Abstract
Background:
Paliperidone palmitate 3-monthly (PP3M) formulation is a long-acting, injectable antipsychotic treatment approved in many countries worldwide for the maintenance treatment of adult patients with schizophrenia. This single-arm, open-label, phase IIIb study evaluated the efficacy and safety of converting patients with schizophrenia stabilized with paliperidone palmitate 1-month (PP1M) to PP3M in a naturalistic clinical setting.
Methods:
After screening (days –7 to 1), patients were converted from PP1M (50–150 mg eq.) to PP3M (175–525 mg eq.), and entered a 52-week, flexible-dose PP3M treatment period. The primary efficacy endpoint was symptomatic remission (SR) (Andreasen criteria) at last observation carried forward (LOCF) endpoint.
Results:
Patients (n = 305) received PP3M, of whom 291 (95.4%) completed the study. Doses of PP3M remained stable during the 12-month treatment period, and changes in dose were uncommon. Overall, 56.8% of patients [95% confidence interval (CI): 51.0, 62.4] achieved SR, and 31.8% achieved both symptomatic and functional remission (Personal and Social Performance scale total score > 70) at LOCF endpoint. Secondary endpoint results were generally consistent with primary endpoint results. There were improvements in Positive and Negative Syndrome Scale total, subscale and Marder factor scores, and also Clinical Global Impression-Severity and -Change scores from baseline to LOCF endpoint. Carer burden was reduced, and the proportion of patients requiring hospitalization for psychiatric reasons decreased from 13.5% in the 12 months prior to baseline to 4.6% during the treatment period. No new safety signals were identified.
Conclusion:
Results from this naturalistic study were similar to those observed in previous randomized clinical trials of PP3M and underline the importance of continuous maintenance treatment in patients with schizophrenia.
Keywords: functional remission, paliperidone palmitate 3-monthly formulation, paliperidone palmitate 1-monthly formulation, schizophrenia, symptomatic remission
Introduction
Schizophrenia is a complex and heterogeneous mental disorder, with a median global lifetime prevalence of approximately 0.5%.1 It is characterized by positive symptoms (hallucinations, delusions, disorganized thinking), negative symptoms (social withdrawal, apathy, anhedonia, affect flattening, and alogia), and general psychopathology (poor attention, anxiety, depression).2–4 Continuous maintenance treatment with antipsychotics is essential for effective symptom control and relapse prevention.5,6 A large study of almost 30,000 people with schizophrenia found that use of antipsychotics reduced mortality by approximately 50% when compared with no use of antipsychotics.7
For people with schizophrenia, the achievement of symptomatic remission (SR) – defined as a score of mild or better (⩽3) for eight core Positive and Negative Syndrome Scale (PANSS) items for at least 6 months – is an important treatment goal that is associated with significant improvements in psychosocial functioning and quality of life,8 as well as decreased healthcare resource utilization (HCRU).9–11 However, it can be argued that functional remission might be an even more important objective, as it is associated with real-world outcomes such as being able to work and live independently.12–14
Suboptimal adherence to daily oral antipsychotic medication is common in people with schizophrenia and is associated with poorer outcomes,15–18 including an increased risk of relapse or hospitalization.19,20 In light of this, long-acting injectable antipsychotic treatments (LATs) were developed to overcome the need for people with schizophrenia to take daily oral antipsychotic medication. LATs help to ensure that people with schizophrenia receive a known quantity of medication at appropriate dosing intervals, thereby promoting lower variability in plasma drug concentrations.21 In addition, as LATs are administered by a healthcare provider, non-adherence is more easily identified due to the necessity for clinic attendance to receive treatment.21 The long apparent elimination half-life of these medications also provides a wider window for healthcare professionals to intervene before plasma drug levels drop below therapeutic thresholds.22
The regular drug delivery afforded by LATs has translated into improved clinical outcomes versus daily oral antipsychotic treatment in people with schizophrenia, including a reduced risk of relapse and hospitalization,11,19,23–25 improved quality of life,26–28 and a reduced risk of mortality.7
Paliperidone palmitate 3-monthly (PP3M) is a LAT formulation that is approved in many countries and regions worldwide, including the United States and Europe, for the maintenance treatment of adults with schizophrenia who have been stabilized with paliperidone 1-monthly (PP1M) formulation.29,30 PP3M offers greater convenience compared with other LAT formulations that are typically administered monthly or bi-monthly. Moreover, in two pivotal, randomized controlled trials, PP3M demonstrated favorable efficacy and safety in the treatment of schizophrenia. In the first trial, Berwaerts et al. showed that PP3M significantly delayed the time to first relapse versus placebo [hazard ratio = 3.45; 95% confidence interval (CI): 1.73, 6.88; p < 0.001].31 In the second trial, Savitz et al. demonstrated non-inferiority of PP3M versus PP1M in relapse rates (8% versus 9%, respectively) during the 48-week double-blind period; additionally, SR rates were similar for PP3M compared with PP1M (58% versus 59%, respectively).32
However, as with all randomized controlled trials, these studies included stringent eligibility criteria and a fixed dose treatment pattern, not reflecting usual daily clinical practice. In order to provide clinically meaningful information for the optimal use of PP3M in people with schizophrenia, it is important to complement randomized clinical trials with real-world data from a broader patient cohort that is more representative of routine clinical practice.
With this in mind, the objectives of the present study were to perform a comprehensive assessment of the efficacy and safety of flexibly dosed PP3M administered for 52 weeks to adult patients with clinically stable schizophrenia, in a real-world setting that included patients with concomitant medications, and mild-to-moderate substance abuse.
Materials and methods
Study design
This was an international, multicenter, prospective, single-arm, open-label, 52-week, phase IIIb study of clinical outcomes in adult patients with schizophrenia treated with PP3M in clinical practice (ClinicalTrials.gov identifier: NCT02713282; EudraCT number: 2015-004835-10; REMISSIO). The study was conducted between May 2016 and March 2018 at 57 sites across Europe, the Middle East, Africa and the Asia Pacific region.
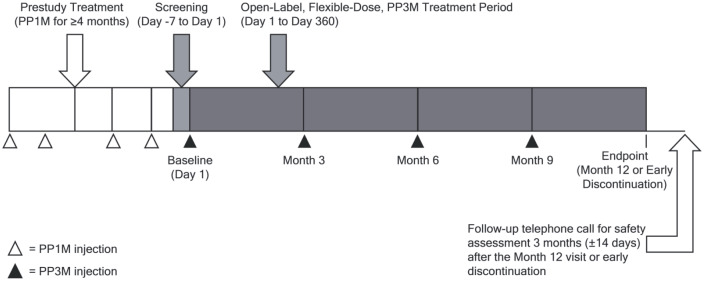
The study consisted of a screening period (day –7 to day 1) and a 52-week, open-label, flexible-dose PP3M treatment period (Figure 1). Study assessments were conducted at 3-monthly intervals during the treatment period. A follow up for safety assessments was made by telephone approximately 3 months after Month 12 or 3 months after early study discontinuation (±14 days).
Figure 1.
Study design.
PP1M, paliperidone palmitate 1-month formulation; PP3M, paliperidone palmitate 3-month formulation.
The study protocol and amendments were reviewed by an Independent Ethics Committee or Institutional Review Board, as appropriate, for each site. The study was conducted in compliance with the Declaration of Helsinki and was consistent with Good Clinical Practice and applicable regulatory requirements. Written informed consent was obtained from all patients before enrollment.
Patients
Adults aged 18–50 years with a confirmed diagnosis of schizophrenia [Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5)] and a baseline PANSS total score < 70, who were likely to benefit from switching to PP3M (based on their physician’s opinion), and who had received treatment with PP1M (50–150 mg eq.) for ⩾4 months (with the last two doses being the same, in order to ensure that they were stabilized on PP1M and establish an appropriate dose of PP3M) were included in the study.
