Abstract
This study was a phase III, multicenter, double-blind, randomized, placebo-controlled trial to evaluate the safety and immunogenicity of a seasonal trivalent split, inactivated influenza vaccine (TIV) in healthy Serbian adults between the ages of 18 and 65 years. This egg-based vaccine was manufactured by the Institute of Virology, Vaccines and Sera, Torlak, Belgrade, Serbia. A total of 480 participants were assigned randomly in a ratio of 2:1 to receive a single intramuscular dose (0.5 ml) of the vaccine (15 µg of hemagglutinin per strain) or placebo (phosphate-buffered saline). Participants were monitored for safety, including solicited and unsolicited adverse events (AEs) and serious adverse events (SAEs). No SAEs related to vaccination were reported. Injection site pain (51.3%), injection site tenderness (40.4%), tiredness (17.0%), and headache (15.1%) were the most commonly reported solicited events in the vaccine group. Incidence of related unsolicited AEs was low (1.3%) among vaccinees. Hemagglutinin inhibition (HAI) titers were measured before and 21 days after vaccination in 151 participants. Overall, HAI seroconversion rates to H1 and H3 were observed in 90.1% and 76.2% of vaccinees, respectively. For B antigen, it was 51.5%, likely due to high pre-vaccination titers. Post-vaccination seroprotection rates were in the range of 78.2–95.0% for the three antigens. Post-vaccination geometric mean titers (GMT) were at least 3.8 times higher than baseline levels for all the three strains among vaccinees. Overall, the study showed that the vaccine was safe and well tolerated, and induced a robust immune response against all three vaccine strains.
ClinicalTrials.gov identifier: NCT02935192, October 17, 2016
Keywords: clinical trial, seasonal influenza vaccine, Serbia, trivalent inactivated split, Torlak
Introduction
Influenza virus affects individuals of all ages, causing repeated infections throughout life, and is responsible for annual worldwide epidemics with high morbidity and mortality in populations at risk.1–4 Vaccination is currently the most effective means of preventing influenza. Multiple manufacturers around the globe have developed seasonal trivalent influenza vaccines. The licensed vaccines are used yearly before the influenza season, and this capacity can be switched to pandemic vaccine production when needed. Serbia participates in the “World Health Organization (WHO) Global Action Plan for Influenza Vaccines” (GAP), an initiative launched in 2006 in response to inequitable distribution and availability of influenza vaccines in the event of an influenza pandemic. The GAP strategy was implemented to increase and maintain influenza vaccine supply globally. Biomedical Advanced Research and Development Authority (BARDA) of the US Department of Health and Human Services and the Government of Japan provided funds for an international collaborative program that would increase the supply of influenza vaccines by developing country vaccine manufacturers (DCVMs), thereby leading to local capacity build up and availability of vaccines during outbreaks or pandemics in low- and middle-income countries.5,6 Through extensive and sustained collaboration between BARDA, WHO, and PATH, 14 manufacturers received assistance. Institute Torlak has been participating in GAP’s technology transfer project since 2009. Through the GAP program, the Institute Torlak has established a process for the production of a seasonal trivalent split inactivated influenza vaccine (TIV) produced in embryonated eggs; the vaccine was shown to be safe and immunogenic in animal studies.7 A phase I trial of the Institute Torlak seasonal trivalent split inactivated influenza vaccine was conducted in healthy adults 18–45 years of age. The study did not identify any safety concerns, and demonstrated the vaccine to be highly immunogenic.8
In the current phase III clinical trial, we further evaluated a single intramuscular dose of vaccine in healthy adults aged 18–65 years. The goal was to expand the safety data of the vaccine and to confirm the immunogenicity findings observed in the phase I trial to seek regulatory approval for indication in adults 18–65 years of age based on the European Medicine Agency (EMA) serological criteria for assessing seasonal influenza vaccines in adults 18–60 years old.9
Materials and methods
Study design
This was a phase III, double blinded, randomized, placebo-controlled study to evaluate the safety and immunogenicity of a single dose of the Institute Torlak seasonal trivalent, split inactivated influenza vaccine (A/H1N1; A/H3N2 and B) versus placebo (phosphate buffered saline) in healthy male and female adults, ages 18–65 years old. The study was performed at six centers in Serbia.
Study participants were randomized in 2:1 ratio to one of the two treatment allocations (320 to vaccine and 160 to placebo); 25% of the participants were ⩾45 years of age (80 vaccine and 40 placebo recipients), whereas 75% of all participants were 18–44 years old (240 vaccine and 120 placebo recipients).
Screening was conducted on volunteers who gave their informed consent to join the study. Participants were randomized to receive either vaccine or placebo on day 1. Blood was collected for baseline immunogenicity analyses prior to vaccination. After administration of the study vaccine, all participants were observed for any immediate adverse events (AEs) at the study clinic for 30 min. During the 5 days following vaccination, participants were asked to record local solicited AEs (hardness, pain, redness, swelling, and tenderness) and systemic solicited AEs (chills, headache, joint aches, muscle aches, nausea, fever, and tiredness) using pre-printed memory aids, a thermometer, and a small ruler. The participants returned to the clinic 7 days after vaccination. At that time, the study staff reviewed the memory aids and transcribed all solicited reactogenicity and other adverse events onto the case report forms. In addition to solicited events, participants were asked to report any other unsolicited AEs for 21 days after the vaccination. Participants were followed up for 90 days post vaccination with a final phone call on day 91 (± 7 days). Blood was analyzed for immune response pre-vaccination (day 1) and post-vaccination (day 22). Safety was assessed clinically in all participants who were randomized and received a study product, whereas immunogenicity was assessed in a subset of these participants: 101 individuals randomized to study vaccine and 50 placebo recipients, for whom serum samples were obtained at baseline and 21 days after vaccination.
The vaccine was used in accordance with Research Protocol of Vaccine Clinical Trial, Protocol Number: TORLAK-300.
