Abstract
Rationale & Objective
Quantification of residual native kidney function is rarely performed in patients receiving hemodialysis. Methods of estimating residual kidney urea clearance that use commonly available laboratory and clinical data, with or without urine volume information, may be useful tools.
Study Design
Retrospective, predictive modeling and model validation.
Setting & Participants
Initial timed urine collections in 604 incident in-center hemodialysis patients on thrice-weekly treatments from a single academic center in which residual kidney urea clearance is measured in usual care.
Predictors
Models using a combination of serum creatinine and urea levels, age, weight, height, sex, race, fluid weight gains, and with and without 24-hour urine volume.
Outcomes
Residual kidney urea clearance.
Analytic Approach
Generalized linear model was used for model development for residual kidney urea clearance using the first urine collection in 604 patients, as both a continuous and binary outcome (for >2.5 mL/min). Model validation was done by bootstrap resampling of the development cohort and with 1,093 follow-up measurements.
Results
Urine volume alone was the strongest predictor of residual kidney urea clearance. The model that included 24-hour urine volume with common clinical data had high diagnostic accuracy for residual kidney urea clearance > 2.5 mL/min (area under the curve, 0.91 in both development and bootstrap validation) and R2 of 0.56 with outcome as a continuous residual kidney urea clearance value. Our model that did not use urine volume performed less well (eg, area under the curve, 0.75). Analyses of follow-up urine collections in these same participants yielded comparable or improved performance.
Limitations
Data were retrospective from a single center, no external validation, not validated in 2- or 4-times-weekly hemodialysis patients.
Conclusions
Estimation equations for residual kidney urea clearance that use commonly available data in dialysis clinics, with and without urine volume, may be useful tools for evaluation of hemodialysis patients who still have residual kidney function for individualization of dialysis prescriptions.
Index Words: Estimation equation, hemodialysis, prediction model, residual kidney function, residual renal function, urea clearance, ESRD, dialysis
Editorial, p. 332
Residual kidney function (RKF) in long-term dialysis patients is associated with lower morbidity and mortality in both peritoneal dialysis and hemodialysis (HD) patients.1, 2, 3, 4 Improved fluid management, anemia control, bone mineral metabolism parameters, and quality of life are a few of the benefits of RKF noted in observational studies.2,5,6 In patients receiving peritoneal dialysis, RKF is routinely measured and taken into account in the dialysis prescription. In stark contrast, HD patients rarely have RKF quantified or incorporated into the final dialysis prescription.
Despite the infrequency of RKF measurement in those receiving HD, a large fraction of incident HD patients have substantial residual native kidney function.7 In addition, the rate of RKF decline in HD may be slow in many cases, with patients garnering the clinical benefits of RKF for longer than what was historically expected.8
The ideal method of quantifying RKF requires a timed urine collection followed by blood tests with appropriate computations to take into account the duration of collected urine and timing of the last HD treatment.9,10 We speculate that the logistical challenges of obtaining, analyzing, and reporting timed urine collections are some of the reasons why RKF is not used more often in routine clinical care of HD patients. Acknowledging the difficulty of quantifying RKF through timed urine collections in patients receiving HD, investigators have examined the use of nontraditional serum markers, such as cystatin C and β2-microglobulin levels, in equations that estimate RKF as alternatives to a complete urine study.11,12
Our goal was to develop and evaluate the performance of RKF estimation equations using commonly available laboratory and clinical parameters, with and without 24-hour urine volume information, in clinical dialysis settings that do not require new tests or extensive additional dialysis clinic labor. We specifically aimed at modeling residual native kidney urea clearance (KRU) in HD patients undergoing thrice-weekly HD because this information has relevant clinical use and can be readily added to dialyzer clearance, providing a total weekly clearance.9 Our model development used a large database of 24-hour urine collections of in-center HD patients from a single center that has been quantifying KRU as part of standard care for more than 25 years. We validated the models developed internally.
