Abstract
Hepatocellular carcinoma (HCC) is one of the most severe diseases worldwide. For the different stages of HCC, there are different clinical treatment strategies, such as surgical therapy for the early stage, and transarterial chemoembolization (TACE) and selective internal radiation therapy (SIRT) for intermediate-stage disease. Systemic treatment, which uses mainly targeted drugs, is the standard therapy against advanced HCC. Sorafenib is an important first-line therapy for advanced HCC. As a classically effective drug, sorafenib can increase overall survival markedly. However, it still has room for improvement because of the heterogeneity of HCC and acquired resistance. Scientists have reported the acquired sorafenib resistance is associated with the anomalous expression of certain genes, most of which are also related with HCC onset and development. Combining sorafenib with inhibitors targeting these genes may be an effective treatment. Combined treatment may not only overcome drug resistance, but also inhibit the expression of carcinoma-related genes. This review focuses on the current status of sorafenib in advanced HCC, summarizes the inhibitors that can combine with sorafenib in the treatment against HCC, and provides the rationale for clinical trials of sorafenib in combination with other inhibitors in HCC. The era of sorafenib in the treatment of HCC is far from over, as long as we find better methods of medication.
Keywords: combination, hepatocellular carcinoma, inhibitor, resistance, sorafenib
Background
Humans have not yet overcome cancer, which is the second most common disease causing death worldwide. Liver cancer is the sixth most common malignancy among all types of cancer. Liver cancer with a 5-year survival of 18% is the second most lethal tumor after pancreatic cancer. In 2018, there were 841,080 new liver cancer cases and 781,631 deaths. WHO estimates that more than 1 million patients will die from liver cancer in 2030. The most common type of liver cancer is hepatocellular carcinoma (HCC). In 2012, 782,000 patients were diagnosed with HCC and 746,000 patients died from it.1–4
For patients with early stage HCC [Barcelona Clinic Liver Cancer (BCLC) stage 0/A], surgical therapy is the main choice. Usually, resection of tumors can result in more than 60% survival at 5 years. Some patients who are not candidates for resection because of tumor location or other reasons can also choose liver transplantation if they have a limited tumor burden. Ablation is another treatment for patients with BCLC stage 0/A disease. For patients with intermediate-stage (BCLC stage B), transarterial chemoembolization (TACE) and selective internal radiation therapy (SIRT) are the main treatment methods. For the patients who have advanced HCC (BCLC stage C), whose tumors cannot be resected, systemic therapies are recommended. Systemic therapies are based on targeted drugs.1,4,5
Sorafenib is the first United Stated Food and Drug Administration (US FDA)-approved first-line systemic therapy, and is also the standard therapeutic agent for advanced HCC. In fact, until lenvatinib was approved as a frontline therapy in 2018, sorafenib was the only first-line therapy in the last 10 years. It is a multi-kinase inhibitor targeting vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR), and is also an activator of AMP-activated protein kinase (AMPK), which can suppress tumors.6 Sorafenib brings an obvious survival benefit to patients with HCC. According to two clinical trials, sorafenib could improve overall survival (OS) significantly compared with placebo (10.7 months versus 7.9 months, p < 0.001; and 6.5 months versus 4.2 months, p < 0.001).6,7
However, there are several factors that prevent more patients benefitting from sorafenib (Figure 1). Only about 40% of patients with HCC can benefit from sorafenib because of the genetic heterogeneity of HCC and other reasons. Some studies showed that sorafenib had more benefits for specific groups of patients. The two clinical trials above involved strictly selected patients, that is, patients with well-preserved liver function, and these patients were named SHARP eligible patients (named after the original SHARP clinical trial). A retrospective study showed sorafenib could only benefit SHARP eligible patients.8 Besides, the benefit from sorafenib is higher in patients with hepatitis C patients than in others.9 However, other groups are resistant to sorafenib from the beginning. This phenomenon is also named primary resistance, whose mechanism is still unknown. However, some studies have revealed possible reasons. Gene polymorphism may be a key factor that can influence the function of sorafenib. Scientists have showed that polymorphisms of ATP binding cassette (ABC) subfamily B member 1 (ABCB1), ATP binding cassette subfamily G member 2 (ABCG2), endothelial nitric oxide synthase (eNOS), and solute carrier family 15 member 2 (SLC15A2) may be associated with the effect of sorafenib.10–12 Silvia et al. showed that using β-caryophyllene oxide can inhibit ABC proteins and induce the chemosensitization of HCC cells to sorafenib.13 However, there is insufficient evidence indicating an exact relationship between these factors and the response to sorafenib. A phase III trial showed that none of ten common biomarkers could predict the response of a patient with HCC to sorafenib.14
Figure 3.
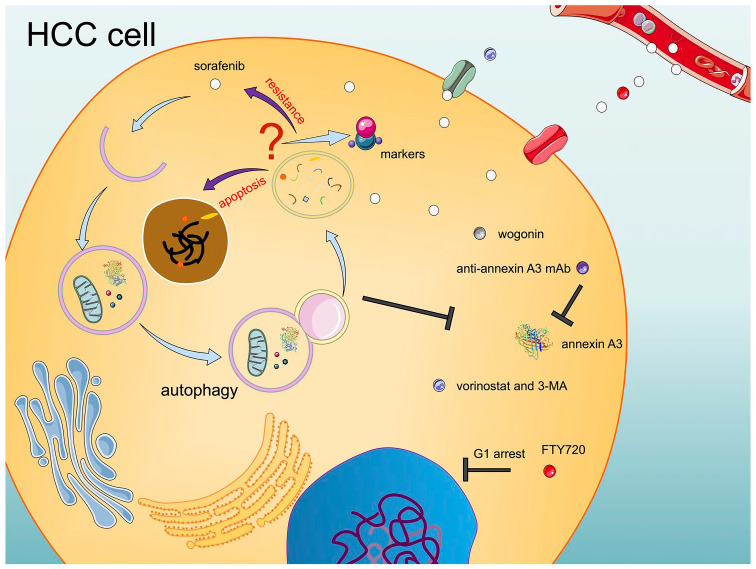
Roles and solutions of autophagy in HCC development and sorafenib resistance. The role of autophagy in HCC development and sorafenib resistance remains controversial. Most studies showed that inhibiting autophagy could enhance the effect of sorafenib through multiple pathways. A few research studies have reported that autophagy can induce cell apoptosis and plays a synergistic role with sorafenib.
HCC, hepatocellular carcinoma; 3-MA, 3-methyladenine; mAb, monoclonal antibody.
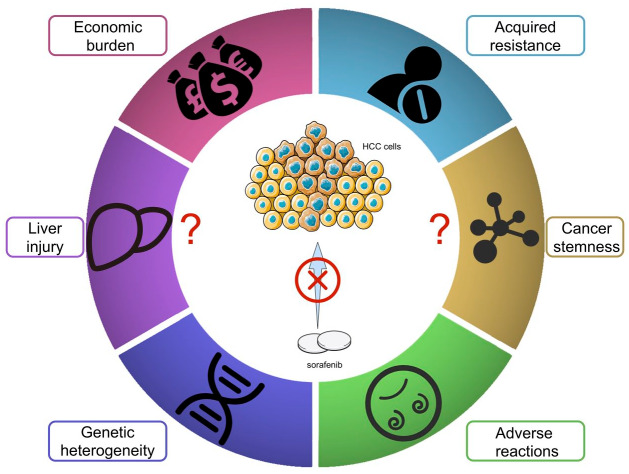
Figure 1.
Factors preventing more patients benefitting from sorafenib. To date, about six factors have been identified to interfere with the effect of sorafenib. Economic burden, acquired resistance, genetic heterogeneity, and adverse reaction are widely accepted factors. The liver is the main metabolic site of sorafenib; therefore, the status of the liver can also influence the effect of sorafenib. Sorafenib cannot kill cancer stem cells effectively; therefore, the existence of cancer stemness is another important factor.
HCC, hepatocellular carcinoma.
Apart from the low response rate, another common problem of sorafenib is the acquired resistance of HCC cells, and patients who are sensitive to sorafenib at the beginning usually develop resistance within 6 months.6,15 These shortcomings, plus the emergence of new drugs, have made scientists propose that the era of sorafenib is over. These weaknesses of sorafenib have prompted many researchers to find novel and effective methods to treat HCC using sorafenib. One important solution is to identify the genetic changes before and after sorafenib resistance, and then use drugs targeting these molecules. Scientists have shown that several pathways, such as glycolysis and autophagy, are related to resistance to sorafenib.16,17 Meanwhile, most targets that are related to resistance are also associated with HCC development. Thus, combinations of sorafenib and other drugs might play synergistic roles, which represent a novel strategy against HCC. This review focuses on the combinations of sorafenib with other inhibitors to treat HCC via increasing the sensitivity to sorafenib and enhance the effect of therapy. We summarize the preclinical and clinical trials, and provide a theoretical basis for the treatment of HCC.
Glycolysis-related HCC development and sorafenib resistance
Glycolysis is the main source of energy for cancer cells. In normal cells, the energy source is glucose oxidative phosphorylation (OXPHOS).18 OXPHOS involves slower ATP production compared with glycolysis. Therefore, glycolysis can support the faster growth of more tumor cells.19 This phenomenon, named the Warburg effect, was reported to be related closely to cell proliferation and drug resistance.16 Sorafenib can inhibit angiogenesis, which will induce hypoxia and glycolysis. Therefore, combinations of sorafenib and glycolytic inhibitors could significantly reduce sorafenib resistance, suppress cell reproduction, and improve the effect of killing HCC cells. This part mainly summarizes studies of the combinations of sorafenib and glycolytic inhibitors to treat HCC.
