Abstract
The important role of proper sanitation in maintaining good public health has been confirmed in the past years. Wastewater treatment plants (WWTPs) serve as efficient processes in removing pathogens, organic pollutants, nutrients, and pharmaceuticals from wastewaters. However, the advance systems of treatment that we use today are the result of a series of inventions that have been performed since 19th century. This chapter explains the evolution of the wastewater origin and the treatment processes along with the developments in microbiology and pathology that led to the present-day scenario of research and advance facilities. Pharmaceuticals can easily enter the environment due to their incomplete degradation in the treatment processes and because of their adverse effects on organisms and environment they are becoming a matter of great concern. A brief discussion on the presence of pharmaceutical compounds in different environment sectors such as wastewater, WWTPs, and the natural aquatic environment has been provided.
Keywords: Hospital wastewater, wastewater treatment plant, microbiology, pharmaceuticals, antibiotics, pollutants
1.1. Introduction
Water is an inevitable part of the daily routine of almost all living beings. During recent years, most parts of the earth have begun to face severe water scarcity and there is a need to adapt the methods for reuse of wastewater [1]. Freshwater availability is not sufficient to fulfill the consumption needs of the entire planet, and most of the freshwater is in the form of ice and snow in polar regions [1]. Besides emerging, chemical pollutants and pharmaceuticals contamination in the aquatic environment raises the concern. The population increase requires a constant supply of clean water for drinking, sanitation, irrigation, and various other uses. The presence of pathogens, microbial toxins, and spores in natural water bodies also affects the day-to-day water requirements.
Moreover, climate change also affects the quality of water and aquatic biodiversity, which is discussed in detail in the latter chapters of this book. Besides the water cycle maintenance, at present, the reuse of wastewater is mandatory to meet the water requirement and to protect the environment health through strict wastewater treatment standards [2]. However, the reuse of poorly treated or untreated wastewater can result in numerous health risks to environmental and public health. The wastewater origin from different sources and its release to the environment through various human activities are depicted in Fig. 1–1 .
Figure 1–1.
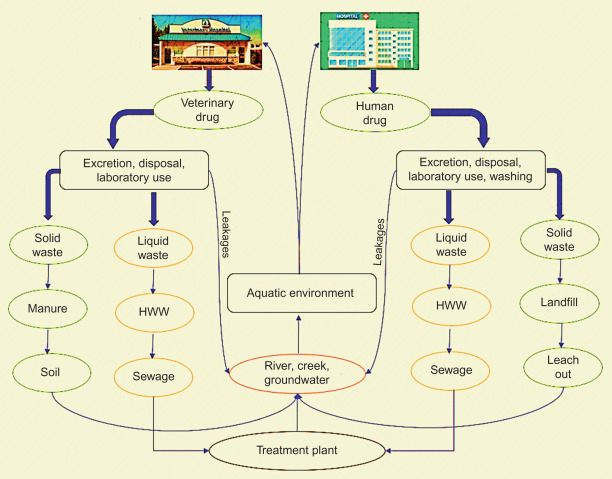
Mode of release of wastewater into environment.
Treated water hygiene is essential to maintain both the biotic and abiotic environment that could support human life on Earth. The recent occurrence in disease outbreaks throughout the world due to the emergence of antibiotic-resistant bacterial strains raised a concern. Most of the drug-resistant development occurs in wastewater collection and treatment points [3], and few similar events are also reported to occur in the human gut [3]. The emerging pathogens enter into wastewater treatment plant (WWTP) through (1) pathogens dwelling in human gut released by municipal wastewater, (2) introduction of decontaminated wastewater, (3) release of poorly treated hospital wastewater (HWW), (4) illicit activities, and (5) surface water runoff.
Hospitals and drug-manufacturing industries release a significant portion of pharmaceuticals through wastewater or by decontamination of unused/expired compounds, which cause extreme hazards to both aquatic and terrestrial environments. Various contaminants such as pharmaceutical compounds and resistant microbes are introduced into the environment by these sources. Coliforms and enterococci are indicators of fecal contamination which are also used to identify antibiotic resistance [4], [5], [6]. HWW has been reportedly shown to have Shigella and Salmonella species of 14.6% and 33.3%, respectively [7]. Besides, the presence of bacteria, yeasts, and filamentous fungal species such as Penicillium and Aspergillus has also been reported in hospital effluents [8]. Hence, efficient techniques and treatment plants are needed to remove such contaminants to ensure the public health and environmental safety. Biological treatment processes are known to amplify antibiotic resistance in bacteria as the microbes continuously interact with subinhibitory concentrations of antibiotics [9], [10]. Conventional approaches are not efficient in the removal of active pharmaceuticals and pathogenic microbes in hospital effluents. Thus advanced methods such as ozonation, photo-Fenton process, and pulsed-electric field are being used as a treatment process [11], [12]. Antibiotics are the most extensively used drugs in the prevention and treatment of human and animal diseases, and their increased use is creating concern regarding resistance development in bacteria [13], [14]. Methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus species, fluoroquinolones, carbapenems-resistant Gram-negative bacteria (enterobacteria, Pseudomonas, and Acinetobacter), and pathogens resistant to broad-spectrum of beta-lactam are the current concern to public health [15]. Genetic elements such as gene cassettes/operon, integrons, plasmids, and heavy metals resistant genes undergo gene transfer mechanism to retain resistance property and to survive in the unfavorable environment [16].
The constant exposure of bacteria to antibiotics in water systems leads to the development of antibiotic-resistant genes (ARGs) against more than one drug (multidrug resistance) [14], [17]. The constant discharge of high load of multidrug-resistant bacteria (such as Proteus vulgaris, Mycobacteria) from HWW, domestic sewage, and animal-farm drainage increases the threat to both environmental and human health [18], [19], [20]. Antibiotics are not only affecting the microbial community but also generate adverse effects on both aquatic and terrestrial environments [20], [21], [22].
The objective of this book was to provide the holistic summary on (1) pharmaceuticals release, (2) their impact on environment, (3) factors governing microbial evolution in the environment due to the presence of pharmaceuticals, and (4) different treatment processes specifically employed for HWW treatment in details. Furthermore, this book concludes using all the possible recent discoveries by a thorough and exhaustive discussion on current research, and future perspectives on advanced treatment methods, microbial evolution, pathogens, antibiotic resistance development, and future directions to maintain and protect the environment through microbiological, molecular biology, and by policymaking. Additionally, this book provides comprehensive insight on microbial aspects of HWW, including pharmaceuticals, pathogens, and viruses, antibiotic-resistance development from the source to sink [23]. This book updates and comprises microbial evolution caused by hospital waste to fulfill the current needs.
1.2. History of wastewater origin and treatment
It is important to highlight the tremendous amount of work done behind the modern WWTP or the sewage system used today [24]. The ancient customs and behavior of a society toward its waste products is an incredible state of human civilization. The word wastewater did not exist before and the word sewer was used in 1892. The importance of sanitation for human health protection was not understood clearly until the 19th century. For many centuries, there was no wastewater management; thus, the wastewater was disposed in the public areas like streets. Such situation created a serious health impacts on both public and environment which is quite evident by various epidemics that occurred until 19th century in Europe [25]. Throughout the history, it is evident that the wastewater management was presented with several technical and political challenges. The progress in wastewater management was driven by scientific events, socioeconomic events such as the two World Wars, and political coalitions uniting municipalities, industrialists, and reformers. Soon after the development and construction of sewer lines, the number of deaths caused by the waterborne pathogenic microbes was significantly reduced.
The evolution of sanitation practices in early periods (Fig. 1–2 ) can be divided into five main stages: (1) early history, (2) Roman period, (3) sanitary dark age, (4) age of sanitary enlightenment and the industrial age, and (5) the age of stringent environmental standards [24].
Figure 1–2.
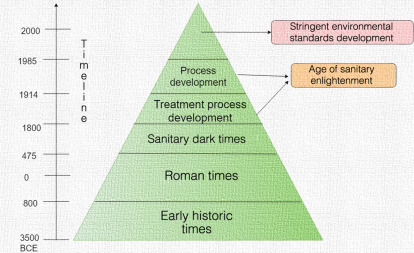
The evolution of sanitation practices throughout the different time periods.
1.2.1. Early history
The early historic times comprise the first humans that were scattered over, and the waste produced was returned to land and decomposed using natural cycles. Before the settlement of first advanced civilization, the disposal of waste was done by holes in ground and was covered after the use. Tribes as early as 10,000 BCE were Nomadic and they may have been the first to recognize that waste products should be separated from living places [25]. There is a lack of records about the human health using such systems; however, they must have serious public problems due to the lack of proper wastewater management. Various civilizations existed as ancient civilizations such as Mesopotamian Empire, Indus civilization, Egyptian civilization, and Greek Empire that addressed the sanitation problems in their own ways [24], [26], [27], [28].
For example, in Mesopotamian Empire, the remains of homes were found that were connected to drainage system to carry away the wastes. In Indus civilization, houses were connected to drainage channels and systems, and the wastewater was said to be treated. The wastewater was passed through pipes in a sump, where solids were settled and accumulated and the liquids overflowed into drainage channels (when sump was 75% full) [26]. The drainage channels could be covered by bricks and stones and such an attempt was the first in treatment record. Rich people in Herakopolis (Egyptian civilization) had bathrooms and toilet seats, and the drainage of wastewater was done by setting a basin underneath or by drainage channels running into a vessel or into the desert sand.
The Greek civilization was the forerunners of modern technologies of wastewater management. They had public latrines that drained the wastewater and storm water into pipes and then, into a collection basin outside city from where it was used in agricultural fields to fertilize crops and orchards [27], [28]. Emphasis on construction, operation, and management of sewage and storm systems was given during 2nd millennium BCE, that is, Minoan period. The hygienic facilities during this period were so advanced that it can be compared with the modern urban water management systems that were developed in the late 19th century CE. The advanced technologies were also used in the other parts of Greece in later Greek civilizations such as Mycenaen, Archaic, Classical, and Hellenistic [27]. They cleverly understood the connection between human health and water quality and used user charge system to cover management expense. This system has been viable for about 800 years [25].
1.2.2. Roman period
The Roman period consisted of great managers and engineers and provided with the first integrated water service to manage water cycle from collection to disposal. They provided dual networks to collect spring water, recycling wastewaters from spas, and dispose of storm water and wastewater. They also built public latrines, baths, and water fountains. They had systems of aqueducts that provided water to cities for household needs and public baths and flush sewers. The sewage system of ancient Rome was very complex that included many small sewers [29], for example, Cloaca Maxima was spread throughout the city center. This central sewer system was initially used to drain the marsh on which Rome was later built. Water was supplied by aqueduct systems that carried sewage from public baths and latrines to sewers that were beneath the city and the streets were regularly washed with aqueduct water and the waste was washed into the water [30]. Repairs, extensions, additions, and renovations were constantly done throughout the canals and drainage systems. Variety of engineering and construction methods depending on the geology of slopes and distance of receiving water body were used.
1.2.3. Sanitary dark age
By CE 226, around 300 million gallons of water passed through aqueduct system per day. With the end of this period, there was deterioration of aqueducts, sanitation systems and the drainage supplies, and coastal roads were not usable which led to the beginning of sanitary dark ages that lasted for over thousand years. The drainage systems and facilities built by Romans and other civilizations before were neglected. Water was used from rivers and wells, and the wastewater was discharged without treatment that resulted in the spread of epidemics. Household did not have sanitary facilities, the practice to empty the chamber pots in streets was followed, and wastewater would run open along the walkways. Few regulations came that made public understood the advantage of using wastewater as fertilizer and prohibiting the emptying of contents of cesspits into streets or other rivers in the city. In 1539 cesspools for sewage collection were built and used until late 1700s that helped to reduce contamination of drinking water. Around 1800, collection systems appeared in various cities driven by city dwellers. In Amsterdam, cart vehicles were driven in which buckets could be emptied.
However, the spilling during this transportation and process was unavoidable and by then, certain plans for sewer systems were made. But the high investment cost, maintenance of sewers and uncertainties of the system put the implementation on hold [30]. In 1883, 25,000–30,000 wells for municipal drinking water in Paris were polluted due to the leaching of cesspools during the rainfalls. Only half of the Italian people had pipes for drinking water and 77% had no sewers by the end of 19th century [24].
1.2.4. Sanitary enlightenment
The industrialization and urbanizations brought into sanitary development and realization of the importance of waste and wastewater disposal. Different countries began experimenting to improve their environmental conditions. In Britain, collection of sewers and pumping stations were built to convey wastewater from streets and discharge to Thames River. Thames became highly polluted and by then also, there was no understanding of removal of pollutants before discharge into the river. In Germany, general introduction of sewers started with construction in Frankfurt in 1867 [31]. In France, extensive reorganization of networks was done for sewers present in the city and collectors were installed. New laws came into action that did not allow building owners to increase the amount of wastewater being discharged as well as discharge it further downstream of the river. In the United States, in early 1800s, new community sewers were installed for storm water, and privies and leaching cesspools were used for human wastes. Cities like Boston and Chicago started installing sewers in the 1700s [24].
Attempts to identify agents that cause diseases were also made. John Snow showed that cholera was transmitted by contaminated water, William Budd showed typhoid fever to be caused by disease agent by sewage and not by bad odors, and Ignaz Semmelweis demonstrated that puerperal fever was transmitted by doctors who did not sanitize their hands when operating between different patients [32]. In 1900s Liernur came with a plan for separate collection of toilet water and gray and storm water. A vacuum sewer was used called the Liernur system that was also found to be used in various European towers. The collected sewage was spread out on land as a fertilizer which resulted in water logging and soon the lack of land seemed to be one of the major problems [30].
1.2.5. Age of stringent environmental standards
The beginning of 20th century came as an age of stringent environmental standards. Scientific discoveries, societal priorities, government interest in environmental science, and societal views toward pollution caused a revolution in wastewater management system [33]. The concept of various established standards and tests, such as biochemical oxygen demand (BOD), came in action and was applied to sewage and effluents and was also followed by various countries. Government made it mandate to treat wastewaters.
World War I and II delayed development of wastewater until 1948 thus increasing pollution in water bodies [31], [33]. The end of the World War II led to rapid progress in wastewater treatment in the United States. In the 19th century, there were outbreaks of cholera that brought the first centralized wastewater treatment technology such as septic tanks (1860), trickling sand filters (1868), and septic tanks effluent treated with sand filters (1893), and they were few of the earliest biological filters used [25]. In the United States and United Kingdom, biological filters that used biofilms on rocks were being applied. The rapid application of these biological filters had a negative impact on the later implementation of activated sludge process in the United Kingdom after its invention in 1913 due to the invested money in filters [30]. By the beginning of 20th century, there was an understanding about chemical water pollution, toxicity, and environmental contamination which led to the development of waste management policy.
Acceptance for the use of centralized and decentralized water systems was strongly driven by environmental concerns, safety issues, and social responsibility related to the water quality. It was believed that the lack of public acceptance toward the use of alternative water sources was based on irrational attitude of water reuse rather than technological or safety issues. Also, the cost of establishment and maintenance was possible barriers to adoption of such systems. Perceptions toward clean and alternative water supplies along with reluctance to use wastewater for portable applications suggest communities to have culturally predetermined emotional responses toward water [34]. However, with the improvements in research and technology, better acceptance and adoption of water systems and management has been observed.