Study population
A total of 503 healthy Serbian male and female adult volunteers aged 18–65 years were screened for inclusion in the study. Inclusion criteria were that participants were literate (by self-report); healthy, as established by the medical history and physical examination; capable and willing to complete a memory aid; and willing to follow the study procedures. In addition, the women had to have a negative pregnancy test, not be breastfeeding, and be willing to use reliable birth control measures (e.g. intrauterine device, hormonal contraception, condoms) for 3 weeks from the day of vaccination. Exclusion criteria included: presence of any acute or chronic illness or any neoplastic of haematological malignancy; hypersensitivity to any vaccine, chicken or egg protein; taking medication, immunoglobulin, blood or blood products that could interfere with assessment of safety or immunogenicity; participating in any other clinical trial; drug addiction or alcohol dependence. Participants who had received influenza vaccine in the previous 10 months were also excluded.
This study was approved by the ethics review committees of the participating centers, the WHO and the Medicines and Medical Devices Agency of Serbia (ALIMS). Full details of the approval dates/IDs from the relevant Institutional Review Boards (IRBs) can be found in Supplemental Table S2. Written informed consent was obtained from all participants before any study-related activities took place.
Treatments
Two randomization schedules were prepared for this trial, with the first list including 120 participants (25% of all 480 participants enrolled) between the age of 45–65 years, and the second consisting of 360 participants (75% of all 480 participants enrolled) between the ages of 18 through 45 years. For both randomization schedules, a ratio of 2:1 vaccine to placebo was maintained. Randomization was conducted by an independent statistician at Comac Medical who was not involved in the day-to-day conduct of the study.
The seasonal trivalent influenza vaccine tested was manufactured by the Institute Torlak using embryonated chicken eggs and supplied in prefilled, single-dose, disposable syringes. It was a split virion vaccine, inactivated with Beta-propiolactone (BPL) and the virion split with Triton X-100. The vaccine contained at least 15 μg of hemagglutinin antigen of each virus strain recommended for the 2016–2017 season in the northern hemisphere: X-181 reassortant of H1/A/California/7/2009; X-263B reassortant of H3/Hong Kong/4801/2014; and BX-35 reassortant of B/Brisbane/60/2008. The placebo was manufactured by Institute Torlak and consisted of phosphate-buffered saline (PBS), pH 7.4. It was also supplied in prefilled, single-dose, disposable syringes. The vaccine and placebo were stored in a refrigerator between 2°C and 8°C or were supplied daily by Institute Torlak during the vaccination period. The shelf-life was estimated to be 9 months under these conditions. Storage temperature was monitored daily and documented. Back-up power or storage was available in case of primary power failure. The vaccine and placebo syringes were identically packaged and labelled so that the study could be conducted in a double-blind manner. In the case of any unblinding, researchers were required to report this in writing to the overseeing Ethics Committee. The allocation code of the product injected into each participant was recorded on the case report form. The study vaccine and placebo were administered as single dose (0.5 ml), intramuscularly (IM) in the deltoid muscle of the arm.
Safety assessment
Solicited (local and systemic) reactions, unsolicited AEs and serious adverse events (SAEs) were collected and recorded. Solicited reactions are local or systemic AEs, typically associated with intramuscular influenza vaccination, that were specifically asked about during the 5-day post-vaccination period. All solicited reactions were regarded as related to product administration. Unsolicited events were reported by the participant or observed by study staff during clinic visits. Such events were recorded up to 21 days post-vaccination, except for SAEs, which were recorded throughout the entire study period of 90 days. All clinical safety evaluations were made by a qualified clinician. Any clinical sign, symptom or laboratory finding that at any time met the established criteria for an SAE was categorized as such.
The study was conducted in accordance with the guidance for International Conference on Harmonisation good clinical practice (ICH GCP), including ICH guidelines, directive 2001/20/EC of the European Parliament, and the most recent version of the Declaration of Helsinki.
Assessment of immunogenicity
Hemagglutination inhibition (HAI) is the most frequently used serological test for determining immunological responses to influenza vaccination.10 Serum specimens obtained before and 21 days after vaccination were tested with a validated assay at the Institute Torlak Clinical Research Laboratory for the presence and titers of HAI antibodies to each of the three influenza antigens (H1, H3, and B) in the vaccine. Details of the method of testing has been described in the publication of phase I results of the vaccine.8
Please refer to Supplemental Table S1 for detailed study schema.
Statistical methods
The full analysis (FA) population included all participants in the enrolled population who were randomized and received a study vaccination. All safety analysis was conducted on this population. The analysis based on a subset of this population for whom immunogenicity was assessed served as the supportive results for all immunogenicity objectives. The per protocol (PP) population included all participants in the FA population for whom immunogenicity was assessed and who had valid post vaccination immunogenicity measures with no major protocol violations that were determined to potentially interfere with the immunogenicity assessment of the study vaccine. This population was the primary analysis population for all immunogenicity objectives. A two-sided significance level of 0.05 was used in the comparisons. All statistical analyses were performed using SAS® version 9.4.
The sample size was selected based safety requirement to analyze the common adverse events in the study group and also considering probability of meeting EMEA/Committee for Proprietary Medicinal Products (CPMP) serological criteria for assessing seasonal influenza for licensure for adults 18–60 years of age.9 With more than 300 evaluable participants who were randomized to receive vaccine, the study had 95% power to detect at least one vaccine-associated AE if true incidence is 1.0%. With a sample size of 100 evaluable participants who received study vaccine and had immunogenicity measures, there was 91% probability that the point estimate for seroconversion to all three strains would be ⩾40%. If the true response to each antigen was to be ⩾60%, there was 92% probability that the lower bound of the exact 95% CI for seroconversion to all strains would be ⩾40% with the same sample size. Likewise, with a sample size of 100 evaluable participants for immunogenicity, there was 92% probability that the point estimate for seroprotection to all three strains would be ⩾70% if the true response to each strain was ⩾78%. If the true response to each strain is ⩾ 87%, there was 95% probability that the lower bound of the exact 95% CI for seroprotection to all strains would be ⩾70% with the same sample size.
Safety analysis
The vaccine safety profile was assessed in terms of the number and percentage of participants with: (a) adverse events occurring within 30 min of vaccination; (b) solicited local and systemic reactions within 5 days post-vaccination; (c) unsolicited AEs within 21 days post-vaccination; and (d) SAEs occurring within 90 days post-vaccination. Two-sided exact 95% confidence intervals (CIs) of the percentage were calculated using the Clopper-Pearson method. For solicited AEs, Fisher’s exact test was used to compare the proportions of reactions in the two treatment groups.