Glycolysis inhibitors that function by activating AMPK
As the main source of energy in cancer cells, glycolysis produces ATP and promotes the growth and reproduction of tumor cells. Suppressing this metabolic progress can slow down the growth rate of tumor cells. Besides, a central metabolic switch, AMPK, is also activated by an increased AMP/ATP ratio, which can be induced by the absorption of glycolytic inhibitors. AMPK can promote catabolic pathways and inhibit cell proliferation. Meanwhile, AMPK can also inhibit the function of mammalian target of rapamycin (mTOR), which is also closely related to drug resistance.20
Combining sorafenib with the glycolytic inhibitor 2-deoxyglucose (2DG) could drastically inhibit the viability of HCC cells, including sorafenib-sensitive and -resistant cells. The mechanism is that the combination of the two drugs inhibits ATP production, and then activates AMPK, which inhibits mTOR, and finally suppresses the cell cycle.21 Tomizawa et al. also revealed that 2DG combined with sorafenib could also suppress the motility of HCC cells.22 Similarly, all-trans retinoic acid (ATRA) can sensitize HCC cells to sorafenib by inhibiting glycolysis and activating AMPK pathways.23
Glycolysis inhibitors that function by inhibiting HIFs
Hypoxia-inducible factors (HIFs) including HIF-1α and HIF-2α are important transcription factors that regulate the induction of several enzymes involved in glycolysis. In cancer cells, increased HIFs and glycolysis activities are observed, which help tumors grow.24,25 As important regulators of glycolysis, HIFs can be ideal targets in the glycolysis-related treatment against HCC. Meanwhile, apart from glycolysis, HIFs are also involved in sorafenib resistance through multiple other downstream factors. Some autophagy processes in HCC cells depend on the stabilization of HIFs. Autophagy was widely reported as being able to promote tumor growth and drug resistance.26 HIFs can also stabilize multidrug resistance protein 1 (MDR1), glucose transporter 1 (GLUT-1), galectin-1, and other drug-resistance-associated proteins and induce sorafenib resistance.27–30 Besides, some upstream factors of HIFs can regulate the expression of HIFs and induce sorafenib resistance. Qiu et al. reported that 14-3-3η can promote the function of HIF-1α and maintain sorafenib resistance in HCC.31 A similar molecule is β-2 adrenergic receptor (ADRB2).32 In a word, HIFs and their upstream and downstream factors are closely related to sorafenib resistance. Some inhibitors can suppress HIFs and related factors, and combinations of sorafenib and these inhibitors can treat HCC via multiple targets.
Li et al. found a natural anti-tumor compound, genistein, that could suppress the levels of HIFs and glycolysis. Genistein combined with sorafenib increased the sensitivity of sorafenib-resistant cells or mice to sorafenib.33 The natural products Rhizoma Paridis saponins (RPS) can also suppress the levels of HIFs and glycolysis, and suppresses lipid synthesis. Thus, RPS combined with sorafenib could improve the effect of sorafenib in the treatment of HCC cells.34 Melatonin was reported to improve HCC cells sensitivity to sorafenib by inhibiting HIF-1α and mTOR.35 Other HIFs/glycolysis axis inhibitors that can improve sorafenib effect include: EF24,28 metformin,36 2-methoxyestradiol37 and PT-2385.38 It is worth mentioning that ICI118551, the inhibitor of ADRB2 mentioned above, can inhibit HIF-1α through inhibiting ADRB2, and thus play a synergistic role with sorafenib.
OXPHOS activators
Notably, most current research has focused on inhibitors targeting glycolysis rather than activators targeting OXPHOS. Shen et al. reported that by reducing lactate production and increasing reactive oxygen species (ROS) and ATP, dichloroacetate (DCA) could activate OXPHOS and inhibit glycolysis simultaneously. This combination can also induce the reversal of sorafenib resistance similarly to glycolytic inhibitors.39 There are few studies on this aspect and it is expected to become a research hot spot in the future. Drugs that can both inhibit glycolysis and activate OXPHOS might be more effective in the treatment of HCC.
Other glycolysis inhibitors
There are also some inhibitors can target glycolysis directly. Sorafenib combined with the β-catenin inhibitor FH535 could simultaneously suppress mitochondrial respiration and glycolytic flux, which plays an important role in cell proliferation and apoptosis.40 Using resveratrol can reduce the expression of hexokinase 2 (HK2), which plays a key role in glycolysis. Thus, combining resveratrol with sorafenib could potentially target sorafenib-resistant HCC cells.41
Summary and discussion
Changes to the metabolic pathways in tumor cells have been recognized as a feature of malignancies, and the Warburg effect is representative of these changes. Glycolysis, which can be activated using sorafenib, is closely related to hepatocarcinogenesis and drug resistance. Most research attempting to inhibit glycolysis showed the feasibility of combining sorafenib and glycolysis inhibitors. However, little research has investigated restoring OXPHOS in tumor cells. Theoretically, targeting OXPHOS is another strategy to solve the problems with sorafenib. OXPHOS depends on mitochondrial function, and activation of OXPHOS also switches on the mitochondria-mediated apoptosis pathway, whereas mitochondrial inhibition induces cell proliferation and cyclin D1 expression.42 Therefore, targeting both OXPHOS and glycolysis is expected to be a reliable way to treat HCC and ameliorate sorafenib resistance. In addition to DCA, Sun et al. showed that overexpression of encoding pyruvate dehydrogenase E1 alpha 1 subunit (PDHA1) could inhibit glycolysis and activate OXPHOS simultaneously, and then enhance the mitochondria-mediated apoptosis pathway.43 Thus, PDHA1 may be a potential target for new drugs.
PI3K/AKT/mTOR-related HCC development and sorafenib resistance
The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mTOR pathway is an important metabolic pathway in cells. It is related closely to cell proliferation, cell growth, protein translation, and other important life processes in organisms. This pathway is overactivated in 30–50% of patients with HCC, and is associated with the occurrence and development of HCC.44–46 Inhibiting the PI3K/AKT/mTOR pathway is a vital anti-cancer therapy, and inhibitors targeting the pathway have been confirmed as an effective way to treat HCC.47
Sorafenib has been reported to suppress cell proliferation and cell growth by inhibiting the PI3K/AKT/mTOR pathway; however, the inhibition of this pathway is not dominant in sorafenib therapy.48,49 Interestingly, research has shown that this pathway is reactivated in sorafenib-resistant cells.50–52 Simultaneously, sorafenib can downregulate mTOR, and then induce autophagy. Autophagy is also a potential cause of resistance to sorafenib.17 Thus, the PI3K/AKT/mTOR pathway is instrumental in HCC and sorafenib resistance. Combinations of sorafenib with inhibitors targeting this pathway are potential treatments for HCC (Figure 2).
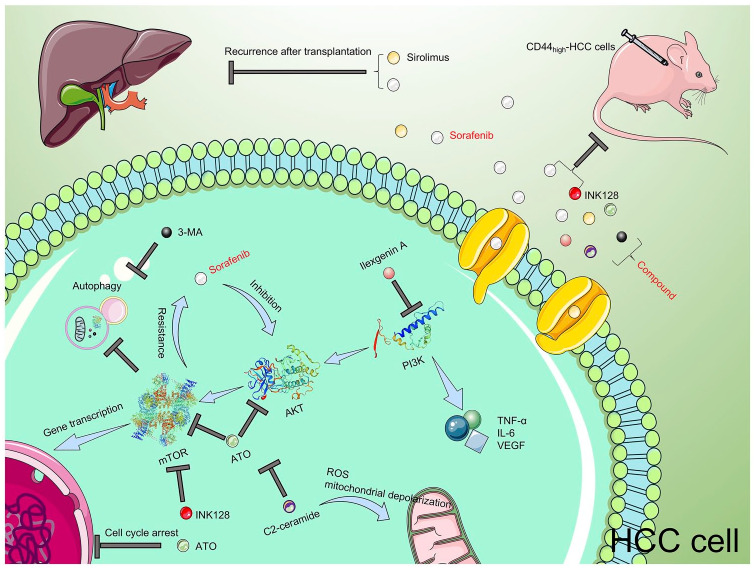
Figure 2.
Theoretical research and clinical application of PI3K/AKT/mTOR inhibitors and sorafenib. The PI3K/AKT/mTOR signal pathway plays a key role in the occurrence and development of HCC, and resistance to sorafenib. Combinations of PI3K/AKT/mTOR inhibitors and sorafenib showed satisfactory results in the treatment of HCC. The two inhibitors above plus 3-MA (an autophagy inhibitor) demonstrated a better treatment effect. As a type of immune inhibitor, mTOR inhibitors combined with sorafenib may also prevent post-transplant HCC.
AKT, phosphatidylinositol 3-kinase (PI3K)/protein kinase B; ATO, arsenic trioxide; HCC, hepatocellular carcinoma; IL-6, interleukin-6; INK128, Sapanisertib; 3-MA, 3-methyladenine; mTOR, mammalian target of rapamycin; ROS, reactive oxygen species; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
PI3K/AKT/mTOR pathway inhibitors
Cotreatment with C2-ceramide and sorafenib could significantly enhance ROS levels and mitochondrial depolarization, which cause caspase-dependent cell apoptosis. An intensive study found that the phenomenon is induced by targeting the PI3K/AKT/mTOR pathway and the extracellular regulated protein kinase (ERK) signaling pathway. This combination therapy could markedly suppress cell growth, cell migration, and cell proliferation in HCC.53 Badawi et al. found that cluster differentiation 44 (CD44) could enhance cellular proliferation and migration in HCC cells, and CD44 is also overexpressed in sorafenib-resistant cells. INK128 is an ATP-competitive mTOR inhibitor, and INK128 combined with sorafenib was efficacious at blocking tumor growth in CD44high xenograft mice.54 However, the relationship between CD44 and mTOR should be explored further. Arsenic trioxide (ATO) given in combination with sorafenib can act synergistically to treat HCC by inhibiting AKT activation. Meanwhile, ATO also inhibits the activation of AKT downstream factors, including mTOR. Besides, both ATO and sorafenib could suppress the expression of cyclin D1, causing cells to arrest at the G0/G1 phase. Thus, this therapy is expected to represent a new type of HCC treatment.52 Ilexgenin A downregulates the levels of inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and vascular endothelial growth factor (VEGF) by inhibiting signal transducer and activator of transcription 3 (STAT3) and PI3K pathways. Ilexgenin A combined with sorafenib is a promising therapeutic candidate that could modulate inflammation, angiogenesis, and HCC growth, as well as treating the hepatotoxicity caused by sorafenib.55
In addition to the above-mentioned combinations, there are also many inhibitors that play a synergistic effect with sorafenib by inhibiting the PI3K/AKT/mTOR pathway, including emodin,56 SB-3CT,57 20(S)-Ginsenoside Rg3,58 bufalin,59 capsaicin,60 silibinin,61,62 amentoflavone,63 MLN8237,64 KU-55933,65 melatonin,35 PT-2385,38 Valproic acid,66 PF-03084014,67 and ARQ 092.68
Multiple inhibitors targeting more targets to improve the curative effect
Notably, mTOR inhibitors combined with sorafenib might induce high level of autophagy, and autophagy could cause resistance. Suppressing autophagy on the basis of the combinations of sorafenib and mTOR inhibitors might be a solution. Ling et al. found that the anti-type II diabetes agent metformin could suppress mTORC1, one component of mTOR, while activating mTORC2. Sorafenib effectively reverses the activation status of mTORC2 when combined with metformin. However, the suppression of mTORC1 induces autophagy, which interferes with the treatment effect. Using a pharmacological inhibitor of autophagy, such as 3-methyladenine (3-MA) or chloroquine, could sensitize HCC cells to sorafenib and metformin. Thus, anti-autophagy treatment might be considered in sorafenib and mTOR inhibitor-based therapy.69 Similarly, targeting AKT could significantly suppress the growth of sorafenib-resistant cells; however, inhibiting AKT activates the mesenchymal–epithelial transition factor (c-Met) pathway, which also induces drug resistance. Therefore, using an AKT inhibitor and a c-Met inhibitor might be a feasible solution. Han et al. combined the AKT inhibitor MK2206 with the c-Met inhibitor capmatinib in sorafenib-resistant HCC cells and xenografts in mice, and found that the combination had a significant anti-cancer effect.70 Although the authors did not combine the three inhibitors together to treat HCC, it represents a guide for future research and clinical trials. Many inhibitors that target this pathway showed effects in the preclinical stage; however, they require further testing and exploration in clinical trials.