Patients were excluded from the study if they had: a DSM-5 diagnosis of other psychiatric disorders or severe substance use disorder within 6 months of screening (patients with mild or moderate substance abuse were not excluded); a psychiatric diagnosis secondary to drug abuse, medication, or a general medical condition (e.g., clinically notable hypothyroidism, organic brain disorder); a serious unstable medical condition (e.g., with clinically relevant laboratory abnormalities); an imminent risk of suicide; experience of intolerable side effects with PP1M; stabilization on PP1M 25 mg eq. (owing to there being no corresponding dose of PP3M); the use of any LAT other than PP1M in the 4 months prior to the first administration of PP3M; treatment with clozapine within 3 months of screening; a history of lack of response or known hypersensitivity to risperidone or paliperidone; or a history or current symptoms of tardive dyskinesia or neuroleptic malignant syndrome.
Treatment
Consistent with routine clinical practice, patients were treated in an open-label manner. The first dose of PP3M was based on the previous PP1M dose using a 3.5-fold dose multiplier (PP1M 50, 75, 100, 150 mg eq. converted to PP3M 175, 263, 350, 525 mg eq., respectively). PP3M was administered (into either the deltoid or gluteal muscle) 1 month after the last PP1M dose (±7 days) initially, and every 3 months (±14 days) thereafter. After the first PP3M administration, investigators could adjust the dose of PP3M for an individual patient at their discretion, based on efficacy and/or the individual patient’s tolerability, according to the product label.29,30 The last dose of PP3M was administered on Day 270 (±14 days). Concomitant psychotropic medications were permitted for comorbid illnesses and symptomatic patients could receive supplemental oral antipsychotic treatment during the study.
Efficacy assessments
The primary efficacy endpoint was the proportion of patients who achieved SR at last observation carried forward (LOCF) endpoint, according to the 2-dimensional Andreasen criteria, that is, 1. (symptom severity criterion): a score of mild or better (⩽3) for the eight PANSS items P1 (delusions), P2 (conceptual disorganization), P3 (hallucinatory behavior), N1 (blunted affect), N4 (passive/apathetic social withdrawal), N6 (lack of spontaneity and flow of conversation), G5 (mannerisms and posturing) and G9 (unusual thought content), which is 2. (duration criterion): to be maintained for a minimum of 6 months as per the Andreasen criteria but with a ±14-day visit window.8 Secondary efficacy endpoints presented here included: achievement of SR at Months 6, 9, and 12; time to SR; maintenance of SR; change from baseline during the 12-month treatment period in PANSS total, subscale (positive, negative, and general psychopathology), and the five Marder factor scores (positive symptoms, negative symptoms, disorganized thoughts, uncontrolled hostility/excitement, anxiety/depression); Clinical Global Impression-Severity (CGI-S) and -Change (CGI-C) scores; Personal and Social Performance (PSP) total and subscale scores, and achievement of functional remission (PSP total score > 70); patient satisfaction with medication [Medication Satisfaction Questionnaire (MSQ); seven-point scale]; physician satisfaction with medication (seven-point scale, for three domains: overall, efficacy and safety); Involvement Evaluation Questionnaire (IEQ) score comprising total score and scores for the domains Tension, Supervision, Worrying and Urging; and Health Care Resource Utilization (HCRU, number and duration of hospital stays for psychiatric reasons, number of emergency room visits, and reasons for visits in the 12 months before and after the first injection of PP3M).
Safety assessments
Safety evaluations included assessment of treatment-emergent adverse events (TEAEs), including potentially prolactin-related TEAEs; extrapyramidal symptoms (EPS), which were evaluated using the Extrapyramidal Symptom Rating Scale (ESRS); weight and body mass index (BMI) changes; and vital signs (systolic and diastolic blood pressure, heart rate). Safety assessments were made during each study visit. In line with clinical practice, subjects were also instructed to report adverse events as they emerge.
Statistical analysis
For the primary analysis a modification to intent-to-treat analysis was applied, meaning that all patients who provided written consent and who received at least one dose of PP3M during the 52-week treatment period were included (mITT analysis set). The mITT efficacy analysis set comprised all patients from the mITT analysis set who had at least one post-baseline efficacy assessment. The mITT safety analysis set comprised all patients from the mITT analysis set who had at least one post-baseline safety assessment.
The sample size for the study was calculated based upon the clinical relevance of the 95% CI of the proportion of patients predicted to achieve the primary endpoint of SR. Under the conservative assumption that 50% of patients would achieve SR (based on the results of Savitz et al.),32 the sample size, calculated as 300 patients, was determined to be sufficient for exploratory subgroup analyses.
The following subgroups were defined: patients with/without SR at LOCF endpoint; patients who met/did not meet the first criterion of remission by Andreasen et al., namely the PANSS remission symptom severity criterion at baseline [a score of mild or better (⩽3) for the 8 PANSS items specified within the Andreasen criteria [P1 (delusions), P2 (conceptual disorganization), P3 (hallucinatory behavior), N1 (blunted affect), N4 (passive/apathetic social withdrawal), N6 (lack of spontaneity and flow of conversation), G5 (mannerisms and posturing), and G9 (unusual thought content)]; patients with >6 months/4–6 months of previous PP1M treatment; and patients who switched to PP3M from PP1M monotherapy/PP1M polytherapy. PP1M polytherapy was defined PP1M plus at least one additional antipsychotic medication that was taken in the period 5 or more days before the first PP3M injection. PP3M polytherapy was defined as comprising PP3M plus an additional antipsychotic therapy that was started or ongoing at the time of the first PP3M injection.
For efficacy outcomes, both observed case and LOCF values were used for analysis. The Wilcoxon signed rank test was used to examine within-group changes from baseline in outcome measures. The maintenance of efficacy was investigated using Schuirmann’s test to evaluate the change from baseline to LOCF endpoint in PANSS total score, with a non-inferiority margin of 5 points on the PANSS scale. Efficacy and safety results were analyzed descriptively; no statistical hypothesis testing was carried out.
Results
Patient disposition and baseline characteristics
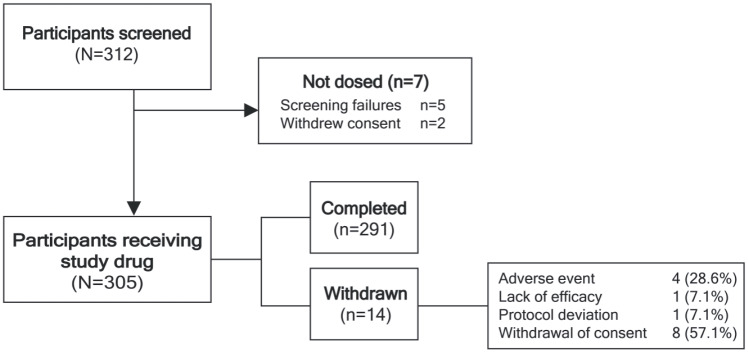
A total of 305 patients were enrolled and received PP3M, of whom 291 (95.4%) completed the 12-month study. The reasons for discontinuation were withdrawal of consent (n = 8; 2.6%), TEAEs (n = 4; 1.3%), lack of efficacy (n = 1; 0.3%), and protocol deviation (n = 1; 0.3%) (Figure 2). Approximately two-thirds of the patients were male, mean [standard deviation (SD)] age was 36.5 (8.0) years and mean (SD) time since first diagnosis of schizophrenia was 9.2 (7.3) years (Table 1). Prior to treatment with PP3M, all patients had received ⩾4 months treatment with PP1M, with the last two doses being the same, indicating clinical stability. The majority of patients (n = 236; 77%) had been treated with PP1M for >6 months, 58 patients (19.0%) were treated with PP1M for between 4 and 6 months, and 11 patients (3.6%) had an undefined PP1M duration; however, it was confirmed that the PP1M duration was ⩾4 months.
Figure 2.
Patient disposition.
Table 1.
