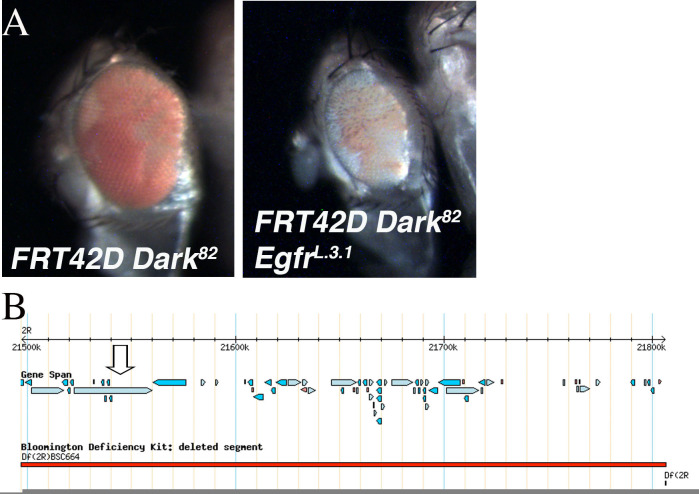
Figure 1.
A. Mosaic (FRT42D Dark82), and Dark82 EgfrL.3.1 (FRT42D Dark82EgfrL.3.1) eyes. In both eyes the homozygous mutant tissue is pigmented (w+mC). B. Region of chromosome 2R that failed to complement L.3.1 by deficiency mapping (2R:21,497,290..21,806,350). Arrow denotes location of Egfr gene. B is adapted from flybase.org (Gramates et al., 2017).
Description
An EMS screen was conducted utilizing the Flp/FRT system to identify mutations that caused an array of phenotypic alterations in the size of the eye including the ratio of mutant to wild type tissue (red over white) or the developmental patterning of the mosaic eye. This screen was done in the genetic background of blocked apoptosis in the homozygous mutant cells to identify conditional regulators of cell growth and eye development (Kagey et al., 2012). The block in apoptosis in the mosaic mutant tissue was achieved by using a FRT42D Dark82 chromosome as a starting point for the EMS mutagenesis (Akdemir et al., 2006). The Dark82 allele was generated by an imprecise excision of the P{lacW}ArkCD4, this allele retains the w+mC (Akdemir et al., 2006) One of the mutants identified was L.3.1 which generated a small rough eye mosaic phenotype, with a smaller percentage of pigmented tissue than the FRT42D, Dark82 control (Figure 1A). The Dark82 mosaic eye is approximately 60% pigmented tissue, while the Dark82 L.3.1 mosaic eye was smaller overall and approximately 50% mutant tissue (w+mC). The ‘rough eye’ phenotype indicates a disruption in the ommatidial organization. In both images the pigmented (w+mC) tissue is homozygous mutant and the unpigmented tissue is homozygous wild type.
The genetic mapping of the location of mutant L.3.1 was done by two independent groups of undergraduate researchers at the University of Detroit Mercy and University of Evansville in undergraduate genetics laboratory courses as part of the Fly-CURE consortium (Bieser et al., 2018). Complementation mapping was conducted independently and the results confirmed between groups. Virgin females from the FRT42D L.3.1 Dark82/CyO stock were mated in series to male flies from the 87 deficiency stocks that comprise the Bloomington Stock Center 2R Deficiency Kit (only stocks distal to the FRT42D site were used for mapping) (Cook et al., 2012). Mutant L.3.1 failed to complement Deficiency stock Df(2R)BSC664 (2R:21,341,647..21,872,028), while complementing the flanking overlapping deficiencies Df(2R)BSC821 and Df(2R)BSC597. This left a region of failure to complement of 2R:21,497,290..21,806,350, which is pictured above in Figure 1 B. Lethal alleles of candidate genes within this region were mated independently to L.3.1 to test for complementation. L.3.1 failed to complement an apparent loss of function allele Egfrk05115 (Dworkin et. al. 2006), indicating that L.3.1 is likely a novel Egfr allele, EgfrL.3.1.
Reagents
FRT42D Dark82/CyO (Akdemir et al., 2006)
FRT42D Dark82 EgfrL.3.1/CyO (this manuscript)
Ey>Flp; FRT42D (BDSC 5616)
y1 w67c23; P{w+mC=lacW}Egfrk05115/CyO (BDSC 10385)
Bloomington Drosophila Stock Center 2R Deficiency Kit (Cook et al., 2012):
w1118; Df(2R)BSC664/SM6a
w1118; Df(2R)BSC821, P+PBac{ w+mC =XP3.RB5}BSC821/SM6a
w111]; Df(2R)BSC597/SM6a
References
- Akdemir F, Farkas R, Chen P, Juhasz G, Medved'ová L, Sass M, Wang L, Wang X, Chittaranjan S, Gorski SM, Rodriguez A, Abrams JM. Autophagy occurs upstream or parallel to the apoptosome during histolytic cell death. Development. 2006 Mar 15;133(8):1457–1465. doi: 10.1242/dev.02332. [DOI] [PubMed] [Google Scholar]
- Bieser Kayla L., Stamm Joyce, Aldo Ayala A., Bhaskara Suneil, Clairborne Makayla, Coronel Gómez Joselyn N., Dean Ron, Dowell Aaron, Dowell Evan, Eissa Mathew, Fawaz Ahmad A., Fouad-Meshriky Michael M., Godoy Dustin, Gonzalez Krista, Hachem Malak K., Hammoud Malak F., Huffman Anthony, Ingram Hunter, Jackman Alex B., Karki Bibek, Khalil Natalia, Khalil Houda, Ha Tran Khanh, Kharel Arjun, Kobylarz Izabell, Lomprey Hunter, Lonnberg Adam, Mahbuba Safa, Massarani Hend, Minster Madeline, Molina Krystina, Molitor Lynette, Murray Taylor, Patel Payal M., Pechulis Sydney, Raja Architha, Rastegari Gladys, Reeves Skylar, Sabu Niveda, Salazar Rafael, Schulert Devan, Senopole Matthew D., Sportiello Kristen, Torres Claudia, Villalobos Jade, Wu Joseph, Zeigler Stacy, Kagey Jacob D. The mapping of Drosophila melanogaster mutant A.4.4. microPublication Biology. 2018 Dec 17; doi: 10.17912/micropub.biology.000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RK, Christensen SJ, Deal JA, Coburn RA, Deal ME, Gresens JM, Kaufman TC, Cook KR. The generation of chromosomal deletions to provide extensive coverage and subdivision of the Drosophila melanogaster genome. Genome Biol. 2012;13(3):R21–R21. doi: 10.1186/gb-2012-13-3-r21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin I, Gibson G. Epidermal growth factor receptor and transforming growth factor-beta signaling contributes to variation for wing shape in Drosophila melanogaster. Genetics. 2006 Apr 28;173(3):1417–1431. doi: 10.1534/genetics.105.053868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates LS, Marygold SJ, Santos GD, Urbano JM, Antonazzo G, Matthews BB, Rey AJ, Tabone CJ, Crosby MA, Emmert DB, Falls K, Goodman JL, Hu Y, Ponting L, Schroeder AJ, Strelets VB, Thurmond J, Zhou P, the FlyBase Consortium. FlyBase at 25: looking to the future. Nucleic Acids Res. 2016 Oct 30;45(D1):D663–D671. doi: 10.1093/nar/gkw1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey JD, Brown JA, Moberg KH. Regulation of Yorkie activity in Drosophila imaginal discs by the Hedgehog receptor gene patched. Mech Dev. 2012 Jun 15;129(9-12):339–349. doi: 10.1016/j.mod.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]