Clinical intervention
Inhibitors of mTOR combined with sorafenib have been tested in some clinical trials. Everolimus is a potent mTOR inhibitor, and Koeberle et al. performed a randomized multicenter, multinational phase II trial concerning sorafenib with or without everolimus in HCC. The OS when using sorafenib alone was 10 months, whereas the combination resulted in an OS of 12 months. The authors concluded that there was no evidence proving that the combination could improve efficacy compared with sorafenib alone. Meanwhile, the combination was more toxic than sorafenib alone.71 A phase I study produced similar results.72 One early-stage clinical trial pointed out that the combination of sorafenib and temsirolimus was safe, but the effect of the combination was similar to sorafenib alone.73 Based on current evidence, further testing of these drug combinations in HCC is unwarranted. New drug combinations still need to be explored.
As classical immune inhibitors, mTOR inhibitors are used widely after liver transplantation. One of the most severe problems of liver transplantation is the recurrence of HCC. Several cases using sorafenib and mTOR inhibitors together showed surprising results in the treatment against post-transplant HCC, and provided a novel method in the post-transplant drug delivery strategy. Wang et al. reported a patient with HCC with pulmonary recurrence after liver transplantation. The patient was treated with sorafenib after relapse; however, his α-fetoprotein (AFP) continued rising and HCC was still developing. Given the positive data of mTOR inhibitors in HCC, the patient’s immunosuppressant was switched from tacrolimus to sirolimus (an mTOR inhibitor). Dramatically, since then, there has been no evidence of HCC progression. This case showed us that a combination of an mTOR inhibitor and sorafenib might be useful in the treatment of post-transplant HCC.74 Another case also showed a synergistic effect of this combination, resulting in a complete radiological response.75 There was also a small retrospective study, and the result was consistent with cases above: the coadministration of sorafenib and mTOR inhibitors could be effective in the treatment against recurrent HCC after liver transplantation.76 Though there is no large trial confirming this conclusion further, the cases and study above provided the possibility of treating recurrent HCC; the strategy still needs further investigation.
Summary and discussion
The PI3K/AKT/mTOR pathway plays different roles at diverse stages in HCC cells. At the beginning, when HCC cells are still sensitive to sorafenib, sorafenib can downregulate the PI3K/AKT/mTOR pathway; however, this action also induces the activation of autophagy. The role of autophagy in drug resistance remains controversial; however, most evidence shows that autophagy can induce drug resistance. Briefly, the use of sorafenib might actuate HCC cells from sorafenib-sensitive to sorafenib-resistant via autophagy. In sorafenib-resistant cells, the PI3K/AKT/mTOR pathway is reactivated, which proves the feasibility of the combinations of sorafenib and PI3K/AKT/mTOR inhibitors. Considering the heterogeneity of HCC cells, using sorafenib, PI3K/AKT/mTOR inhibitors, and autophagy inhibitors simultaneously could kill more HCC cells at different stages, which would benefit more patients.
Autophagy-related HCC development and sorafenib resistance
Autophagy is a mechanism for the degradation of unwanted or damaged organelles in cells. Autophagy is important to maintain cellular homeostasis. Various stimulations can induce autophagy, such as drug treatment and nutrient deprivation.77 Based on current research, the roles of autophagy in the progression of cancer and the reaction to drugs remain controversial. Research has shown that sorafenib can induce autophagy in HCC.78,79 Most studies have demonstrated that autophagy contributes to the resistance of HCC cells to sorafenib, whereas a few scientists hold the opinion that autophagy can enhance the toxicity of sorafenib.17,80 Based on these theories, the combinations of drugs are also divided into two groups; those that activate autophagy and those that inhibit autophagy (Figure 3).
Most research concluded that autophagy leads to drug resistance. Rong et al. found that wogonin combined with sorafenib could suppress the autophagy induced by sorafenib, and enhance cell death.81 Annexin A3, which is enriched in sorafenib-resistant HCC cells, can induce autophagy for cell survival. This contributes to the sorafenib resistance of HCC cells. Using an anti-annexin A3 monoclonal antibody (mAb) could significantly increase HCC cell sensitivity to sorafenib. Another study also stated that annexin A3 could be a potential target for immunotherapy to treat HCC.82,83 Ahmed et al. combined sorafenib with FTY720 (Fingolimod, used to treat multiple sclerosis), and found this combination could markedly enhance HCC cell apoptosis, and could result in G1 arrest. The phenomenon is related to the inhibition of sorafenib-induced autophagy by FTY720.84 In addition, based on the combination of sorafenib and vorinostat, a histone deacetylase (HDAC) inhibitor, autophagy inhibitor 3-MA could cause further inhibition of HCC cells, which demonstrated that inhibiting autophagy could enhance the synergistic effect of the combination of sorafenib and vorinostat.85
Almost all studies showed that autophagy has a negative effect on treatment of HCC with sorafenib; however, one study by Tai et al. reported that autophagy induced by sorafenib could increase the cell death rate.80 Although the authors did not conduct a study on the combinations of sorafenib and autophagy activators, this conclusion provides a new perspective that requires further research to determine the role of autophagy in the therapeutic intervention in HCC by sorafenib.
Autophagy plays roles in both cell survival and cell death. Similarly, autophagy also plays a dual role in drug resistance. The function of autophagy depends on the tumor type and the treatment characteristics.86 There are only a few reports in various tumors of the anti-tumor effect of excessive autophagy induced by targeted drugs. For HCC, only one study mentioned the positive role of autophagy in treatment against HCC. Lee et al. suggested that an HDAC inhibitor, suberoylanilide hydroxamic acid (SAHA), represents a new strategy to treat tamoxifen-resistant human breast cancer. The mechanism is that SAHA increase the expression of autophagic cell death markers, LC3-II and beclin-1, which induces autophagic cell death.87 Similar results were found in colon cancer cells.88 However, there has been no further research on this drug combination.
Almost all studies stand by the opinion that autophagy induces drug resistance. Apart from the combinations of sorafenib and autophagy inhibitors in HCC mentioned above, there are also many reports in other kinds of tumors, for example, gemcitabine and 3-MA in lung cancer.89 These studies combined two or more inhibitors together and showed ideal results, and are expected to contribute to the treatment of cancer in the future.
In conclusion, it could be speculated that autophagy participates mainly in sorafenib resistance, and, in only very few cases, excessive autophagy can induce cell death. This is consistent with the duality of autophagy in cell survival. Some reports showed there are different markers in the stages of autophagy and subsequent apoptosis in sorafenib-treated HCC cells. Autophagy has been generally acknowledged to suppress tumor by inhibiting inflammation. So, changes in some inflammation-related factors can be markers of the roles of autophagy in suppressing tumor. HIFs and reactive oxygen species (ROS) have been reported related with tumor-promoting role of autophagy.26,90 Another study found the dynamic features of specific markers of autophagy after treating sorafenib, and revealed important roles of endoplasmic reticulum stress in the transformation of autophagy to excessive autophagy.91 So, finding specific markers may clarify specific roles of autophagy in sorafenib resistance and provide better treatment strategies.
Defining the role of autophagy in drug resistance and cancer treatment, and determining the conditions leading to the appearance of excessive autophagy are challenges in future research.
Immune checkpoint-related HCC development
Inhibiting immune checkpoints is a novel and promising way to treat HCC and other cancers. Two inhibitors of immune checkpoint, nivolumab, and pembrolizumab, have been approved as second-line treatments for HCC by the US FDA. They showed satisfactory response rate according to phase I/II clinical trials.92,93 In following phase III trials, though the primary endpoints did not achieve statistical significances both in CheckMate 459 (nivolumab versus sorafenib in first-line) and KEYNOTE-240 (pembrolizumab versus placebo in second-line), nivolumab and pembrolizumab still showed some beneficial improvements compared with sorafenib and placebo. The effects of nivolumab and pembrolizumab in phase III trials still need further research.94,95
Though immune checkpoint inhibitors showed meaningful results in some clinical trials, the effects of them might be influenced by immunosuppression.96 Scientists have proven that the VEGF/VEGFR pathway leads to immunosuppression, such as inhibition of the maturation of dendritic cells (DCs) and accumulation of immunosuppressive inflammatory cells.97,98 Inhibiting the VEGF/VEGFR pathway using sorafenib could modulate the immune microenvironment and enhance the activity of inhibitors of immune checkpoints. Theoretically, combinations of sorafenib and immune checkpoint inhibitors could have synergistic roles.
Currently, there are only a few reports on this aspect. Chen et al. found that inhibition of programmed cell death 1 (PD-1) alone has anti-cancer activity; however, the combination of anti-PD-1 antibodies, sorafenib, and AMD3100, which targets hypoxia, showed additional anti-cancer activity. However, anti-PD-1 antibodies combined with sorafenib alone did not have this effect.99 These results encourage further research into combinations of sorafenib and immune checkpoint inhibitors.
There has also been a clinical case of the use of sorafenib and immune checkpoint inhibitors. Adcock et al. reported that a patient showed a significant response after consecutive treatment with transarterial radioembolization (TARE), sorafenib, and nivolumab. The authors speculated that TARE and sorafenib might create an advantageous environment for immunogenic cell death, which subsequently enhanced the effect of nivolumab.100 This is consistent with the theory about the function of sorafenib in the immune microenvironment, as mentioned above. Although there is no report on simultaneous cotreatment, the evidence provides a new direction for targeted therapy and immunotherapy in HCC.