1.2.6. Evolution of sanitation process
Today wastewater is typically classified according to its origin: domestic, industrial, commercial [23], or urban. Domestic wastewater represents from residential places including sinks, toilets, and laundry containing a range of chemicals, soaps, detergents along with bacteria, and pathogenic microbes. Industrial wastewater comes from manufacturing facilities or industries, and commercial wastewater is discharged from hotels, offices, and other building or stores. Urban or municipal wastewater is the mixture of all the abovedescribed wastewaters, that is, domestic, industrial, and commercial. Storm water in case of combined sewer system of the community is also included in the mixture of wastewater [23].
It is important to know the most significant developments in the wastewater treatment techniques that led to its evolution into the complex and effective system that we use today (Fig. 1–3 ). The early form of primary treatment was trenches and pits that removed heavier solids before their application so that the load on land could be reduce to avoid clogging. In 1860s cesspit with inlet and outlet pipes dipped below the water surface was designed that formed a water seal [35]. In 1906 Karl Imhoff designed Imhoff tank that was advanced and is still used worldwide. With increase in organic loads, the rivers were not able to cope up and there was a requirement to reduce oxygen demand in rivers and eliminate ammonia. This resulted in construction of various low-loaded trickling filter process plants for organic removal and nitrification in many countries [30]. Clean Water Act in 1972 mandated the secondary treatment before which primary treatment was the most common form of treatment of wastewater in the United States [24]. The secondary treatment uses microbes to convert the organic matter in the wastewater into carbon dioxide, water, and energy. There can be attached growth (biofilms) and suspended growth (activated sludge) treatment types in secondary treatment.
Figure 1–3.
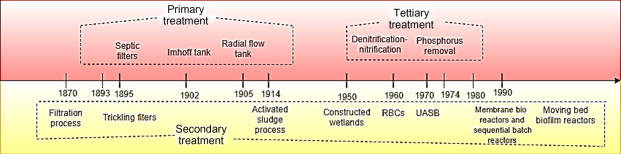
A timeline representing the evolution of wastewater treatment plants.
In attached growth systems, microbes attach and grow on a fixed substrate, wastewater flows over this aerated film and there is a reduction of BOD; however, in suspended growth systems, biomass and wastewater are mixed resulting in the BOD reduction, following which the solids are removed in a sedimentation step and majority is returned in the process [24]. In 1870 principles of filtration through soil were established that became the base for the successive developments which led to trickling filters [36].
The first trickling filter was installed in 1893 in England and since then it has been used to treat wastewater. The first patent for the concept of attached growth method was on a concept of moving cylinder with wooden slats. Another one was based on a porous brick fashioned as hollow cylinder that rotated on its horizontal axis. These designs led to another growth process called rotating biological contactors (RBCs). The wooden slats were replaced by metal disks that were further replaced by polystyrene disks. RBCs were first installed in 1960 in Germany and later introduced in the United Kingdom and United States [24]. The activated sludge process was patented in 1913 in the United Kingdom by Edward Arden and W.T. Lockett [37]. Experiments on treating sewage in a draw-fill reactor resulted in treated effluent, and believing at that time that the sludge had been activated (like activated charcoal), the process was called “activated sludge” [30]. In the United Kingdom due to wide use of trickling filters, activated sludge process use was not rapid. In the United States, the activated sludge process plants were among the first form of treatment system and much faster than Europe.
Then a new problem of eutrophication (the explosive growth of algae and other plants due to effect of nitrogen and phosphorus discharge) came in notice. It was concluded that after the secondary treatment, there is a requirement of removal of nitrogen, phosphorus, or both. In 1964 Downing et al. [38] showed that nitrification process depends on maximum specific growth rate of autotrophic nitrifying microbes which is slow as compared with the heterotrophic organisms. Therefore the sludge age has to be long to achieve low-ammonia concentrations in effluent.
In 1964 it was found that the nitrate produced by nitrification can also be used by bacteria (heterotrophic) instead of oxygen and converted into nitrogen gas which led to the nitrification– denitrification activated sludge system. In 1962 the use of anoxic zone to achieve biological denitrification in activated sludge process was introduced. Barnard patented the single sludge system for the removal of nitrogen and phosphorus [38]. Membrane systems are also used to produce high-quality effluent and they have been used for many years in industries. Ultrafiltration was first described in 1969 [39] followed by which, in 1970 the technology entered in Japan. The use of external membrane bioreactors for sanitary applications was reported in Europe which followed the work in developing systems for industrial wastewater [24]. Phosphorus removal by chemical precipitation came during the 1970s. In water-scarce areas, there was already indirect use of surface water and removing chemical phosphate would cause surface water salinity, in addition, salinity reduces agricultural use of water and impacts the durability of water distribution and management systems.
Therefore water policy in South Africa was formed which said that if the high cost of chemical phosphate removal was going to be incurred then the water may be reclaimed and returned to distribution system rather than environment. The first indication of phosphate removal came from India in 1959 where they observed sludge in a treatment plant showed excessive phosphate uptake when it was aerated showing the process to be biological process and later enhanced biological phosphate removal was developed [30].
In the 1970s, with an increased demand for industrial wastewater treatment, the attention shifted from aerobic to anaerobic wastewater treatment. One of the limitations in the process development was the slow growth rate of methane-producing bacteria. But the development of upflow anaerobic sludge blanket reactors served as a breakthrough in anaerobic treatment making the technology feasible for industrial wastewater treatment and anaerobic treatment of low-strength municipal wastewater. In the 1970s a whole range of new processes such as biological aerated filters, suspension reactors, biorotors, fluid bed reactors, and granular sludge process were developed. In addition, activated sludge process with membrane separation instead of settlers was also developed [30]. Similarly, various research problems resulted in many innovations in the treatment facilities such as anaerobic ammonia oxidation process for improved nitrogen removal and crystallization process for phosphorus precipitation for its recovery and reuse [30].
In the 19th century, it was believed that odor caused disease so chemicals like chlorine were used. Chloride of lime was used to deodorize wastewater in London in 1854. In 1859 it was shown that calcium chloride 400 lb/mg dosage could delay raw wastewater purification for 4 days [40]. Later, scientists began to understand pathogens and the diseases they cause. In 1893, in Germany, chlorine was used at plant scale for disinfection; in 1906, ozone was used in France; and in 1909, compressed, liquefied chlorine was commercially available. In 1961 first chlorine residual disinfection system was available. Ultraviolet (UV) light for disinfection was first used in France in 1916, slowly replacing chlorine gas for disinfection practice [24]. Recently, more renewed attention has been given to the disinfection processes due to the advancement in wastewater treatment processes. Also, sludge disposal to agricultural lands is becoming limiting and its handling becoming more important, making this topic a strong research area.
Today, we are well aware of the sanitary practices and the underlying consequences if not followed. This success is attributed to the new discoveries in the field of medical, surgeries, pharmacology, and environmental science. Local regulations and demands make it economically profitable to use wastewater effluent for various purposes. All the developments take time and efforts and after a century of separate development of wastewater and drinking water treatment, these technologies are growing closer to each other.
1.3. History of microbiology and pathogens discovery
Microbiology refers to the study of microbes, their biology and their activities. The word microorganism originates from the Greek word “micro” which means small [41]. The major groups of microorganisms include bacteria, algae, fungi, protozoa, and viruses. Their study is required for the investigation of genetic, physiological, and biochemical reactions that serves the basis of life. Microbiologists today have remarkably explained useful and harmful microbes. However, this was not the condition few centuries before, owning to the small size of microorganism or pathogen, their existence was not recognized.
1.3.1. Waterborne outbreaks
The initial work in the field of environmental microbiology was focused on water quality and fate of pathogens in water systems and environment. The start of regulating the water quality goes back to the 20th century (as discussed earlier), when treatment of water and its disinfection resulted in decrease in the occurrence of typhoid fever and cholera. Application of such processes eliminated the waterborne bacterial disease. Other agents such as viruses and protozoa were seen to be more resistant to disinfection than the enteric bacteria. Studies have demonstrated the discovery of outbreaks caused by protozoan parasite like Giardia and Norovirus which were found in disinfected drinking water. Another documented major outbreak occurred in 1993 where more than 400,000 people became ill and 100 died in Wisconsin, by the waterborne protozoan parasite Cryptosporidium [42].
The first recorded plague that could be referred was in 3180 BCE in Egypt’s First Dynasty which resulted in very high morbidity and mortality due to the widespread of the disease [43]. It is clear from the history, the evolution of connection formed by medicine-microbe and environment before we even knew the role of water in diseases spread. In ancient times, medicine addressed diagnosis of illness by the description of symptoms and recorded the first epidemic that took place in Egypt. It was proposed that bad air, rotten waters, and crowd were associated with disease and its spread. One of the major plagues occurred in Athens in 430 BCE. It killed about 30,000 people and the illness was described by symptoms that included fever, inflammation, open sores, blisters, ulcers and diarrhea and death [43]. Various hypotheses have been given on etiology such as influenza, smallpox, plague, typhus, and Staphylococcus. Recent molecular evidence supported typhoid fever as a probability as well a spread of some viral pathogens and Staphylococcus that resulted in the plague [44]. Journey of finding the cause and cure of diseases started with the association of medicine with microbiology.
1.3.2. Birth of bacteriology
Girolomo Fracastoro suggested germ theory in 1547 and gave the idea that disease is contagious and transmitted from one person to another. He suggested that the transmission is done by “particles” that are very small to comprehend but with appropriate media or surroundings can grow and reproduce. He also claimed that they can be transmitted over long distances and survive well but cannot resist extreme condition (such as heat or cold) [44]. The birth of bacteriology was delayed because of the lack of an important instrument, the microscope. It was not until the microscope was invented in 1590 and refined in 1668 that bacteria were described in 1773 by Otto Frederik Muller [45].
The first observation and recognizable period in the history of bacteriology is credited to Antonie van Leeuwenhoek in 1675 and extending up till 1885 where a large part of modern bacteriological techniques were developed. He used a very simple form of microscope and saw objects which he called “animalcules” harvested from his own teeth which could now be associated with various germs, microbes, or bacteria.
However, a lot of controversies came to oppose his ideas. These arguments gave conflicting results such as bacteria were found in sealed containers of meat which had been heated but later was said that it was due to the insufficiency to sterilize by Spallanzi. It has also been suggested that Athanasius Kircher observe microbes before Leeuwenhoek. He stated doctrine of “contagium animatum” and suggested worms are present in putrefying materials. It was not until 200 years later that the era of microbiology began which was dominated by the works of Robert Koch and Louis Pasteur. The germ theory further set in 1849 and the second period of microbiology began with the concept of “microbe hunters”[45].
John Snow was able to show that cholera was transmitted by water. In 1856 it was suggested that typhoid fever was spread by feces, and in 1876 Robert Koch’s theories to identify contagious agents helped to move the studies forward [46].
Thus it was the germ theory credited to Pasteur and Koch that set a new baseline for studying the infectious diseases. Pasteur helped further in finding the vastness of different microbes and important pathogenic bacteria that we recognize nowadays. He found the microorganisms responsible for fermentation of sugars into alcohols and souring of milk and also developed pasteurization that killed these microbes in milk. He also investigated a silk worm disease which was impacting the silk industry and showed that living organisms (microscopic) can spread from worm to worm and cause the disease. These observations led forward the germ theory.
Koch stated certain experimental criteria that are now known as “Koch’s Postulates” which gave researchers’ the proof about a bacterium to cause a disease. His one of the most important investigations could no doubt be the anthrax. It was found that the blood of animals suffering from anthrax contained rod-shaped bacteria but it was not proved that they were the cause of disease. Pasteur later confirmed this by his theory that under certain conditions disease-causing bacteria could be made harmless (called the attenuated strains of bacteria) and will be unable to produce the disease when given to a susceptible animal. He gave injections of attenuated anthrax bacteria to sheep and was able to prevent them from developing anthrax. This, in addition to the development of vaccination (milkmaids exposed to cowpox did not contract smallpox) by Edward Jenner (1796), led to the beginning of prevention of diseases by the means of vaccines [46]. Koch also identified bacteria that caused tuberculosis and cholera, ultimately leading to the formulation of his famous postulates that started the legacy of systematic research, thus marking the beginning of golden age of microbiology [44]. Lister, a Professor of Surgery, believed that the bacteria present in air might be responsible for causing sepsis postoperation. He sprayed phenol solutions over the operative areas and showed the reduction in infection which eventually led to the modern aseptic surgery.
1.3.3. Era of identifying and characterizing bacteria
Inventions of culture techniques using salts and yeast in 1872, plating techniques in 1881 using gelatin and use of sterilization techniques by Robert Koch contributed in the microbiological advancements [43]. Escherichia coli and gram staining came (1884–85) but took more than 25 years for addressing the application for assessing water, health risks and fecal contamination. In 1884 Koch isolated pure culture of Vibrio and Gaffky isolated bacteria that caused typhoid. In 1607 massive outbreak occurred that is now believed to be typhoid with up to 85% of mortality in Jamestown, Virginia. William Budd in 1856 suggested that typhoid was contracted through contaminated water but it was not accepted until 1884. This growing search was beginning to go beyond bacteria and it was found after several years that some infectious diseases are not caused by bacteria. In 1892 and 1898 two scientists Dmitri Ivanowski and Martinus Beijerinck, respectively, discovered tiny infectious particle that could pass through bacteria-stopping filters and described them as “filterable [sic] viruses” [44].
The two decades, from 1880 to 1900s, agents of several diseases along with their mode of transmission, ways to avoid infections or overcome them were also revealed. Most of the work in this period was inspired by medical and industrial interest. However, few attempts were made to know about the biology of nonpathogenic bacteria. They cared less about the morphology of microbes and their relations with each other and were interested in their activities and how they affected humans. Ultimately, bacteriology began to slow up. The older methods were not anymore sufficient and the new tools for biology, industry, and pathology were needed. The International Joint Commission (IJC) between the United States and Canada comprehended various bacteriological studies in 1914. They promoted use of advanced technological methods and set up various laboratories for the testing. Studies indicated 242 outbreaks with 9367 cases of waterborne typhoid, 84,345 cases of dysentery associated with these outbreaks between 1920 and 1930 in the United States and Canada. This high mortality rates finally changed the way wastewater was handled. In 1880 more than half a million people disposed their waste in Lake Michigan. Until 1922, Chicago Drainage canal was completed that could take all the wastewater downstream into the Chicago River and to Mississippi and this period resulted in a significant decrease in typhoid and finally leading to its elimination [43].
Massive outbreaks were seen in India in 1768 that spread across Europe, England and then the United States by 1832. John Snow’s advancement on transmission by water played an important role in moderating the disease spread and by 1854, Vibrio cholerae was described. With the beginning of disinfection of drinking water, cholera was eliminated in the developed countries.
Another key discovery was of British physician Fredrick Griffith in 1928 who started the hunt for “transforming factor” by his experiment of pathogenic strain of pneumococcus that transformed a harmless strain into pathogenic strain [46]. Slowly, relations such as virulence and immunity began to come up in existence. Not only bacteriology, but immunology was also gaining popularity. Considerations with relation of bacteria to each other, chemical constitution of antigenic agents, physicochemical mechanisms in relation to immunity or recovering from disease were studied [45]. Fig. 1–4 shows the timeline of the important events in the history that led to the discovery of bacteriology and infectious diseases.
Figure 1–4.