Demographic and baseline characteristics (mITT population).
| Characteristic | Total (n = 305) | LOCF endpoint SR |
|
|---|---|---|---|
| Yes (n = 172) | No (n = 133) | ||
| Age, years | n = 305 | n = 172 | n = 133 |
| Mean (SD) | 36.5 (8.0) | 35.9 (8.0) | 37.2 (8.0) |
| Males, n (%) | 200 (65.6) | 107 (62.2) | 93 (69.9) |
| Weight, kg | n = 303 | n = 171 | n = 132 |
| Mean (SD) | 80.8 (18.1) | 79.8 (16.6) | 82.2 (19.9) |
| BMI, kg/m 2 | n = 303 | n = 171 | n = 132 |
| Mean (SD) | 27.4 (5.2) | 27.2 (4.8) | 27.7 (5.7) |
| Schizophrenia duration from diagnosis, years | n = 304 | n = 171 | n = 133 |
| Mean (SD) | 9.2 (7.3) | 8.6 (6.9) | 10.0 (7.7) |
| Previous hospitalizations for psychiatric reasons, n (%) | n = 304 | n = 171 | n = 133 |
| Yes | 255 (83.9) | 140 (81.9) | 115 (86.5) |
| No | 47 (15.5) | 29 (17.0) | 18 (13.5) |
| Unknown | 2 (0.7) | 2 (1.2) | 0 |
| Total number of previous hospitalizations for psychiatric reasons, n (%) | n = 255 | n = 140 | n = 115 |
| Mean (SD) | 3.3 (3.8) | 3.1 (3.6) | 3.4 (4.0) |
| Last PP1M dose category, n (%) | n = 305 | n = 172 | n = 133 |
| 50 mg | 27 (8.9) | 16 (9.3) | 11 (8.3) |
| 75 mg | 74 (24.3) | 43 (25.0) | 31 (23.3) |
| 100 mg | 114 (37.4) | 63 (36.6) | 51 (38.3) |
| 150 mg | 90 (29.5) | 50 (29.1) | 40 (30.1) |
BMI, body mass index; LOCF, last observation carried forward; mITT, modified intent-to-treat; PP1M, paliperidone palmitate 1-month formulation; SD, standard deviation; SR, symptomatic remission.
In total, 100 patients (32.8%) had ongoing comorbidities at baseline, including gastrointestinal (5.2%) and metabolism and nutrition (7.2%) somatic disorders, as well as nervous system (6.6%) and psychiatric disorders (3.3%). Ongoing nervous system and psychiatric disorders at baseline are listed in Supplemental Table S1.
PP3M treatment
Most patients (83%) were switched from PP1M monotherapy to PP3M monotherapy (Supplemental Table S2). The mean (SD) study follow-up time was 352.7 (52.3) days, and the mean (SD) exposure to PP3M treatment was 263.0 (42.5) days. PP3M doses remained stable during the 12-month treatment period from baseline [mean (SD) 363.6 (115.4) mg] to 12 months [362.5 (118.8) mg] (Table 2). Changes in dose were uncommon; 11 patients (3.6%) had at least one dose increase, whereas 15 patients (4.9%) had at least one dose decrease (Table 2). The most common reasons for dose increases were worsening of schizophrenia (n = 3; 1.0%) and PP3M efficacy not within expectations (n = 3; 1.0%). The most common reasons for dose decreases were tolerability not within expectations (n = 5; 1.6%) and improvement of condition (n = 2; 0.7%).
Table 2.
PP3M exposure and dosing (mITT population).
| Parameter | Total (n = 305) | LOCF endpoint SR |
|
|---|---|---|---|
| Yes (n = 172) | No (n = 133) | ||
| Follow-up duration, days, mean (SD) | 352.7 (52.3) | 362.4 (11.5) | 340.1 (76.5) |
| Exposure duration, days, mean (SD) | 263.0 (42.5) | 271.7 (7.4) | 251.6 (62.1) |
| Baseline dosea (Day 1) Mean (SD) | 363.6 (115.4) | 362.84 (116.4) | 364.6 (114.5) |
| Month 3 dose, mg | |||
| Mean (SD) | 366.7 (117.7) | 361.8 (117.3) | 373.3 (118.4) |
| Month 6 dose, mg | |||
| Mean (SD) | 362.6 (119.8) | 357.8 (117.7) | 369.3 (122.9) |
| Month 9 dose, mg | |||
| Mean (SD) | 363.6 (119.5) | 356.2 (117.2) | 374.2 (122.6) |
| Month 12 dose, mg | |||
| Mean (SD) | 362.5 (118.8) | 356.2 (117.2) | 370.5 (120.7) |
| Patients with ⩾1 dose increase, n (%) b | 11 (3.6) | 1 (0.6) | 10 (7.5) |
| Patients with ⩾1 dose decrease, n (%) b | 15 (4.9) | 10 (5.8) | 5 (3.8) |
One patient received an incorrect PP1M to PP3M dose conversion.
Some patients had two or more dose increases/decreases.
LOCF, last observation carried forward; mITT, modified intent-to-treat; PP1M, paliperidone palmitate 1-month formulation; PP3M, paliperidone palmitate 3-month formulation; SD, standard deviation; SR, symptomatic remission.
Concomitant medication
There were 130 patients who used at least one additional psychotropic medication at baseline that was continued with variable duration at the discretion of the investigator. The most commonly used psychotropic medications were biperiden (8.2%), olanzapine (5.2%), and diazepam (3.6%) (Supplemental Table S3). After initiation of PP3M, 92 patients (30.2%) started a new psychotropic medication with variable duration at the discretion of the investigator; the most frequently used were lorazepam (5.2%), paliperidone [4.6%, mean duration (SD) 66.3 days (88.1)], and risperidone [3.9%, mean duration (SD) 104.1 days (76.3)]. (Supplemental Table S4). It is important to note that the 130 patients with continued use of psychotropic medication at baseline, and the 92 patients starting a new psychotropic agent, are not two separate groups of patients. The group of 92 patients includes 54 patients who had therapy that was ongoing at baseline; the other 38 patients started a new therapy during PP3M use who did not have therapy ongoing at baseline.
Primary efficacy endpoint
A total of 172 of 303 patients in the mITT efficacy analysis set [56.8% (95% CI: 51.0, 62.4)] achieved SR at LOCF endpoint. Of these patients, 142 (46.9%) met the PANSS remission symptom severity criterion at baseline and all timepoints during the treatment period; 17 patients (5.6%) met this criterion at Months 3 through 12. Moreover, 11 (3.6%) patients met the criterion at Months 6 through 12, and 1 patient (0.3%) met the criterion at baseline, lost it at Month 3, then regained it for Months 6 through 12; and 1 patient (0.3%) met the criterion from baseline through Month 9.
