Figure 1.
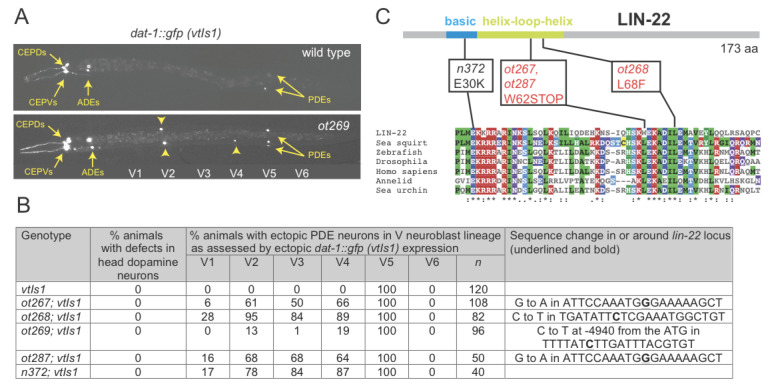
Alleles of lin-22. (A) lin-22 mutant alleles display an ectopic expression of dat-1::gfp (vtIs1; Nass et al., 2002). One representative example is shown. (B) Quantification of lin-22 mutant defects and sequence changes. (C) Sequence change in protein coding sequences. Sequences of Hairy homologs from different animal phyla are shown.
Description
We screened for mutants that affect expression of dopaminergic neuron identity, using a transcriptional reporter for expression of the dopamine transporter dat-1. We previously published and characterized a number of mutants that affect dat-1 expression in different neuron types (Doitsidou et al., 2008). Four alleles that we did not publish in our original screening paper are described here. While wild-type animals only display a single dat-1::gfp(+) neuron pair in the midbody region, the PDE neuron pair from the postdeirid lineage, all 4 mutant alleles display ectopic dat-1::gfp expression along the anterior/posterior axis of the animal (Fig.1A,B). Postdeirid lineage duplication defects were previously described in animals lacking the bHLH transcription factor lin-22/Hairy (Wrischnik and Kenyon, 1997). We find that the canonical lin-22 allele, n372, indeed displays dat-1::gfp expression defects similar to those observed in our mutants (Fig.1B). We sequenced the lin-22 locus in all of our four, independently isolated alleles. Two of them are premature stop codons, one is a missense mutation affecting a conserved leucine residue and all display a similar penetrance of defects (Fig.1B,C). The fourth and weakest allele, ot269, displayed no sequence alteration in the lin-22 coding sequence or in exon/intron boundaries. ot269 failed to complement ot267, ot268, ot287 and the canonical lin-22 allele n372. Furthermore, the ot269 phenotype was rescued by injection of the fosmid WRM0627dG07, which contains lin-22 and one additional complete gene. We found that ot269 harbors a single nucleotide change in the upstream intergenic region of lin-22, almost 5kb away from the start of the gene (sequence change shown in Fig.1B). Subsequent work has shown that this mutation affects a binding site for a GATA transcription factor (Katsanos et al. 2017).
Reagents
OH4265 lin-22(ot267);vtIs1
OH4270 lin-22(ot268);vtIs1
OH4271 lin-22(ot269);vtIs1
OH4320 lin-22(ot287);vtIs1
Strains are available at the CGC.
Acknowledgments
Acknowledgments
We thank Chi Chen for injections, Andrea J. Pretorian and Albert Lee for help with screening, Alexander Boyanov and Berta Vidal for help with sequencing.
Funding
This work was funded was funded by the US National Institutes of Health (R01NS050266), the Howard Hughes Medical Institute and a European Molecular Biology Organization long-term fellowship to M.D.
References
- Doitsidou M, Flames N, Lee AC, Boyanov A, Hobert O. Automated screening for mutants affecting dopaminergic-neuron specification in C. elegans. Nat Methods. 2008 Aug 31;5(10):869–872. doi: 10.1038/nmeth.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanos D, Koneru SL, Mestek Boukhibar L, Gritti N, Ghose R, Appleford PJ, Doitsidou M, Woollard A, van Zon JS, Poole RJ, Barkoulas M. Stochastic loss and gain of symmetric divisions in the C. elegans epidermis perturbs robustness of stem cell number. PLoS Biol. 2017 Nov 01;15(11):e2002429–e2002429. doi: 10.1371/journal.pbio.2002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM 3rd, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002 Feb 26;99(5):3264–3269. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrischnik LA, Kenyon CJ. The role of lin-22, a hairy/enhancer of split homolog, in patterning the peripheral nervous system of C. elegans. Development. 1997 Aug 01;124(15):2875–2888. doi: 10.1242/dev.124.15.2875. [DOI] [PubMed] [Google Scholar]