Immunogenicity analysis
Immunogenicity assessments included: number and percentage of participants with a serum HAI antibody titer ⩾1:40 (seroprotection titer) 21 days post-vaccination for each of the three antigens; number and percentage of participants seroconverting for each of the three antigens (seroconversion was defined as a serum HAI titer on day 22 meeting one of the following criteria: (a) pre-vaccination titer <1:10 and post-vaccination titer ⩾1:40, or (b) pre-vaccination titer ⩾1:10 and at least a four-fold increase in post-vaccination titer); geometric mean titers (GMTs) of serum HAI antibodies pre- and post-vaccination for each of the three antigens; rise in GMTs of serum HAI antibodies for each of the three antigens.
Immunological responses were also calculated separately in participants with pre-vaccination antibody titers of <1:10 or ⩾1:10. The seroprotection and seroconversion rates, as well as the GMTs of HAI immune responses were also analyzed separately for the two age subgroups (18–44 and 45–65 years old) and compared.
The percentages of participants with an immune response were calculated, along with the corresponding two-sided exact 95% (Clopper-Pearson) CIs. GMT was summarized by treatment group, along with the corresponding two-sided 95% CIs, by exponentiation of the corresponding log-transformed means and their 95% CIs. Fisher’s exact test and t test were used to compare age groups as appropriate. No multiplicity adjustment was made because the primary analysis for immunogenicity was descriptive.
Results
Demographic and other baseline characteristics
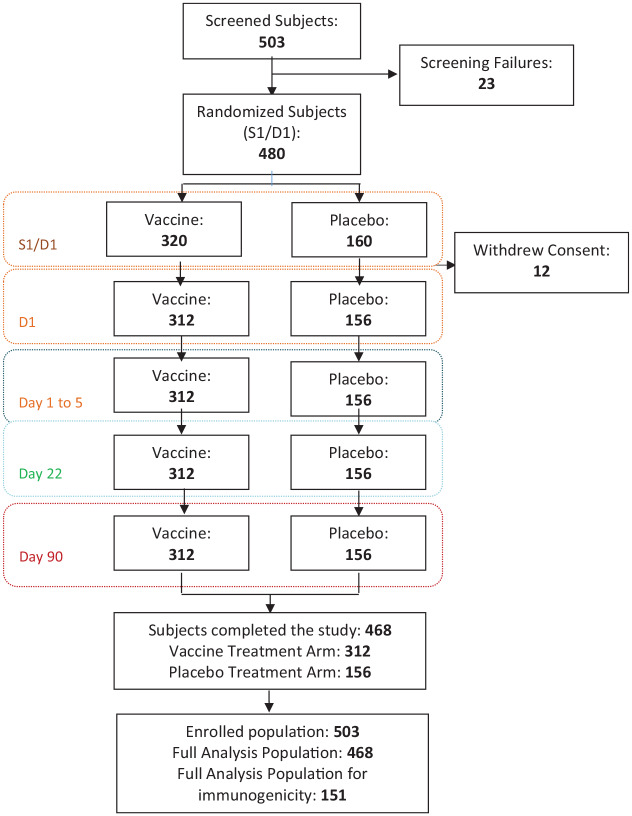
Participants were enrolled across six sites in Serbia between 28 November and 23 December 2016. A total of 503 participants were screened; of these, 23 were considered as screening failures; 480 participants between the age of 18–65 years were randomized in a ratio of 2:1 to receive TIV or placebo. Among the randomized participants, 360 (75%) were 18–44 and 120 (25%) were 45–65 years of age. In all, 12 participants withdrew consent before vaccine administration, with 468 receiving a dose of the study vaccine; 312 (66.7%) received TIV and 156 (33.3%) received placebo and were included in the FA population for evaluation of safety (Figure 1: participant flow diagram). In the FA population, 222 (47.4%) participants were female and 246 (52.6%) were male, with similar distributions in vaccine and placebo groups. The overall mean age [standard deviation (SD)] was 38.2 years (11.6), 32.6 years (7.3) in the 18–44 year age group and 54.4 years (5.5) in the 45–65 years age group). Mean age was similar for participants treated with vaccine or placebo in all groups and subgroups. Basic demographic characteristics are presented in Table 1. Immunogenicity was tested in 151 participants randomly selected (101 from the vaccine and 50 from placebo group), stratifying by age. with 100 (66.23%) being from participants aged 18–44 and 51 (33.77%) from participants aged 45–65 years. The immunogenicity results from the PP population are reported.
Figure 1.
Participant flow chart.
Table 1.
Demographic characteristics of study subjects.
| FA population | Vaccine | Placebo | |
|---|---|---|---|
| No. (%) of subjects | 468 | 312 (66.7) | 156 (33.3) |
| Male (%) | 246 (52.6) | 167 (53.5) | 79 (50.6) |
| Female (%) | 222 (47.4) | 145 (46.5) | 77 (49.4) |
| Mean age in years (SD) | 38.2 (11.6) | 38.4 (11.8) | 37.6 (11.6) |
| Distribution by age group | |||
| 18–44 years | 32.6 (7.3) | 32.8 (7.4) | 32.0 (7.1) |
| 45–65 years | 54.4 (5.5) | 54.7 (5.7) | 53.8 (5.2) |
No statistically significant difference was found between the treatment groups in terms of age and sex by analysis of variance and Cochran–Mantel–Haenszel tests, respectively; p-value was > 0.05 for both comparisons.
FA, full analysis; SD, standard deviation.
Immunogenicity
Immune responses based on HAI assay against seasonal influenza strains H1N1, H3N2, and B are presented in Table 2. In the vaccine group, out of 101 participants tested by the HAI assay, seroconversion rates were 90.1% for H1, 76.2% for H3, and 51.5% for B HA antigen. Of the 50 participants tested in the placebo group, only 1 participant presented a seroconversion response to the H1 antigen, whereas 9 participants seroconverted to B antigen. None of the participants in the placebo group seroconverted to the H3 antigen. Pre-vaccination GMTs ranged between 6.9 and 27.4 for the vaccine and placebo recipients with higher titers against the H1 and B antigens. Pre-vaccination GMTs were similar in the two study groups. Similarly, seroprotection rate at baseline were high for the B and H1 antigens ranging between 23.8% and 52.5%, whereas only 6.9% and 6.0% participants had seroprotective titers to the H3 antigen in vaccine and placebo groups, respectively. At 3 weeks after receiving the vaccine, a robust increase in GMT was seen among vaccine recipients with GMTs of 336.3 (95% CI 268.2–421.7) against H1; 88.4 (95% CI 65.9–118.5) against H3 and 103.5 (95% CI 86.9–123.3) against the B antigens. There were minimal rises in post-vaccination GMTs among placebo recipients. Seroprotection rates post-vaccination were very high among vaccine recipients and ranged from 78.2% to 95.0% for the three antigens, whereas in the placebo group it remained the same for the H1 and H3 antigens and increased from 36.0% to 52.0% for the B antigen. GMTs rose substantially in the vaccine group from baseline (pre-immunization) to 21 days after immunization with calculated GMT fold rise (GMFR) of 22.5 (H1), 12.9 (H3), and 3.8 (B), indicating a strong immune response to all three strains evoked by Torlak’s TIV. In the placebo group the GMFR was 1.6 for B antigen and 1.0 for H1 and H3 antigens.