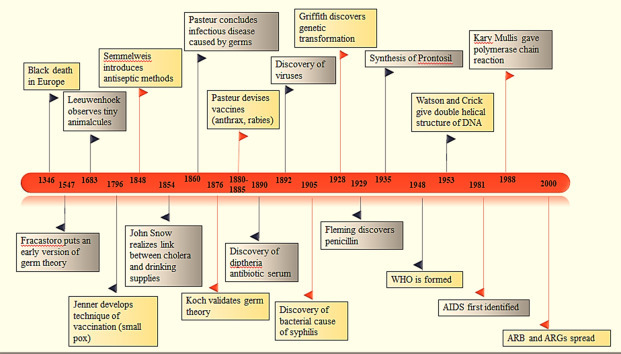
Major events and discoveries in bacteriology and infectious diseases.
1.3.4. Wastewater treatment and disease control
In ancient times, aesthetics played a major role to come up with the need to separate wastes from drinking water. During epidemic in France in 580, King burned all the tax lists as he believed that wealth was responsible for epidemic. The major turning point was in 19th century in 1846 John Snow observed cholera to be transmitted by drinking water and was able to test his theory by removing the handle from water pump which he suspected the cause of outbreak in London. In 1884 isolation of the bacteria that caused cholera by Robert Koch enforced his achievements. In 1894 came the discovery of bacteria that caused bubonic plague and in the same period findings of microbes of malaria, sleeping sickness, and other diseases were also given.
Similar theory was also suggested by William Bud and proposed eliminating exposure to contaminated water supply which initiated the approach of disinfection. Disinfection of drinking water started to prevent the spread of diseases. Chlorine was accepted for this purpose due to its cost-effectiveness in treatment. In 1800 William Cruikshank suggested that chlorine could kill germs in water, when the germ theory was yet to be substantiated. The Royal Sewage Commission added chloride of lime for deodorizing sewage in London as at that time odor was considered the cause of disease spread. In 1896 hypochlorite was first used as a disinfectant during typhoid epidemic in Austria–Hungary naval base in Pola.
Bleach solutions were also used as temporary measures for disinfection. First continuous chlorination of water supply was done in Belgium in 1902, after which this method was accepted as a primary measure of disinfection throughout the world. In 1983 chorine was used as a disinfectant of sewage effluent in Germany. However, chlorination of sewage was not widely performed because the treatment of wastewater was considered important as compared to drinking water treatment. UV light as disinfection techniques was discovered in 1801 and used first for the municipal drinking water in France in 1901. Ozone was introduced in Netherlands in 1893, to deodorize water [43].
1.3.5. Age of genomics
With genomics entering the scenario nowadays, new evidence for the evolutionary charts is being discovered. Functional analysis of various genes is being explored for new therapeutic targets. We are able to show intricate relationships of hosts’ and parasites’ physiological pathways or channels. More versatile platforms to develop new responses against infectious diseases are being developed leading to development of new vaccines, antibiotics, and immune modulators.
A list of infectious diseases has emerged over the past years such as acquired immunodeficiency syndrome, Ebola virus disease, Cryptosporidium parvum diarrhea, Legionnaires’ disease, avian influenza, and severe acute respiratory syndrome. Enhancing the prevention as well as control of epidemics requires development of effective therapeutic agents and vaccines. The current scenario of the public health chart includes challenges in the prevention and control of diseases and their association with the communicable diseases. Essential infrastructures to provide potable water, sewage disposal, sanitation, and safe food supplies are being greatly stressed upon. Our understanding toward examining the transmission and potential of waterborne disease potential will allow us to upgrade the necessary engineering advancements for community health protection. The most significant areas in this field consist of new advances to be applied to wastewater for pathogen discovery and characterization of diseases, further analysis of these data for water quality, health status, and the assessment of approaches and actions focusing toward sewage, drainage and treatment of wastewaters as well as the analysis and comparison of household versus community-based water and wastewater systems.
1.4. Antibiotic discovery
Antibiotics have been in use since a long time in ancient Egypt, China, and Greece. Extracts from filamentous fungi, herbs, and organic compounds were used for the healing of wounds and treating ailments. In 1870 Sir John Scott Burdon-Sanderson mentioned about the inhibition of bacterial growth in culture mold [47]. Joseph Lister in 1871 was able to cure an injury with Penicillium glaucum extracts, discovering the inhibitory effects of the fungus on bacterial growth. A similar observation was mentioned by John Tyndall in 1875 regarding the inhibitory effects of Penicillium on bacterial growth [48]. The antibiotic term was coined from the word antibiose used in publication of Paul Vuillemin in 1890. The word antibiose was used to describe the antagonistic relation between bacteria versus protozoa or fungi versus bacteria. However, the term antibiotic was first used by Selman Waksman in 1942 and described the antibiotic as a small molecule having antagonistic effect against other microorganisms [49].
Bartolomeo Gosio discovered the first natural antibiotic, mycophenolic acid in 1893, when he studied the fungal contaminated corn which was causing Pellagra in people of Southern Europe and Southern United States. He identified the fungus as Penicillium brevicompactum with a crystalline filtrate within the culture, which inhibited the growth of Bacillus anthracis [50]. In the later years, the antibiotic (mycophenolic acid) was also identified to possess antifungal, antitumor, and antiviral effects. However, this discovery of mycophenolic acid remained unknown until it was rediscovered again in the United States in 1913. The structure of mycophenolic acid was recognized in 1952 and total synthesis was obtained in 1969 [51].
Ernest Duchesne discovered that P. glaucum has the ability to inhibit E. coli in 1897. This antagonism between microorganisms was a strategy for survival. However, despite the presence of several evidences of antagonisms, the extraction of the antimicrobial substance was not achieved until 1909 when Paul Ehrlich discovered the arsphenamine, the first sulfa drug [52]. Arsphenamine antibiotic was first man made antibacterial agent, commercialized in 1911 and is an arsenic derivative against Treponema pallidum. Another antibiotic sulfanilamide was discovered in 1930 and was commercialized 5 years later under the name Protonsil [53].
1.4.1. Discovery of penicillin
Alexander Fleming, a bacteriologist in Paddington (London) hospital discovered that S. aureus culture left on bench for few days was contaminated with Penicillium notatum. On careful examination, he found that the colonies of S. aureus present in proximity to P. notatum underwent lysis; however, other colonies present far away remained unaffected [54]. He then checked its effect on other gram-positive bacterial strains concluded that fungus releases some substances, which can kill bacterial colonies present in its vicinity and penicillin was named in 1929 [55].
Fleming further collaborated with several researchers for identification of the substance, but was unsuccessful for many years. Howard Walter Florey (pathologist), Norman Heatly, and Ernst Chain (biochemists) in 1939 started to work on isolation of substance, and thus, even successfully tested on mouse at Oxford University. Thereafter penicillin was used for treatment of sepsis on a policeman named Albert Alexander in 1941. The treatment improved his condition for 5 days, but ultimately the patient died due to limited supply of drugs; however, the penicillin was proved to be a significant discovery having many promising affects. Thus Penicillium chrysogenum was the strain used for the industrial production of this antibiotic by Howard Walter Florey and Ernst Chain in 1940 and in 1943 during World War II, Florey made a successful attempt at testing the antibiotic on wounded soldiers [56]. In the same year, René Dubos isolated an antibiotic gramicidin from Bacillus brevis that inhibited gram-positive bacteria [57]. In the year of 1945, the penicillin was available for the use of public and was no longer restricted for use of military, thus, it was able to save life of millions. In the same year, Howard Walter Florey, Alexander Flaming, and Ernst Boris Chain shared the Nobel Prize for penicillin discovery and its use for treatment of various diseases. In 1957 John Sheehan reported the first total synthesis of penicillin V, although it was not useful for industrial production due to low overall yield, but the structural synthesis paved the way for preparation of penicillin analog from 6-aminopenicillanic acid [49]. The penicillin acts against enzyme transpeptidase, which helps in formation of peptide bonds to make the bacterial cell wall strong. In the absence of this enzyme, integrity of cell wall is not maintained, and thus, affecting the osmotic pressure and causing cell death [58].
1.4.2. Golden era for discovery of antibiotics
Guiseppe Brotsu in 1945 discovered the cephalosporin C from Cephalosporium acremonium, which was further rediscovered in 1955 [59]. The structure elucidation and analysis of cephalosporin C by X-ray crystallography was performed in 1961, while its complete synthesis was reported in 1965 by Robert B. Woodward. The structure and synthesis of cephalosporin C led to design of several analogs with powerful antibacterial properties and first cephalosporin-based clinically approved antibacterial drug was cefalotin [60].
After discovery of penicillin, the next 20 years were golden age for discovery of antibiotics. The discovery of streptomycin by Selmen Waksman and Albert Schatz was another milestone in history of antibiotics in 1943. The streptomycin was effective against both gram-positive and gram-negative bacterial infections. In addition, the streptomycin was also effective against Mycobacterium tuberculosis, and thus, offered a potential hope for treatment against tuberculosis for the first time [61]. The first systematic and repetitive study of the antimicrobial activities of soil bacteria led to the discovery of several antibiotics such as streptomycin (from Streptomyces griseus), actinomycin (from Streptomyces spp.), neomycin (from Streptomyces fradiae), clavacin (from Aspergillus clavatus), and fumigacin (from Aspergillus fumigatus). Streptomycin was one of the first aminoglycosides to be isolated and the first antibiotic remedy for tuberculosis [62]. Bacteria and fungi are known to be the greatest producers with the genus Streptomyces as the largest contributor of antimicrobial agents for human medicine. The mode of action for streptomycin involves binding to 30S subunit of ribosome during translation, which leads to the inhibition of protein synthesis in bacteria [63]. Due to the efforts of Waksman, an industrial revolution in the field of antibiotic production took place between 1940 and 1980 when 24 novel classes of antibiotics were discovered.
Tetracycline was another important class of antibiotics, which was discovered in 1945 by Benjamin M. Duggar from Streptomyces aureofaciens. Amongst the several samples screened for safe alternative for streptomycin, finally a gold colored actinomycete was found, which was able to produce aureomycin. After its structural synthesis, it was renamed as chlortetracycline. The identification of tetracycline lead to competition between different pharmaceutical companies due to concern over price decline of penicillin, and thus, Pfizer discovered oxytetracycline after screening of 100,000 samples collected from over the world [64]. The oxytetracycline produced by Streptomyces rimosus was found to have similar properties as chlortetracycline. In the meantime, Bristol labs also discovered tetracycline produced by Streptomyces virdifaciens. Pfizer also converted chlortetracycline into tetracycline, thus patent was granted to both Pfizer and Bristol labs. The structure of chlortetracycline was determined from time to time using chemical derivatization methods and X-ray crystallographic techniques in 1959 and 1963 by Woodward and total synthesis of chlortetracycline was achieved in 1973 by Hans Muxfeldt. Several other tetracyclines have also been discovered over the years [65]. The mechanism of action for tetracycline also involves cessation of translation by binding to 30S ribosome unit in the bacteria [66].
Another important discovery in 1947 was chloramphenicol antibiotic, which was first antibiotic to be used systemically or oral route. It was discovered by screening 7000 soil samples and culture was named as Streptomyces venezuelae. The isolation of active compounds was done by Prake Davis pharmaceutical company and in 1949 large amount of chloramphenicol was manufactured [67]. The sale of this drug was drastic and Prake Davis became largest pharmaceutical company in the world; however, some cases of aplastic anemia were detected due to side effects of chloramphenicol which decreased its use with time. The mechanism of action of chloramphenicol antibiotic involves binding to 50S ribosome unit during translation, and thus inhibits protein synthesis in bacteria [68].
The scientist team led by James McGuire at Eli Lilly identified another important antibiotic Erythromycin from Saccharopolyspora erythraea strain isolated from soil sample in 1952. The six molecule complex produced by strain contained only one effective molecule Ilotycin, which is now called erythromycin A. This molecule was having potency against gram-positive bacteria. The structure of erythromycin was resolved in 1956 and contained 14 or 16 membered rings [49]. The bitter taste, acid instability, and insolubility were some of the problems associated with this antibiotic. Thus Upjohn decided not to continue with erythromycin considering it a weak antibiotic; however, Abbott Laboratories and Eli Lilly achieved a huge success with commercialization of erythromycin. The mechanism of action of erythromycin involves binding to 50S ribosome unit during translation process, which inhibits bacterial protein synthesis [69].
After a year later, in 1953, another important discovery of glycopeptides from Streptomyces orientalis was done. The antibiotic was named as vancomycin and was effective against gram-positive bacteria. In addition, the antibiotic was having ability to act against penicillin-resistant Staphylococci; however, it tend to cause side effects, thus was later improved [67]. The structure of vancomycin was elucidated in 1977 by chemical studies and X-ray crystallography.
The total synthesis of antibiotic was achieved in 1999 by Evans group. The mechanism of action of vancomycin involves inhibiting the cell wall synthesis. It binds with d-alanyl-d-alanine molecule, which further prevents the formation of backbone strands during cell wall synthesis [70]. The use of vancomycin is restricted to intravenous treatment and is still considered as an effective antibiotic.
In the year of 1959, Lepetit pharmaceutical company examined several soil samples to isolate the Streptomyces mediterranei for activity against M. tuberculosis [71]. The identified antibiotic rifamycin was very complex and it was difficult to determine its structure. Thus Oppolzer and Prelog, two chemists were able to solve the complexity of the structure with chemical degradation studies and with the help of X-ray crystallography, the rifamycin B was also determined. The rifamycin B was found to be inactive component, while its intermediate molecule, rifamycin O generated rifamycin S, which was active and effective against tuberculosis. The rifampicin was further generated with the help of collaboration with Ciba-Geigy Company. The rifampicin inhibits DNA-dependent RNA polymerase, which further inhibits protein synthesis [63]. The rifampicin has high in vitro activity against staphylococci; however, resistance against antibiotic has decreased its use for treating tuberculosis.
The linezolid, novel class of synthetic antibiotics was originated in 1970 in Dupont as a result to discover chemicals which have potent effect against fungal and bacterial pathogens for plants. Thus one of the oxazolidinones was found to have in vitro activity, which turned out to be false information; however, during in vivo testing, the compounds were successful and were active against fungal and bacterial pathogens [72]. The research was continued by team led by Walter Gregory at Dupont and two analogs (DuP-721 and DuP-105) were successfully tested in vitro against Bacteroides fragilis, streptococci, and staphylococci. However, liver toxicity was reported in rats, and thus, research was terminated. During ICAAC (Interscience conference on Antimicrobial agents and Chemotherapy), Steve Brickner (Upjohn chemist) noticed the results on posters by Dupont, and thus, started to make improved oxazolidinones and also early testing was started at same time. Thus, in 1995, two analogs Eperezolid and Linezolid were selected for human testing in phase 1 and based upon the results. Linezolid was further selected for phase 2 evaluation. In 2002 final Food and Drug Administration approved the Linezolid as a novel class of antibiotics [67]. The mechanism of action for Linezolid also involves binding to 50S ribosome unit during protein synthesis causing cessation of protein translation [73].
After this, in vitro synthesis was the new approach preferred by industries for discovery of antibiotics. However, not a huge success has been achieved in this respect as only four new classes, the nitrofuran, the quinolone, the sulfonamide, and oxazolidinones have been discovered till 1987. Recently, cephalosporin called cefiderocol [74] and semisynthetic compounds like ketolides [75] or metronidazole [53] have been observed to have antimicrobial properties. The 20th century witnessed the discovery and large-scale production of antibiotics which proved to be one of the greatest achievements in the field of medicine.