Secondary efficacy endpoints
Achievement of SR over time
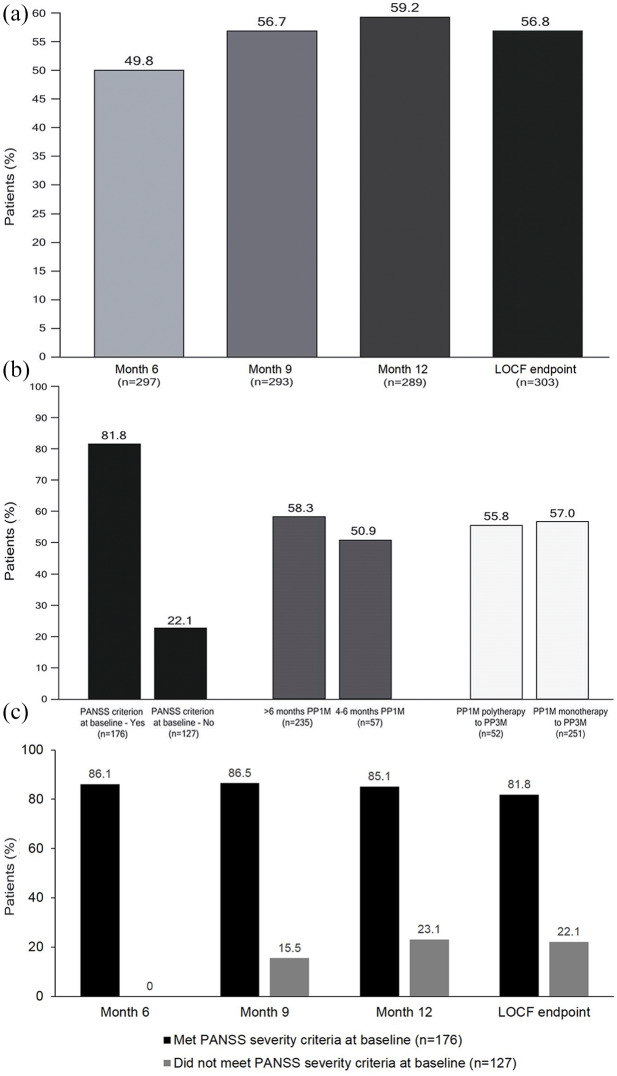
A total of 184 of 303 patients (60.7%) achieved SR during the 12-month treatment period. Of these, five patients (1.7%) who had met the SR criteria at Month 6 lost SR at Month 9, and seven patients (2.3%) who achieved SR at Month 9 lost it at Month 12. The proportion of patients achieving SR increased over time from 49.8% (148 of 297 patients) at 6 months, 56.7% at 9 months (166 of 293 patients), to 59.2% at 12 months (171 of 289 patients) (Figure 3A). The Kaplan–Meier estimate of median time to achievement of SR was 247 days (95% CI: 189, 275).
Figure 3.
Achievement of SR during the treatment period in the whole population over time (A) and in subgroups at LOCF endpoint: PANSS remission symptom severity criterion at baseline – yes versus no; >6 months prior PP1M versus 4–6 months prior PP1M; and switched from PP1M polytherapy versus PP1M monotherapy (B), and in patients who met and did not meet the PANSS severity criterion for remission at baseline (C) (mITT population).
LOCF, last observation carried forward; mITT, modified intent-to-treat; PANSS, positive and negative syndrome scale; PP1M, paliperidone palmitate 1-month formulation; SR, symptomatic remission.
The achievement of SR at LOCF endpoint was higher for patients who had >6 months of previous PP1M treatment than for patients with 4–6 months of PP1M treatment (58.3% versus 50.9%), but was comparable for those switching to PP3M from PP1M monotherapy (57.0%) or polytherapy (55.8%) (Figure 3B).
For patients who met the PANSS remission symptom severity criterion at baseline (58.1%), the proportion who achieved SR remained stable from Month 6 (the first timepoint for assessing SR) to Month 12, was over 85%. Of the patients who did not meet the PANSS severity criterion at baseline, the proportion who achieved SR increased from 15.5% at Month 9, to 23.1% at Month 12 (Figure 3C). Failure to fulfill the PANSS remission symptom severity criterion was most often due to not meeting a score of ⩽3 for items N1 (blunted affect) and N4 (passive/apathetic social withdrawal), and this was seen at all timepoints during the treatment period (Table 3).
Table 3.
PANSS items missed most frequently by patients who did not meet the PANSS remission severity criterion.*
| n = 303 | PANSS criterion not met, n (%) | P1 score > 3, n (%) | P2 score > 3, n (%) | P3 score > 3, n (%) | N1 score > 3, n (%) | N4 score > 3, n (%) | N6 score > 3, n (%) | G5 score > 3, n (%) | G9 score > 3, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 127 (100.0) | 21 (16.54) | 11 (8.66) | 20 (15.75) | 62 (48.82) | 61 (48.03) | 25 (19.69) | 1 (0.79) | 9 (7.09) |
| Month 3 | 111 (100.0) | 17 (15.32) | 15 (13.51) | 21 (18.92) | 58 (52.25) | 53 (47.75) | 26 (23.42) | 1 (0.90) | 11 (9.91) |
| Month 6 | 104 (100.0) | 12 (11.54) | 17 (16.35) | 20 (19.23) | 50 (48.08) | 55 (52.88) | 16 (15.38) | 2 (1.92) | 12 (11.54) |
| Month 9 | 91 (100.0) | 12 (13.19) | 17 (18.68) | 21 (23.08) | 44 (48.35) | 48 (52.75) | 17 (18.68) | 3 (3.30) | 11 (12.09) |
| Month 12 | 91 (100.0) | 15 (16.48) | 15 (16.48) | 14 (15.38) | 41 (45.05) | 48 (52.75) | 17 (18.68) | 2 (2.20) | 17 (18.68) |
| LOCF endpoint | 95 (100.0) | 16 (16.84) | 15 (15.79) | 15 (15.79) | 41 (43.16) | 50 (52.63) | 17 (17.89) | 2 (2.11) | 18 (18.95) |
PANSS severity criterion is only met when all of the 8 PANSS items have a score ⩽3.
P1, delusions; P2, conceptual disorganization; P3, hallucinatory behavior; N1, blunted affect; N4, passive-apathetic social withdrawal; N6, lack of spontaneity and flow of conversation; G5, mannerisms and posturing; G9, unusual thought content.
LOCF, last observation carried forward; PANSS, positive and negative syndrome scale.
PANSS total, subscale, and Marder factor scores
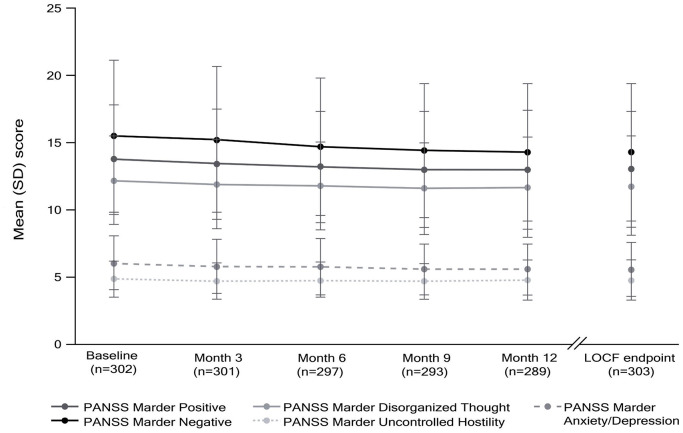
At baseline, mean (SD) baseline PANSS total score was 52.4 (10.6), reflecting mild/moderate symptomatology in this stable patient group. At LOCF endpoint, mean (SD) PANSS total score was 49.4 (12.3), representing a mean change from baseline of –3.1 points (95% CI: –4.1, –2.0), and indicating maintained efficacy with PP3M (Schuirmann’s test p < 0.0001) (Table 4). There were improvements in PANSS positive, negative, and general subscale scores (Table 4) and PANSS Marder positive, negative, disorganized thoughts, and anxiety/depression factor scores (Table 4, Figure 4) from baseline to LOCF endpoint.
Table 4.