Table 2.
Immune response by HAI assay in vaccine and placebo recipients.
| Group strain | n | Seroprotection % (95% CI) | SC % (95% CI) | GMT value (95% CI) | GMFR value (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Vaccine group | |||||||
| A/H1N1 | 101 | Pre | 23.8 (15.9–33.3) | 90.1 (82.5–95.1) | Pre | 15.0 (12.3–18.1) | 22.5 (17.5–28.9) |
| Post | 95.0 (88.8–98.4) | Post | 336.3 (268.2–421.7) | ||||
| A/H3N2 | 101 | Pre | 6.9 (2.8–13.8) | 76.2 (66.7–84.1) | Pre | 6.9 (6.0–7.8) | 12.9 (9.8–17.0) |
| Post | 78.2 (68.9–85.8) | Post | 88.4 (65.9–118.5) | ||||
| B | 101 | Pre | 52.5 (42.3–62.5) | 51.5 (41.3–61.6) | Pre | 27.4 (21.6–34.8) | 3.8 (3.0–4.8) |
| Post | 92.1 (85.0–96.5) | Post | 103.5 (86.9–123.3) | ||||
| Placebo group | |||||||
| A/H1N1 | 50 | Pre | 42.0 (28.2–56.8) | 2.0 (0.1–10.6) | Pre | 24.1 (16.9–34.5) | 1.0 (0.9–1.2) |
| Post | 44.0 (30.0–58.7) | Post | 25.0 (17.7–35.2) | ||||
| A/H3N2 | 50 | Pre | 6.0 (1.3–16.5) | 0.0 (0.0–7.1) | Pre | 8.0 (6.4–10.0) | 1.0 (1.0–1.1) |
| Post | 6.0 (1.3–16.5) | Post | 8.4 (6.6–10.5) | ||||
| B | 50 | Pre | 36.0 (22.9–50.8) | 18.0 (8.6–31.4) | Pre | 21.9 (15.8–30.3) | 1.6 (1.3–2.1) |
| Post | 52.0 (37.4–66.3) | Post | 36.1 (26.7–48.7) | ||||
n, Number of participants tested in PP population in the group.
SC defined as a serum HAI antibody titer meeting the following criteria:
Pre-vaccination titer <1:10 and a post-vaccination titer measured on day 22 of ⩾1:40, or
Pre-vaccination titer ⩾1:10 and at least a four-fold increase in post-vaccination measured on day 22.
CI, confidence interval; GMFR, GMT fold rise; GMT, geometric mean titer; HAI, hemagglutinin inhibition; PP, per protocol; SC, seroconversion.
Immune response was also calculated according to the level of pre-existing antibodies (Table 3). As expected, the proportion of participants who had pre-vaccination (baseline) titers ⩾1:10 was higher for H1 (67.3% of all participants in the vaccine group) and B (75.2% of all participants in the vaccine group) antigens, than for H3, with only 22.8% of all participants in the vaccine group showing titers above this level.
Table 3.
Immune response in vaccine group by pre-vaccination HAI antibody titers.
| Group strain | n | SC* % (95% CI) | GMT value (95% CI) | GMFR value (95% CI) | |
|---|---|---|---|---|---|
| Pre-vaccination HAI antibody titer of <1:10 | |||||
| A/H1N1 | 33 | 87.9 (71.8–96.6) | Pre | 5.0 (NA) | 38.1 (23.2–62.3) |
| Post | 190.3 (116.2–311.7) | ||||
| A/H3N2 | 78 | 73.1 (61.8–82.5) | Pre | 5.0 (NA) | 13.5 (9.6–18.8) |
| Post | 67.3 (48.1–94.2) | ||||
| B | 25 | 88.0 (68.8–97.5) | Pre | 5.0 (NA) | 14.1 (10.6–18.8) |
| Post | 70.6 (53.1–94.0) | ||||
| Pre-vaccination HAI antibody titer of >=1:10 | |||||
| A/H1N1 | 68 | 91.2 (81.8–96.7) | Pre | 25.5 (21.3–30.4) | 17.4 (13.2–22.9) |
| Post | 443.4 (357.1–550.5) | ||||
| A/H3N2 | 23 | 87.0 (66.4–97.2) | Pre | 20.0 (15.6–25.7) | 11.1 (7.2–17.2) |
| Post | 222.9 (141.4–351.4) | ||||
| B | 76 | 39.5 (28.4–51.4) | Pre | 48.0 (39.9–57.8) | 2.4 (1.9–3.1) |
| Post | 117.3 (95.3–144.5) | ||||
n, Number of participants tested in PP population in the group.
SC defined as a percentage of participants with 4-fold rise in titer (pre-vaccination titer <1:10 and a post-vaccination titer of ⩾1:40 on day 22 or a four-fold rise in case pre-vaccination titer was ⩾1:10).
CI, confidence interval; GMFR, GMT fold rise; GMT, geometric mean titer; HAI, hemagglutinin inhibition; PP, per protocol; SC, seroconversion.
Seroresponse rates were independent of pre-existing titers for H1 and H3, whereas, for B, it was lower for participants with baseline antibody titer ⩾1:10 compared with participants with baseline titer <1:10. Thus, for B antigen 88.0% of participants with pre-vaccination titer below 1:10 had post vaccination titers ⩾1:40, whereas only 39.5% of participants with pre-vaccination titer ⩾1:10 had ⩾4 fold rises. In the vaccine group, for the three antigens tested, post-vaccination GMTs were higher in the subgroup of participants with pre-vaccination antibody titers of ⩾1:10 (117.3–443.4) compared with those with pre-vaccination antibody titers of <1:10 (67.3–190.3). Although the post-vaccination GMT titers were higher in the cohort with pre-vaccination antibody titers of ⩾1:10, the GMFR was lower (2.4–17.4) as compared with those with pre-vaccination antibody titers of <1:10 (13.5–38.1).