Methods
Study Cohort: Development and Validation
In this retrospective study, we analyzed residual native KRU in incident adult patients with end-stage kidney disease undergoing thrice-weekly in-center HD in 5 dialysis clinics affiliated with an academic nephrology practice. It is customary at our center to measure KRU in all HD patients who still make urine, repeating the timed urine collection every 3 months (or sooner if ordered by the nephrologist) until 24-hour urine volume is <100 mL or anuria is self-reported by the patient. In this study, we used the deidentified urine collection database spanning 14 years that was used in a former study (consent not required due to retrospective deidentified data) approved by the University of California, Davis Institutional Review Board (protocol number 806282-1) and an administrative review of the dialysis provider (Dialysis Clinic, Inc) on evaluation of KRU in incident HD patients and theoretical twice-weekly HD.7
For this study, KRU was determined by multiplying urine volume (Vol) in milliliters with the concentration of urine urea (Uurea) in milligrams per deciliter, divided by an adjusted plasma urea (Purea) in milligrams per deciliter, which was the pre-HD plasma urea collected on the day that the urine collection terminated. The adjustment factor of 0.92 or 0.98 used was based on whether the 24-hour urine volume was collected on the last day of the short or long interdialytic period, respectively. These conversion factors have been determined by Daugirdas10 in a recent study. To convert KRU into the more familiar units of milliliters per minute, a factor of 1,440 was used:
Given the retrospective nature of this study, we cannot attest to the accuracy of these measures; however, the correlation between calculated and formal kinetically modeled KRU in a subset of 112 of the patients was excellent, with correlation coefficient of 0.998.7 The other collected clinical variables were from chart queries.
After excluding outliers or implausible values (KRU > 15 mL/min, intradialytic weight gain ≥ 20 kg, and urine volume ≥ 10 L), we took the first 24-hour urine collections in a cohort of nonoliguric incident patients with end-stage kidney disease receiving thrice-weekly HD as the basis of our model development, as well as bootstrap validation. In addition, we used 1,093 follow-up measurements obtained from the same patient cohort as additional validation in an exploratory manner.
Statistical Analyses
Patient characteristics were summarized as mean and standard deviation for continuous variables, median and interquartile range added when useful, and frequency and percentage for categorical variables. We developed 3 models that used commonly available demographic and clinical information: (1) model that used 24-hour urine volume only; (2) model that used urine volume with additional demographic, laboratory, and clinical factors; and (3) model that did not include urine volume and used only demographic, laboratory, and clinical factors. We selected candidate predictors guided by scientific literature and reached the final model based on statistical significance. Generalized linear models were used for KRU as a continuous outcome, as well as a dichotomized outcome for KRU > 2.5 mL/min (eg, logistic regression). We used scatter plots along with locally estimated scatterplot smoothing fit before model development to determine the need for transformation of variables.
In the model for the mean of KRU, we used a generalized linear model with gamma distribution and natural log link. Among predictors, urine volume was transformed into a log scale and all other variables were used in their original untransformed scale. Of note, urine volume and KRU had positive values. We explored serum urea level centered by mean value and squared to address nonlinearity and collinearity. The multifactor models were derived based on the backward elimination principle on multiple regression, together with clinical knowledge; thus, manual and automatic searches were used jointly. With the goal of developing relatively simple, transparent, and easily reproducible and implementable models, we focused on main effects, not interaction or high-order nonlinearity (such as cubic polynomials or splines).
For model evaluation/validation, we used the coefficient of determination (denoted by R2), root mean squared error (RMSE), and calibration (a plot of observed vs predicted value, and average difference and interquartile range) for the continuous outcome.13 For the binary outcome of KRU > 2.5 mL/min, the area under the receiver operating characteristic curve (AUC, or C statistic) and calibration plot (eg, observed vs predicted using deciles) were used. We used 100 bootstrap resamples for internal validation to address optimism/overfit issue,14 partly because the total sample size was not large enough for split sample. We computed 95% bootstrap confidence intervals for R2, RMSE, and AUC. All analyses were performed using SAS, version 9.4 (SAS Institute, Inc).
Results
A total of 604 unique patients who were eligible and screened from 1,067 incident HD patients were included in our analyses (Fig 1). Details of patient characteristics in the cohort are outlined in Table 1.
Figure 1.
Flow chart of incident patients. Incomplete urine collection includes missing urine volume or missing urine urea concentration. Patients who self-reported no daily urine output or had a collected 24-hour urine volume < 100 mL were considered to have no significant residual kidney function. Abbreviation: HD, hemodialysis.
Table 1.
