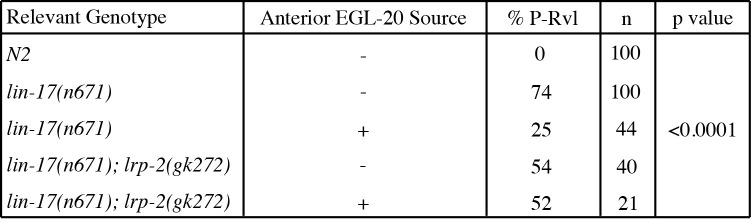
Table 1. LRP-2 is downstream of EGL-20/Wnt.
Data illustrating the proportion of posterior-reversed vulval lineage (% P-Rvl). By ectopically expressing EGL-20 from the anterior side of P7.p using the Pfos-1a promoter, P5.p and P7.p reorient to face the anterior gradient. The anterior source of EGL-20 suppresses the lin-17(n671) phenotype. Because we do not see further suppression of the lin-17(n671) lrp-2(gk272) phenotype when EGL-20 is ectopically expressed from the anterior side of P7.p we conclude that LRP-2 is downstream of EGL-20.
Description
The C. elegans vulva is formed from divisions of three vulval precursor cells (VPCs) – P5.p, P6.p, and P7.p – arranged along the anteroposterior axis in the ventral epithelium (Sulston and Horvitz, 1977). Previous analyses show the orientation of P5.p and P7.p descendants is determined by the interaction of multiple Wnt signals. egl-20/Wnt is expressed in the tail (Whangbo and Kenyon, 2000) and forms a posterior-to-anterior concentration gradient (Coudreuse et al., 2006). It has previously been shown that EGL-20 acts instructively during vulva development by imparting directional information, as opposed to being permissive, where it would only be required for polarization (Green et al., 2008; Minor et al., 2013). By moving the source of egl-20 expression from the posterior of the worm to the anchor cell, the axis of symmetry of the developing vulva, we can reorient the daughter cells of P5.p and P7.p toward the center in a wild-type configuration.
Expression of egl-20 from the center of the axis of symmetry partially suppresses the lin-17(n671) phenotype (Green et al., 2008; Table 1). To test whether LRP-2 acts downstream of EGL-20, we ectopically expressed egl-20 from the anchor cell in a lin-17(n671); lrp-2(gk272) double mutant background and compared it to a lin-17(n671); lrp-2(+) strain. If LRP-2 acts downstream of EGL-20, then anteriorly-expressed EGL-20 will not be able to suppress the lin-17 phenotype, with is the result observed (Table 1). Thus, like CAM-1 and VANG-1, LRP-2 likely acts downstream of EGL-20.
Reagents
Strains:
VC543: lrp-2(gk272). Strain obtained from the CGC and provided by the C. elegans Reverse Genetics Core Facility at the University of British Columbia, which is part of the international C. elegans Gene Knockout Consortium.
MT1306: lin-17(n671) (Ferguson and Horvitz, 1985)
PS5800: lin-17(n671); syEx1031[Pfos-1a::EGL-20::GFP] (Green et al., 2008)
MT1488: lin-17(n671); unc-13(e1091)
The lin-17(n671); lrp-2(gk272) double mutant constructed by crossing lrp-2(gk272) males with strain MT1488 hermaphrodites.
Acknowledgments
Funding
Howard Hughes Medical Institute, with whom PWS was an Investigator. The National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number 1F32NS098658-01A1 awarded to PJM.
References
- Coudreuse DY, Roël G, Betist MC, Destrée O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006 Apr 27;312(5775):921–924. doi: 10.1126/science.1124856. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Horvitz HR. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics. 1985 May 01;110(1):17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JL, Inoue T, Sternberg PW. Opposing Wnt pathways orient cell polarity during organogenesis. Cell. 2008 Aug 22;134(4):646–656. doi: 10.1016/j.cell.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor PJ, He TF, Sohn CH, Asthagiri AR, Sternberg PW. FGF signaling regulates Wnt ligand expression to control vulval cell lineage polarity in C. elegans. Development. 2013 Aug 14;140(18):3882–3891. doi: 10.1242/dev.095687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977 Mar 01;56(1):110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Whangbo J, Harris J, Kenyon C. Multiple levels of regulation specify the polarity of an asymmetric cell division in C. elegans. Development. 2000 Nov 01;127(21):4587–4598. doi: 10.1242/dev.127.21.4587. [DOI] [PubMed] [Google Scholar]