HAI immune responses of the vaccine were analyzed separately for the two age subgroups (18–44 and 45–65 years old) and compared (Table 4). In the vaccine group, pre-vaccination seroprotection rates for the three antigens were similar across the two age groups. There was no statistically significant difference in the post-vaccination seroprotection rate between the two age groups for H1 and H3 antigens. For the B antigen, a higher post-vaccination seroprotection rate was observed for the age group 45–65 years when compared with 18–44 years (100% versus 88%; p = 0.049). Seroconversion rates were not statistically significant across the two age groups for all three strains, ranging from 50.7% to 88.1% for age group of 18–44 years old and 52.9% to 94.1% for age group of 45–65 years old. The lowest seroconversion rate in both age groups was observed for the B strain.
Table 4.
Immune response by HAI assay in vaccine and placebo groups by age group.
| Group strain | Seroprotection | SC | GMT | GMFR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | GMT titer (95% CI) | Ratio (95% CI) | |||||||
| 18–44 years. n = 67 (Vaccine) 34 (Placebo) |
45–65 years. n = 34 (Vaccine) 16 (Placebo) |
18–44 years. n = 67 (Vaccine) 34 (Placebo) |
45–65 years. n = 34 (Vaccine) 16 (Placebo) |
18–44 years. n = 67 (Vaccine) 34 (Placebo) |
45–65 years. n = 34 (Vaccine) 16 (Placebo) |
18–44 years. n = 67 (Vaccine) 34 (Placebo) |
45–65 years. n = 34 (Vaccine) 16 (Placebo) |
|||
| Vaccine group | ||||||||||
| A/H1N1 | Pre | 26.9 (16.8–39.1) | 17.6 (6.8–34.5) | 88.1 (77.8–94.7) |
94.1 (80.3–99.3) |
Pre | 16.1 (12.7–20.5) | 12.9 (9.3–17.9) | 22.9 (16.6–31.7) |
21.6 (14.4–32.4) |
| Post | 92.5 (83.4–97.5) | 100.0 (89.7–100.0) | Post | 369.9 (276.0–495.7) | 278.9 (195.2–398.3) | |||||
| A/H3N2 | Pre | 9.0 (3.4–18.5) | 2.9 (0.1–15.3) | 80.6 (69.1–89.2) |
67.6 (49.5–82.6) |
Pre | 7.7 (6.4–9.1) | 5.5 (4.8–6.3) | 13.8 (10.2–18.5) |
11.3 (6.3–20.5) |
| Post | 82.1 (70.8–90.4) | 70.6 (52.5–84.9) | Post | 105.8 (76.1–147.1) | 62.0 (34.4–111.9) | |||||
| B | Pre | 46.3 (34.0–58.9) | 64.7 (46.5–80.3) | 50.7 (38.2–63.2) |
52.9 (35.1–70.2) |
Pre | 22.3 (16.8–29.5) | 41.2 (26.9–63.1) | 3.7 (2.8–5.0) |
3.9 (2.6–5.8) |
| Post | 88.1 (77.8–94.7) | 100.0 (89.7–100.0) | Post | 82.9 (67.5–101.9) | 160.0 (120.6–212.2) | |||||
| Placebo group | ||||||||||
| A/H1N1 | Pre | 51.5 (33.5–69.2) | 23.5 (6.8–49.9) | 3.0 (0.1–15.8) |
0.0 (0.0–19.5) |
Pre | 32.4 (21.4–49.1) | 13.6 (7.1–25.8) | 1.0 (0.9–1.2) |
1.0 (1.0–1.1) |
| Post | 54.5 (36.4–71.9) | 23.5 (6.8–49.9) | Post | 33.5 (22.7–49.3) | 14.1 (7.4–26.9) | |||||
| A/H3N2 | Pre | 6.1 (0.7–20.2) | 5.9 (0.1–28.7) | 0.0 (0.0–10.6) |
0.0 (0.0–19.5) |
Pre | 9.1 (6.8–12.2) | 6.3 (4.5–8.8) | 1.1 (1.0–1.2) |
1.0 (NA) |
| Post | 6.1 (0.7–20.2) | 5.9 (0.1–28.7) | Post | 9.7 (7.2–13.1) | 6.3 (4.5–8.8) | |||||
| B | Pre | 30.3 (15.6–48.7) | 47.1 (23.0–72.2) | 18.2 (7.0–35.5) |
17.6 (3.8–43.4) |
Pre | 18.0 (12.4–26.1) | 32.0 (16.6–61.5) | 1.5 (1.1–2.1) |
2.0 (1.3–3.1) |
| Post | 39.4 (22.9–57.9) | 76.5 (50.1–93.2) | Post | 26.8 (18.7–38.6) | 63.9 (40.4–101.1) | |||||
n, Number of participants tested in PP population in the group.
SC defined as a serum HAI antibody titer meeting the following criteria: pre-vaccination titer <1:10 and a post-vaccination titer measured on day 22 of ⩾1:40, or pre-vaccination titer ⩾1:10 and at least a four-fold increase in post-vaccination measured on day 22.
The comparison for post-vaccination seroprotection rate between the two age groups within vaccine group is made using Fisher’s exact test; p values obtained were 0.165 for H1N1 strain, 0.209 for H3N2 strain, and 0.049 for B strain.
The comparison of SC between the two age groups within vaccine group is made using Fisher’s exact test; p values were 0.488 for H1N1 strain, 0.215 for H3N2 strain, and 1.000 for B strain on day 22.
The comparison of post vaccination GMT between the two age groups within vaccine group was made using t test; p values were 0.244 for H1N1 strain, 0.088 for H3N2 strain, and <0.001 for B strain.
CI, confidence interval; GMFR, GMT fold rise; GMT, geometric mean titer; HAI, hemagglutinin inhibition; PP, per protocol; SC, seroconversion.