Change in PANSS total, subscale and Marder factors scores (mITT efficacy analysis set).
| Characteristic | Total (n = 302) | Achieved SR at LOCF endpoint (n = 172) | Did not achieve SR at LOCF endpoint (n = 130) |
|---|---|---|---|
| PANSS total score | |||
| Baseline | 52.4 (10.6) | 48.4 (10.0) | 57.7 (8.8) |
| LOCF endpoint | 49.4 (12.3) | 43.7 (8.4) | 56.9 (12.6) |
| Mean change from baseline (95% CI) | −3.1 (–4.1, –2.0) | −4.8 (–5.8, –3.7) | −0.9 (–2.8, 1.1) |
| PANSS positive subscale | |||
| Baseline | 10.7 (3.2) | 9.8 (2.4) | 12.0 (3.6) |
| LOCF endpoint | 10.0 (3.5) | 8.7 (1.9) | 11.6 (4.4) |
| Mean change from baseline (95% CI) | −0.8 (–1.1, –0.4) | −1.1 (–1.4, –0.8) | −0.4 (–1.0, 0.3) |
| PANSS negative subscale | |||
| Baseline | 16.2 (5.4) | 14.2 (4.3) | 18.8 (5.5) |
| LOCF endpoint | 15.1 (5.1) | 12.9 (3.6) | 18.0 (5.3) |
| Mean change from baseline (95% CI) | −1.1 (–1.5, –0.7) | −1.33 (–1.8, –0.9) | −0.8 (–1.5, 0.0) |
| PANSS general subscale | |||
| Baseline | 25.6 (5.2) | 24.5 (5.4) | 27.0 (4.6) |
| LOCF endpoint | 24.3 (5.9) | 22.1 (4.4) | 27.3 (6.4) |
| Mean change from baseline (95% CI) | −1.2 (–1.8, –0.6) | −2.3 (–3.0, –1.7) | 0.3 (–0.8, 1.3) |
| PANSS Marder positive symptoms | |||
| Baseline | 13.8 (4.0) | 12.6 (3.4) | 15.3 (4.3) |
| LOCF endpoint | 13.0 (4.3) | 11.4 (3.0) | 15.0 (4.9) |
| Mean change from baseline (95% CI) | −0.8 (–1.2, –0.5) | −1.2 (–1.6, –0.8) | −0.3 (–1.0, 0.4) |
| PANSS Marder negative symptoms | |||
| Baseline | 15.5 (5.6) | 13.6 (4.3) | 18.2 (6.1) |
| LOCF endpoint | 14.3 (5.1) | 12.0 (3.4) | 17.2 (5.5) |
| Mean change from baseline (95% CI) | −1.3 (–1.7, –0.9) | −1.5 (–2.0, –1.1) | −1.0 (–1.7, –0.2) |
| PANSS Marder disorganized thoughts | |||
| Baseline | 12.2 (3.3) | 11.5 (3.2) | 13.1 (3.3) |
| LOCF endpoint | 11.8 (3.7) | 10.5 (2.7) | 13.5 (4.1) |
| Mean change from baseline (95% CI) | −0.4 (–0.7, –0.1) | −1.0 (–1.3, –0.7) | 0.3 (–0.2, 0.8) |
| PANSS Marder uncontrolled hostility | |||
| Baseline | 4.9 (1.4) | 4.8 (1.4) | 4.9 (1.5) |
| LOCF endpoint | 4.8 (1.5) | 4.5 (0.9) | 5.1 (2.0) |
| Mean change from baseline (95% CI) | −0.1 (–0.3, 0.1) | −0.3 (–0.5, –0.1) | 0.2 (–0.1, 0.6) |
| PANSS Marder anxiety/depression | |||
| Baseline | 6.1 (2.1) | 6.0 (2.0) | 6.2 (2.1) |
| LOCF endpoint | 5.6 (2.0) | 5.3 (1.5) | 6.1 (2.4) |
| Mean change from baseline (95% CI) | −0.4 (–0.7, –0.2) | −0.7 (–1.0, –0.4) | −0.1 (–0.5, –0.3) |
Values are mean (standard deviation) unless otherwise indicated.
CI, confidence interval; LOCF, last observation carried forward; mITT, modified intent-to-treat; PANSS, positive and negative syndrome scale; SR, symptomatic remission.
Figure 4.
PANSS Marder positive, negative, and disorganized thoughts; uncontrolled hostility; and anxiety/depression factor scores over time with LOCF endpoint (mITT efficacy analysis set).
Only patients with both baseline and at least one post-baseline assessment were included in the analysis.
LOCF, last observation carried forward; mITT, modified intent-to-treat; PANSS, positive and negative syndrome scale.
CGI-S and -C scores
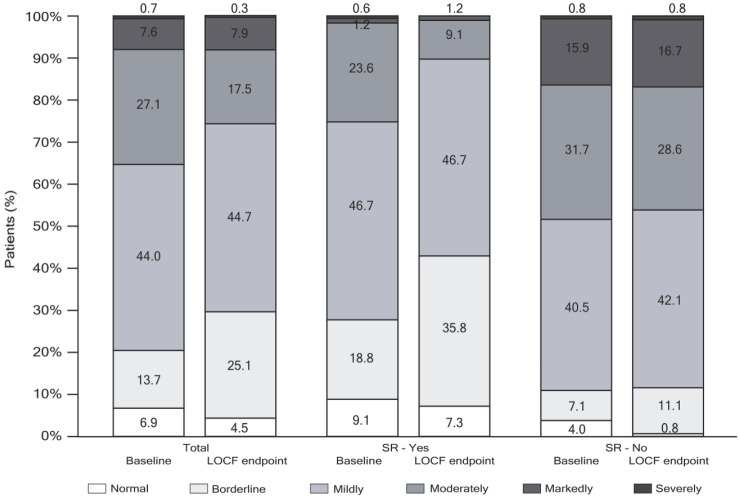
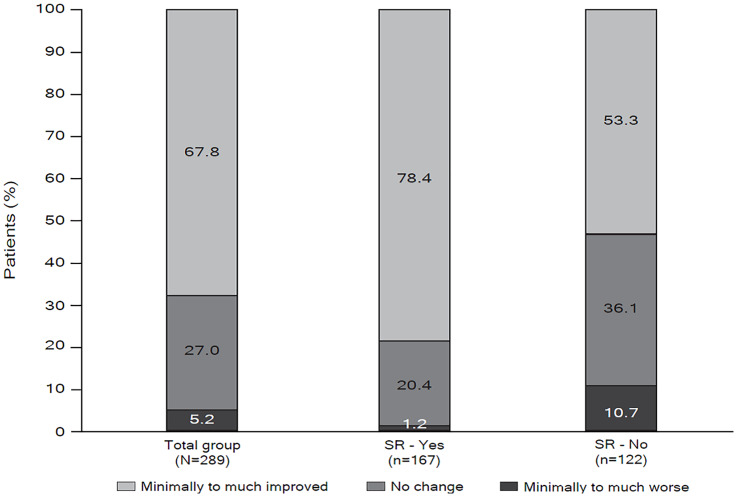
Mean (SD) CGI-S was 3.2 (1.0) at baseline and 3.0 (1.0) at LOCF endpoint (Figure 5), and the percentage of patients considered to be moderately-to-severely ill decreased from baseline to LOCF endpoint (from 35.4% to 25.7%). According to the CGI-C, at LOCF endpoint the condition of more than two-thirds (67.8%) of patients had improved, with 27.0% showing no change, and only 5.2% considered to be worse as compared with baseline (Figure 6).
Figure 5.
CGI-S frequency distribution score categories at baseline and LOCF endpoint for all patients and according to achievement of SR at LOCF (Yes/No) (mITT efficacy analysis set).
CGI-S ranges from 1 = Normal to 7 = extremely ill. Only patients with both baseline and at least one post-baseline assessment were included in the analysis.
CGI-S, clinical global impression of severity; LOCF, last observation carried forward; mITT, modified intent-to-treat; SR, symptomatic remission.
Figure 6.
Distribution of CGI-C score categories at LOCF endpoint for total population and subgroups with or without LOCF endpoint SR (mITT population efficacy analysis set).
CGI-C ranges from 1 = very much improved to 7 = very much worse
CGI-S, clinical global impression of change; LOCF, last observation carried forward; mITT, modified intent-to-treat; SR, symptomatic remission.