The discovery of antibiotics was an incredible achievement in the field of medicine but the development of resistance in various bacteria and lack of return after investment has resulted in a reduction in interest of the industries toward investment in antibiotic research. This statement is proved by the data that around 20 companies invested in research toward antibiotic in 1980 and only 5 are left in 2015. Thus more research and investment is focused on drugs to deal with chronic diseases [53].
1.5. Pharmaceuticals in hospital wastewater
Emerging pollutants are compounds which do not have a regulatory status and whose effects and fate in humans and environment are poorly understood. Pharmaceuticals are emerging pollutants which, when released, pose a possible nonquantifiable risk on living beings and environment [76], [77], [78]. The discharges from hospital can contain disinfectants, detergents, contagious feces/excreta, biological liquids, drug residues, metal radio elements, and many other chemicals with a genotoxic or cytotoxic activity, toxic or hazardous chemicals or pharmaceutical residues, and radioactive and/or infectious agents [79]. Metabolites and substrates utilized in clinical applications are potent enough to cause mutagenic and genotoxic effects in the aquatic environment as well as in organisms dependent on such water bodies [80]. Hospital effluents contain pollutants as a result of the various activities occurring in a hospital such as surgery, drug treatments, and cleaning. These activities cause emission of pollutants into the aquatic and terrestrial environments through sewers and WWTPs. Several studies have been focused on the characterization of the contaminants present in HWW to determine their ecotoxicity. The discharges from hospital can be domestic (laundries and toilets of normal wards) or specific (generated by care, analysis, or research activities). Pharmaceuticals administered to the patients pass through the body in metabolized or nonmetabolized state later combining with HWW [81], [82].
Antibiotics specifically are known to be microbial-resistant compound excreted in HWWs and domestic effluent and discharged into the water system causing disruption to the microbial ecology. Pharmaceuticals used for veterinary administration are also released into the environment making their way through municipal wastewater streams [76]. WWTPs are less effective in eliminating pharmaceutical compounds which may be due to their recalcitrant and nonbiodegradable nature. Other reason for poor degradation might be the high concentration of these compounds in HWW which treated with the municipal wastewater in WWTPs, many pharmaceuticals are resistant to conventional treatments so the elimination efficiencies of these compounds in municipal WWTPs is overall average removal rates range between 10% and 90%, which leads to their presence in effluents of WWTPs and further in ground and surface water bodies [82], [83], [84]. Some countries consider HWW as domestic and allow their discharge into municipal sewer network without any prior treatment or standard limit while there are few countries where HWW is considered industrial and, hence, is pretreated before discharge into municipal sewers.
Active or inactive metabolites in the excreta of patients on medication are the major source by which micropollutants make their way into the municipal sewer streams and further into the water bodies. The water from such polluted sources acts as a medium for transport of these hazardous pollutants into other living organisms. Disposal of unused drugs into drains or toilets is another way by which pharmaceuticals mix into the aquatic system unaltered. Besides these, hospitals have laboratories for research activities and sample testing where various body fluids and samples are treated with chemicals and metabolites which when later discarded into the sewer system and ultimately discharged into larger water bodies.
The production of HWW depends on several factors including the number of inpatients and outpatients, facilities available, drugs prescribed, geographical location, predominant disease, and equipments or machines used for various medical activities [83], [85]. The total load on a WWTP in a city will also be affected by the total number of hospitals and diseased population density in a city. Considering that there are approximately 2.6 hospitals for every 100,000 inhabitants in Europe (ranging from 1 in the Netherlands to almost 6 in Finland) [86], with on average 530 hospital beds (ranging from approximately 320 in Spain and little more than 800 in Germany) and that HWW discharge is approximately 0.3–0.7 m3/bed/day [87], [88], [89], the total effluents produced from these facilities are approximately 265 m3/day (from an average of 0.5 m3/day for hospital bed) in a city with 100,000 inhabitants.
The indicators (NH4, NOx, oil and grease, tensioactives, phosphorus, and chlorines) to measure the biodegradable and nonbiodegradable oxidizable pollutants from HWW detected their high values in the effluents. Concerns about the macropollutants such as ammonium ions have increased due to their high concentration in HWW than in the urban wastewater (UWW). The data of microbial indicators show that the E. coli load is high in UWW than HWW, due to dilution of wastewater in hospitals in which water consumption per bed approximately 700 L/day [90]. The fecal and total coliforms are higher in concentration in UWW than in HWW.
1.5.1. Chemical hazards
The major chemicals that can be found in HWW are antibiotics, analgesics, β-blockers, anesthetics, chemicals from labs, and X-ray contrast media with maximum concentration excreted in urine (55%–80%) followed by feces (4%–30%) as unmetabolized substances [91]. The chemicals generally present in HWW show varying behavior in WWTP due to their different physical and chemical properties such as solubility, volatility, and biodegradability and often pass in the effluent of WWTP as they are not neutralized during the treatment process. The complexity in the degradation of such chemicals is enhanced due to their wide physical and chemical diversities.
Worldwide pharmaceutical consumption was increasing and, specifically, antibiotics consumption, therefore, the concentration of those compounds in surface water and other wastewaters was found to be high. They are main chemical hazard as they cause endocrine disruption and fish feminism in aquatic life [86], [91]. Few substances such as diclofenac, 17β-oestradiol, and 17α-etinylestradiol are included in European and US priority list [92].
There are several different sources by which contamination of surface water with pharmaceuticals compounds can occur such as livestock, agriculture, aquacultures, and slaughterhouses [15]. Several studies considered risk quotient (RQ) as a standard evaluation for ecotoxicological compounds in HWW [93]. The RQ value derives the predicted effect concentration (PEC) of the substance and its non-PEC where that concentration has no adverse effects on environment.
The PEC value in the case of HWW is derived from the amount of each active compound consumed in the hospital M (g), the excreted fraction in feces and urine (F excreted), and volume of the pharmaceuticals are consumed V (L) (Eq. 1.1) [93].
| (1.1) |
Certain compounds such as ofloxacin, erythromycin, and sulfamethoxazole were analyzed and their RQ values were above 1 in hospital effluent [91]. World Health Organization (WHO) stated that in the United Kingdom, mercury emission was 50% high from dental amalgam and from medical device industries [79]. Certain countries prohibited the discharge of dental amalgam in WWTPs and certain metals such as copper, zinc, and tin. Among them, mercury is on the top of the list of dangerous substances in the European Dangerous substances.
There are several hazardous substances present in HWW such as chlorine, metal ions, and ammonium, and they deactivate Legionella bacteria in the warm water system. In some healthcare facilities, formalin was be used as a disinfectant [79]. The adsorbable organic halide compounds from radiography laboratory are toxic for the fish and aquatic organisms even at low concentrations. The radioisotope contamination was mainly from the excreta of patients. Its radioactive half-life of 8 days is a significant risk of 131I radioisotope accumulation after its discharge into the sewer network [94].
1.5.2. Physical hazards
Besides the chemical hazards, certain hospital wastes also pose physical hazards to environment. Nuclear medicine therapies utilize radioactive isotopes like 131I or β-emitters (phosphorus-32, strontium-89, and yttrium-90) which when derived from patient’s excreta cause contamination up to 90% of the radioactive dose administered. 131I is known to accumulate in the sewer network and environment and its natural decomposition in holding tanks (half-life: 8 days) is the best way to eliminate its radioactivity [94], [95].
Drugs are supposed to have physiological effect in small quantities and waterways containing concentrations in part per billion (ppb) to less than one part per trillion (ppt) can prove to be hazardous if consumed [96].
Besides human, veterinary drugs also cause contamination of surface water and groundwater with the animal excreta or slurry moving along with run off from fields especially during rain [97]. Pharmaceuticals are grouped into various classes depending on the therapeutic action and effects they impose. Though there is a large diversity among the classes, a few of them include the nonsteroidal antiinflammatory drugs (NSAIDs), antibiotics, β-blockers, antiepileptics, blood lipid lowering agents, and antidepressants.
1.5.2.1. Nonsteroidal antiinflammatory drugs
Ibuprofen, naproxen, and diclofenac are the most commonly used NSAIDs that relieve pain by blocking enzyme cyclooxygenase. Cyclooxygenase catalyzes prostaglandins biosynthesis from arachidonic acid [98]. NSAIDs generally show low acute toxicities and only diclofenac and acetylsalicylic acids have been classified as potentially harmful to aquatic organisms by the European Union [99]. Some crustaceans, rotifer and green algae have been reportedly used to test for the ecotoxicity of ibuprofen, naproxen, diclofenac, and acetylsalicylic acid, where the individual EC50 value of the latter two (<100 mg/L) categorizes them as potentially harmful to aquatic organisms. Environmental presence of diclofenac affects the renal and immunological aspects in brown trout [100]. The mass killing of three vulture species in Indian subcontinent have been attributed to feeding on carcasses of diclofenac treated cattle’s [101].
1.5.2.2. β-Blockers
These are one of the most important cardiovascular drugs prescribed in cases of hypertension and angina. Norvasc, diltiazem, and amlodipine are some drugs of this class are excreted in urine and bile that are being metabolized in the body. Diltiazem has been shown to have higher persistence and toxicity in aquatic environment [102]. Amlodipine is excreted in urine mostly as inactive metabolites as a result of about 90% conversion of the intact drug into metabolites. Propranolol with an EC50 of <1 mg/L is very toxic followed by metoprolol and atenolol. Propranolol has been reported to inhibit photosynthesis in Desmosdesmus subspicatus with a 24 h exposure chlorophyll fluorescence assay [103].
1.5.2.3. Antibiotics
These are antimicrobial drugs which are used in large quantities for treatment of human and animal diseases. Up to 90% of these drugs pass into aquatic environment because they are less metabolized in the body [104]. Six classes of antibiotics including β-lactam, macrolide, sulfonamide, fluoroquinolone, and tetracycline are widely used in human and animal medicine. β-Lactam (e.g., amoxycillin) is one of the most widely used antibiotics in humans which are followed by macrolides (e.g., azithromycin and clarithromycin) and sulfonamides (e.g., sulfamethoxazole). Despite of the greatest use of β-lactam antibiotics, they are not detected in the environment due to their easy hydrolysis in mild acidic or basic conditions. Besides the direct contamination of water, antibiotics have been known to generate resistance in bacterial strains even with exposure to small quantities [105]. Their long half-lives cause accumulation in aquatic and terrestrial environment in detectable quantities such as erythromycin, sulfamethoxazole, and sulfamethazine which can persist in the environment for more than a year. In one study, three strains of E. coli and an unidentifiable bacterium were isolated from a sewage treatment plant (STP) and assessed for resistance to antibiotics: erythromycin, ampicillin, tetramycine, trimethoprim, ciprofloxacin, and sulfamethoxazole. All the bacteria showed resistance to erythromycin followed by ampicillin, tetracycline, ciprofloxacin, trimethoprim, and sulfamethoxazole [106]. Chlortetracycline, oxytetracycline, and tetracycline have been reported in surface waters in the United States but no amounts of doxycycline were traced [107]. Antibiotics such as amoxicillin, ciprofloxacin, and erythromycin have been reported to influence the growth, structure, and function of many algal species [108], [109]. Phytotoxicity has been shown by antibiotics such as fluoroquinolone, sulfonamide, and tetracycline below 1 ppm [110].
1.5.2.4. Antiepileptics
Antiepileptics are central nervous system drugs that reduce the abnormal signal trigger by neurons. Carbamazepine (CBZ) and gabapentine are two antiepileptics drugs which act by controlling the ions channels. CBZ blocks the sodium channels of excitatory neurons. Gabapentine is used for neuropathic pain and is a gamma-aminobutyric acid analog. It modulated the action of calcium channels and neurotransmitter release. Groundwater and rivers in the United States, Canada, and Germany have been frequently detected with CBZ and its metabolites [111], [112].
1.5.2.5. Blood lipid lowering agents
Statins and fibrates are cardiovascular drugs which reduce cholesterol and triglyceride. Clofibric (CLF) acid is a metabolite of fibrate which is known to have a very high persistence in aquatic environments and is found in surface water and groundwater reservoir [113]. Statins have hypolipidemic effect which reduce the level of low-density lipoprotein and enhance the production of high-density lipoproteins. Both fibrates and statins have been reported to show toxicity in fishes, algae, and plants [114], [115].
1.5.2.6. Antidepressants
Selective serotonin reuptake inhibitors (SSRIs), and selective serotonin and norepinephrin reuptake inhibitors are class of antidepressants for treating depression, anxiety, panic disorder, obsessive compulsive disorder, eating disorders, and social phobia. Fluoxetine and sertraline are SSRIs which along with their metabolites were detected at 0.1 ng/g and higher levels in fish residing in a municipal effluent dominated stream [116], [117]. Long-term exposure to antidepressants in rivers and streams has been shown to cause developmental and behavioral changes in fishes and frogs [117].
1.5.3. Biological hazards
The HWW contains various pathogens and infectious agents such as bacteria, protozoa, helminths, and viruses that are from different excretions and body fluids of human beings. According to the Environmental Protection Agency data of the concentration of infectious agent such as Shigella, Salmonella, Campylobacter, Giardia, and Cryptosporidium, Enterovirus, Nororvirus, Adenovirus, Rotavirus, and Hepatitis A virus are potentially high in raw domestic wastewater compare with the pathogens in HWW.
A critical situation arose in last few years regarding bacterial resistance to antibiotics. It became an issue due to excess use of antibiotics. In such a case, WWTP represents a hub of antibiotic-resistant bacteria (ARB) and ARGs. The ARB can be a primarily link to release from patients and hospital equipments. In acute hospitals, there is 20%–30% high antimicrobial and antibiotic treatment on inpatients of Europe [118].
The antibiotic class fluoroquinolones and β-lactams are widely used antibiotics. However, β-lactams are rarely detected in wastewater because the β-lactam ring is readily cleaved by hydrolysis [90]. By contrast, high concentrations of fluoroquinolones are found in HWW, WWTPs, and downstream from the WWTP, in rivers [94]. This is also the case for macrolides and sulfonamides. The ARB represents a risk to humans and animals because they can reduce the therapeutic potential against pathogens.
1.5.4. Guidelines for management of hospital effluents
The WHO published guidelines in 1999 “Safe Management of Wastes from Health-care Activities” and updated them in 2013. It describes the disposal of health waste management. And it also states that the healthcare facilities generate very high-risk infectious wastewater than domestic water which also depends on the healthcare facility, and the water might contain chemicals, pharmaceuticals, and contagious biological agents as well as radioisotopes [79].
The guidelines describe the hazardous characteristics of wastewaters that agree with majority of studies reviewed. In general, pretreatment of wastewater is necessary for medical laboratories and includes neutralization, filtering, and autoclaving. Moreover, the minimum requirements to discharge the HWW into municipal sewerage system are as follows: the treatment plant should have primary, secondary, and tertiary treatment facilities. The sewers need to be connected to central treatment to remove 95% of bacterial load. The waste management system maintains very high standards to ensure low quantity of toxic chemicals, pharmaceuticals, radionuclides, cytotoxic drugs, and antibiotics in the discharges sewage.
The wastewater disinfection will be based on the discharge of local areas residents, hospitals, and industries. For example, one of the European country’s appetite was raw sell fish and they discharged residual shell fish to local water bodies. So, to disinfect the contaminants, chlorine was used as an disinfectant for wastewater. There are several factors need to be considered for discharge of wastewater such as leakages between entry points like sinks, toilets, and drains and on-site treatment plant or discharge to sewage plant.