Sorafenib is an inhibitor targeting VEGF/VEGFR pathway. Recently, a phase III clinical trial showed exciting progress in the combination therapy for HCC. The phase III IMbrave150 study showed the combination of atezolizumab (targeting programmed cell death ligand 1, PD-L1) and bevacizumab (targeting VEGF) can improve OS significantly compared with sorafenib [not evaluable (NE) versus 13.2 months].101 This result showed a positive effect and it was consistent with the previous phase I study whose objective response rate (ORR) was 36%.102 Gratifyingly, tolerable safety profiles were observed in both studies and no new safety signal was identified. This combination is the first strategy that showed a better effect than sorafenib in phase III trials in advanced HCC. And it may be a promising treatment for patients with HCC because of its effect and safety.
Another phase Ib trial of the combination of lenvatinib (targeting VEGFR) and pembrolizumab (targeting PD-1) also showed promising antitumor activity in HCC; ORR was 44.8% compared with using lenvatinib alone (24.1%) in another study. An acceptable safety profile was also observed.103,104 A phase III trial of this combination is also ongoing (NCT03713593). These results show the great potential of the combinations of VEGF/VEGFR inhibitors and immune checkpoint inhibitors. In addition, there are also several ongoing phase III clinical trials of combinations of VEGF/VEGFR inhibitors and immune checkpoint inhibitors. These combinations include: apatinib and SHR-1210 (NCT03764293); IBI305 and sintilimab (NCT03794440); bevacizumab and durvalumab (NCT03847428); and cabozantinib and atezolizumab (NCT03755791).
Therapy using combinations of VEGF/VEGFR inhibitors with immune checkpoint inhibitors has showed significant effects in several kinds of tumors, including HCC. Sorafenib is a representative drug targeting VEGFR; however, there have been few studies about sorafenib plus immune checkpoint inhibitors to treat HCC. We speculate that using immune checkpoint inhibitors could be an important method to allow more patients with HCC to benefit from sorafenib. In summary, targeting immune checkpoints and VEGF/VEGFR is expected to become a treatment with great potential against HCC. These molecular pathways have complex connections; therefore, targeting multiple targets together might produce better treatment results.
Other pathways-related HCC development and sorafenib resistance
The targets above are important factors in the development of HCC. They also play important roles in the resistance of HCC cells to sorafenib. In addition, there are other pathways related to HCC and sorafenib resistance.
Wnt/β-catenin
The Wnt/β-catenin pathway is a de-regulation pathway in HCC and is one of the pathways that is difficult to treat.105 This pathway is also reported to be closely linked to sorafenib resistance in HCC.106,107 Scientists combined sorafenib with inhibitors targeting the Wnt/β-catenin pathway and observed positive therapeutic effects. Galuppo et al. found that the Wnt/β-catenin inhibitor FH535 combined with sorafenib could produce a synergistic effect in HCC, and induce apoptosis in HCC cell lines.108 Other Wnt/β-catenin inhibitors that can be combined with sorafenib to treat HCC are ICG-001,107 pargyline, GSK2879552,106 iCRT3,109 and destruxin B.110
c-Met/HGF
c-Met is a receptor tyrosine kinase that is involved in epithelial–mesenchymal transition. It is regulated by hepatocyte growth factor (HGF). Overexpression of c-Met increases cell proliferation, survival, mobilization, and invasiveness. Aberrant c-Met/HGF activity is associated with the development of HCC and resistance to sorafenib.70,111 Thus, the c-Met/HGF pathway is a potential target to treat HCC, and combining sorafenib with c-Met/HGF inhibitors may be an effective strategy against HCC. Sorafenib and vitamin K can have a synergistic effect on the suppression of HCC cell migration and metastasis by inhibiting the c-Met/HGF pathway, and could suppress HCC cell growth. These conclusions provided a theoretical basis for subsequent clinical trials.112,113 Jiang et al. found a novel c-Met inhibitor, DE605, which has a synergistic effect with sorafenib. They detected that DE605 could inhibit c-Met, but activate the fibroblast growth factor receptor 3 (FGFR3)/ERK pathway, which is inhibited by sorafenib. This explained the synergistic effect of the combination in suppressing HCC.114
Tivantinib, an inhibitor targeting c-Met, has been proved to play a synergistic role with sorafenib in treating HCC in a phase I trial. This therapy strategy showed preliminary evidence of anticancer activity in 10% of HCC patients, including those refractory to sorafenib. Though there was no phase II/III trial of the combination of tivantinib and sorafenib in HCC, this result still indicated the feasibility of the combination strategy and provided impetus for further research.115
HDAC
HDAC is a novel target in the treatment of cancer. High HDAC activity correlates with higher incidence of HCC. HDAC inhibitors have been proven as a novel treatment against cancer. HDAC inhibitors can induce apoptosis, autophagy, differentiation, and inhibition of tumor vascularization via different mechanisms.116,117 HDAC is frequently involved in resistance to anti-cancer drugs.118 Therefore, it is vital to explore combinations of HDAC inhibitors with other anti-cancer drugs such as sorafenib. Lachenmayer et al. showed that the HDAC inhibitor panobinostat could induce strong anti-tumor effects that could be enhanced by sorafenib. The combination demonstrated high preclinical efficacy, which provided the rationale for clinical trials.119 Similarly, other HDAC inhibitors, such as quisinostat,120 resminostat,121,122 and vorinostat123 can also synergistically suppress HCC together with sorafenib. As described above, the autophagy inhibitor 3-MA can enhance the toxicity of the combination of sorafenib and vorinostat.85
Resminostat is an HDAC inhibitor that has promising anti-tumor activity in HCC. A SHELTER study invested the role of resminostat in sorafenib resistance. The results showed that the OS of patients treated with the combination of sorafenib and resminostat was 8 months, compared with 4.1 months after sorafenib treatment alone.122 This trial provided evidence that using sorafenib with HDAC inhibitors can treat HCC more effectively. Further testing of this strategy appears necessary.
Clinical trials performed to date
Leveraging basic research, scientists have conducted clinical trials to test the effects of drug combinations. Most of these clinical trials are in phase I or phase II trials. Though some trials produced a positive conclusion, they still need more research because of their small sample size.124 Some trials did not set a group of sorafenib alone, and some did not calculate the OS, and these factors might lead to inaccuracy. Some drugs can inhibit HCC markedly when combined with sorafenib; however, the trials finally failed because of their poor safety. Some drugs in the trials are targeted drugs and have specific treatment mechanisms; however, for most of these drugs, the anti-tumor mechanisms have not been determined. In conclusion, in the future, more clinical trials should be conducted to find better combinations, and the mechanisms of some effective combinations should be further clarified (Table 1).
Table 1.
Recent clinical trials investigating the combinations of drugs and sorafenib in HCC.
| Drug combined with sorafenib (target) | Research stage | Dose of drug | Dose of sorafenib | OS of sorafenib | OS of combination | Result | Reference |
|---|---|---|---|---|---|---|---|
| 5-fluorouracil | phase II | 200 mg/m2 day 1–14 every 3 weeks | 400 mg twice daily | / | 13.7 months | Effective | Petrini et al.125 |
| Lenalidomide (immune system) | phase I | 10 mg daily | 400 mg daily | / | 5.9 months | Ineffective | Shahda et al.126 |
| Selumetinib (RAS/RAF/MAPK) | phase Ib | 75 mg twice daily | 400 mg twice daily | / | 14.4 months | Effective | Tai et al.127 |
| Everolimus (mTOR) | phase II | 5 mg daily | 800 mg daily | 10 months | 12 months | Ineffective | Koeberle et al.71 |
| TRC105 (CD105) | phase I | 3,6,10,15 mg/kg every 2 weeks | 400 mg twice daily | / | 15.5 months | Effective | Duffy et al.128 |
| Codrituzumab (glypican-3) |
phase Ib | 1600 mg every 2 weeks | 400 mg twice daily | / | / | Ineffective | Abou-Alfa et al.129 |
| Capecitabine | phase II | 500–850 mg/m2 daily | 200–400 mg daily | / | 12.7 months | Effective | Patt et al.124 |
| Gemcitabine | phase II | 1000 mg/m2 on day 1 and day 8 of a four-week cycle | 400 mg twice daily | / | / | Ineffective | Naqi et al.130 |
| Tivantinib (Met) | phase I | 240 mg twice daily | 400 mg twice daily | / | / | Effective | Puzanov et al.115 |
| Mapatumumab (TRAIL) | phase II | 30 mg/kg on day 1 per 21-day cycle | 400 mg twice daily | 10.1 months | 10 months | Ineffective | Ciuleanu et al.131 |
| AEG35156 (XIAP) | phase II | 300 mg weekly | 400 mg twice daily | 5.4 months | 6.5 months | Effective | Lee et al.132 |
| Resminostat (HDACs) | phase I/II | 200–600 mg daily | 400–800 mg daily | 4.1 months | 8 months | Effective | Bitzer et al.122 |
| Refametinib (MEK) | phase II | 50 mg twice daily | 600 mg daily | / | 9.6 months | Effective | Lim et al.133 |
| S-1* | phase I/II | 64 mg/m2 daily | 800 mg daily | / | 10.5 months | Effective | Ooka et al.134 |
| Trebananib (Ang-1, Ang-2) | phase II | 10 mg/kg or 15 mg/kg weekly | 400 mg twice daily | / | 11/17 months | Ineffective | Abou-Alfa et al.135 |
| Bevacizumab (VEGF) | phase I/II | phase I: 1.25 mg/kg day 1 and 15; phase II: 2.5 mg/kg weekly | phase I: 400 mg twice daily days 1–28; phase II: 200 mg daily twice days 1–28 | / | 13.3 months | Ineffective | Hubbard et al.136 |
S-1 is an anticancer drug comprising three components: Tegafur, 5-chloride-2,4-dihydroxypyridine, and oteracil potassium (molar concentration ratio = 1:0.4:1).
HCC, hepatocellular carcinoma; HDAC, histone deacetylase; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; OS, overall survival; VEGF, vascular endothelial growth factor.
Perspectives
Systemic treatment is the standard therapy against advanced HCC. This treatment depends mainly on targeted drugs. However, a key problem is that there are too few of these drugs and their effects are unsatisfactory. Sorafenib was approved as the first frontline drug against advanced HCC, and was the only one until 2018. Sorafenib has two problems; one is primary resistance, whose mechanism remains unclear, which leads to a low response rate at the start of sorafenib therapy. The other is acquired resistance, which means that, as HCC progresses, the potency of sorafenib becomes lower and lower until the cancer is completely resistant. Patients encountering these problems will be offered other treatments. Lenvatinib, which was approved as the second first-line treatment against advanced HCC, was used in a randomized phase III non-inferiority trial, which showed that it was non-inferior to sorafenib for OS in untreated advanced HCC (13.6 months with lenvatinib and 12.3 months with sorafenib). And lenvatinib showed statistically significant improvements in progression-free survival, time to progression, and ORR compared with sorafenib.103 In general, both drugs have reasonable safety profiles. For lenvatinib, more hypertension, proteinuria, dysphonia, and hypothyroidism occurred; and more palmar-plantar erythrodysaesthesia, diarrhoea, and alopecia were observed in the patients who received sorafenib. These differences can provide reference for the choices of first-line treatment and subsequent second-line treatment against HCC.
