and emotional context insensitivity hypotheses of depression, increased anhedonia predicted blunted self-reported emotional reactivity to both positive and negative images. In other words, individuals reporting heightened anhedonia found positive stimuli less positive and negative stimuli less negative, relative to neutral images, than those reporting lower levels of anhedonia. These results are consistent with the findings of the meta-analysis conducted by Bylsma et al. (2008) on ER in MDD, which found reductions in responsivity to both positive and negative stimuli in depressed groups. However, they are not consistent with evidence found by Grimm et al. (2009) and Kellough et al. (2008) which supported the negative potentiation theory of MDD. Yet, individual differences in depressed mood were associated with potentiated negative ER, such that those individuals reporting greater depressed mood provided more negative ratings of negatively valenced images. These findings are consistent with the negative potentiation hypothesis of depression (Grimm et al. 2009; Kellough et al. 2008). However, it is important to note that that depressed mood no longer significantly predicted more negative ratings of negatively valenced imaging when current negative affect was included in the model. This suggests the possibility that the relationship between depressed mood and negative ratings of negative images is operating through negative affect at the time that the individual is processing the stimulus.
Our findings generate new information that clarifies how individual differences in dissociable components of emotion function, anhedonia and depressed mood, influence ER to both positive and negative stimuli. Further, our results are consistent with all three theories of altered ER associated with depression, but importantly point to the need to conceptualize these theories at the level of specific symptoms, rather than examining depression as a homogeneous construct. The observed relations between ER and Anhedonia are consistent with the blunted reactivity posited by the positive attenuation and ECI hypotheses of depression, while the effects of depressed mood are consistent with the negative potentiation hypothesis. Critically, these findings may also help us to explain inconsistencies in the literature exploring emotional reactivity in depression. Although anhedonia and depressed mood will co-occur in some portion of the population and may be more likely to co-occur in actively depressed than non-depressed groups, the relative distributions of these symptoms and their severity may also vary among depressed populations. As such, studies recruiting depressed populations with heightened anhedonia vs. depressed mood may be more likely to support ECI, for example, than studies where the depressed group exhibits lower levels of anhedonia, either by chance or design.
This relative distribution of symptomology is especially important, given that in this study depressed mood and anhedonia both significantly predicted negative emotional reactivity, but anhedonia accounted for 21% and depressed mood accounted for only 3% of the variance, providing some evidence that the relationships between emotional reactivity and anhedonia are stronger than the relationships between emotional reactivity and depressed mood. This is a potentially important finding, but additional work will need to be done to both replicate this result and clarify its source. For example, it is possible that the stronger relationship of emotional reactivity to anhedonia than depression reflects stronger psychometric characteristics of the anhedonia measure given that it was a composite of several measures. However, if so, it will also be important to determine if the stronger psychometric characteristics reflect something inherent about the construct (e.g., anhedonia is a more stable or more reliable characteristic in humans than depression), or something about the measurement approach (e.g., a composite of depression measures would show stronger relationships to emotional reactivity.
Research concerning anhedonia in both healthy and clinical populations often focuses on behavioral and neural reactivity to reward (Pizzagalli et al. 2005, 2008). Thus, it is important to note that the current study extends the literature to link anhedonia to normative individual differences in self-reported emotional reactivity to non-incentive affective stimuli. Furthermore, much of the anhedonia literature has focused on responses to positive outcomes/stimuli. However, the current study adds to the emerging literature showing blunted responses to both positive and negative stimuli with elevated anhedonia (Berenbaum and Oltmanns 1992; Burbridge and Barch 2007; Dowd and Barch 2010; Luking et al. 2015). These findings suggest that it may be useful to explore the construct of anhedonia as a blunting of responses to all salient stimuli, not just positive stimuli.