There are some most common parameters that can be considered for effluent quality such as temperature, pH, chemical oxygen demand (COD), BOD5, nitrate, total phosphorus, total solids, and concentrations of E. coli. The regular survey is necessary on in-flow and out flow points to know how efficient the treatment plant is to reduce the concentration of contaminants.
1.6. Presence of pharmacological substances in aquatic environment
The presence of pharmaceutical compounds in aquatic environment as a result of their release in industrial, domestic, and municipal effluents is a cause of major concern. The presence of such contaminants in aquatic environments creates adverse effects on the living organisms within aquatic ecosystem. These wastes alter the BOD and COD values of the water bodies, increasing the toxicity to various aquatic organisms [119]. Small streams are particularly affected as the pharmaceuticals are released from point sources like WWTPs. Several pharmacological substances have also been identified in run-offs from agricultural fields which ultimately merge into lakes and rivers. Even with the availability and constant improvement (development) of advanced treatment technologies used in WWTP failed to achieve the complete removal of emerging pharmaceutical compounds. The constant loading of water bodies including the freshwater reservoirs with the effluents of WWTPs, hospital, industrial, and domestic wastes containing pharmaceutical compounds is creating strain on the potable water sources [120]. A number of studies have detected various amounts of pharmaceutical compounds like antibiotics in aquatic environments in recent years [121] (Table 1–1 ). The environmental and human risk associated with these contaminants is still understudied and requires proper attention to diminish their release and accumulation in environment and its living members. Most of the studies on pharmaceuticals have been conducted in Germany and Spain, followed by the United States and Canada, because these countries are known for their environmental research and host researchers with outstanding and distinguished expertise in the field [131].
Table 1–1.
Common antibiotics detected around the world in different sources of water.
| Antibiotic | Concentration (ng/L) | Source | Region | Reference |
|---|---|---|---|---|
| Clarithromycin | 1.7±0.1 | Surface water | United States | [121], [122] |
| 40.1−54.4 | River | Spain | ||
| 524 | River | United Kingdom | ||
| Erythromycin | 21–33 | River | Spain | [123] |
| 2000 | River | United Kingdom | ||
| Sulfamethoxazole | 4 | Surface water and groundwater | France | [124], [125] |
| 2626.3 | Hospital effluent | Greece | ||
| 0.6 | Southeast Asia | |||
| Amoxicillin | 261±3 | Surface water | Hong Kong | [124], [126] |
| 150 | Southeast Asia | |||
| 10,000,000 | Italy | |||
| Chloramphenicol | 3 | River | Spain | [124] |
| 2.0 | Southeast Asia | |||
| Norfloxacin | 48±19 | Surface water | Hong Kong | [127] |
| Indomethacin | 3 | River | Spain | [128] |
| Metronidazole | 145 | Hospital effluents | Spain | [129] |
| Azithromycin | 163 | River | UK | [123] |
| Ciprofloxacin | 2610 | Municipal wastewater | Western Balkan region | [130] |
| 30 | Surface water | United States | ||
| DiOH-CBZ | 3400–4000 | WWTP | Many countries | [131] |
| Fenofibric acid | 394 | WWTP | Spain | [131] |
| CX-IBU | 10,600–38,400 | WWTP | Many countries | [131] |
| OH-IBU | 1130–6840 | WWTP | Many countries | [131] |
| Aminophen | 90–6000 | WWTP | Many countries | [132] |
| Salicylic acid | 2014.7 | Groundwater | China | [132] |
1.6.1. Natural water bodies: rivers and oceans
The presence of 36 pharmaceuticals in urban river water samples was investigated in Beijing, Changzhou, and Shenzhen in China [133], [134]. The compounds such as sulfadimethoxine, sulpiride, atenolol, and indomethacin were detected with concentrations ranging from 50.9 to 164 ng/L. Another study analyzed 34 pharmaceuticals present in 86 individual water samples obtained from coastal regions and surface water in Costa Rica [132]. The compounds detected with high frequency (34%–77%) were doxycycline, sulfadimethoxine, salicylic acid (SA), and triclosan (TCS). The compounds detected in higher concentrations (10–74 µg/L) were acetaminophen, doxycycline, ibuprofen, gemfibrozil, and ketoprofen.
One of the studies observed the presence of 22 pharmaceuticals in 3 major rivers of South Korea [135]. The rivers received effluents from secondary treatment plants located in industrialized areas. The concentrations of compounds such as iopromide (20–361 ng/L) and caffeine (10–194 ng/L) were highest. The target pharmaceuticals were found in all sampling sites with detection frequencies of 17%–53%. Compounds such as tris(2-chloroethyl) phosphate (TCEP), caffeine, naproxen, iopromide, and CBZ were frequently observed in surface water samples. Drugs like CBZ are frequently detected in surface waters at relatively higher concentrations (ng/L–µg/L). This is because CBZ is a stable drug which is difficult to remove in conventional treatment plants and is expected to be in similar concentration in influent, effluent, and downstream of the receiving body [132].
Zhang et al. [136] detected the presence of erythromycin, sulfamethoxazole, and trimethoprim along with nine other antibiotics in the coastal bays of Yellow sea and in treatment plant effluents discharged into the sea. Erythromycin, sulfamethoxazole, and trimethoprim were detected at the highest concentration in coastal waters and fresh waters at <0.23–50.4 and <0.25–663.1 ng/L, respectively [136]. There have been reports regarding the potential toxic effects of active pharmaceutical compounds on aquatic organisms like fishes and algae. Also, sustained exposure to antibiotics has been known to develop resistance in bacteria toward these contaminants creating a hindrance in the disinfection processes of freshwater bodies. Toxicity effects of amoxicillin, erythromycin, norfloxacin, levofloxacin, and tetracycline on cyanobacteria Anabaena CPB4337 and green alga Pseudokirchneriella subcapitata were studied by González-Pleiter et al. [137] and cyanobacterium was found to be more sensitive to the toxic effects of the various antibiotics [137]. Although the individual compounds have been known to be present in low concentration in water bodies, the interactive effects between two or more of these compounds can result in synergistic effects with enhanced ecotoxicity risks. Certain transformed products are converted into toxic compounds during the treatment process in WWTPs, and hence, increases the necessity of quantitative risk assessment of the transformed products and metabolites. Evgenidou et al. [131] conducted investigations on the occurrence and fate of transformed products of pharmaceuticals and personal care products and illicit drugs in influents and effluents of different WWTPs in regions of Europe, the United States, and Canada. Transformed products and metabolites were found in ranges of ng/L to µg/L in wastewaters with no specific pattern of their occurrence in municipal, industrial, and hospital wastewaters [131]. Trace number of pharmaceuticals are present in domestic wastewater as a result of human consumption. Treatment of such wastewater can be performed using conventional activated sludge process [138]. Chlorination has also been found effective in the removal of sulfonamides and sulfamethoxazole.
The presence of 25 pharmaceuticals was investigated in Taihu Lake, China [139]. A high detection frequency (>70%) in the Taihu Lake was observed for nearly all the 25 pharmaceutics. Chlortetracycline (234.7 ng/L), chloramphenicol (27.1 ng/L), erythromycin (72.6 ng/L), paracetamol (71.7 ng/L), and carbamazepine (23.6 ng/L) were detected with a high frequency (100%) and high concentrations, indicating their wide use in the Taihu Basin. Concentration of pharmaceuticals was observed higher in dry season than in wet season, probably due to the poor flow conditions of the lake in winter and the properties of pharmaceuticals. The contamination levels of antibiotic pharmaceuticals (0.2–74.9 ng/L) in the Taihu Lake were lower than or comparable to those reported worldwide.
1.6.2. Groundwater
The pharmaceutical concentration in groundwater differs from region to region. The frequencies and concentrations of pharmaceuticals are lower in groundwater than in surface water [132]. Out of the 52 pharmaceuticals detected in 70 groundwater samples collected in China, most frequently detected were bisphenol, erythromycin, fluconazole, methyl paraben, SA, and sulfamethoxazole, and their concentration was around 70 ng/L. On the basis of studies conducted in 14 countries, mean concentrations of commonly found pharmaceuticals in groundwater were CBZ (5 µg/L), sulfamethoxazole (252 ng/L), ibuprofen (1.5 µg/L), caffeine (9.8 µg/L), and diclofenac (121 ng/L). Pharmaceuticals do not display significant seasonal variations, whereas their concentrations in reservoirs are higher during spring than in other seasons indicating maximum use of pharmaceuticals by patients during spring season.
One of the studies investigated the presence of pharmaceutical contaminants in groundwater in Taiwan [140]. These authors detected many pharmaceuticals and perfluorinated chemicals at the ng/L level, except for 17α-ethinylestradiol (1882 ng/L), sulfamethoxazole (1820 ng/L), and acetaminophen (1036 ng/L). It was found that pharmaceutical concentrations in groundwater were consistent with the results obtained in other countries. The most commonly detected antiinflammatory drugs and analgesics in groundwater include CBZ, diclofenac, ibuprofen paracetamol, and SA because they are widely and frequently consumed [132]. Many pharmaceuticals and their metabolites, such as ibuprofen, diclofenac, and ketoprofen, have been found at concentrations of up to mg/L in China. SA was found with a detection frequency of 98% while its concentration ranged from 43.7 to 2014.7 ng/L. According to a national survey of pharmaceuticals and organic pollutants in the United States, N,N-diethyl-meta-toluamide (DEET) (35%) and sulfamethoxazole (23%) were the most frequently detected pharmaceuticals in 47 groundwaters across 18 states [132]. Pharmaceuticals have ecological risks which could be a serious concern for groundwater near landfill sites.
1.6.3. Wastewater treatment plant
Among the various sources of pharmaceutical wastewater release in the aquatic environment or as influent in WWTPs, pharmaceutical industry and hospital effluent are the main contributors. Municipal and domestic water also contain active pharmaceutical ingredients (APIs) but in a lower concentration. Pharmaceutical manufacturing industries dispose effluents with active pharmaceutical compounds which are either released directly into the water bodies or treated in WWTPs. Even after WWTPs processing, some of these compounds pass out in active or untreated form and are nonbiodegradable causing adverse effects in receiving waters [141]. The concentration of pharmaceuticals and other metabolites in hospital effluents varies with time and region. This may be due to the occurrence of different diseases and differences in drugs prescribed in various parts of the world. HWW is potentially more toxic than other as they contain pathogens that have become antibiotic resistant over time [142]. The scarcity of water resources has created a need to develop methodologies to eliminate pharmaceutical and other contaminants allowing reuse of water to favor water management.
Hydroxylated derivative of the antibiotic metronidazole (METR-OH) has been identified in hospital effluents in Portugal. In hospital effluents, hydroxylated derivative of METR-OH reaches concentration up to 11 μg/L [131]. A pilot scale WWTP for HWW was operated by Kovalova et al. [78], [89] to test the plant efficiency in removal of 56 micropollutants. The plant consisted of membrane bioreactor with five posttreatment technologies [O3, O3/H2O2, powdered activated carbon (PAC), and low pressured UV light with or without TiO2] where the removal efficiency for pharmaceuticals and metabolites was 90% and 86% by O3 and PAC, respectively [78], [89].
1.6.3.1. Municipal
Municipal wastewater containing APIs is generally treated in conventional WWTPs. Many antibiotics are released into the environment without being metabolized. Sulfonamides, fluoroquinolones, and macrolides are frequently observed in sewage. Among these antibiotics, sulfamethoxazole, ciprofloxacin, azithromycin, and tylosin are the most frequently detected in STP effluent. Trimethoprim and tetracycline exhibit high presence in both influent and effluent, indicating low removal efficiencies in STPs [143]. β-Blockers are common pharmaceuticals which are used in the treatment of cardiovascular diseases, such as angina and hypertension, and they were observed in European waters in 1995 study [132]. Subgroups of β-blockers include atenolol, metoprolol, and propranolol and they were detected in the influents and effluents of STPs [132]. Hormones have also been detected in influent and effluent of STPs. The concentration of estrone, 17β-estradiol, estriol, progesterone, and testosterone in influent was 41, 8.6, 13, 10, and 7 ng/L in Czech Republic, respectively, while their effluent concentration was <2.5, <1, <10, <0.5, and <0.5 ng/L [144]. Pharmaceuticals, such as antiepileptic drugs and blood lipid regulators, have also been detected in sewage at relatively low concentrations. Analgesics (painkillers) and antipyretic (fever reducing drugs) like aminophen (up to 6000 ng/L) have been detected in different countries. Diclofenic, ibuprofen, and naproxen were found in relatively lower concentration (ng/L) [132].
The presence of 14 pharmaceuticals in two conventional WWTPs was investigated in South Korea [132]. In the Seoul WWTP, six pharmaceuticals were detected in raw water at concentration range of 2–143 ng/L. Moreover, their concentrations were below the detection limits in treated water (<1 ng/L). Only oxybenzone (sunscreen) was detected, at a very low level (i.e., 1.2 ng/L) in Gwangju WWTPs. In most of the studies conducted on WWTPs, ERY-H2O (erythromycin-H2O) was frequently detected antibiotic in influents and effluents of WWTPs [131]. ERY-H2O is dehydrated metabolite of ERY as ERY is not stable in aquatic environment and is converted into dehydrated product. In another study, seasonal variation of ERY-H2O was studied, and it was observed that higher frequencies of ERY-H2O were obtained in fall. In influents, the maximum concentration of ERY-H2O observed was 1978 ng/L while in effluents, maximum concentration of ERY-H2O observed was 838 ng/L. METR-OH has been identified in WWTPs influents and effluents in Portugal [131]. The concentration of METR-OH in WWTP influent was observed in the range of 0–158 ng/L.
In most WWTP, SA had high frequency levels of detection (100%) and was persistently detected in all samples (WWTP influents, effluents, and in surface water samples collected near WWTP) [131]. The concentrations of SA in influents and effluents range up to 89.1 µg/L and 6825 ng/L, respectively. Ibuprofen is among the most widely used pharmaceuticals in the world. After administration of ibuprofen, 15% is excreted as the parent compound while 26% is excreted as hydroxy-ibuprofen (OH-IBU) and 43% as carboxy-ibuprofen (CX-IBU) with conjugates and carboxy-hydratropic acid (2-phenylpropanoic acid) in minor amounts. OH-IBU was detected in WWTP influents and effluents at concentrations reaching 6840 and 1130 ng/L, respectively. CX-IBU was detected at concentration of 38.4 μg/L in the influents and 10.6 μg/L in the effluents [131]. CBZ is predominantly metabolized in liver and it is metabolized into DiOH-CBZ after hydration. The metabolite 10,11-trans-dihydroxy-carbamazepine (DiOH-CBZ) has been identified in influents up to concentration in 4000 ng/L and effluents up to concentration in 3400 ng/L [131]. Fenofibric (FEN) acid was detected in WWTPs of Spain at concentration of 349 ng/L. FEN was detected in many samples and had low removal efficiency after secondary treatment (1.3%). O-Desmethyl-venlafaxine (up to 5500 ng/L), OH-bupropion (up to 2000 ng/L), and erythro-threo hydrobupropion (up to 5700 ng/L) were detected in high concentration in wastewater. Desmethyl-citalopram was detected with a high frequency but with lower concentration (up to 426.7 ng/L).