If patients cannot benefit from first-line treatments, they have to turn to second-line drugs. Up to now, there are five second-line treatments (Table 2).137 Regorafenib is a second-line drug approved by the FDA for use by patients showing progression on sorafenib therapy. It is also a multi-kinase inhibitor targeting STAT3, nuclear factor-κB (NF-κB) and other HCC-related factors.138–140 The RESORCE trial showed that regorafenib could improve OS significantly compared with placebo (10.6 months versus 7.8 months) in HCC patients progressing on sorafenib treatment. An exploratory analyses from the RESORCE trial reached the conclusion that the sequence of sorafenib followed by regorafenib for HCC may extend survival compared with sorafenib followed by placebo (26 months versus 19 months).141,142 In addition to regorafenib, another two US FDA-approved second-line drugs showed exciting therapeutic effects in phase III trials. Cabozantinib was evaluated in a population of second-line and third-line HCC patients in a trial named CELESTIAL. The OS was 10.2 months with cabozantinib and 8 months with placebo. In the subgroup of second-line patients (previous systemic therapy was only sorafenib), the OS was 11.3 months with cabozantinib and 7.2 months with placebo. These results indicate that the effect of cabozantinib is not affected by patient characteristics to some extent, and that cabozantinib may benefit more HCC patients who have disease progression after other systemic treatments.143 Ramucirumab was established in advanced HCC patients with AFP⩾400 ng/ml who had used sorafenib before in the REACH-2 trial, and the OS was 8.5 months with ramucirumab and 7.3 months with placebo. It is the first biomarker-driven drug to show positive data in a phase III trial. It was approved for use on advanced HCC patients who have an AFP⩾400 ng/ml and previously received sorafenib.144 Biomarker-driven therapy may develop a larger impact in the future. Nivolumab is an anti-PD-1 antibody, with an ORR of 20% in a phase I/II trial.92 Another anti-PD-1 antibody named pembrolizumab also showed a response rate of 17% in a phase II trial.93 And they were also approved as second-line treatment for HCC because of the meaningful results above. The effects of nivolumab and pembrolizumab have also been verified in phase III clinical trials. In the CheckMate 459 trial, OS was 16.4 months with nivolumab and 14.7 months with sorafenib. In the KEYNOTE-240 trial, OS was 13.9 months with pembrolizumab and 10.6 months with placebo. Although these results did not show statistical significance, they still showed some clinically meaningful improvements. Nivolumab and pembrolizumab demonstrated favorable safety profiles in these phase III trials, but their effects in phase III trials still need more studies.94,95 These second-line drugs also have similar shortcomings, such as low response rate and acquired resistance, and if these drugs are still not effective in some HCC patients, these patients will face the dilemma that no treatment is suitable for them. Therefore, it is urgent to develop more treatment strategies. Identifying and testing more drug combinations is feasible.
Table 2.
US FDA-approved targeted drugs for HCC.
| Drug | Approval date | Recommended dose | Target | Indication |
|---|---|---|---|---|
| Sorafenib (first-line) | 16 November 2007 | 400 mg twice daily | Multiple kinases | Unresectable HCC |
| Lenvatinib (first-line) | 16 August 2018 | 12 mg once daily (actual body weight ⩾ 60 kg); 8 mg once daily (actual body weight < 60 kg) | Multiple kinases | Unresectable HCC |
| Regorafenib (second-line) | 27 April 2017 | 160 mg once daily for the first 21 days of each 28-day cycle | Multiple kinases | HCC treated previously with sorafenib |
| Cabozantinib (second-line) | 14 January 2019 | 60 mg once daily | Multiple tyrosine kinases | HCC treated previously with sorafenib |
| Ramucirumab (second-line) | 10 May 2019 | 8 mg/kg every 2 weeks | VEGFR2 | HCC treated previously with sorafenib and AFP ⩾400 ng/mL |
| Nivolumab (second-line) | 22 September 2017 | 240 mg every 2 weeks | PD-1 | HCC treated previously with sorafenib |
| Pembrolizumab (second-line) | 9 November 2018 | 200 mg every 3 weeks | PD-1 | HCC treated previously with sorafenib |
AFP, α-fetoprotein; HCC, hepatocellular carcinoma; PD-1, programmed cell death 1; US FDA, United States Food and Drug Administration; VEGFR2, vascular endothelial growth factor receptor 2.
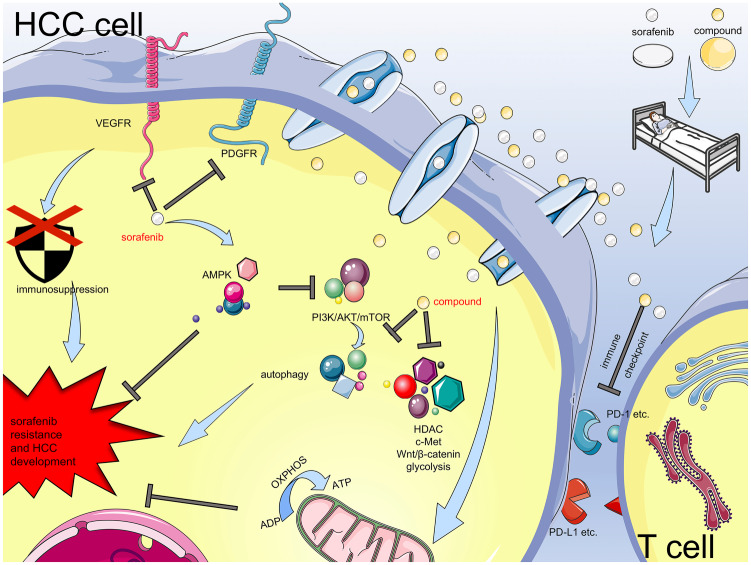
There are many reports about the combinations of sorafenib and other drugs to treat HCC (Figure 4). To date, most research has investigated small molecule targeted drugs, and several clinical trials concerning their combination with sorafenib are in progress. Inhibiting immune checkpoints has been proven as a promising method to treat HCC and other types of cancer. Theoretically, sorafenib could enhance the effects of immune checkpoint inhibitors. However, research on combining sorafenib and immune checkpoint inhibitors is still at an early stage. Notably, the combination of sorafenib and one inhibitor cannot produce satisfactory results because of complex connections between pathways; therefore, it is critical to determine their molecular mechanisms and test more combinations comprising several drugs.
Figure 4.
Mechanisms of sorafenib resistance and HCC development, and combination therapies to treat HCC. Current research about the combinations of sorafenib and other inhibitors demonstrates the feasibility of drug combination. Through mechanistic research and clinical trials, scientists can find more treatments against HCC.
Notably, although some reports did not use the identified inhibitors with sorafenib, the results showed that regulating these molecules could play synergistic roles with sorafenib. Therefore, it is worth seeking further inhibitors of these molecules in future research. Lu et al. found that cluster differentiation 24 (CD24)-induced autophagy is associated with resistance to sorafenib.145 Multidrug resistance-associated protein 3 (MRP3) is expressed at significantly higher levels in resistant clones than in parent cells, and knocking down MPR3 could reverse the sensitivity to sorafenib.146 Li et al. reported that the receptor for advanced glycation end products (RAGE) is related to the development of HCC and autophagy, inducing sorafenib resistance. Thus, RAGE might be a potential target for the treatment of HCC.147 Similarly, targeting galectin-1 (GAL-1),148 stress-inducible protein Sestrin2 (SESN2),149 a disintegrin and metalloproteinase 10 (ADAM10),150 also showed anti-tumor activity when combined with sorafenib. Our previous research showed that ubiquitin specific peptidase 22 (USP22) induces multidrug resistance of HCC via the Sirtuin 1 (SIRT1)/AKT/multidrug resistance associated protein 1 (MRP1) signaling pathway151; and recently we also explored the relationship among USP22, sorafenib resistance, and cancer stemness.152
Precision therapy is the future direction of cancer treatment. Through profiling cell line cultures’ gene expression and comparing it with that of patients with cancer, machine learning could predict the efficiency of targeted drugs. Sorafenib has been used in this research.153 Drug combination also depends on the gene expressions of different patients. Using probabilistic modeling, scientists presented an artificial intelligence method to predict different synergistic personalized two-drug combinations for different patients. This system could help more patients benefit from precision therapy.154 Surprisingly, another group developed an in silico prediction system that identifies new combinations, which solved another problem of combination therapy, finding new combinations.155 With the help of machine learning and artificial intelligence, drug combinations containing sorafenib could benefit more HCC patients.
Previous research and future prospects promise significant progress in the exploration and application of drug combinations. Machine learning predicts targets that might be involved in HCC development and drug resistance, and provides potential drug combinations. Preclinical studies show positive results in cell models and animal models, and clinical trials will confirm their validity and safety further. Finally, based on genetic testing, patients will be treated with specific drug combinations.
In summary, therapy using combinations of sorafenib and other inhibitors is a promising strategy to treat HCC. After further in-depth studies, they are expected to play important roles in fighting HCC in the future. The promising results of combination therapies mean that sorafenib has more possibilities depending on new technologies and new methods.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Our work is supported by the National Science and Technology Major Project (2017ZX100203205), National Natural Science Funds for Distinguished Young Scholars of China (81625003), Yangtze River Scholar Project, Zhejiang Provincial Natural Science Foundation of China (LQ17H160006).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Guanghan Fan  https://orcid.org/0000-0003-0107-1794
https://orcid.org/0000-0003-0107-1794
Contributor Information
Guanghan Fan, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine; NHC Key Laboratory of Combined Multi-organ Transplantation; Key Laboratory of the diagnosis and treatment of organ Transplantation, CAMS; Key Laboratory of Organ Transplantation, Zhejiang Province, Hangzhou, China.
Xuyong Wei, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine; NHC Key Laboratory of Combined Multi-organ Transplantation; Key Laboratory of the diagnosis and treatment of organ Transplantation, CAMS; Key Laboratory of Organ Transplantation, Zhejiang Province, Hangzhou, China.