Functioning
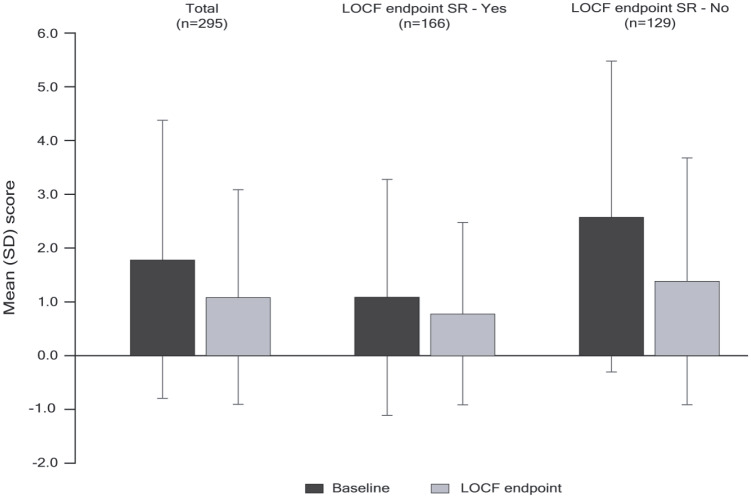
On average, patients exhibited a mild degree of functional impairment at baseline, as evidenced by a mean (SD) baseline PSP total score of 65.9 (14.0) and median score of 68. The mean change in PSP total score from baseline to LOCF endpoint for all patients was 1.04 (95% CI: –0.3, 2.3) (Table 5). At baseline, 38.4% of patients were in a state of functional remission (PSP total score > 70) and this was observed in 39.8% of patients at LOCF endpoint. At LOCF endpoint, 31.8% of patients had achieved both symptomatic and functional remission.
Table 5.
Change in PSP Scale total and subscale scores (mITT efficacy analysis set).
| Characteristic | Total (n = 294) | Achieved SR at LOCF endpoint (n = 166) | Did not achieve SR at LOCF endpoint (n = 128) |
|---|---|---|---|
| PSP total score | |||
| Baseline | 65.85 (14.03) | 70.42 (10.95) | 59.94 (15.35) |
| LOCF endpoint | 66.89 (14.28) | 72.36 (10.33) | 59.80 (15.55) |
| Mean change from baseline (95% CI) | 1.04 (–0.3, 2.3) | 1.95 (0.5, 3.4) | −0.14 (–2.5, 2.2) |
| Social useful activities | |||
| Baseline | 2.62 (1.13) | 2.28 (0.91) | 3.07 (1.22) |
| LOCF endpoint | 2.59 (1.01) | 2.19 (0.78) | 3.09 (1.06) |
| Mean change from baseline (95% CI) | −0.04 (–0.1, 0.1) | −0.08 (–0.2, 0) | 0.02 (–0.2, 0.2) |
| Personal and social relationships | |||
| Baseline | 2.63 (0.99) | 2.37 (0.88) | 2.98 (1.02) |
| LOCF endpoint | 2.59 (1.00) | 2.20 (0.81) | 3.09 (1.00) |
| Mean change from baseline (95% CI) | −0.04 (–0.1, 0.1) | −0.16 (–0.3, 0) | 0.12 (0, 0.3) |
| Self-care | |||
| Baseline | 1.79 (0.86) | 1.58 (0.74) | 2.06 (0.92) |
| LOCF endpoint | 1.79 (0.87) | 1.61 (0.76) | 2.02 (0.95) |
| Mean change from baseline (95% CI) | 0 (–0.1, 0.1) | 0.04 (–0.1, 0.2) | −0.05 (–0.2, 0.1) |
| Disturbing and aggressive behavior | |||
| Baseline | 1.14 (0.37) | 1.13 (0.35) | 1.16 (0.39) |
| LOCF endpoint | 1.11 (0.40) | 1.04 (0.23) | 1.20 (0.54) |
| Mean change from baseline (95% CI) | −0.03 (–0.1, 0) | −0.08 (–0.1, 0) | 0.05 (–0.1, 0.2) |
Values are mean (standard deviation) unless otherwise indicated. Only patients with both baseline and at least one post-baseline assessment were included in the analysis.
CI, confidence interval; LOCF, last observation carried forward; mITT, modified intent-to-treat; PSP, personal and social performance; SR, symptomatic remission.
Satisfaction with medication
Most patients (80.8%) were at least somewhat satisfied with medication at baseline; at LOCF endpoint, the proportion was 82.1%. Almost all physicians were at least somewhat satisfied with all aspects of medication at both baseline (95.9%) and LOCF endpoint (94.2%).
Carer burden
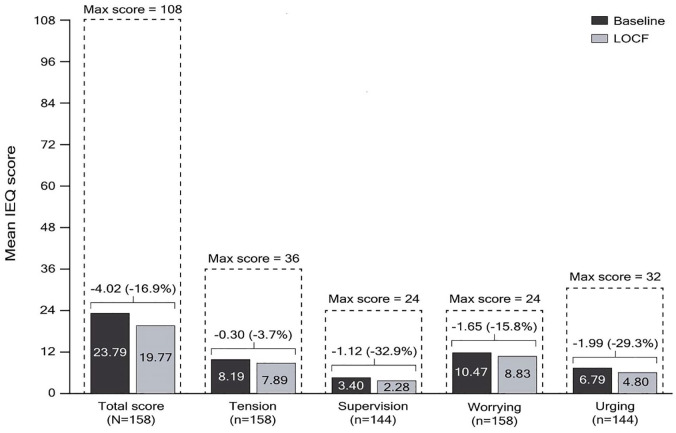
A total of 158 carers completed at least one section of the IEQ and 54.1% of these had >32 h per week of contact with their relative/friend at baseline. The mean (SD) IEQ total score of 23.8 (12.6) at baseline decreased by 16.8% [4.0 points (95% CI: –5.9, –2.1)] at LOCF endpoint. The largest subscale score improvement from baseline to LOCF endpoint was observed for the supervision subscale (–32.9%), followed by urging (–29.3%), worrying (–15.8%), and tension (–3.7%) (Figure 7).
Figure 7.
Change from baseline to LOCF endpoint in IEQ total and domain scores (mITT efficacy analysis set).
Only patients with both baseline and at least one post-baseline assessment were included in the analysis. Wilcoxon signed rank p-values for change from baseline are shown.
IEQ, involvement evaluation questionnaire; LOCF, last observation carried forward; mITT, modified intent-to-treat.
Health-care resource utilization
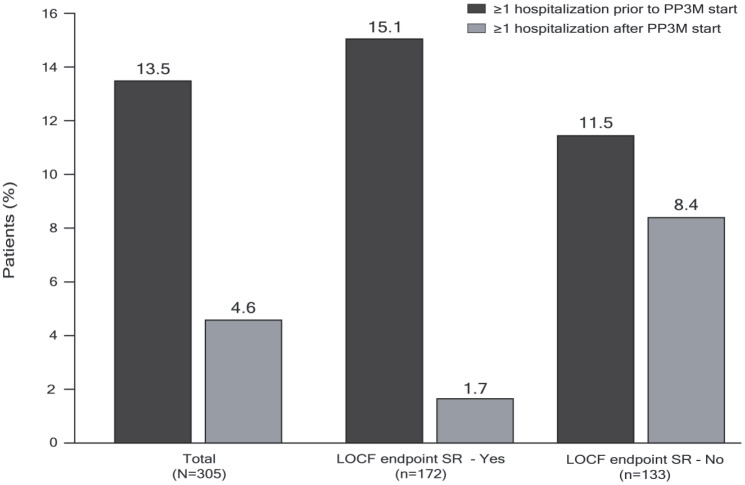
The proportion of patients requiring hospitalization for psychiatric reasons decreased from 13.5% of the total group in the 12 months prior to baseline to 4.6% during the treatment period (Figure 8), whereas the mean (SD) total number of days spent in the hospital decreased from 33.2 (22.4) to 15.2 (10.8). A small number of patients (n = 19; 6.3%) had visited the emergency department at least once during the 12 months prior to baseline (11 of these for psychiatric reasons), and this decreased to 9 patients (3.0%) (3 for psychiatric reasons) during the treatment period.
Figure 8.
Number of patients with at least one hospitalization prior to PP3M start and during PP3M treatment (mITT population).