Occurrence of pharmaceuticals was detected in municipal wastewater of Saudi Arabia [145]. Drugs such as acetaminophen (38.9 ng/mL), metformin (15.2 ng/mL), norfluoxetine (7.07 ng/mL), atenolol (2.04 ng/mL), and cephalexin (1.88 ng/mL) were detected in influent samples. Meanwhile, their concentrations in effluents were acetaminophen (31.2 ng/mL), metformin (3.19 ng/mL), norfluoxetine (7.25 ng/mL), atenolol (0.545 ng/mL), and cephalexin (1.53 ng/mL). The presence of four pharmaceutical active compounds such as naproxen, ibuprofen, ketoprofen, and diclofenac was detected in WWTPs in coastal environment of Algiers [146]. The target compounds were detected in influents and effluents with concentrations range of 155.5–6554 ng/L, implying removal efficiencies of WWTPs, between 30.3% and 95%. The surface water was also had diclofenac (72.9 ng/L) and naproxen (228.3 ng/L). The occurrence of ibuprofen and ketoprofen was observed in drinking water, at concentrations of 142.1 and 110.9 ng/L, respectively.
The conventional approach incorporates the use of activated sludge process for the removal of contaminants [147]. However, typical activated sludge treatments are not highly effective in removal of micropollutants and often result in their discharge into surface water. The removal efficiency of pharmaceuticals vary from <20% to 99% depending upon the class and nature of the compounds [148]. Considering the drawbacks associated with conventional activated sludge treatment method, advanced processes are either being used solely or in incorporation with activated sludge process to enhance the removal efficiency of pharmaceuticals from municipal wastewater. Karelid et al. [149] achieved a goal of 95% removal of pharmaceutically active compounds such as carbamazepine, clarithromycin, and diclofenac (present in the range of 1–20 ng/L) from municipal wastewater using activated carbon in granular and powdered form [149].
1.6.3.2. Industrial
Industrial wastewater is often polluted by toxic nonbiodegradable micropollutants like pharmaceutical compounds. Antibiotics are of major concern as they are responsible for developing resistance in bacteria even at low concentrations. Wastewater from industries is a result of either upstream or downstream processes and contains a wide variety of compounds. Clofibrate and etofibrate are pharmaceuticals (lipid regulators) that are hydrolyzed within the body to yield the metabolite CLF acid. CLF acid has been frequently detected in municipal, industrial, and hospital wastewaters with variable concentrations [131]. It has also been detected in rivers, groundwater, or tap water. Discrepancies have been observed depending on the prescription rates in different countries. CLF acid was not observed in Norway since its parent compounds are not prescribed in Norway.
In one of the studies, the presence of pharmaceuticals and metabolites in raw water and purified water was investigated in DWTPs and industrial water purification plants of Japan [150]. The concentration of pharmaceuticals in the source water was mostly below 50 ng/L. Iopamidol was found in high concentration (>1000 ng/L) at most of treatment plants. Another study, the presence of 20 pharmaceutical compounds was investigated in WWTPs of the largest industrial city of Korea [151]. Acetaminophen (7460 ng/L), ibuprofen (2265 ng/L), naproxen (2584 ng/L), caffeine (2349 ng/L), atenolol (7801 ng/L), and lincomycin (8176 ng/L) were detected in high concentration in influent, while atenolol (2772 ng/L) and lincomycin (9089 ng/L) were detected in high concentration in effluent.
Conventional activated sludge has long been a method of choice for pharmaceutical removal from industrial wastewater but nonbiodegradable compounds are not adequately removed by activated sludge process and hence alternative methods like advanced oxidation processes are employed for the efficient removal of such pollutants [152]. These advanced treatment processes are applied at either pre- or poststage of conventional treatments to enhance the micropollutant removal efficiency. Photo-Fenton process has been used as a pretreatment process prior to biological degradation to increase the biodegradability of nalidixic acid [153]. Sequential batch reactors and membrane bioreactors have been studied to enhance the effectiveness activated sludge process for pharmaceutical removal. Study on the effect of redox environment on the degradation of effluent consisting pharmaceuticals and their transformed products in four sequential batch reactors was done by Stadler et al. [154]. Conjugated products of sulfamethoxazole and desvenlafaxine were reported in the influent which retransformed into parent compounds during the reaction cycle mentioning a necessity to track the presence and effects of transformed products on overall degradation. Among the six pharmaceuticals in the influent, atenolol, and trimethoprim showed highest degradation in aerobic reactor, sulfamethoxazole loss was highest in microaerobic sequential batch reactor while phenytoin remains unaffected in all the reactors [154].
1.6.3.3. Drinking water treatment plants
In 20 US DWTPs, 36 pharmaceuticals have been detected in the source and finished water [120]. Among 36 pharmaceuticals, 12 compounds were detected in 50% of source water samples: atrazine (28 ng/L), caffeine (27 ng/L), carbamazepine (<10 ng/L), DEET (<10 ng/L), gemfibrozil (<10 ng/L), ibuprofen (<10 ng/L), iopromide (<10 ng/L), meprobamate (<10 ng/L), naproxen (<10 ng/L), phenytoin (<10 ng/L), sulfamethoxazole (<10 ng/L), and TCEP (13 ng/L). Out of 36 pharmaceuticals, 8 compounds were detected in 50% of finished drinking water sample: atrazine (29 ng/L), caffeine (23 ng/L), carbamazepine (<10 ng/L), DEET (<10 ng/L), ibuprofen (<10 ng/L), iopromide (<10 ng/L), meprobamate (<10 ng/L), and phenytoin (<10 ng/L).
One of the studies investigated the presence of 52 pharmaceuticals in 19 source waters, 18 finished waters, and 15 distribution system water in the United States [120]. Eleven compounds were detected in 50% of source water samples: atenolol (<10 ng/L), atrazine (32 ng/L), naproxen (<10 ng/L), carbamazepine (<10 ng/L), estrone (<10 ng/L), gemfibrozil (<10 ng/L), meprobamate (<10 ng/L), phenytoin (<10 ng/L), sulfamethoxazole (<10 ng/L), trimethoprim (<10 ng/L), and TCEP (120 ng/L). In finished drinking water treatment samples, atrazine (49 ng/L), meprobamate (<10 ng/L), and phenytoin (<10 ng/L). The presence of pharmaceuticals in finished water is due to occurrence of pharmaceuticals in source water.
In other study, pharmaceuticals in drinking water sources (from five major river watersheds) were detected in China [155]. Samples were collected from various sampling sites of rivers and reservoirs. Out of 70 pharmaceuticals, lincomycin, sulfamethoxazole, acetaminophen, and paraxanthine were present in high concentration (44–134 ng/L) while concentration of metoprolol, diphenhydramine, venlafaxine, nalidixic acid, and androstenedione was less than 1 ng/L. Most frequently detected compounds were DEET and carbamazepine with their concentration range of 0.8–10.2 and 0.01–3.5 ng/L, respectively.
In one of the studies, 31 pharmaceuticals were detected in Lisbon’s drinking water supply system [156]. Erythromycin and atenolol were quantified in 8%–15% of the drinking water samples and 40%–56% of the raw water samples, respectively. Mean concentrations of erythromycin and atenolol were 5.69 and 0.21 ng/L, respectively. Caffeine and carbamazepine were detected with high frequency in drinking water (81%–96%) and raw water (69%–84%), respectively. Caffeine and carbamazepine’s mean concentrations were 4.02 and 1.90 ng/L, respectively. In another study, the distribution of 25 pharmaceuticals in 2 DWTPs and a constructed wetland was evaluated [139]. High detection frequencies of 25 pharmaceuticals were observed in 2 DWTPs (100%) and the wetland (>60%) except for florfenicol and sulfapyridine.
1.7. Conclusion and perspectives
The concept of waste management and wastewater treatment started from the 19th century and the increase in the pollution of water bodies (such as river Thames) sought the attention from world which made the development of stringent guidelines for discharge of wastewater into the environment in early 20th century. The emergences of infectious diseases due to the polluted water led to the development of different treatment strategies (septic tank, activated sludge process, and membrane filtration). Thus it was not wrong to say that the progress in waste and wastewater management leads to the discovery and identification of pathogens present in wastewater system. Today, all the processes and technologies are mixed to help us create complex treatment systems which required the means of models and other new methods to handle the full complexity of the systems.
The industrialization, urbanization, demand, and desire of a healthy life resulted in increased production and consumption of pharmaceutical compounds throughout the world. However, the partial metabolism of pharmaceutical in human and animal body, the inappropriate use of drugs (such as for animal growth and agriculture) and improper disposal of medicine resulted in presence of these compound in various environment sectors (soil, water, and air). Various sections of the chapter provide an overview on the presence of these substances in varying aquatic environments. The contribution of point and diffuse sources of pharmaceuticals pollutant in the environment, including hospitals, industries, agriculture runoff, urban runoff, and WWTP indicates the requirement of efficient treatment process for the removal and neutralization of drug residues. The study of detailed and complete transformation pathway of frequently detected pharmaceuticals, namely, ibuprofen, carbamazepine, diclofenac, tetracycline, clarithromycin, acebutolol, ketoprofen, SA, and others will provide an insight chemistry of these compounds which could aid in the development of effective treatment process.
However, the fate and behavior of many pharmaceuticals present in environment and their interaction with other substances and microbes are still unknown. In addition, there is a shortcoming in assessing pathogen concentration in the effluent. The lack of studies in chemical and pathogen characteristics of wastewaters could be because of absence of specific guidelines regarding pharmaceutical residues and pathogen limitation before discharging the wastewater in WWTP or in water bodies. Collective knowledge on the epidemiology community profiles has to be quantified depending on the pathogen type, different effluents from healthcare centers or hospitals, and geographic regions. It is highly important for countries to monitor their concentrations of pharmaceutical residues, pathogen circulation and resistance profile of ARB in HWWs as well as WWTP. This can help in mitigating the risk of contamination in future and maintain a safer public health and environment protection.
Abbreviations
- API
Active pharmaceutical ingredients
- ARB
Antibiotic resistance bacteria
- ARGs
Antibiotic-resistant genes
- BOD
Biochemical oxygen demand
- CBZ
Carbamazepine
- CLF
Clofibric
- COD
Chemical oxygen demand
- CX-IBU
Carboxy-ibuprofen
- DEET
N,N-Diethyl-meta-toluamide
- DiOH-CBZ
10,11-Trans-dihydroxy-carbamazepine
- DWTP
Drinking water treatment plants
- ERY-H2O
dehydrated erythromycin
- FEN
Fenofibric
- HWW
Hospital wastewater
- METR-OH
Hydroxylated derivative of the antibiotic metronidazole
- NSAIDs
Nonsteroidal antiinflammatory drugs
- OH-IBU
Hydroxy-ibuprofen
- PAC
Powdered activated carbon
- PEC
Predicted effect concentration
- ppb
Part per billion
- ppt
Part per trillion
- RBCs
Rotating biological contactors
- RQ
Risk quotient
- SA
Salicylic acid
- SSRIs
Serotonin reuptake inhibitors
- STP
Sewage treatment plant
- TCEP
Tris(2-chloroethyl) phosphate
- TPs
Transformative products
- UV
Ultraviolet
- UWW
Urban wastewater
- WHO
World Health Organization
- WWTPs
Wastewater treatment plants
References
- 1.Shiklomanov I.A. Water in Crisis: A Guide to the World’s Fresh Water Resources. Oxford University Press; New York, NY: 1993. World freshwater resources. [Google Scholar]
- 2.Larsson D.G.J., de Pedro C., Paxeus N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 2007;148:751–755. doi: 10.1016/j.jhazmat.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 3.S. Chattopadhyay, S. Taft, Exposure Pathways to High-Consequence Pathogens in the Wastewater Collection and Treatment Systems, U.S. Environmental Protection Agency, Washington, DC, 2018.
- 4.Araújo C., Torres C., Silva N., Carneiro C., Gonçalves A., Radhouani H. Vancomycin-resistant enterococci from Portuguese wastewater treatment plants. J. Basic Microbiol. 2010;50:605–609. doi: 10.1002/jobm.201000102. [DOI] [PubMed] [Google Scholar]
- 5.Figueira V., Serra E., Manaia C.M. Differential patterns of antimicrobial resistance in population subsets of Escherichia coli isolated from waste-and surface waters. Sci. Total Environ. 2011;409:1017–1023. doi: 10.1016/j.scitotenv.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Sabaté M., Prats G., Moreno E., Ballesté E., Blanch A.R., Andreu A. Virulence and antimicrobial resistance profiles among Escherichia coli strains isolated from human and animal wastewater. Res. Microbiol. 2008;159:288–293. doi: 10.1016/j.resmic.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Tsai C.T., Lai J.S., Lin S.T. Quantification of pathogenic micro-organisms in the sludge from treated hospital wastewater. J. Appl. Microbiol. 1998;85:171–176. doi: 10.1046/j.1365-2672.1998.00491.x. [DOI] [PubMed] [Google Scholar]
- 8.Arvanitidou M., Kanellou K., Constantinides T.C., Katsouyannopoulos V. The occurrence of fungi in hospital and community potable waters. Lett. Appl. Microbiol. 1999;29:81–84. doi: 10.1046/j.1365-2672.1999.00583.x. [DOI] [PubMed] [Google Scholar]
- 9.Auerbach E.A., Seyfried E.E., McMahon K.D. Tetracycline resistance genes in activated sludge wastewater treatment plants. Water Res. 2007;41:1143–1151. doi: 10.1016/j.watres.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 10.Da Silva M.F., Tiago I., Veríssimo A., Boaventura R.A.R., Nunes O.C., Manaia C.M. Antibiotic resistance of enterococci and related bacteria in an urban wastewater treatment plant. FEMS Microbiol. Ecol. 2006;55:322–329. doi: 10.1111/j.1574-6941.2005.00032.x. [DOI] [PubMed] [Google Scholar]
- 11.Sim W.-J., Lee J.-W., Lee E.-S., Shin S.-K., Hwang S.-R., Oh J.-E. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere. 2011;82:179–186. doi: 10.1016/j.chemosphere.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Gusbeth C., Frey W., Volkmann H., Schwartz T., Bluhm H. Pulsed electric field treatment for bacteria reduction and its impact on hospital wastewater. Chemosphere. 2009;75:228–233. doi: 10.1016/j.chemosphere.2008.11.066. [DOI] [PubMed] [Google Scholar]
- 13.Bouki C., Venieri D., Diamadopoulos E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: a review. Ecotoxicol. Environ. Saf. 2013;91:1–9. doi: 10.1016/j.ecoenv.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A., Pal D. Antibiotic resistance and wastewater: correlation, impact and critical human health challenges. J. Environ. Chem. Eng. 2018;6:52–58. [Google Scholar]
- 15.Rizzo L., Manaia C., Merlin C., Schwartz T., Dagot C., Ploy M.C. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci. Total Environ. 2013;447:345–360. doi: 10.1016/j.scitotenv.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Moura A., Pereira C., Henriques I., Correia A. Novel gene cassettes and integrons in antibiotic-resistant bacteria isolated from urban wastewaters. Res. Microbiol. 2012;163:92–100. doi: 10.1016/j.resmic.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Korzeniewska E., Korzeniewska A., Harnisz M. Antibiotic resistant Escherichia coli in hospital and municipal sewage and their emission to the environment. Ecotoxicol. Environ. Saf. 2013;91:96–102. doi: 10.1016/j.ecoenv.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Fent K., Weston A.A., Caminada D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006;76:122–159. doi: 10.1016/j.aquatox.2005.09.009. Find this article online. [DOI] [PubMed] [Google Scholar]
- 19.Khetan S.K., Collins T.J. Human pharmaceuticals in the aquatic environment: a challenge to green chemistry. Chem. Rev. 2007;107:2319–2364. doi: 10.1021/cr020441w. [DOI] [PubMed] [Google Scholar]
- 20.Watkinson A.J., Murby E.J., Costanzo S.D. Removal of antibiotics in conventional and advanced wastewater treatment: implications for environmental discharge and wastewater recycling. Water Res. 2007;41:4164–4176. doi: 10.1016/j.watres.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Liu P., Zhang H., Feng Y., Yang F., Zhang J. Removal of trace antibiotics from wastewater: a systematic study of nanofiltration combined with ozone-based advanced oxidation processes. Chem. Eng. J. 2014;240:211–220. [Google Scholar]
- 22.Su H.-C., Ying G.-G., Tao R., Zhang R.-Q., Zhao J.-L., Liu Y.-S. Class 1 and 2 integrons, sul resistance genes and antibiotic resistance in Escherichia coli isolated from Dongjiang River, South China. Environ. Pollut. 2012;169:42–49. doi: 10.1016/j.envpol.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Water Environment Federation, Design of Municipal Wastewater Treatment Plant, WEF Manual of Practice No. 8, 1992.