Robust increase in GMT from baseline to 21 days post-vaccination was observed against all the three strains in both age groups among vaccine recipients. Post-vaccination GMTs were comparable between the two age groups for both the H1 and H3 strains, whereas for the B strain, it was higher in the 45- to 65-year-old group compared with the 18- to 44-year-old group; 160.0 (95% CI 120.6–212.2) versus 82.9 (95% CI 67.5–101.9), respectively (p value < 0.001). GMFR rates were similar for both age groups across all thee antigens.
Adverse events
No immediate AEs, such as shock or anaphylaxis, were reported within 30 min post-vaccination. A total of 29 participants in the vaccine group and 4 participants in the placebo group reported a solicited event during this period, redness at the injection site being the most common (22 participants in the vaccine group and 3 participants in the placebo group). During the 5-day post-vaccination period, solicited AEs (local or systemic) were experienced by at least 209 (67.0%) participants in the vaccine group and 47 (30.1%) participants in the placebo group (Table 5). Solicited local AEs were reported by 191 (61.2%) of the participants in the vaccine group and 26 (16.7%) participants in the placebo group. Pain [160 participants (51.3%) in the vaccine group and 17 participants (10.9%) in the placebo group] and tenderness (126 participants (40.4%) in the vaccine group and 11 participants (7.1%) in the placebo group) at the injection site were the most common. Overall, local reactions were reported more frequently in the vaccine recipients than in placebo recipients and this difference was found to be statistically significant (p < 0.05). Most of the local events were mild to moderate in intensity, with only one event of pain and swelling categorized as severe. None of the local solicited events were ongoing beyond the 5-day solicitation period.
Table 5.
Solicited symptoms in the 5-day period after immunization with Torlak Institute seasonal influenza vaccine.
| Solicited AEs | Vaccine (N = 312) | Placebo (N = 156) | p value** | ||
|---|---|---|---|---|---|
| n | % (95% CI)* | n | % (95% CI)* | ||
| Participants with at least one solicited AE | 209 | 67.0 (61.5–72.2) | 47 | 30.1 (23.1–38.0) | <0.0001 |
| Participants with at least one local solicited AE | 191 | 61.2 (55.6–66.7) | 26 | 16.7 (11.2–23.5) | <0.0001 |
| Hardness | 29 | 9.3 (6.3–13.1) | 0 | 0 (0.0–2.3) | <0.0001 |
| Pain | 160 | 51.3 (45.6–57.0) | 17 | 10.9 (6.5–16.9) | <0.0001 |
| Redness | 40 | 12.8 (9.3–17.0) | 3 | 1.9 (0.4–5.5) | <0.0001 |
| Swelling | 20 | 6.4 (4.0–9.7) | 0 | 0 (0.0–2.3) | 0.0004 |
| Tenderness | 126 | 40.4 (34.9–46.1) | 11 | 7.1 (3.6–12.3) | <0.0001 |
| Participants with at least one systemic solicited AE | 87 | 27.9 (23.0–33.2) | 27 | 17.3 (11.7–24.2) | 0.0121 |
| Chills | 11 | 3.5 (1.8–6.2) | 1 | 0.6 (0.0–3.5) | 0.0693 |
| Headache | 47 | 15.1 (11.3–19.5) | 19 | 12.2 (7.5–18.4) | 0.4815 |
| Joint aches | 16 | 5.1 (3.0–8.2) | 5 | 3.2 (1.0–7.3) | 0.4784 |
| Muscle aches | 27 | 8.7 (5.8–12.3) | 6 | 3.8 (1.4–8.2) | 0.0576 |
| Nausea | 18 | 5.8 (3.5–9.0) | 6 | 3.8 (1.4–8.2) | 0.5058 |
| Temperature | 6 | 1.9 (0.7–4.1) | 1 | 0.6 (0.0–3.5) | 0.4330 |
| Tiredness | 53 | 17 (13.0–21.6) | 16 | 10.3 (6.0–16.1) | 0.0541 |
N, total number of FA population in the group; n, the number of the participants with event.
% Percentage is calculated using total number of participants in FA population in that group as a denominator (n/N × 100).
Clopper–Pearson exact confidence interval.
Fisher’s exact test.
AE, adverse event; FA, full analysis
Systemic AEs were less commonly reported and occurred more frequently in the vaccine recipients than placebo recipients; 87 participants (27.9%) in the vaccine group and 27 participants (17.3%) in the placebo group reported at least one solicited systemic adverse event. The most common were tiredness and headache, reported by 17.0% and 15.1% participants, respectively, in the vaccine group. In the placebo group, 12.2% participants reported headache, and 10.3% participant reported tiredness. The incidence of all other solicited systemic symptoms was less than 10% in both study groups. The majority of these symptoms were mild and moderate in intensity, with only four participants in the vaccine group and none in the placebo group reporting grade 3 (severe) systemic reactions within 5 days of vaccination. All solicited systemic symptoms resolved within 5 days, with only one episode of headache and muscle aches in the vaccine group and one episode of tiredness in the placebo group that continued beyond the solicitation period of 5 days.
Unsolicited AEs were analyzed through 21 days post vaccination. A total of 56 (12.0%) participants experienced 68 unsolicited events during the specified period. The incidence of unsolicited AEs was similar for the two groups, with 41 (13.1%) participants reporting 52 events in the vaccine group and 15 (9.6%) participants reporting 16 events in the placebo group. The most frequently reported unsolicited AE in the vaccine group was respiratory tract infection [7 (2.2%) participants in the vaccine group and 1 (0.6%) participant in the placebo group]. All the unsolicited AEs recovered before the 90-day contact with the participant. The majority of the events were mild to moderate, with only once case of vomiting in the vaccine group reported as grade 3 (severe). No life-threatening unsolicited event was reported in the study. All the events except for three (one case each of vomiting, pharyngitis, and muscle spasms) in the vaccine group and one case of dizziness in the placebo group were considered unrelated to the vaccination by the investigators.
Serious AEs were recorded for 90 days after vaccination. During this period, a total of two (0.4%) participants reported SAEs. These cases occurred between days 21 and 90 post vaccination in the vaccine recipients and included one case each of acute lymphocytic leukemia and varicocele. Both were deemed as not related to the vaccine by the investigator. The case of varicocele was resolved at the time of last contact, whereas the disease of acute lymphocytic leukemia was still ongoing. A pregnancy was reported during the follow up period in a placebo recipient. This participant was followed-up for the outcome of the pregnancy, which was uneventful with the mother delivering a healthy baby without any congenital malformation. Therefore, this was not considered and reported as a SAE. No deaths were reported in this study.