Only patients with both baseline and at least one post-baseline assessment were included in the analysis.
LOCF, last observation carried forward; mITT, modified intent-to-treat; PP3M, paliperidone palmitate 3-month formulation; SR, symptomatic remission.
Analysis of data according to achievement of SR at LOCF endpoint
Baseline characteristics were comparable, although patients who achieved SR tended to be younger and to have a shorter duration of schizophrenia since diagnosis (mean, 8.6 and 10.0 years, respectively) than those who did not achieve SR (Table 1). Additionally, although the overall number of dose changes were low in the study, substantially fewer patients who achieved SR at LOCF endpoint had a PP3M dose increase than those who did not achieve SR (0.6% versus 7.5%) (Table 2).
Patients who achieved SR at LOCF endpoint had consistently lower baseline scores for PANSS total, positive, negative, and general symptoms, as well as Marder positive, negative, and disorganized thoughts factor scores relative to those patients who did not achieve SR (Table 4). At LOCF endpoint, the improvements observed in PANSS total, subscale and Marder factor scores were all greater in the subgroup of patients who achieved SR at LOCF endpoint than in those who did not, although these differences were small.
Based upon results from the CGI-S, fewer patients who achieved SR at LOCF endpoint were moderately to severely ill at baseline compared with those who did not achieve SR (25.4% and 48.4%, respectively) (Figure 5). At least a minimal improvement in the CGI-C was observed in 78.4% of patients who achieved SR at LOCF endpoint, and in 53.3% of patients who did not (Figure 6).
Mean (SD) baseline PSP total score in patients who achieved SR at LOCF endpoint was higher than in patients who did not achieve SR [70.42 (11.0) versus 59.9 (15.4)], although subscale scores were comparable (Table 5). Functional remission (PSP total score > 70) at baseline was observed in 53.6% and 21.9% of patients who achieved SR at LOCF endpoint versus patients who did not, respectively.
Among patients who achieved and those who did not achieve SR at LOCF endpoint, similar percentages were at least somewhat satisfied with medication at both baseline (81.6% and 79.7%) and LOCF endpoint (82.8% and 81.2%). Overall, more than 90% of physicians were at least somewhat satisfied with medication in both subgroups at baseline and LOCF endpoint. For the two subgroups, similar improvements in IEQ total and subscale scores were observed (Figure 7).
The proportion of patients hospitalized decreased in both subgroups but the extent of the decrease was greater in those patients who achieved SR at LOCF endpoint (from 15.1% to 1.7%) than in those who did not (from 11.5 to 8.4%). The mean (SD) number of hospitalization days was also lower in patients achieving SR at LOCF endpoint than in those who did not [3.7 (1.2) versus 18.7 (9.9)] (Figure 8).
Safety
A total of 161 patients (53.1%) reported at least one TEAE, with the most common being weight increase (8.6%), injection site pain (5.9%), viral upper respiratory infection (3.6%), insomnia (3.6%), schizophrenia (3.3%), and weight decrease (3.3%). No deaths were reported during the study period. Most TEAEs were mild (74.6%) or moderate (22.8%) in intensity; seven patients (2.6%) experienced severe TEAEs: thrombocytopenia, abdominal pain, and colon adenocarcinoma; delusion and schizophrenia; delusion and psychiatric decompensation; vomiting; injection site pain; blunted affect; and orchidectomy. Serious TEAEs were experienced by 18 patients (5.9%). TEAEs were considered to be at least possibly related to study medication in 91 patients (30.0%). In total, 4 patients (1.3%) withdrew from the study due to TEAEs. Most TEAEs (88.4%) did not result in a PP3M dose change. All TEAEs, TEAEs possibly related to treatment, and serious AEs were all more common in patients who did not achieve SR at LOCF endpoint. With regard to common TEAEs of special interest, 26 patients (8.6%) experienced weight gain and 14 patients (4.6%) experienced one or more possibly prolactin-related TEAEs (Table 6). No clinical laboratory evaluations were performed as part of the study.
Table 6.
Summary of TEAEs (mITT safety analysis set).
| Characteristic, n (%) | Total (n = 303) | Achieved SR at LOCF endpoint (n = 172) | Did not achieve SR at LOCF endpoint (n = 131) |
|---|---|---|---|
| Patients with ⩾1 TEAE | 161 (53.1) | 79 (45.9) | 82 (62.6) |
| TEAEs possibly related to study medication | 91 (30.0) | 39 (22.7) | 52 (39.7) |
| Serious TEAEs | 18 (5.9) | 2 (1.2) | 16 (12.2) |
| TEAEs leading to treatment/study withdrawal | 4 (1.3) | 0 | 4 (3.1) |
| Thrombocytopenia | 1 (0.3) | 0 | 1 (0.8) |
| Delusion | 2 (0.7) | 0 | 2 (1.5) |
| Hallucination, auditory | 2 (0.7) | 0 | 2 (1.5) |
| TEAEs experienced by ⩾3% of patients | |||
| Weight increased | 26 (8.6) | 12 (7.0) | 14 (10.7) |
| Injection site pain | 18 (5.9) | 11 (6.4) | 7 (5.3) |
| Viral upper respiratory tract infection | 11 (3.6) | 9 (5.2) | 2 (1.5) |
| Insomnia | 11 (3.6) | 9 (5.2) | 2 (1.5) |
| Schizophrenia | 10 (3.3) | 1 (0.6) | 9 (6.9) |
| Weight decreased | 10 (3.3) | 2 (1.2) | 8 (6.1) |
| Potentially prolactin-related TEAEs | 14 (4.6) | 10 (5.8) | 4 (3.1) |
| Amenorrhea | 6 (2.0) | 4 (2.3) | 2 (1.5) |
| Sexual/erectile dysfunction | 3 (1.0) | 2 (1.2) | 1 (0.8) |
| Galactorrhea | 1 (0.3) | 1 (0.6) | 0 |
| Hyperprolactinemia/blood prolactin increased | 8 (2.6) | 5 (2.9) | 3 (2.3) |
| Loss of libido | 1 (0.3) | 0 | 1 (0.8) |
| Menstruation irregular | 2 (0.7) | 2 (1.2) | 0 |
LOCF, last observation carried forward; mITT, modified intent-to-treat; SR, symptomatic remission; TEAE, treatment-emergent adverse event.
The mean (SD) ESRS score at baseline was 1.76 (2.60), indicating minimal EPS. There was a small but clinically relevant improvement in ESRS total score from baseline to LOCF endpoint (–0.69; 95% CI: –1.0, –0.4) and the change was greater in patients who did not achieve SR (Figure 9).
Figure 9.
ESRS score at baseline and LOCF endpoint (mITT safety analysis set).
ESRS, extrapyramidal symptom rating scale; LOCF, last observation carried forward; mITT, modified intent-to-treat; SD, standard deviation.
Mean (SD) weight and BMI showed a small change from baseline [80.5 (17.9) kg and 27.3 (5.0) kg/m2, respectively] to LOCF endpoint [81.1 (18.3) kg and 27.5 (5.3) kg/m2]. Weight increases of ⩾7% from baseline (not always recorded as a TEAE) were observed in 11 patients (10.4%) with a baseline BMI < 25 kg/m2, in 13 patients (11.6%) with a baseline BMI 25 to <30 kg/m2, and in 7 patients (9.3%) with a baseline BMI ⩾30 kg/m2. There were no significant changes in vital signs during the study.
Discussion
This study assessed the impact of converting patients with schizophrenia stabilized with PP1M to PP3M in a naturalistic clinical setting that included patients with concomitant medications and mild-to-moderate substance abuse (providing that these patients met study criteria), and allowed flexible dosing after the first administration of PP3M if required based on clinical judgement. However, only a low number of patients required dose changes during the study, confirming the real-world clinical utility of the recommended dosing regimen for converting stabilized patients from PP1M to PP3M.