- 24.Lofrano G., Brown J. Wastewater management through the ages: a history of mankind. Sci. Total Environ. 2010;408(22):5254–5264. doi: 10.1016/j.scitotenv.2010.07.062. [DOI] [PubMed] [Google Scholar]
- 25.Brown J.A. The early history of wastewater treatment and disinfection. Impact Global Clim. Change. 2005:1–7. [Google Scholar]
- 26.Webster C. The sewers of Mohenjo-Daro. J. Water Pollut. Control Federat. 1962:116–123. [Google Scholar]
- 27.Angelakis A.N., Koutsoyiannis D., Tchobanoglous G. Urban wastewater and stormwater technologies in ancient Greece. Water Res. 2005;39(1):210–220. doi: 10.1016/j.watres.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Angelakis A.N., Savvakis Y., Charalampakis G. Aqueducts during the Minoan era. Water Sci. Technol. Water Supply. 2007;7(1):95–101. [Google Scholar]
- 29.Hopkins J.N. The Cloaca Maxima and the monumental manipulation of water in archaic Rome. Waters Rome. 2007;4:1–15. [Google Scholar]
- 30.Henze M., van Loosdrecht M.C., Ekama G.A., Brdjanovic D. Biological Wastewater Treatment. IWA Publishing; 2008. [Google Scholar]
- 31.Seeger H. The history of German waste water treatment. Euro. Water Manage. 1999;2:51–56. [Google Scholar]
- 32.Aiello A.E., Larson E.L., Sedlak R. Hidden heroes of the health revolution Sanitation and personal hygiene. Am. J. Infect. Control. 2008;36(10):S128–S151. doi: 10.1016/j.ajic.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Tsuzuki Y. Water and Sanitation in Developing Countries, Pollutant Discharge and Water Quality in Urbanisation. Springer; 2014. pp. 69–88. [Google Scholar]
- 34.Mankad A., Tapsuwan S. Review of socio-economic drivers of community acceptance and adoption of decentralised water systems. J. Environ. Manage. 2011;92(3):380–391. doi: 10.1016/j.jenvman.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Vuorinen H.S., Juuti P.S., Katko T.S. History of water and health from ancient civilizations to modern times. Water Sci. Technol. Water Supply. 2007;7(1):49–57. [Google Scholar]
- 36.Cooper P. Decentralised Sanitation and Reuse: Concepts, Systems and Implementation. IWA Publishing; 2001. Historical aspects of wastewater treatment. [Google Scholar]
- 37.Ardern E., Lockett W.T. Experiments on the oxidation of sewage without the aid of filters. J. Soc. Chem. Ind. 1914;33(10):523–539. [Google Scholar]
- 38.Downing, A. L. Painter, HA; and Knowles, G.(1964) Nitrification in the activated sludge process. J. Inst. Sew. Purification, 130.
- 39.C. Smith Jr, The use of ultrafiltration membrane for activated sludge separation, Proceedings of the 24th Annual Purdue Industrial Waste Conference, 1968, pp. 1300–1310.
- 40.Handcock W., Douglas D.C. English Historical Documents, 1874-1914. Psychology Press; 1996. [Google Scholar]
- 41.Csuros M. Microbiological Examination of Water and Wastewater. CRC Press; 2018. [Google Scholar]
- 42.Maier R.M., Pepper I.L., Gerba C.P. Environmental Microbiology. Academic Press; 2009. [Google Scholar]
- 43.Rose J., Masago Y. A toast to our health: our journey toward safe water. Water Sci. Technol. Water Supply. 2007;7(1):41–48. [Google Scholar]
- 44.Laskin A.I., Bennett J.W., Gadd G.M. Advances in Applied Microbiology. Academic Press; 2003. [Google Scholar]
- 45.Brown J.H. The biological approach to bacteriology. J. Bacteriol. 1932;23(1):1. doi: 10.1128/jb.23.1.1-10.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lederberg J. Infectious history. Science. 2000;288(5464):287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- 47.Williams C. Medicinal Plants in Australia, Vol. 4: An Antipodean Apothecary. Rosenberg Publishing; 2013. [Google Scholar]
- 48.Tyndall J. II. The optical deportment of the atmosphere in relation to the phenomena of putrefaction and infection. Philos. Trans. Royal Soc. Lond. 1876;166:27–74. [Google Scholar]
- 49.Nicolaou K.C., Rigol S. A brief history of antibiotics and select advances in their synthesis. J. Antibiot. 2018;71(2):153. doi: 10.1038/ja.2017.62. [DOI] [PubMed] [Google Scholar]
- 50.Gosio B. Richerche batteriologiche e chemiche sulle alterazoni del mais. Riv d’Igiene Sanita Pub. Ann. 1896;7:825–849. [Google Scholar]
- 51.Kitchin J.E.S., Pomeranz M.K., Pak G., Washenik K., Shupack J.L. Rediscovering mycophenolic acid: a review of its mechanism, side effects, and potential uses. J. Am. Acad. Dermatol. 1997;37(3):445–449. doi: 10.1016/s0190-9622(97)70147-6. [DOI] [PubMed] [Google Scholar]
- 52.Jones H.W. Report of a series of cases of syphilis treated by Ehrlich’s arsenobenzol at the Walter Reed General Hospital, District of Columbia. Boston Med. Surg. J. 1911;164(11):381–383. [Google Scholar]
- 53.Lewis K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013;12(5):371. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 54.Zaffiri L., Gardner J., Toledo-Pereyra L.H. History of antibiotics. From salvarsan to cephalosporins. J. Investig. Surgery. 2012;25(2):67–77. doi: 10.3109/08941939.2012.664099. [DOI] [PubMed] [Google Scholar]
- 55.Fleming A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzæ. Br. J. Exp. Pathol. 1929;10(3):226–236. [Google Scholar]
- 56.Grossman C.M. The first use of penicillin in the United States. Ann. Intern. Med. 2009;150(10):737. doi: 10.7326/0003-4819-150-10-200905190-00022. [DOI] [PubMed] [Google Scholar]
- 57.Dubos R.J., Hotchkiss R.D. The production of bactericidal substances by aerobic sporulating bacilli. J. Exp. Med. 1941;73(5):629–640. doi: 10.1084/jem.73.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brem J., Cain R., Cahill S., McDonough M.A., Clifton I.J., Jiménez-Castellanos J.-C. Structural basis of metallo-β-lactamase, serine-β-lactamase and penicillin-binding protein inhibition by cyclic boronates. Nat. Commun. 2016;7:12406. doi: 10.1038/ncomms12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bo G. Giuseppe Brotzu and the discovery of cephalosporins. Clin. Microbiol. Infect. 2000;6:6–8. doi: 10.1111/j.1469-0691.2000.tb02032.x. [DOI] [PubMed] [Google Scholar]
- 60.Kalesse M., Böhm A., Kipper A., Wandelt V. Synthesis of Antibiotics, How to Overcome the Antibiotic Crisis. Springer; 2016. pp. 419–445. [DOI] [PubMed] [Google Scholar]
- 61.Lee L.-H., Chan K.-G., Stach J., Wellington E.M., Goh B.-H. The search for biological active agent (s) from actinobacteria. Front. Microbiol. 2018;9:824. doi: 10.3389/fmicb.2018.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durand G.A., Raoult D., Dubourg G. Antibiotic discovery: history, methods and perspectives. Int. J. Antimicrob. Agents. 2019;53(4):371–382. doi: 10.1016/j.ijantimicag.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 63.Das G., Baek K.-H., Patra J.K. Biofabrication of streptomycin-conjugated calcium phosphate nanoparticles using red ginseng extract and investigation of their antibacterial potential. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0217318. e0217318. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Chopra I., Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001;65(2):232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun C., Xiao X.-Y. Antibacterials. Springer; 2017. Fully synthetic tetracyclines: increasing chemical diversity to combat multidrug-resistant bacterial infections; pp. 55–95. [Google Scholar]
- 66.Stepanek J.J., Lukežič T., Teichert I., Petković H., Bandow J.E. Dual mechanism of action of the atypical tetracycline chelocardin. Biochim. Biophys. Acta. 2016;1864(6):645–654. doi: 10.1016/j.bbapap.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 67.White R.J. The early history of antibiotic discovery: empiricism ruled. In: Dougherty T.J., Pucci M.J., editors. Antibiotic Discovery and Development. Springer US; Boston, MA: 2012. pp. 3–31. [Google Scholar]
- 68.Schlunzen F., Zarivach R., Harms J., Bashan A., Tocilj A., Albrecht R. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001;413(6858):814–821. doi: 10.1038/35101544. [DOI] [PubMed] [Google Scholar]
- 69.Jelić D., Antolović R. From erythromycin to azithromycin and new potential ribosome-binding antimicrobials. Antibiotics. 2016;5(3):29. doi: 10.3390/antibiotics5030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barbour A., Tagg J., Abou-Zied O.K., Philip K. New insights into the mode of action of the lantibiotic salivaricin B. Sci. Rep. 2016;6:31749. doi: 10.1038/srep31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sensi P., Margalith P., Timbal M.T. Rifomycin, a new antibiotic; preliminary report. Farmaco Sci. 1959;14(2):146–147. [PubMed] [Google Scholar]
- 72.Batts D.H., Koleff M., Lipsky B.A., Nicolau D.P., Weigelt J.A. Creation of a Novel Class: The Oxazolidinone Antibiotics. Innova Institute for Medical Education; Tampa: 2004. [Google Scholar]
- 73.Livermore D.M. Linezolid in vitro: mechanism and antibacterial spectrum. J. Antimicrob. Chemother. 2003;51(Suppl. 2):ii9–ii16. doi: 10.1093/jac/dkg249. [DOI] [PubMed] [Google Scholar]
- 74.Falagas M.E., Skalidis T., Vardakas K.Z., Legakis N.J. Activity of cefiderocol (S-649266) against carbapenem-resistant Gram-negative bacteria collected from inpatients in Greek hospitals. J. Antimicrob. Chemother. 2017;72(6):1704–1708. doi: 10.1093/jac/dkx049. [DOI] [PubMed] [Google Scholar]
- 75.Bhullar K., Waglechner N., Pawlowski A., Koteva K., Banks E.D., Johnston M.D. Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034953. e34953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Emmanuel E., Keck G., Blanchard J.-M., Vermande P., Perrodin Y. Toxicological effects of disinfections using sodium hypochlorite on aquatic organisms and its contribution to AOX formation in hospital wastewater. Environ. Int. 2004;30:891–900. doi: 10.1016/j.envint.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Suarez S., Lema J.M., Omil F. Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresour. Technol. 2009;100:2138–2146. doi: 10.1016/j.biortech.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 78.Le Corre K.S., Ort C., Kateley D., Allen B., Escher B.I., Keller J. Consumption-based approach for assessing the contribution of hospitals towards the load of pharmaceutical residues in municipal wastewater. Environ. Int. 2012;45:99–111. doi: 10.1016/j.envint.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Y. Chartier, Safe management of wastes from health-care activities, WHO, 2014.
- 80.S.Y. Chan, Potential environmental hazards of wastewater from hospitals and theirmitigation, Hong Kong University Dissertation, 2005, pp. 0–1.
- 81.Kümmerer K. Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—a review. Chemosphere. 2001;45:957–969. doi: 10.1016/s0045-6535(01)00144-8. [DOI] [PubMed] [Google Scholar]
- 82.Langford K.H., Thomas K.V. Determination of pharmaceutical compounds in hospital effluents and their contribution to wastewater treatment works. Environ. Int. 2009;35:766–770. doi: 10.1016/j.envint.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Verlicchi P., Galletti A., Petrovic M., Barceló D. Hospital effluents as a source of emerging pollutants: an overview of micropollutants and sustainable treatment options. J. Hydrol. 2010;389:416–428. [Google Scholar]
- 84.Vazquez-Roig P., Andreu V., Blasco C., Picó Y. Risk assessment on the presence of pharmaceuticals in sediments, soils and waters of the Pego–Oliva Marshlands (Valencia, eastern Spain) Sci. Total Environ. 2012;440:24–32. doi: 10.1016/j.scitotenv.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 85.Diwan V., Lundborg C.S., Tamhankar A.J. Seasonal and temporal variation in release of antibiotics in hospital wastewater: estimation using continuous and grab sampling. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068715. e68715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carraro E., Bonetta S., Bertino C., Lorenzi E., Bonetta S., Gilli G. Hospital effluents management: chemical, physical, microbiological risks and legislation in different countries. J. Environ. Manage. 2016;168:185–199. doi: 10.1016/j.jenvman.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 87.Boillot C., Bazin C., Tissot-Guerraz F., Droguet J., Perraud M., Cetre J.C. Daily physicochemical, microbiological and ecotoxicological fluctuations of a hospital effluent according to technical and care activities. Sci. Total Environ. 2008;403:113–129. doi: 10.1016/j.scitotenv.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 88.Escher B.I., Baumgartner R., Koller M., Treyer K., Lienert J., McArdell C.S. Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res. 2011;45:75–92. doi: 10.1016/j.watres.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 89.Kovalova L., Siegrist H., Von Gunten U., Eugster J., Hagenbuch M., Wittmer A. Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV. Environ. Sci. Technol. 2013;47:7899–7908. doi: 10.1021/es400708w. [DOI] [PubMed] [Google Scholar]
- 90.Bréchet C., Plantin J., Sauget M., Thouverez M., Talon D., Cholley P. Wastewater treatment plants release large amounts of extended-spectrum β-lactamase–producing Escherichia coli into the environment. Clin. Infect. Dis. 2014;58(12):1658–1665. doi: 10.1093/cid/ciu190. [DOI] [PubMed] [Google Scholar]
- 91.Aukidy M. Al, Verlicchi P., Voulvoulis N. A framework for the assessment of the environmental risk posed by pharmaceuticals originating from hospital effluents. Sci. Total Environ. 2014;493:54–64. doi: 10.1016/j.scitotenv.2014.05.128. [DOI] [PubMed] [Google Scholar]
- 92.US EPA . National Recommended Water Quality Criteria. United States Environmental Protection Agency’, Office of Water, Office of Science and Technology; 2009. [Google Scholar]
- 93.Orias F., Perrodin Y. Characterisation of the ecotoxicity of hospital effluents: a review. Sci. Total Environ. 2013;454:250–276. doi: 10.1016/j.scitotenv.2013.02.064. [DOI] [PubMed] [Google Scholar]
- 94.Rodríguez J.C. BioChroma—a new and patented technology for processing radioactive wastewater from nuclear medicine therapy facilities in hospitals and clinics. World J. Nucl. Med. 2012;11:12. doi: 10.4103/1450-1147.98735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tavakoli M.B. Radioactive discharge from patients with thyroid cancer under 131I treatment and its safe disposal to the public sewer system. Wspólczesna Onkologia. 2005;9:38. [Google Scholar]
- 96.Stackelberg P.E., Furlong E.T., Meyer M.T., Zaugg S.D., Henderson A.K., Reissman D.B. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci. Total Environ. 2004;329:99–113. doi: 10.1016/j.scitotenv.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 97.Daughton, C.G., and T.L. Jones-Lepp. Pharmaceuticals and Personal Care Products in the Environment. American Chemical Society, 2001.