Xiao Xu, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine; NHC Key Laboratory of Combined Multi-organ Transplantation; Key Laboratory of the diagnosis and treatment of organ Transplantation, CAMS; Key Laboratory of Organ Transplantation, Zhejiang Province, 79 QingChun Road, Hangzhou, 310003, China.
References
- 1. Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019; 380: 1450–1462. [DOI] [PubMed] [Google Scholar]
- 2. Tang A, Hallouch O, Chernyak V, et al. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY) 2018; 43: 13–25. [DOI] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 4. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018; 391: 1301–1314. [DOI] [PubMed] [Google Scholar]
- 5. Marquardt JU, Thorgeirsson SS. SnapShot: hepatocellular carcinoma. Cancer Cell 2014; 25: 550e1. [DOI] [PubMed] [Google Scholar]
- 6. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 7. Cheng AL, Kang YK, Chen ZD, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 8. Labeur TA, Ten Cate DWG, Bart Takkenberg R, et al. Are we SHARP enough? The importance of adequate patient selection in sorafenib treatment for hepatocellular carcinoma. Acta Oncol 2018; 57: 1467–1474. [DOI] [PubMed] [Google Scholar]
- 9. Bruix J, Cheng AL, Meinhardt G, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol 2017; 67: 999–1008. [DOI] [PubMed] [Google Scholar]
- 10. Tandia M, Mhiri A, Paule B, et al. Correlation between clinical response to sorafenib in hepatocellular carcinoma treatment and polymorphisms of P-glycoprotein (ABCB1) and of breast cancer resistance protein (ABCG2): monocentric study. Cancer Chemother Pharmacol 2017; 79: 759–766. [DOI] [PubMed] [Google Scholar]
- 11. Casadei Gardini A, Marisi G, Faloppi L, et al. eNOS polymorphisms and clinical outcome in advanced HCC patients receiving sorafenib: final results of the ePHAS study. Oncotarget 2016; 7: 27988–27999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee YS, Kim BH, Kim BC, et al. SLC15A2 genomic variation is associated with the extraordinary response of sorafenib treatment: whole-genome analysis in patients with hepatocellular carcinoma. Oncotarget 2015; 6: 16449–16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Giacomo S, Briz O, Monte MJ, et al. Chemosensitization of hepatocellular carcinoma cells to sorafenib by β-caryophyllene oxide-induced inhibition of ABC export pumps. Arch Toxicol 2019; 93: 623–634. [DOI] [PubMed] [Google Scholar]
- 14. Llovet JM, Pena CE, Lathia CD, et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2012; 18: 2290–2300. [DOI] [PubMed] [Google Scholar]
- 15. Zhu YJ, Zheng B, Wang HY, et al. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin 2017; 38: 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pelicano H, Martin DS, Xu RH, et al. Glycolysis inhibition for anticancer treatment. Oncogene 2006; 25: 4633–4646. [DOI] [PubMed] [Google Scholar]
- 17. Shimizu S, Takehara T, Hikita H, et al. Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J Cancer 2012; 131: 548–557. [DOI] [PubMed] [Google Scholar]
- 18. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Del Rey MJ, Valin A, Usategui A, et al. Hif-1α knockdown reduces glycolytic metabolism and induces cell death of human synovial fibroblasts under normoxic conditions. Sci Rep 2017; 7: 3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab 2018; 27: 299–313. [DOI] [PubMed] [Google Scholar]
- 21. Reyes R, Wani NA, Ghoshal K, et al. Sorafenib and 2-deoxyglucose synergistically inhibit proliferation of both sorafenib-sensitive and -resistant HCC cells by inhibiting ATP production. Gene Expr 2017; 17: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomizawa M, Shinozaki F, Motoyoshi Y, et al. 2-Deoxyglucose and sorafenib synergistically suppress the proliferation and motility of hepatocellular carcinoma cells. Oncol Lett 2017; 13: 800–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishijima N, Kanki K, Shimizu H, et al. Activation of AMP-activated protein kinase by retinoic acid sensitizes hepatocellular carcinoma cells to apoptosis induced by sorafenib. Cancer Sci 2015; 106: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang M, Zhao X, Zhu D, et al. HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res 2017; 36: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013; 496: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yazdani HO, Huang H, Tsung A. Autophagy: dual response in the development of hepatocellular carcinoma. Cells 2019; 8: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Méndez-Blanco C, Fondevila F, García-Palomo A, et al. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med 2018; 50: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liang Y, Zheng T, Song R, et al. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in hepatocellular carcinoma. Hepatology 2013; 57: 1847–1857. [DOI] [PubMed] [Google Scholar]
- 29. Xu H, Zhao L, Fang Q, et al. MiR-338-3p inhibits hepatocarcinoma cells and sensitizes these cells to sorafenib by targeting hypoxia-induced factor 1α. PLoS One 2014; 9: e115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yeh CC, Hsu CH, Shao YY, et al. Integrated stable isotope labeling by amino acids in cell culture (SILAC) and isobaric tags for relative and absolute quantitation (iTRAQ) quantitative proteomic analysis identifies galectin-1 as a potential biomarker for predicting sorafenib resistance in liver cancer. Mol Cell Proteomics 2015; 14: 1527–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qiu Y, Shan W, Yang Y, et al. Reversal of sorafenib resistance in hepatocellular carcinoma: epigenetically regulated disruption of 14-3-3η/hypoxia-inducible factor-1α. Cell Death Discov 2019; 5: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu FQ, Fang T, Yu LX, et al. ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1α. J Hepatol 2016; 65: 314–324. [DOI] [PubMed] [Google Scholar]
- 33. Li S, Li J, Dai W, et al. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br J Cancer 2017; 117: 1518–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yao J, Man S, Dong H, et al. Combinatorial treatment of rhizoma paridis saponins and sorafenib overcomes the intolerance of sorafenib. J Steroid Biochem Mol Biol 2018; 183: 159–166. [DOI] [PubMed] [Google Scholar]
- 35. Prieto-Dominguez N, Mendez-Blanco C, Carbajo-Pescador S, et al. Melatonin enhances sorafenib actions in human hepatocarcinoma cells by inhibiting mTORC1/p70S6K/HIF-1α and hypoxia-mediated mitophagy. Oncotarget 2017; 8: 91402–91414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. You A, Cao M, Guo Z, et al. Metformin sensitizes sorafenib to inhibit postoperative recurrence and metastasis of hepatocellular carcinoma in orthotopic mouse models. J Hematol Oncol 2016; 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma L, Li G, Zhu H, et al. 2-Methoxyestradiol synergizes with sorafenib to suppress hepatocellular carcinoma by simultaneously dysregulating hypoxia-inducible factor-1 and -2. Cancer Lett 2014; 355: 96–105. [DOI] [PubMed] [Google Scholar]
- 38. Xu JJ, Zheng LB, Chen J, et al. Increasing AR by HIF-2α inhibitor (PT-2385) overcomes the side-effects of sorafenib by suppressing hepatocellular carcinoma invasion via alteration of pSTAT3, pAKT and pERK signals. Cell Death Dis 2017; 8: e3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shen YC, Ou DL, Hsu C, et al. Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma. Br J Cancer 2013; 108: 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Turcios L, Vilchez V, Acosta LF, et al. Sorafenib and FH535 in combination act synergistically on hepatocellular carcinoma by targeting cell bioenergetics and mitochondrial function. Dig Liver Dis 2017; 49: 697–704. [DOI] [PubMed] [Google Scholar]
- 41. Dai W, Wang F, Lu J, et al. By reducing hexokinase 2, resveratrol induces apoptosis in HCC cells addicted to aerobic glycolysis and inhibits tumor growth in mice. Oncotarget 2015; 6: 13703–13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Antico Arciuch VG, Elguero ME, Poderoso JJ, et al. Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal 2012; 16: 1150–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun J, Li J, Guo Z, et al. Overexpression of pyruvate dehydrogenase E1α subunit inhibits Warburg effect and induces cell apoptosis through mitochondria-mediated pathway in hepatocellular carcinoma. Oncol Res 2019; 27: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Minguez B, Tovar V, Chiang D, et al. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr Opin Gastroenterol 2009; 25: 186–194. [DOI] [PubMed] [Google Scholar]
- 45. Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol 2009; 27: 2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ge P, Cui Y, Liu F, et al. L-carnitine affects osteoblast differentiation in NIH3T3 fibroblasts by the IGF-1/PI3K/Akt signalling pathway. Biosci Trends 2015; 9: 42–48. [DOI] [PubMed] [Google Scholar]
- 47. Villanueva A, Chiang DY, Newell P, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 2008; 135: 1972–1983, 1983.e1971–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu LP, Ho RL, Chen GG, et al. Sorafenib inhibits hypoxia-inducible factor-1alpha synthesis: implications for antiangiogenic activity in hepatocellular carcinoma. Clin Cancer Res 2012; 18: 5662–5671. [DOI] [PubMed] [Google Scholar]
- 49. Singh AR, Joshi S, Burgoyne AM, et al. Single agent and synergistic activity of the “First-in-Class” dual PI3K/BRD4 inhibitor SF1126 with sorafenib in hepatocellular carcinoma. Mol Cancer Ther 2016; 15: 2553–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Masuda M, Chen WY, Miyanaga A, et al. Alternative mammalian target of rapamycin (mTOR) signal activation in sorafenib-resistant hepatocellular carcinoma cells revealed by array-based pathway profiling. Mol Cell Proteomics 2014; 13: 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen KF, Chen HL, Tai WT, et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther 2011; 337: 155–161. [DOI] [PubMed] [Google Scholar]
- 52. Zhai B, Jiang X, He C, et al. Arsenic trioxide potentiates the anti-cancer activities of sorafenib against hepatocellular carcinoma by inhibiting Akt activation. Tumour Biol 2015; 36: 2323–2334. [DOI] [PubMed] [Google Scholar]
- 53. Jiang S, Wang Q, Feng M, et al. C2-ceramide enhances sorafenib-induced caspase-dependent apoptosis via PI3K/AKT/mTOR and Erk signaling pathways in HCC cells. Appl Microbiol Biotechnol 2017; 101: 1535–1546. [DOI] [PubMed] [Google Scholar]