Characteristics of Patients
| Age, y | 59.2 (15.0) |
| Women | 228 (37.8%) |
| Race | |
| Asian | 73 (12.1%) |
| Black/ African American | 152 (25.2%) |
| Other | 51 (8.4%) |
| White | 165 (27.3%) |
| White-Hispanic | 100 (16.6%) |
| Unknown | 63 (10.4%) |
| Weight, kg | 78.7 (22.7) |
| Height, cm | 168.5 (11.4) |
| Body mass index, kg/m2 | 27.6 (7.0) |
| BSA, m2 | 1.9 (0.3) |
| Intradialytic weight gain, kg | 2.4 (1.3) |
| Diabetes mellitus; yes | 288 (47.7%) |
| Vintage, d | 153 (179) [median, 61; IQR, 30-213] |
| Serum creatinine, mg/dL | 6.8 (2.8) |
| Pre-HD serum urea, mg/dL | 56.7 (19.6) |
| Urine volume, mL | 943 (611) |
| Phosphorus, mg/dL | 5.3 (1.6) |
| KRU, mL/min | 3.6 (2.6) |
| KRU ≥ 2.5 mL/min | 354 (58.6%) |
| KRU with BSA adjustment,a mL/min | 1.9 (1.4) |
Note: n = 604; Values for categorical variables are given as number (percent); values for continuous variables are given as mean (standard deviation) unless otherwise noted. Two missing observations in phosphorus and 1 missing observation in height and variables using height.
Abbreviations: BSA, body surface area; HD, hemodialysis; IQR, interquartile range; KRU, kidney urea clearance.
KRU/BSA, where BSA = 0.007184 × (weight in kg)0.425 × (height in cm)0.725.
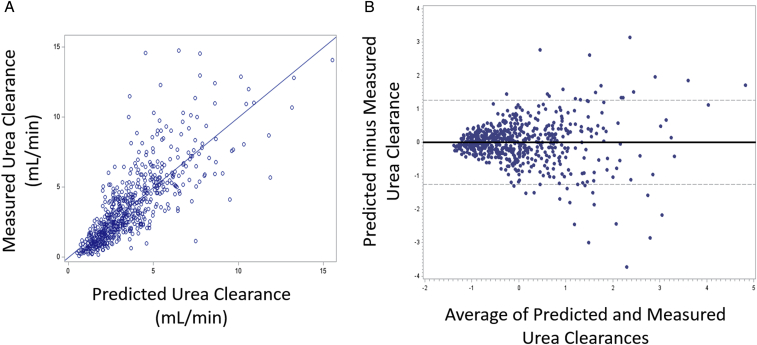
Models for KRU as either a continuous outcome or a binary outcome >2.5 mL/min are shown in Table 2. Model 1 used only 24-hour urine volume as the predictor variable. Model 2, the most comprehensive of our 3 models, used urine volume with 5 and 4 additional predictors for continuous and binary KRU, respectively. To determine how well a non–urine-based model could predict KRU, our third model did not include urine volume and used commonly available clinical and laboratory factors. A correlation plot of measured versus predicted KRU and Bland-Altman plot with no major systemic bias are shown in Figure 2.
Table 2.
Models and Equations for Continuous KRU and Binary Outcome of KRU ≥ 2.5 mL/min
| Continuous KRU | |
|---|---|
| Model and Predictors | Outcome = Mean of KRU |
|
Model 1 Urine volume, mL |
exp(−4.2437 + log(Urin_vol) × 0.8133) |
|
Model 2 Urine volume, mL Weight, kg Race (black = 1, others = 0) Sex (female = 1, male = 0) Serum creatinine, mg/dL Serum urea, mg/dL |
exp[−3.4640 + log(Urin_vol) × 0.7726 + (weight) × 0.0036 + (race) × 0.1483 + (gender) × (−0.1043) + (serum_Cr) × (−0.1055) + (serum_urea) × (−0.0022)] |
|
Model 3 BSA, m2 Intradialytic weight gain, kg Age, y Race (black = 1, others = 0) Sex (female = 1, male = 0) Serum creatinine, mg/dL |
exp[1.6275 + (BSA) × 0.7013 + (intradialytic weight gain) × (−0.0944) + (age) × (−0.0079) + (race) × 0.1176 + (gender) × (−0.2114) + (serum_Cr) × (−0.1471)] |
| Binary Outcome of KRU ≥ 2.5 mL/min | |
|---|---|
| Model and Predictors | Outcome = Probability of KRU ≥ 2.5 mL/min |
|
Model 1 Urine volume, mL |
1/[1 + exp(−xβ)] where xβ = −17.1422 + log(Urin_vol) × 2.6575 |
|
Model 2 Urine volume, mL Weight, kg Race (black = 1, others = 0) Sex (female = 1, male = 0) Serum creatinine, mg/dL |
1/[1 + exp(−xβ)] where xβ = −19.5374 + log(Urin_vol) × 3.2907 + (weight) × 0.0195 + (race) × 1.0061 + (gender) × (−0.4479) + (serum_Cr) × (−0.4850) |
|
Model 3 BSA, m2 Intradialytic weight gain, kg Age, y Race (black = 1, others = 0) Sex (female = 1, male = 0) Serum creatinine, mg/dL |
1/[1 + exp(−xβ)] where xβ = 1.0751 + (BSA) × 1.9848 + (intradialytic weight gain) × (−0.3035) + (age) × (−0.0200) + (race) × 0.5117 + (gender) × (−0.5529) + (serum_Cr) × (-0.3536) |
Note: BSA = 0.007184 × (weight in kg)0.425 × (height in cm)0.725. Log is natural logarithm.