Discussion
Vaccination is currently the most effective means of preventing influenza. However, global influenza vaccine coverage is low and vaccine production capacity is concentrated mostly in industrialized countries. In addition to assuring supplies, local production of vaccines also supports socioeconomic development, controls cost, and enhances national vaccine security and preparedness towards early response in case of local epidemics.11
This phase III study was conducted with the objective to further investigate and confirm the safety and immunogenicity profile of seasonal trivalent split, inactivated influenza vaccine produced by Institute Torlak, Serbia, with the aim of generating data to support licensure of the vaccine for adults 18–65 years of age.
The vaccine was shown to be safe and well-tolerated in adults. The reactogenicity profile observed, particularly the frequency and severity of solicited and unsolicited adverse events, is similar to those of previously licensed inactivated seasonal influenza vaccines and was in line with the results obtained in the phase I study. Reported local reactions were mostly mild and of short duration, as normally seen after licensed influenza vaccinations.12–15 Systemic AEs were not commonly reported, and most of them were mild to moderate and of short duration. Severe cases were rare. None of the SAEs observed during the follow up was considered by the investigators to be related to the study vaccine.
The immunogenicity assessment employed the HAI assay, which is an established correlate of protection for influenza. The trial results indicate that the vaccine is capable of eliciting robust immune responses, both in naïve participants as well as in those previously primed by infection or prior vaccination as assessed by baseline HAI titers.
The high rate of participants with elevated pre-vaccination titers to the B strain (approximately half of the participants tested) and for the H1N1 strain (approximately one-third of the participants tested) exhibiting seroprotective titers at baseline was in line with a recent publication that provides surveillance data for influenza disease in Serbia from the years 2009 to 2015, showing that A viruses were predominant in the first two seasons (2010/11 and 2011/12), with A/H1N1pdm09 in the 2010/11 season; A/H3N2 in the 2011/12 season and B viruses predominant in the 2012/13 season, where both A/H3N2 and A/H1N1 pdm09 were also present.16 Our study findings, which also showed that 18% of the participants in the placebo group also seroconverted by day 22, suggest that influenza B infection was ongoing at the time of the study. However, recent data from WHO FLUNET (a global web-based tool for influenza virological surveillance) shows influenza circulating predominately from December 2016 (week 50) to early January (week 4) 2017 with prevalence of A/H3N2 and absence of influenza B activity during the study period, which did not corroborate our observation.17
The immunogenicity criteria required for licensure by the EMA for seasonal vaccines with regard to seroconversion (>40%), seroprotection (>70%), and GMT increase (GMFR > 2.5) were met convincingly.
An exploratory analysis of the immune response in the two age groups of 18–44 years and 45–65 years showed similar immune responses for H1 and H3 antigens. Further testing needs to be conducted in other populations, anticipating that different responses to the vaccine may occur in response to administration in children, pregnant women, or in individuals with health problems (e.g. immune deficiency, diabetes, chronic renal disease, etc.).
The study also evaluated the immunological response in participants with or without preexisting antibodies. The vaccine showed good response in both the groups. In conclusion, the tested seasonal, trivalent influenza vaccine was shown to be safe and well tolerated and induced high levels of seroconversion and seroprotection rates in immunologically naïve populations and those with pre-existing HAI antibodies to all three strains tested.
The immune response of the vaccine was similar across the age groups of 18–44 years and 45–65 years for H1 and H3 antigens. These clinical data provide convincing evidence that it is feasible to produce safe and immunogenic influenza vaccine locally.
Supplemental Material
Supplemental material, Supplementary_tables for Safety and immunogenicity of a seasonal trivalent inactivated split influenza vaccine: a double blind, phase III randomized clinical trial in healthy Serbian adults by Goran Stevanovic, Aleksandar Obradovic, Snezana Ristic, Dragan Petrovic, Branislava Milenkovic, Danilo Mitrovic, Svetlana Filipovic Vignjevic, Katarina Ilic, Vera Stoiljkovic, Lidija Lavadinovic, Mijomir Pelemis, Svetlana Petrovic, Ana Vidmanic, Olga Popovic, Natasa Eremic, Erin Sparrow, Guido Torelli, Muriel Socquet, Renée Holt, Yordanka Ilieva-Borisova, Yuxiao Tang, Francesco Berlanda Scorza, Jorge Flores and Niraj Rathi in Therapeutic Advances in Vaccines and Immunotherapy
Acknowledgments
The authors wish to acknowledge support from Joseph Chiu and Iksung Cho for their work on protocol development and Rahnuma Wahid for her dedication to this program; the clinical and administrative staff at each of the study sites; Comac Medical, Ltd., for providing the clinical monitoring, data management, and analysis services, in particular Vanja Vucetic and Sava Jovanovic for their operational efforts; WHO: Florence Barthelemy, Martin Friede, and Marie-Paule Kieny; Biomedical Advanced Research and Development Authority (BARDA), US Department of Health and Human Services (BARDA): Rick Bright, Sheng Li, Chuong Huynh and Julia Schafer; and most importantly the trial participants in Serbia who volunteered for the study. We are grateful to them.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Disclaimer: The authors alone are responsible for the views expressed in this article, which do not necessarily represent the views, decisions or policies of the institutions with which the authors are affiliated.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for the study was provided to WHO and to PATH from the US Department of Health and Human Services/BARDA (grant Numbers: IDSEP130015-01 and IDSEP130018-01-06).
ORCID iD: Niraj Rathi  https://orcid.org/0000-0002-5560-5504
https://orcid.org/0000-0002-5560-5504
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Goran Stevanovic, Clinical Center of Serbia, Clinic for Infectious and Tropical Diseases, Belgrade Faculty of Medicine, Belgrade, Serbia.
Aleksandar Obradovic, Jevremova Special gynecology hospital with maternity, Belgrade, Serbia.
Snezana Ristic, Institute for Students’ Healthcare, Belgrade, Serbia.
Dragan Petrovic, Institute of Health Care of Workers of the Ministry of Internal Affairs, Belgrade, Serbia.