It is also important to note that we operationalized individual differences in anhedonia as reduced hedonic capacity. The current results relating anhedonia to blunted ER to both positive and negative stimuli are similar to those observed in other studies operationalizing anhedonia as low hedonic capacity, for example in schizophrenia and healthy controls (Berenbaum and Oltmanns 1992; Burbridge and Barch 2007; Dowd and Barch 2010) or in the depression literature concerning reward/risk (Chase et al. 2010; Steele et al. 2007). However, anhedonia within the depression literature is frequently assessed via anhedonic-/melan-cholic-depressive symptom scales (e.g. Hughes et al. 2006; Kashdan et al. 2006; Stoy et al. 2012; Tuohy and McVey 2008) that also include items regarding somatic/vegetative symptoms and negative outcomes, such as the Beck depression inventory (Beck et al. 1996) or the Hamilton Depression Rating Scale (Hamilton 1967). As such, measures that more specifically assess anhedonia will be needed in future studies examining MDD to investigate whether the severity of anhedonia in depression is related to blunted ER to both positive and negative stimuli. We also found that a person’s current affective state predicted individual differences in ER beyond the effects of anhedonia and depressed mood. Particularly, more positive affect at the time of the task predicted potentiation of positive responses to positive images, while more negative affective at the time of the task predicted potentiation of negative responses to negative stimuli above and beyond the effect of anhedonia and depressed mood. While exploratory, these results suggest that current affective states vs. self-evaluations of emotional function over longer periods of time are a dissociable and important source of variance to consider when investigating ER. Measures of anhedonia and depressed mood that assess function over several days or even weeks may reflect more trait-level characteristics, which in the current study had unique influences separable from current affective states.
Limitations and future directions
A limitation of the current study is that our measure of ER was based on self-report. It will be important in future studies to examine other ways of assessing ER, such as physiological responses or functional brain responses. The current findings provide novel data on how individual differences in dissociable components of emotion function, anhedonia and depressed mood, influence ER to both positive and negative stimuli in an unselected sample. A next step will be to examine individual differences in symptom severity in a depressed population to help further our understanding of the heterogeneity in alterations in ER associated with depression, which in turn may be useful for informing more effective treatment decisions. This goal is in line with those of the Research Domain Criteria initiative (Insel et al. 2010), recently adopted by the National Institute of Mental Health. Another important future direction will be to further examine the effects of current affective state on ER and its relation to individual differences in anhedonia and depressed mood. For example, it will be useful to examine whether experimental manipulations of current affective state also modulate ER to either or both positive or negative stimuli, and whether such manipulations interact with individual differences in anhedonia or depression mood. This could help to clarify whether the mechanisms underlying effects of current affect are dissociable from the mechanisms mediating the effects of anhedonia and depressed mood on ER.
Supplementary Material
Acknowledgements
D.P., and K.R.L.’s work was supported by a Grant from the National Institute of General Medical Sciences (No. 5T32GM081739). K.R.L.’s work was also supported by a Grant from the National Institute of Health (No. MH097335). The NIH and NIGMS had no further role in the design and conduct of the study (collection, management, analysis, or interpretation of the data) or in the preparation, review, or approval of the manuscript. A.S. had full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis. We thank all participants who provided time and effort to making this study possible. The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Compliance with ethical standards
Conflict of interest All Authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s11031-017-9610-1) contains supplementary material, which is available to authorized users.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5). American Psychiatric Pub. [Google Scholar]
- Beck AT, Steer RA, Ball R, & Ranieri WF (1996). Comparison of beck depression Inventories-IA and -II in psychiatric outpatients. Journal of Personality Assessment, 67(3), 588–597. doi: 10.1207/s15327752jpa670313. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, & Oltmanns TF (1992). Emotional experience and expression in schizophrenia and depression. Journal of Abnormal Psychology, 101(1), 37–44. doi: 10.1037/0021-843X.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, & Lang PJ (1994). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Burbridge JA, & Barch DM (2007). Anhedo-nia and the experience of emotion in individuals with schizophrenia. Journal of Abnormal Psychology, 116(1), 30–42. doi: 10.1037/0021-843X.116.1.30. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, & Rottenberg J. (2008). A met-aanalysis of emotional reactivity in major depressive disorder. Clinical Psychology Review, 28(4), 676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67(2), 319–333. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- Chase HW, Frank MJ, Michael A, Bullmore ET, Sahakian J, & Robbins TW (2010). Approach and avoidance learning in patients with major depression and healthy controls: Relation to anhedonia. Psychological Medicine, 40(03), 433–440. doi: 10.1017/s0033291709990468. [DOI] [PubMed] [Google Scholar]