- 54. Badawi M, Kim J, Dauki A, et al. CD44 positive and sorafenib insensitive hepatocellular carcinomas respond to the ATP-competitive mTOR inhibitor INK128. Oncotarget 2018; 9: 26032–26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang H, Wang J, Fan JH, et al. Ilexgenin A exerts anti-inflammation and anti-angiogenesis effects through inhibition of STAT3 and PI3K pathways and exhibits synergistic effects with sorafenib on hepatoma growth. Toxicol Appl Pharmacol 2017; 315: 90–101. [DOI] [PubMed] [Google Scholar]
- 56. Kim YS, Lee YM, Oh TI, et al. Emodin sensitizes hepatocellular carcinoma cells to the anti-cancer effect of sorafenib through suppression of cholesterol metabolism. Int J Mol Sci 2018; 19: 3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tan W, Zhu S, Cao J, et al. Inhibition of MMP-2 expression enhances the antitumor effect of sorafenib in hepatocellular carcinoma by suppressing the PI3K/AKT/mTOR pathway. Oncol Res 2017; 25: 1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lu M, Fei Z, Zhang G. Synergistic anticancer activity of 20(S)-ginsenoside Rg3 and sorafenib in hepatocellular carcinoma by modulating PTEN/Akt signaling pathway. Biomed Pharmacother 2018; 97: 1282–1288. [DOI] [PubMed] [Google Scholar]
- 59. Wang HY, Zhang CY, Chi HY, et al. Synergistic anti-hepatoma effect of bufalin combined with sorafenib via mediating the tumor vascular microenvironment by targeting mTOR/VEGF signaling. Int J Oncol 2018; 52: 2051–2060. [DOI] [PubMed] [Google Scholar]
- 60. Dai N, Ye R, He Q, et al. Capsaicin and sorafenib combination treatment exerts synergistic antihepatocellular carcinoma activity by suppressing EGFR and PI3K/AKT/mTOR signaling. Oncol Rep 2018; 40: 3235–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mao J, Yang H, Cui T, et al. Combined treatment with sorafenib and silibinin synergistically targets both HCC cells and cancer stem cells by enhanced inhibition of the phosphorylation of STAT3/ERK/AKT. Eur J Pharmacol 2018; 832: 39–49. [DOI] [PubMed] [Google Scholar]
- 62. Gu HR, Park SC, Choi SJ, et al. Combined treatment with silibinin and either sorafenib or gefitinib enhances their growth-inhibiting effects in hepatocellular carcinoma cells. Clin Mol Hepatol 2015; 21: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tsai JJ, Hsu FT, Pan PJ, et al. Amentoflavone enhances the therapeutic efficacy of sorafenib by inhibiting anti-apoptotic potential and potentiating apoptosis in hepatocellular carcinoma in vivo. Anticancer Res 2018; 38: 2119–2125. [DOI] [PubMed] [Google Scholar]
- 64. Zhang K, Wang T, Zhou H, et al. A novel aurora-A inhibitor (MLN8237) synergistically enhances the antitumor activity of sorafenib in hepatocellular carcinoma. Mol Ther Nucleic Acids 2018; 13: 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu JH, Liu YH, Meng LY, et al. Synergistic antitumor effect of sorafenib in combination with ATM inhibitor in hepatocellular carcinoma cells. Int J Med Sci 2017; 14: 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhu W, Liang Q, Yang X, et al. Combination of sorafenib and valproic acid synergistically induces cell apoptosis and inhibits hepatocellular carcinoma growth via down-regulating notch3 and pAkt. Am J Cancer Res 2017; 7: 2503–2514. [PMC free article] [PubMed] [Google Scholar]
- 67. Yang X, Xia W, Chen L, et al. Synergistic antitumor effect of a gamma-secretase inhibitor PF-03084014 and sorafenib in hepatocellular carcinoma. Oncotarget 2018; 9: 34996–35007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jilkova ZM, Kuyucu AZ, Kurma K, et al. Combination of AKT inhibitor ARQ 092 and sorafenib potentiates inhibition of tumor progression in cirrhotic rat model of hepatocellular carcinoma. Oncotarget 2018; 9: 11145–11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ling S, Song L, Fan N, et al. Combination of metformin and sorafenib suppresses proliferation and induces autophagy of hepatocellular carcinoma via targeting the mTOR pathway. Int J Oncol 2017; 50: 297–309. [DOI] [PubMed] [Google Scholar]
- 70. Han P, Li H, Jiang X, et al. Dual inhibition of Akt and c-Met as a second-line therapy following acquired resistance to sorafenib in hepatocellular carcinoma cells. Mol Oncol 2017; 11: 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Koeberle D, Dufour JF, Demeter G, et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): a randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann Oncol 2016; 27: 856–861. [DOI] [PubMed] [Google Scholar]
- 72. Finn RS, Poon RT, Yau T, et al. Phase I study investigating everolimus combined with sorafenib in patients with advanced hepatocellular carcinoma. J Hepatol 2013; 59: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 73. Kelley RK, Nimeiri HS, Munster PN, et al. Temsirolimus combined with sorafenib in hepatocellular carcinoma: a phase I dose-finding trial with pharmacokinetic and biomarker correlates. Ann Oncol 2013; 24: 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang YB, Speeg KV, Washburn WK, et al. Sirolimus plus sorafenib in treating HCC recurrence after liver transplantation: a case report. World J Gastroenterol 2010; 16: 5518–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim R, Aucejo F. Radiologic complete response with sirolimus and sorafenib in a hepatocellular carcinoma patient who relapsed after orthotopic liver transplantation. J Gastrointest Cancer 2011; 42: 50–53. [DOI] [PubMed] [Google Scholar]
- 76. Gomez-Martin C, Bustamante J, Castroagudin JF, et al. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl 2012; 18: 45–52. [DOI] [PubMed] [Google Scholar]
- 77. Parkhitko AA, Favorova OO, Henske EP. Autophagy: mechanisms, regulation, and its role in tumorigenesis. Biochemistry (Mosc) 2013; 78: 355–367. [DOI] [PubMed] [Google Scholar]
- 78. Shi YH, Ding ZB, Zhou J, et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy 2011; 7: 1159–1172. [DOI] [PubMed] [Google Scholar]
- 79. Zhai B, Hu F, Jiang X, et al. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther 2014; 13: 1589–1598. [DOI] [PubMed] [Google Scholar]
- 80. Tai WT, Shiau CW, Chen HL, et al. Mcl-1-dependent activation of Beclin 1 mediates autophagic cell death induced by sorafenib and SC-59 in hepatocellular carcinoma cells. Cell Death Dis 2013; 4: e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rong LW, Wang RX, Zheng XL, et al. Combination of wogonin and sorafenib effectively kills human hepatocellular carcinoma cells through apoptosis potentiation and autophagy inhibition. Oncol Lett 2017; 13: 5028–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pan QZ, Pan K, Wang QJ, et al. Annexin A3 as a potential target for immunotherapy of liver cancer stem-like cells. Stem Cells 2015; 33: 354–366. [DOI] [PubMed] [Google Scholar]
- 83. Tong M, Che N, Zhou L, et al. Efficacy of annexin A3 blockade in sensitizing hepatocellular carcinoma to sorafenib and regorafenib. J Hepatol 2018; 69: 826–839. [DOI] [PubMed] [Google Scholar]
- 84. Ahmed D, de Verdier PJ, Ryk C, et al. FTY720 (Fingolimod) sensitizes hepatocellular carcinoma cells to sorafenib-mediated cytotoxicity. Pharmacol Res Perspect 2015; 3: e00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yuan H, Li AJ, Ma SL, et al. Inhibition of autophagy significantly enhances combination therapy with sorafenib and HDAC inhibitors for human hepatoma cells. World J Gastroenterol 2014; 20: 4953–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li YJ, Lei YH, Yao N, et al. Autophagy and multidrug resistance in cancer. Chin J Cancer 2017; 36: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lee YJ, Won AJ, Lee J, et al. Molecular mechanism of SAHA on regulation of autophagic cell death in tamoxifen-resistant MCF-7 breast cancer cells. Int J Med Sci 2012; 9: 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hu T, Wang L, Zhang L, et al. Sensitivity of apoptosis-resistant colon cancer cells to tanshinones is mediated by autophagic cell death and p53-independent cytotoxicity. Phytomedicine 2015; 22: 536–544. [DOI] [PubMed] [Google Scholar]
- 89. Wu HM, Shao LJ, Jiang ZF, et al. Gemcitabine-induced autophagy protects human lung cancer cells from apoptotic death. Lung 2016; 194: 959–966. [DOI] [PubMed] [Google Scholar]
- 90. Prieto-Domínguez N, Ordóñez R, Fernández A, et al. Modulation of autophagy by sorafenib: effects on treatment response. Front Pharmacol 2016; 7: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rodríguez-Hernández MA, González R, de la Rosa ÁJ, et al. Molecular characterization of autophagic and apoptotic signaling induced by sorafenib in liver cancer cells. J Cell Physiol 2018; 234: 692–708. [DOI] [PubMed] [Google Scholar]
- 92. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 94. Yau T, Park JW, Finn RS, et al. LBA38_PRCheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol 2019; 30. [Google Scholar]
- 95. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol 2020; 38: 193–202. [DOI] [PubMed] [Google Scholar]
- 96. Hato T, Zhu AX, Duda DG. Rationally combining anti-VEGF therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy 2016; 8: 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rivera LB, Meyronet D, Hervieu V, et al. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep 2015; 11: 577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Terme M, Colussi O, Marcheteau E, et al. Modulation of immunity by antiangiogenic molecules in cancer. Clin Dev Immunol 2012; 2012: 492920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen Y, Ramjiawan RR, Reiberger T, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 2015; 61: 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Adcock CS, Puneky LV, Campbell GS. Favorable response of metastatic hepatocellular carcinoma to treatment with trans-arterial radioembolization followed by sorafenib and nivolumab. Cureus 2019; 11: e4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cheng AL, Qin S, Ikeda M, et al. LBA3IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol 2019; 30(Suppl. 9). [Google Scholar]
- 102. Lee M, Ryoo BY, Hsu CH, et al. LBA39Randomised efficacy and safety results for atezolizumab (Atezo) + bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Ann Oncol 2019; 30(Suppl. 5): v851–v934. [Google Scholar]
- 103. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 104. Llovet J, Shepard KV, Finn RS, et al. 747PA phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): updated results. Ann Oncol 2019; 30(Suppl.5). [Google Scholar]