Branislava Milenkovic, Clinical Center of Serbia, Clinic for Pulmonology, Belgrade, Serbia.
Danilo Mitrovic, General Hospital Vrsac, Belgrade, Serbia.
Svetlana Filipovic Vignjevic, Institute of Virology, Vaccines and Sera “Torlak”, Belgrade, Serbia.
Katarina Ilic, Institute of Virology, Vaccines and Sera “Torlak”, Belgrade, Serbia.
Vera Stoiljkovic, Institute of Virology, Vaccines and Sera “Torlak”, Belgrade, Serbia.
Lidija Lavadinovic, Clinical Center of Serbia, Clinic for Infectious and Tropical Diseases, Belgrade Faculty of Medicine, Belgrade, Serbia.
Mijomir Pelemis, Clinical Center of Serbia, Clinic for Infectious and Tropical Diseases, Belgrade Faculty of Medicine, Belgrade, Serbia.
Svetlana Petrovic, Institute for Students’ Healthcare, Belgrade, Serbia.
Ana Vidmanic, Institute of Virology, Vaccines and Sera “Torlak”, Belgrade, Serbia.
Olga Popovic, Institute of Virology, Vaccines and Sera “Torlak”, Belgrade, Serbia.
Natasa Eremic, Institute of Virology, Vaccines and Sera “Torlak”, Belgrade, Serbia.
Erin Sparrow, The World Health Organization, Geneva, Switzerland.
Guido Torelli, The World Health Organization, Geneva, Switzerland.
Muriel Socquet, PATH Switzerland, Geneva, Switzerland.
Renée Holt, PATH US, Seattle, WA, USA.
Yordanka Ilieva-Borisova, PATH US, Washington DC, USA.
Yuxiao Tang, PATH US, Seattle, WA, USA.
Francesco Berlanda Scorza, PATH US, Washington DC, USA.
Jorge Flores, PATH US, Washington DC, USA.
Niraj Rathi, PATH India, 15th Floor, Dr Gopal Das Bhawan, 28, Barakhamba Road, Connaught Place, New Delhi, Delhi 110001, India.
References
- 1. Lafond KE, Nair H, Rasooly MH, et al. Global role and burden of influenza in pediatric respiratory hospitalizations, 1982-2012: a systematic analysis. PLoS Med 2016; 13: e1001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis 2012; 54: 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dawood FS, Fiore A, Kamimoto L, et al. Influenza-associated pneumonia in children hospitalized with laboratory-confirmed influenza, 2003-2008. Pediatr Infect Dis J 2010; 29: 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378: 1917–1930. [DOI] [PubMed] [Google Scholar]
- 5. Grohmann G, Francis DP, Sokhey J, et al. Challenges and successes for the grantees and the technical advisory group of WHO’s influenza vaccine technology transfer initiative. Vaccine 2016; 34: 5420–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wahid R, Holt R, Hjorth R, et al. Chemistry, manufacturing and control (CMC) and clinical trial technical support for influenza vaccine manufacturers. Vaccine 2016; 34: 5430–5435. [DOI] [PubMed] [Google Scholar]
- 7. Unpublished studies: Immunogenicity (mice); Toxicity: single dose, repeated dose (rats); Local Tolerance (rabbits). Belgrade, Serbia: Institute of Virology, Vaccines and Sera “Torlak”, (data on file), 2014. –2015. [Google Scholar]
- 8. Stevanovic G, Lavadinovic L, Filipovic Vignjevic S, et al. Safety and immunogenicity of a seasonal trivalent inactivated split influenza vaccine: a phase I randomized clinical trial in healthy Serbian adults. Hum Vaccin Immunother 2018; 14: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines. CPMP/BWP/214/96. The European Agency for the Evaluation of Medicinal Products (EMEA), 1997. [Google Scholar]
- 10. European Medicines Agency. EMA/CHMP/VWP/457259/2014 committee for medicinal products for human use. Guideline on influenza vaccines non-clinical and clinical module, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500211324.pdf (2016, accessed 11 September 2017).
- 11. Plotkin S, Robinson JM, Cunningham G, et al. The complexity and cost of vaccine manufacturing - an overview. Vaccine 2017; 35: 4064–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee GM, Greene SK, Weintraub ES, et al. H1N1 and seasonal influenza vaccine safety in the vaccine safety datalink project. Am J Prev Med 2011; 41: 121–128. [DOI] [PubMed] [Google Scholar]
- 13. Delore V, Salamand C, Marsh G, et al. Long-term clinical trial safety experience with the inactivated split influenza vaccine, Vaxigrip. Vaccine 2006; 24: 1586–1592. [DOI] [PubMed] [Google Scholar]
- 14. Sequirus. Prescribing Information: Afluria, http://labelingseqiruscom/PI/US/Afluria/EN/Afluria-Prescribing-Informationpdf (accessed 19 July 2018).
- 15. FLUARIX QUADRIVALENT (Influenza Vaccine). Prescribing information: Fluarix Quadrivalent, Germany: GlaxoSmithKline Biologicals, https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm220624.pdf (accessed 19 July 2018).
- 16. Radovanov JMV, Hrnjaković I, Petrović V, et al. Influenza A and B Viruses in the population of Vojvodina, Serbia. Arch Biol Sci, Belgrade 2014; 66: 43–50. [Google Scholar]
- 17. Aliabadi N, Tate JE, Parashar UD. Potential safety issues and other factors that may affect the introduction and uptake of rotavirus vaccines. Clin Microbiol Infect 2016; 22(Suppl. 5): S128–S135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_tables for Safety and immunogenicity of a seasonal trivalent inactivated split influenza vaccine: a double blind, phase III randomized clinical trial in healthy Serbian adults by Goran Stevanovic, Aleksandar Obradovic, Snezana Ristic, Dragan Petrovic, Branislava Milenkovic, Danilo Mitrovic, Svetlana Filipovic Vignjevic, Katarina Ilic, Vera Stoiljkovic, Lidija Lavadinovic, Mijomir Pelemis, Svetlana Petrovic, Ana Vidmanic, Olga Popovic, Natasa Eremic, Erin Sparrow, Guido Torelli, Muriel Socquet, Renée Holt, Yordanka Ilieva-Borisova, Yuxiao Tang, Francesco Berlanda Scorza, Jorge Flores and Niraj Rathi in Therapeutic Advances in Vaccines and Immunotherapy