Abbreviations: BSA, body surface area; Cr, creatinine; KRU, kidney urea clearance.
Figure 2.
Model 2 for residual kidney urea clearance as a continuous outcome: (A) observed versus predicted and (B) standardized Bland-Altman plot.
Bootstrap-based model validation for KRU as a continuous value, using the first 24-hour urine collected from 604 incident patients with end-stage kidney disease, demonstrated R2 values of 0.48, 0.56, and 0.26 for models 1, 2, and 3, respectively. When the outcome was binary for KRU > 2.5 mL/min, AUCs were 0.86, 0.91, and 0.75 for the 3 models, respectively (Table 3). Other criteria (RMSE and calibration) were qualitatively similar. Mean squared error measures the average of the squares of the errors—that is, the average squared difference between the estimated values and what is estimated. Square root of mean squared error, RMSE, has the same unit as the outcome and a lower RMSE suggests a better model fit. Model 2, the most complex with inclusion of urine volume, performed best. The receiver operating characteristic curves of all 3 models are shown in Figure 3A, and the calibration plot of model 2 is noted in Figure 3B. Finally, exploratory validation of 1,093 follow-up urine collections in these 604 patients also provided consistent findings; for example, models 1, 2, and 3 yielded R2 of 0.49, 0.72, and 0.36; RMSE of 0.50, 0.44, and 0.58; and AUC of 0.88, 0.93, and 0.78, respectively.
Table 3.
Performance of the Equations in Development Data Set and Bootstrap Validation
| Model | For Continuous KRU | For Binary KRU |
|---|---|---|
|
Development | ||
| R2/RMSE/mean of O-P (IQR) | AUC | |
| Model 1 | 0.47/0.59/−0.03 (−1.06, 0.70) | 0.86 |
| Model 2 | 0.55/0.55/−0.03 (−0.79, 0.61) | 0.91 |
| Model 3 | 0.26/0.71/−0.01 (−1.43, 1.02) | 0.76 |
| Validation (average from 100 bootstrap resamples) | ||
|---|---|---|
| R2/RMSE (95% CIa) | AUC (95% CIa) | |
| Model 1 | 0.48 (0.41-0.52)/0.59 (0.55-0.63) | 0.86 (0.82-0.89) |
| Model 2 | 0.56 (0.51-0.60)/0.54 (0.51-0.58) | 0.91 (0.89-0.94) |
| Model 3 | 0.26 (0.21-0.32)/0.70 (0.66-0.75) | 0.75 (0.72-0.79) |
Note: Higher AUC implies better discrimination (0.5: random vs 1: perfect). We computed R2 and RMSE from a linear model with observed as outcome and predicted as regressor.
Abbreviations: AUC, area under the receiver operating characteristic curve; CI, confidence interval; IQR, interquartile range; KRU, kidney urea clearance; O, observed; P, predicted; RMSE, root mean squared error.
CI was estimated using bootstrap percentile method.
Figure 3.
Model performance for residual kidney urea clearance as a binary outcome: (A) receiver operator characteristic curves for models 1 to 3 and (B) calibration plot using deciles for best-performing model 2.
Discussion
In this study, we developed formulas to estimate KRU in patients on thrice-weekly in-center HD who still have native kidney function using a relatively large data set of 24-hour urine collections. We developed 2 models that used 24-hour collection volume because in our view timed urine collections in HD patients are feasible. We also developed a model that did not incorporate urine volume (often not easily available in some practices), using only readily available clinical and pre-HD laboratory data that are part of usual patient care.