- Dowd EC, & Barch DM (2010). Anhedonia and emotional experience in schizophrenia: Neural and behavioral indicators. Biological Psychiatry, 67(10), 902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozois DJ, Dobson KS, & Ahnberg JL (1998). A psychometric evaluation of the Beck depression Inventory-II. Psychological Assessment, 10(2), 83. doi: 10.1037/1040-3590.10.2.83. [DOI] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, & Gibbons RD (1983). Assessing anhedonia in psychiatric patients: The pleasure scale. Archives of general psychiatry, 40(1), 79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- Gollan JK, Pane H, McCloskey M, & Coccaro EF (2008). Identifying differences in biased affective information processing in major depression. Psychiatry Research, 159(1–2), 18–24. doi: 10.1016/j.psychres.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, ... Northoff G. (2009). Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 34(4), 932–843. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1967). Development of a rating scale for primary depressive illness. British Journal of Clinical Psychology, 6(4), 278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hughes AA, Heimberg RG, Coles ME, Gibb BE, Liebowitz MR, & Schneier FR (2006). Relations of the factors of the tripartite model of anxiety and depression to types of social anxiety. Behaviour Research and Therapy, 44(11), 1629–1641. doi: 10.1016/j.brat.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, ... Wang P(2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167(7), 748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Elhai JD, & Frueh BC (2006). Anhedonia and emotional numbing in combat veterans with PTSD. Behaviour Research and Therapy, 44(3), 457–467. doi: 10.1016/j.brat.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Kellough JL, Beevers CG, Ellis AJ, & Wells TT (2008). Time course of selective attention in clinically depressed young adults: An eye tracking study. Behaviour Research and Therapy, 46(11), 1238–1243. doi: 10.1016/j.brat.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Steer RA, Teitelman KB, & Villacis L. (2002). Effectiveness of Beck depression inventory-II sub-scales in screening for major depressive disorders in adolescent psychiatric inpatients. Assessment, 9(2), 164–170. doi: 10.1177/10791102009002007. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical report A-8. [Google Scholar]
- Luking KR, Neiman JS, Luby JL, & Barch DM (2015). Reduced hedonic capacity/approach motivation relates to blunted responsivity to gain and loss feedback in children. Journal of Clinical Child & Adolescent Psychology, 1–13. doi: 10.1080/15374416.2015.1012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, & Barch DM (2015). Child gain approach and loss avoidance behavior: Relationships with depression risk, negative mood, and Anhedonia. Journal of the American Academy of Child & Adolescent Psychiatry, 54(8), 643–651. doi: 10.1016/j.jaac.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman A, Downs WR, Barrios FX, Kopper BA, Gutierrez PM, & Chiros CE (1997). Factor structure and psychometric characteristics of the Beck depression inventory-II. Journal of Psychopathology and Behavioral Assessment, 19(4), 359–376. doi: 10.1007/BF02229026. [DOI] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, & Fava M. (2008). Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. Journal of Psychiatric Research, 43(1), 76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, & O’Shea JP (2005). Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biological Psychiatry, 57(4), 319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, & Gotlib IH (2005). Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology, 114(4), 627–639. doi: 10.1037/0021-843x.114.4.627. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Strauss ME, Quirk SW, & Sajatovic M. (1997). Subjective and expressive emotional responses in depression. Journal of Affective Disorders, 46(2), 135–141. doi: 10.1016/S0165-0327(97)00097-9. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, & Trigwell P. (1995). A scale for the assessment of hedonic tone the snaith-hamilton pleasure scale. The British Journal of Psychiatry, 167(1), 99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Steele JD, Kumar P, & Ebmeier KP (2007). Blunted response to feedback information in depressive illness. Brain: A Journal of Neurology, 130(9), 2367–2374. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- Steer RA, Ball R, Ranieri WF, & Beck AT (1999). Dimensions of the Beck depression inventory-II in clinically depressed outpatients. Journal of Clinical Psychology, 55(1), 117–128. doi: . [DOI] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele, Suchotzki K ,... Ströhle A. (2012). Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escit-alopram. Journal of Psychopharmacology, 26(5), 677–688. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Tuohy A, & McVey C. (2008). Subscales measuring symptoms of non-specific depression, anhedonia, and anxiety in the edinburgh postnatal depression scale. The British Journal of Psychiatry, 47(2), 153–169. doi: 10.1111/j.2044-8260.2008.tb00463.x. [DOI] [PubMed] [Google Scholar]
- White DA, Myerson J, & Hale S. (1997). How cognitive is psychomotor slowing in depression? evidence from a meta-analysis. Aging, Neuropsychology, and Cognition, 4(3), 166–174. doi: 10.1080/13825589708256645. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