- 98.Vane J.R., Botting R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998;47:78–87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- 99.Cleuvers M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004;59:309–315. doi: 10.1016/S0147-6513(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 100.Hoeger B., Köllner B., Dietrich D.R., Hitzfeld B. Water-borne diclofenac affects kidney and gill integrity and selected immune parameters in brown trout (Salmo trutta f. fario) Aquat. Toxicol. 2005;75:53–64. doi: 10.1016/j.aquatox.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 101.Risebrough R. Conservation biology: fatal medicine for vultures. Nature. 2004;427:596. doi: 10.1038/nature02365. [DOI] [PubMed] [Google Scholar]
- 102.Kim Y., Choi K., Jung J., Park S., Kim P.-G., Park J. Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int. 2007;33:370–375. doi: 10.1016/j.envint.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 103.Escher B.I., Bramaz N., Maurer M., Richter M., Sutter D., von Känel C. Screening test battery for pharmaceuticals in urine and wastewater. Environ. Toxicol. Chem. Int. J. 2005;24:750–758. doi: 10.1897/04-091r.1. [DOI] [PubMed] [Google Scholar]
- 104.Huang C.-H., Renew J.E., Smeby K.L., Pinkston K., Sedlak D.L. Assessment of potential antibiotic contaminants in water and preliminary occurrence analysis. J. Contemp. Water Res. Educ. 2011;120:4. [Google Scholar]
- 105.Kümmerer K. Resistance in the environment. J. Antimicrob. Chemother. 2004;54:311–320. doi: 10.1093/jac/dkh325. [DOI] [PubMed] [Google Scholar]
- 106.Watkinson A.J., Murby E.J., Kolpin D.W., Costanzo S.D. The occurrence of antibiotics in an urban watershed: from wastewater to drinking water. Sci. Total Environ. 2009;407:2711–2723. doi: 10.1016/j.scitotenv.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 107.Kolpin D.W., Furlong E.T., Meyer M.T., Thurman E.M., Zaugg S.D., Barber L.B. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999−2000: a national reconnaissance. Environ. Sci. Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- 108.Andreozzi R., Caprio V., Ciniglia C., De Champdoré M., Lo Giudice R., Marotta R. Antibiotics in the environment: occurrence in Italian STPs, fate, and preliminary assessment on algal toxicity of amoxicillin. Environ. Sci. Technol. 2004;38:6832–6838. doi: 10.1021/es049509a. [DOI] [PubMed] [Google Scholar]
- 109.Pomati F., Netting A.G., Calamari D., Neilan B.A. Effects of erythromycin, tetracycline and ibuprofen on the growth of Synechocystis sp. and Lemna minor. Aquat. Toxicol. 2004;67:387–396. doi: 10.1016/j.aquatox.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 110.Brain R.A., Johnson D.J., Richards S.M., Sanderson H., Sibley P.K., Solomon K.R. Effects of 25 pharmaceutical compounds to Lemna gibba using a seven-day static-renewal test. Environ. Toxicol. Chem. Int. J. 2004;23:371–382. doi: 10.1897/02-576. [DOI] [PubMed] [Google Scholar]
- 111.Miao X.-S., Yang J.-J., Metcalfe C.D. Carbamazepine and its metabolites in wastewater and in biosolids in a municipal wastewater treatment plant. Environ. Sci. Technol. 2005;39:7469–7475. doi: 10.1021/es050261e. [DOI] [PubMed] [Google Scholar]
- 112.Thacker P.D. Pharmaceutical data elude researchers. Environ. Sci. Technol. 2005;39:193A. [PubMed] [Google Scholar]
- 113.Zuccato E., Calamari D., Natangelo M., Fanelli R. Presence of therapeutic drugs in the environment. Lancet. 2000;355:1789–1790. doi: 10.1016/S0140-6736(00)02270-4. [DOI] [PubMed] [Google Scholar]
- 114.Nunes B., Carvalho F., Guilhermino L. Acute toxicity of widely used pharmaceuticals in aquatic species: Gambusia holbrooki, Artemia parthenogenetica and Tetraselmis chuii. Ecotoxicol. Environ. Saf. 2005;61:413–419. doi: 10.1016/j.ecoenv.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 115.Bach T.J., Lichtenthaler H.K. Inhibition by mevinolin of plant growth, sterol formation and pigment accumulation. Physiol. Plantarum. 1983;59:50–60. [Google Scholar]
- 116.Brooks B.W., Chambliss C.K., Stanley J.K., Ramirez A., Banks K.E., Johnson R.D. Determination of select antidepressants in fish from an effluent-dominated stream. Environ. Toxicol. Chem. Int. J. 2005;24:464–469. doi: 10.1897/04-081r.1. [DOI] [PubMed] [Google Scholar]
- 117.Black M.C., Rogers E.D., Henry T.B. Endocrine Effects of Selective Serotonin Reuptake Inhibitors (SSRIs) on Aquatic Organisms. Environmental Protection Agency; Washington, DC: 2005. [Google Scholar]
- 118.E.C.f.D. Prevention, Control, Suetens C., Hopkins S., Kolman J., Högberg L.D. Point Prevalence Survey of Healthcare-associated Infections and Antimicrobial Use in European Acute Care Hospitals: 2011–2012. Publications Office of the European Union; 2013. [Google Scholar]
- 119.Heberer T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. 2002;266(3-4):175–189. [PubMed] [Google Scholar]
- 120.Snyder S.A., Benotti M.J. Endocrine disruptors and pharmaceuticals: implications for water sustainability. Water Sci. Technol. 2010;61(1):145–154. doi: 10.2166/wst.2010.791. [DOI] [PubMed] [Google Scholar]
- 121.Padhye L.P., Yao H., Kung’u F.T., Huang C.-H. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res. 2014;51:266–276. doi: 10.1016/j.watres.2013.10.070. [DOI] [PubMed] [Google Scholar]
- 122.Boleda M.R., Galceran M.T., Ventura F. Behavior of pharmaceuticals and drugs of abuse in a drinking water treatment plant (DWTP) using combined conventional and ultrafiltration and reverse osmosis (UF/RO) treatments. Environ. Pollut. 2011;159(6):1584–1591. doi: 10.1016/j.envpol.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 123.Singer A.C., Järhult J.D., Grabic R., Khan G.A., Lindberg R.H., Fedorova G. Intra-and inter-pandemic variations of antiviral, antibiotics and decongestants in wastewater treatment plants and receiving rivers. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108621. e108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tran N.H., Chen H., Reinhard M., Mao F., Gin K.Y.-H. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016;104:461–472. doi: 10.1016/j.watres.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 125.Kosma C.I., Lambropoulou D.A., Albanis T.A. Investigation of PPCPs in wastewater treatment plants in Greece: occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014;466:421–438. doi: 10.1016/j.scitotenv.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 126.Secondes M.F.N., Naddeo V., Belgiorno V., Ballesteros F., Jr Removal of emerging contaminants by simultaneous application of membrane ultrafiltration, activated carbon adsorption, and ultrasound irradiation. J. Hazard. Mater. 2014;264:342–349. doi: 10.1016/j.jhazmat.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 127.Leung H.W., Minh T., Murphy M.B., Lam J.C., So M.K., Martin M. Distribution, fate and risk assessment of antibiotics in sewage treatment plants in Hong Kong, South China. Environ. Int. 2012;42:1–9. doi: 10.1016/j.envint.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 128.Carmona E., Andreu V., Picó Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: from waste to drinking water. Sci. Total Environ. 2014;484:53–63. doi: 10.1016/j.scitotenv.2014.02.085. [DOI] [PubMed] [Google Scholar]
- 129.Santos L.H., Gros M., Rodriguez-Mozaz S., Delerue-Matos C., Pena A., Barceló D. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci. Total Environ. 2013;461:302–316. doi: 10.1016/j.scitotenv.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 130.Terzić S., Senta I., Ahel M., Gros M., Petrović M., Barcelo D. Occurrence and fate of emerging wastewater contaminants in Western Balkan Region. Sci. Total Environ. 2008;399(1-3):66–77. doi: 10.1016/j.scitotenv.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 131.Evgenidou E.N., Konstantinou I.K., Lambropoulou D.A. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: a review. Sci. Total Environ. 2015;505:905–926. doi: 10.1016/j.scitotenv.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 132.Yang Y., Ok Y.S., Kim K.-H., Kwon E.E., Tsang Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci. Total Environ. 2017;596:303–320. doi: 10.1016/j.scitotenv.2017.04.102. [DOI] [PubMed] [Google Scholar]
- 133.Milić N., Milanović M., Letić N.G., Sekulić M.T., Radonić J., Mihajlović I. Occurrence of antibiotics as emerging contaminant substances in aquatic environment. Int. J. Environ. Health Res. 2013;23(4):296–310. doi: 10.1080/09603123.2012.733934. [DOI] [PubMed] [Google Scholar]
- 134.Wang Z., Zhang X.-H., Huang Y., Wang H. Comprehensive evaluation of pharmaceuticals and personal care products (PPCPs) in typical highly urbanized regions across China. Environ. Pollut. 2015;204:223–232. doi: 10.1016/j.envpol.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 135.Wood T.P., Duvenage C.S., Rohwer E. The occurrence of anti-retroviral compounds used for HIV treatment in South African surface water. Environ. Pollut. 2015;199:235–243. doi: 10.1016/j.envpol.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 136.Zhang R., Tang J., Li J., Cheng Z., Chaemfa C., Liu D. Occurrence and risks of antibiotics in the coastal aquatic environment of the Yellow Sea, North China. Sci. Total Environ. 2013;450:197–204. doi: 10.1016/j.scitotenv.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 137.González-Pleiter M., Gonzalo S., Rodea-Palomares I., Leganés F., Rosal R., Boltes K. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: implications for environmental risk assessment. Water Res. 2013;47(6):2050–2064. doi: 10.1016/j.watres.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 138.Qiang Z., Macauley J.J., Mormile M.R., Surampalli R., Adams C.D. Treatment of antibiotics and antibiotic resistant bacteria in swine wastewater with free chlorine. J. Agric. Food Chem. 2006;54(21):8144–8154. doi: 10.1021/jf060779h. [DOI] [PubMed] [Google Scholar]
- 139.Hu X.-L., Bao Y.-F., Hu J.-J., Liu Y.-Y., Yin D.-Q. Occurrence of 25 pharmaceuticals in Taihu Lake and their removal from two urban drinking water treatment plants and a constructed wetland. Environ. Sci. Pollut. Res. 2017;24(17):14889–14902. doi: 10.1007/s11356-017-8830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lin Y.-C., Lai W.W.-P., Tung H.-H., Lin A.Y.-C. Occurrence of pharmaceuticals, hormones, and perfluorinated compounds in groundwater in Taiwan. Environ. Monitor. Assess. 2015;187(5):256. doi: 10.1007/s10661-015-4497-3. [DOI] [PubMed] [Google Scholar]
- 141.Vieno N., Tuhkanen T., Kronberg L. Elimination of pharmaceuticals in sewage treatment plants in Finland. Water Res. 2007;41(5):1001–1012. doi: 10.1016/j.watres.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 142.Kümmerer K. Antibiotics in the aquatic environment—a review. Part II. Chemosphere. 2009;75(4):435–441. doi: 10.1016/j.chemosphere.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 143.Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. U.S.A. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vymazal J., Březinová T., Koželuh M. Occurrence and removal of estrogens, progesterone and testosterone in three constructed wetlands treating municipal sewage in the Czech Republic. Sci. Total Environ. 2015;536:625–631. doi: 10.1016/j.scitotenv.2015.07.077. [DOI] [PubMed] [Google Scholar]
- 145.Shraim A., Diab A., Alsuhaimi A., Niazy E., Metwally M., Amad M. Analysis of some pharmaceuticals in municipal wastewater of Almadinah Almunawarah. Arab. J. Chem. 2017;10:S719–S729. [Google Scholar]
- 146.Kermia A.E.B., Fouial-Djebbar D., Trari M. Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants (WWTPs) discharging in the coastal environment of Algiers. Comp. Rend. Chim. 2016;19(8):963–970. [Google Scholar]
- 147.Saunders A.M., Albertsen M., Vollertsen J., Nielsen P.H. The activated sludge ecosystem contains a core community of abundant organisms. ISME J. 2016;10(1):11. doi: 10.1038/ismej.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bueno M.M., Gomez M., Herrera S., Hernando M., Agüera A., Fernández-Alba A. Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: two years pilot survey monitoring. Environ. Pollut. 2012;164:267–273. doi: 10.1016/j.envpol.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 149.Kårelid V., Larsson G., Björlenius B. Pilot-scale removal of pharmaceuticals in municipal wastewater: comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. J. Environ. Manage. 2017;193:491–502. doi: 10.1016/j.jenvman.2017.02.042. [DOI] [PubMed] [Google Scholar]
- 150.Simazaki D., Kubota R., Suzuki T., Akiba M., Nishimura T., Kunikane S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015;76:187–200. doi: 10.1016/j.watres.2015.02.059. [DOI] [PubMed] [Google Scholar]
- 151.Behera S.K., Kim H.W., Oh J.-E., Park H.-S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011;409(20):4351–4360. doi: 10.1016/j.scitotenv.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 152.Comninellis C., Kapalka A., Malato S., Parsons S.A., Poulios I., Mantzavinos D. Advanced oxidation processes for water treatment: advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008;83(6):769–776. [Google Scholar]
- 153.Sirtori C., Zapata A., Oller I., Gernjak W., Agüera A., Malato S. Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment. Water Res. 2009;43(3):661–668. doi: 10.1016/j.watres.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 154.Stadler L.B., Su L., Moline C.J., Ernstoff A.S., Aga D.S., Love N.G. Effect of redox conditions on pharmaceutical loss during biological wastewater treatment using sequencing batch reactors. J. Hazard. Mater. 2015;282:106–115. doi: 10.1016/j.jhazmat.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 155.Sun J., Luo Q., Wang D., Wang Z. Occurrences of pharmaceuticals in drinking water sources of major river watersheds, China. Ecotoxicol. Environ. Saf. 2015;117:132–140. doi: 10.1016/j.ecoenv.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 156.de Jesus Gaffney V., Almeida C.M., Rodrigues A., Ferreira E., Benoliel M.J., Cardoso V.V. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res. 2015;72:199–208. doi: 10.1016/j.watres.2014.10.027. [DOI] [PubMed] [Google Scholar]