- 105. Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008; 48: 1312–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Huang M, Chen C, Geng J, et al. Targeting KDM1A attenuates Wnt/β-catenin signaling pathway to eliminate sorafenib-resistant stem-like cells in hepatocellular carcinoma. Cancer Lett 2017; 398: 12–21. [DOI] [PubMed] [Google Scholar]
- 107. Lin HH, Feng WC, Lu LC, et al. Inhibition of the Wnt/β-catenin signaling pathway improves the anti-tumor effects of sorafenib against hepatocellular carcinoma. Cancer Lett 2016; 381: 58–66. [DOI] [PubMed] [Google Scholar]
- 108. Galuppo R, Maynard E, Shah M, et al. Synergistic inhibition of HCC and liver cancer stem cell proliferation by targeting RAS/RAF/MAPK and WNT/β-catenin pathways. Anticancer Res 2014; 34: 1709–1713. [PMC free article] [PubMed] [Google Scholar]
- 109. Muche S, Kirschnick M, Schwarz M, et al. Synergistic effects of β-catenin inhibitors and sorafenib in hepatoma cells. Anticancer Res 2014; 34: 4677–4683. [PubMed] [Google Scholar]
- 110. Huynh TT, Rao YK, Lee WH, et al. Destruxin B inhibits hepatocellular carcinoma cell growth through modulation of the Wnt/β-catenin signaling pathway and epithelial-mesenchymal transition. Toxicol in Vitro 2014; 28: 552–561. [DOI] [PubMed] [Google Scholar]
- 111. Bouattour M, Raymond E, Qin S, et al. Recent developments of c-Met as a therapeutic target in hepatocellular carcinoma. Hepatology 2018; 67: 1132–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Carr BI, Wang Z, Wang M, et al. c-Met-Akt pathway-mediated enhancement of inhibitory c-Raf phosphorylation is involved in vitamin K1 and sorafenib synergy on HCC growth inhibition. Cancer Biol Ther 2011; 12: 531–538. [DOI] [PubMed] [Google Scholar]
- 113. Ha TY, Hwang S, Hong HN, et al. Synergistic effect of sorafenib and vitamin K on suppression of hepatocellular carcinoma cell migration and metastasis. Anticancer Res 2015; 35: 1985–1995. [PubMed] [Google Scholar]
- 114. Jiang X, Feng K, Zhang Y, et al. Sorafenib and DE605, a novel c-Met inhibitor, synergistically suppress hepatocellular carcinoma. Oncotarget 2015; 6: 12340–12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Puzanov I, Sosman J, Santoro A, et al. Phase 1 trial of tivantinib in combination with sorafenib in adult patients with advanced solid tumors. Invest New Drugs 2015; 33: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mann DA. Epigenetics in liver disease. Hepatology 2014; 60: 1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Witt O, Lindemann R. HDAC inhibitors: magic bullets, dirty drugs or just another targeted therapy. Cancer Lett 2009; 280: 123–124. [DOI] [PubMed] [Google Scholar]
- 118. Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 2010; 141: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lachenmayer A, Toffanin S, Cabellos L, et al. Combination therapy for hepatocellular carcinoma: additive preclinical efficacy of the HDAC inhibitor panobinostat with sorafenib. J Hepatol 2012; 56: 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. He B, Dai L, Zhang X, et al. The HDAC inhibitor quisinostat (JNJ-26481585) supresses hepatocellular carcinoma alone and synergistically in combination with sorafenib by G0/G1 phase arrest and apoptosis induction. Int J Biol Sci 2018; 14: 1845–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Soukupova J, Bertran E, Penuelas-Haro I, et al. Resminostat induces changes in epithelial plasticity of hepatocellular carcinoma cells and sensitizes them to sorafenib-induced apoptosis. Oncotarget 2017; 8: 110367–110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bitzer M, Horger M, Giannini EG, et al. Resminostat plus sorafenib as second-line therapy of advanced hepatocellular carcinoma - The SHELTER study. J Hepatol 2016; 65: 280–288. [DOI] [PubMed] [Google Scholar]
- 123. Hsu FT, Liu YC, Chiang IT, et al. Sorafenib increases efficacy of vorinostat against human hepatocellular carcinoma through transduction inhibition of vorinostat-induced ERK/NF-κB signaling. Int J Oncol 2014; 45: 177–188. [DOI] [PubMed] [Google Scholar]
- 124. Patt Y, Rojas-Hernandez C, Fekrazad HM, et al. Phase II trial of sorafenib in combination with capecitabine in patients with hepatocellular carcinoma: INST 08-20. Oncologist 2017; 22: 1158–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Petrini I, Lencioni M, Ricasoli M, et al. Phase II trial of sorafenib in combination with 5-fluorouracil infusion in advanced hepatocellular carcinoma. Cancer Chemother Pharmacol 2012; 69: 773–780. [DOI] [PubMed] [Google Scholar]
- 126. Shahda S, Loehrer PJ, Clark RS, et al. Phase I study of lenalidomide and sorafenib in patients with advanced hepatocellular carcinoma. Oncologist 2016; 21: 664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tai WM, Yong WP, Lim C, et al. A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC). Ann Oncol 2016; 27: 2210–2215. [DOI] [PubMed] [Google Scholar]
- 128. Duffy AG, Ma C, Ulahannan SV, et al. Phase I and preliminary phase II study of TRC105 in combination with sorafenib in hepatocellular carcinoma. Clin Cancer Res 2017; 23: 4633–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Abou-Alfa GK, Yen CJ, Hsu CH, et al. Phase Ib study of codrituzumab in combination with sorafenib in patients with non-curable advanced hepatocellular carcinoma (HCC). Cancer Chemother Pharmacol 2017; 79: 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Naqi N, Ahmad S, Murad S, et al. Efficacy and safety of sorafenib-gemcitabine combination therapy in advanced hepatocellular carcinoma: an open-label phase II feasibility study. Hematol Oncol Stem Cell Ther 2014; 7: 27–31. [DOI] [PubMed] [Google Scholar]
- 131. Ciuleanu T, Bazin I, Lungulescu D, et al. A randomized, double-blind, placebo-controlled phase II study to assess the efficacy and safety of mapatumumab with sorafenib in patients with advanced hepatocellular carcinoma. Ann Oncol 2016; 27: 680–687. [DOI] [PubMed] [Google Scholar]
- 132. Lee FA, Zee BC, Cheung FY, et al. Randomized phase II study of the X-linked inhibitor of apoptosis (XIAP) antisense AEG35156 in combination with sorafenib in patients with advanced hepatocellular carcinoma (HCC). Am J Clin Oncol 2016; 39: 609–613. [DOI] [PubMed] [Google Scholar]
- 133. Lim HY, Heo J, Choi HJ, et al. A phase II study of the efficacy and safety of the combination therapy of the MEK inhibitor refametinib (BAY 86-9766) plus sorafenib for Asian patients with unresectable hepatocellular carcinoma. Clin Cancer Res 2014; 20: 5976–5985. [DOI] [PubMed] [Google Scholar]
- 134. Ooka Y, Chiba T, Ogasawara S, et al. A phase I/II study of S-1 with sorafenib in patients with advanced hepatocellular carcinoma. Invest New Drugs 2014; 32: 723–728. [DOI] [PubMed] [Google Scholar]
- 135. Abou-Alfa GK, Blanc JF, Miles S, et al. Phase II study of first-line trebananib plus sorafenib in patients with advanced hepatocellular carcinoma. Oncologist 2017; 22: 780–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hubbard JM, Mahoney MR, Loui WS, et al. Phase I/II randomized trial of sorafenib and bevacizumab as first-line therapy in patients with locally advanced or metastatic hepatocellular carcinoma: North Central Cancer Treatment Group Trial N0745 (Alliance). Target Oncol 2017; 12: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jindal A, Thadi A, Shailubhai K. Hepatocellular carcinoma: etiology and current and future drugs. J Clin Exp Hepatol 2019; 9: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Tai WT, Chu PY, Shiau CW, et al. STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin Cancer Res 2014; 20: 5768–5776. [DOI] [PubMed] [Google Scholar]
- 139. Tsai JJ, Pan PJ, Hsu FT. Regorafenib induces extrinsic and intrinsic apoptosis through inhibition of ERK/NF-κB activation in hepatocellular carcinoma cells. Oncol Rep 2017; 37: 1036–1044. [DOI] [PubMed] [Google Scholar]
- 140. Fondevila F, Mendez-Blanco C, Fernandez-Palanca P, et al. Anti-tumoral activity of single and combined regorafenib treatments in preclinical models of liver and gastrointestinal cancers. Exp Mol Med 2019; 51: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 142. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol 2018; 69: 353–358. [DOI] [PubMed] [Google Scholar]
- 143. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018; 379: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20: 282–296. [DOI] [PubMed] [Google Scholar]
- 145. Lu S, Yao Y, Xu G, et al. CD24 regulates sorafenib resistance via activating autophagy in hepatocellular carcinoma. Cell Death Dis 2018; 9: 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tomonari T, Takeishi S, Taniguchi T, et al. MRP3 as a novel resistance factor for sorafenib in hepatocellular carcinoma. Oncotarget 2016; 7: 7207–7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Li J, Wu PW, Zhou Y, et al. Rage induces hepatocellular carcinoma proliferation and sorafenib resistance by modulating autophagy. Cell Death Dis 2018; 9: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhang PF, Li KS, Shen YH, et al. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis 2016; 7: e2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Dai J, Huang Q, Niu K, et al. Sestrin 2 confers primary resistance to sorafenib by simultaneously activating AKT and AMPK in hepatocellular carcinoma. Cancer Med 2018; 7: 5691–5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhang W, Liu S, Liu K, et al. Knockout of ADAM10 enhances sorafenib antitumor activity of hepatocellular carcinoma in vitro and in vivo. Oncol Rep 2014; 32: 1913–1922. [DOI] [PubMed] [Google Scholar]
- 151. Ling S, Li J, Shan Q, et al. USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway. Mol Oncol 2017; 11: 682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ling S, Shan Q, Zhan Q, et al. USP22 promotes hypoxia-induced hepatocellular carcinoma stemness by a HIF1α/USP22 positive feedback loop upon TP53 inactivation. Gut. Epub ahead of print 27 November 2019. DOI: 10.1136/gutjnl-2019-319616. [DOI] [PubMed] [Google Scholar]
- 153. Borisov N, Tkachev V, Suntsova M, et al. A method of gene expression data transfer from cell lines to cancer patients for machine-learning prediction of drug efficiency. Cell Cycle 2018; 17: 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Berlow NE, Rikhi R, Geltzeiler M, et al. Probabilistic modeling of personalized drug combinations from integrated chemical screen and molecular data in sarcoma. BMC Cancer 2019; 19: 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Celebi R, Bear Don’t Walk O, IV, Movva R, et al. In-silico prediction of synergistic anti-cancer drug combinations using multi-omics data. Sci Rep 2019; 9: 8949. [DOI] [PMC free article] [PubMed] [Google Scholar]