The model that used only the 24-hour urine volume performed well with a high diagnostic accuracy for KRU > 2.5 mL/min (AUC, 0.86 in the average of 100 bootstrap resampling validation analysis). Our most inclusive model, which used 24-hour urine volume along with several clinical and common laboratory factors, had the highest performance of all in both estimating KRU as a continuous outcome and for discriminating for KRU > 2.5 mL/min (AUC, 0.91). We also wanted to explore an estimation equation that did not require urine collection and thus we developed our third model that used only clinical and pre-HD laboratory data. Diagnostic accuracy of our third model for KRU > 2.5 mL/min was the lowest of the 3 models but still provided reasonable discrimination with AUC of 0.75 on bootstrap validation. The latter model’s performance was not inferior to those of previously published estimations of KRU or residual glomerular filtration rate in HD patients using only serum creatinine and/or urea level, with reported AUCs ranging from 0.43 to 0.74.11,12,15
RKF in patients receiving HD, even in seemingly trivial amounts, is associated with important clinical benefits, including improved nutrition, reduced erythropoietin requirements, better potassium clearance, and improved quality of life. Preservation of RKF in dialysis patients, including those receiving HD, is considered a reasonable aim in managing dialysis patients.1,2,16, 17, 18 However, < 5% of HD patients in the United States ever have formal RKF evaluation.3 We suspect that the perceived logistical difficulties of timed urine collections, urine laboratory testing, and computation in a usable format are some of the reasons why this important part of the prescribing dialysis prescription is mostly ignored in HD patients.
Currently, the recommended method of obtaining RKF information requires a timed urine collection.19 Typically, the process of the 24- or 48-hour urine collection, urine volume measurement in the clinic, and laboratory sending out of the urine aliquot and pre-HD blood tests with the required calculation can be cumbersome for clinics that do not habitually perform RKF testing. An estimation equation for a urea clearance cutoff of 2.5 mL/min, a level of KRU at which many patients may be able to maintain good total weekly clearance on a twice-weekly HD prescription, could serve as a tool to identify patients for subsequent formal timed urine testing.
Estimation equations for RKF in HD patients have recently been published, with many using less commonly available serum tests such as β2-microglobulin, β-trace protein, and cystatin C. These equations using nontraditional markers have demonstrated better accuracy than those using only serum creatinine and/or urea levels.11,12,15 However, the major drawback is the use of laboratory tests not routinely performed in dialysis provider central laboratories for patient care. Our estimation equation that used only usually available data without urine collection performed fairly well with about the same performance as other published equations using cystatin C level.11
In this study, 24-hour urine volume was the most significant singular factor predicting KRU. For achieving the most accurate prediction, it appeared to be indispensable. Some clinicians may argue that if one were to obtain a timed urine collection from a patient, why not simply do the formal analysis and calculation of KRU, rather than using only the volume in an estimation? From a practical and workflow perspective, we believe that asking a patient for a 24-hour urine collection and measuring the volume in the dialysis clinic is a very simple process that can be implemented without any significant additional training, inconvenience, or new laboratory send-out studies. Therefore, the improved performance of estimation models that include urine volume can be used easily and quickly within just about any dialysis clinic with minimal additional labor needs.
This study has strengths. This study incorporated many urine collections in incident HD patients, a group in which KRU is not routinely formally assessed. In addition, our academic nephrology practice has been measuring KRU in in-center HD patients for decades, so protocols and procedures are well developed and carried out on a frequent basis. We believe that the accuracy is quite good for a usual-care environment, with the calculated and modeled KRU correlating well in a subset of the cohort.7
However, this study also has weaknesses. We cannot be certain that all urine samples were collected in the correct manner due to the retrospective nature of the study. Because the cohort spanned many years, we also cannot be certain that laboratory assay techniques were uniform for all the patients. We also did not have an external validation cohort, so systematic biases and generalizability problems may be inherent in our conclusions; external validations along with assessment of utility and effectiveness using cross-sectional and longitudinal data are clearly warranted in the future. Additionally, we included only long-term dialysis patients who were receiving thrice-weekly HD, so use of the models in patients on different HD frequencies or those with acute kidney injury receiving HD was not tested. The analysis of the models using subsequent urine collections in this cohort yielded results at least as good as our bootstrap resampling validation.
In conclusion, although the non–urine-based method of estimating RKF in HD patients did not perform as well as models that included urine volume, it could be considered as an initial assessment of KRU, especially for the incident patient who may not require thrice-weekly HD. Increased precision of estimating KRU can be achieved by including urine volume without formal urine assessment, something that may be helpful in clinics that do not perform RKF assessment on HD patients routinely. As the clinical benefits of RKF in HD patients are being rediscovered and appreciated, we hope clinicians will increasingly quantify RKF and individualize HD prescriptions.
Article Information
Authors’ Full Names and Academic Degrees
Andrew I. Chin, MD, Vishwa Sheth, MD, Jeehyoung Kim, MD, and Heejung Bang, PhD.
Authors’ Contributions
Research idea and study design: AIC, HB, VS; data acquisition: AIC; statistical analysis: HB, JK; supervision and mentorship: AIC. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Investigator-initiated study. Dr Bang was partly supported by the National Institutes of Health through grant UL1 TR001860. No funders had any role in the current study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received May 3, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form August 12, 2019.
Footnotes
Complete author and article information provided before references.
References
- 1.Brener Z.Z., Kotanko P., Thijssen S., Winchester J.F., Bergman M. Clinical benefit of preserving residual renal function in dialysis patients: an update for clinicians. Am J Med Sci. 2010;339(5):453–456. doi: 10.1097/MAJ.0b013e3181cf7d5b. [DOI] [PubMed] [Google Scholar]
- 2.Shafi T., Jaar B.G., Plantinga L.C. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56(2):348–358. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bargman J.M., Thorpe K.E., Churchill D.N. CANUSA Peritoneal Dialysis Study Group. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12(10):2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 4.Termorshuizen F., Dekker F.W., van Manen J.G. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15(4):1061–1070. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 5.Penne E.L., van der Weerd N.C., Grooteman M.P. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(2):281–289. doi: 10.2215/CJN.04480510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilar E., Wellsted D., Chandna S.M., Greenwood R.N., Farrington K. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant. 2009;24(8):2502–2510. doi: 10.1093/ndt/gfp071. [DOI] [PubMed] [Google Scholar]
- 7.Chin A.I., Appasamy S., Carey R.J., Madan N. Feasibility of incremental 2-times weekly hemodialysis in incident patients with residual kidney function. Kidney Int Rep. 2017;2(5):933–942. doi: 10.1016/j.ekir.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung A.M., Young B.S., Chertow G.M. The decline in residual renal function in hemodialysis is slow and age dependent. Hemodial Int. 2003;7(1):17–22. doi: 10.1046/j.1492-7535.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- 9.Chin A.I., Depner T.A., Daugirdas J.T. Assessing the adequacy of small solute clearance for various dialysis modalities, with inclusion of residual native kidney function. Semin Dial. 2017;30(3):235–240. doi: 10.1111/sdi.12584. [DOI] [PubMed] [Google Scholar]
- 10.Daugirdas J.T. Estimating time-averaged serum urea nitrogen concentration during various urine collection periods: a prediction equation for thrice weekly and biweekly dialysis schedules. Semin Dial. 2016;29(6):507–509. doi: 10.1111/sdi.12554. [DOI] [PubMed] [Google Scholar]
- 11.Shafi T., Michels W.M., Levey A.S. Estimating residual kidney function in dialysis patients without urine collection. Kidney Int. 2016;89(5):1099–1110. doi: 10.1016/j.kint.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong J., Kaja Kamal R.M., Vilar E., Farrington K. Measuring residual renal function in hemodialysis patients without urine collection. Semin Dial. 2017;30(1):39–49. doi: 10.1111/sdi.12557. [DOI] [PubMed] [Google Scholar]
- 13.Bang H., Mazumdar M., Newman G. Screening for kidney disease in vascular patients: SCreening for Occult REnal Disease (SCORED) experience. Nephrol Dial Transplant. 2009;24(8):2452–2457. doi: 10.1093/ndt/gfp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boos D.D., Stefanski L.A. Springer; New York, NY: 2013. Essential Statistical Inference: Theory and Methods. [Google Scholar]
- 15.Vilar E., Boltiador C., Wong J. Plasma levels of middle molecules to estimate residual kidney function in haemodialysis without urine collection. PloS One. 2015;10(12) doi: 10.1371/journal.pone.0143813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(suppl 1):S2–S90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 17.Lee M.J., Park J.T., Park K.S. Prognostic value of residual urine volume, GFR by 24-hour urine collection, and eGFR in patients receiving dialysis. Clin J Am Soc Nephrol. 2017;12(3):426–434. doi: 10.2215/CJN.05520516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong J., Davies M., Mount P. The importance of residual kidney function in haemodialysis patients. Nephrology. 2018;23(12):1073–1080. doi: 10.1111/nep.13427. [DOI] [PubMed] [Google Scholar]
- 19.KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66(5):884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]