The primary endpoint results demonstrate that treatment with PP3M for 1 year after stabilization with PP1M resulted in over half of patients (57%) achieving SR at LOCF endpoint. The increase in SR was 10% from Month 6 to Month 12. These results are in agreement with those of the pivotal trial in which 58% of patients who received PP3M achieved SR during the final 6 months of the double-blind period.32 There are a limited number of comparable studies using the same criteria (for both severity and duration) for SR. In a systematic review by Al Aqueel et al., the proportion of patients achieving SR with first-episode criteria was 17–78%, with a weighted mean of 35.6%. For multiple-episode patients, the proportion achieving SR was 16–62% with a weighted mean of 37%; such variability may be explained by the differing length and frequency of follow ups, and differing drop-out rates.33 The remission status achieved with oral medication decreased over time, whereas remission achieved following treatment with long-acting injectable antipsychotics tended to be increased, with a high level of sustainability (84–94%),33,34 in line with the outcomes of the REMISSIO study. The majority of patients (93.5%) in the current study who achieved SR at Month 6 retained their remission status until the end of study. Of note, more than one-half of patients who achieved SR at LOCF endpoint also achieved functional remission (PSP total score > 70), which is associated with better daily functioning.9–11
More than 50% of patients who did not achieve SR experienced at least some improvement in their condition, as indicated by the decrease in the percentage of patients considered to be moderately to severely ill at LOCF endpoint. However, the proportion of patients in this group who achieved functional remission was low. This is unsurprising, as this group tended to demonstrate higher symptom scores, and almost twice as many of these patients were considered to be moderately to severely ill at baseline compared with patients who achieved SR. Failure to fulfill the PANSS remission symptom severity criterion was most often due to not meeting a score of ⩽3 for items N1 (blunted affect) and N4 (passive/apathetic social withdrawal), though the proportion of patients reaching the threshold for these items increased during the observation period.
The results of the secondary endpoint analyses corroborated those of the primary endpoint. PANSS total, subscale, and Marder factor scores all showed a reduction from baseline to LOCF endpoint, indicating that symptom control was maintained during treatment with PP3M for 1 year. CGI scores also decreased from baseline to LOCF endpoint, indicative of a reduction in the severity of the condition, with over 50% of patients showing at least minimum improvement in the group of patients without SR at study endpoint. In general, the efficacy results of the current study are broadly consistent with those of the two previous randomized trials, which demonstrated that PP3M is effective in preventing relapse in clinically stable patients with schizophrenia previously treated with PP1M.31,32 The overall carer burden also decreased from baseline to endpoint, both in patients who did and did not achieve SR, perhaps as a result of maintained or improved patient well-being.
With regard to hospitalizations, a substantial reduction was observed in the number of patients hospitalized during the 1-year PP3M treatment period compared with the year preceding PP3M, accompanied by a decrease in the mean number of inpatient days. Although the hospitalization rate was already low prior to switching patients from PP1M to PP3M, the reduction in hospitalizations was almost 90% in patients who achieved SR at LOCF endpoint compared with about 40% in patients without SR. The number of emergency department visits was low in the 12 months prior to treatment with PP3M and remained so during the study treatment period. These results are consistent with those reported in one study conducted in the United States using electronic health record data; this study assessed the transition from PP1M to PP3M and showed a decrease in the number of inpatient days and outpatient visits, as well as reduced medical costs.35
The safety profile was consistent with that of the two previous PP3M phase III clinical trials,31,32 and no new safety signals were identified during the current study. There was a slight improvement in EPS during the study from low baseline values, and no changes in vital signs, further supporting the safety profile of PP3M. This was reinforced by the small number of discontinuations due to TEAEs (four patients; 1.3%). Despite the known propensity for paliperidone palmitate to increase prolactin levels,36,37 the incidence of potentially prolactin-related TEAEs was low. Approximately 10% of patients experienced a significant weight increase, which is comparable with that observed with PP3M and PP1M in the phase III randomized non-inferiority study (15% and 16%, respectively).32
A strength of the current study is that the completion rate of 95.4% is one of the highest observed for a 1-year real-world study in schizophrenia.38–41 This supports the overall effectiveness and tolerability of PP3M, although it may also be reflective of the requirement for patients to be stabilized prior to inclusion in this study. The high completion rate is consistent with the extremely high proportion of both patients (82%) and physicians (94%) who were satisfied with PP3M treatment at the end of the study. The geographical spread of the participants is also important.
Study limitations
The primary limitation of this study was that it is a single treatment arm, uncontrolled, open-label study. This did not allow for a direct head-to-head comparison of the safety and efficacy of PP1M and PP3M therapy in patients with schizophrenia. The eligibility requirements for patients with clinically stable schizophrenia and PP1M tolerability may have restricted the patient population somewhat, such that it was not fully representative of routine clinical practice. However, it could be argued that a study population that included patients with concomitant medications and mild-to-moderate substance abuse is reasonably reflective of the real-world clinical setting. We acknowledge that this study excluded patients with comorbid psychiatric and severe substance use disorders; thus, the study results may not be applicable to patients with these conditions. Furthermore, cultural-based differences in patient and treatment characteristics within the geographical locations of the clinical centers (Europe, the Middle East, Africa and the Asia Pacific region) may have introduced bias.
Conclusion
The majority of patients with stable schizophrenia converting from PP1M to PP3M in a naturalistic clinical setting achieved SR and/or maintained symptom stability. Personal and social functioning were also maintained, with some incremental continuous improvements. The safety profile of PP3M was consistent with that of the two previous PP3M phase III clinical trials31,32 and no new safety signals were identified during the current study. High proportions of both patients and physicians were satisfied with PP3M medication at study end, which was also reflected in the very high study completion rate of 95.4%. There was a decrease in the number of psychiatric hospitalizations over the study period, regardless of the achievement of SR, and a reduction in carer burden. Overall, the efficacy and safety results from this naturalistic study were similar to those observed in previous randomized clinical trials of PP3M and underline the importance of continuous maintenance treatment in patients with schizophrenia. Further long-term studies of the efficacy and safety of PP3M in adult patients with schizophrenia are warranted.
Supplemental Material
Supplemental material, Supplementary_Tables for Symptomatic and functional outcomes after treatment with paliperidone palmitate 3-month formulation for 52 weeks in patients with clinically stable schizophrenia by Maria Paz Garcia-Portilla, Pierre-Michel Llorca, Giuseppe Maina, Vasilis P. Bozikas, Halise Devrimci-Ozguven, Sung-Wan Kim, Paul Bergmans, Irina Usankova and Katalin Pungor in Therapeutic Advances in Psychopharmacology
Acknowledgments
This study was sponsored by Janssen. Cello Health MedErgy (Europe) assisted in drafting the manuscript under the direction of the authors and provided editorial support throughout its development. Medical writing and editorial support were funded by Janssen.
Footnotes
Conflict of interest statement: MP Garcia-Portilla has been a consultant to and/or has received honoraria/grants from Angelini, Alianza Otsuka-Lundbeck, Instituto de Salud Carlos III, Janssen-Cilag, Lundbeck, Otsuka, and Pfizer. P-M Llorca has received speaker and consultation fees from Janssen, Eisai, Lundbeck, Otsuka, and Bouchara-Recordatti. G Maina has no conflicts of interest to disclose. V Bozikas has received speaker and consultation fees from Angelini, Janssen, Lilly and Vianex. H Devrimci-Özgüven has no conflicts of interest to disclose. S-W Kim has no conflicts of interest to disclose. P Bergmans is a full-time employee of Janssen and stockholder of Johnson & Johnson. I Usankova is a full-time employee of Janssen. K Pungor is a full-time employee of Janssen and stockholder of Johnson & Johnson.
Data sharing statement: Access to anonymized individual participant level data will not be provided for this trial as it meets one or more of the exceptions described on https://yoda.yale.edu/ under “Data Use Agreement - Janssen Pharmaceuticals DUA”.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Janssen. Medical writing and editorial support was provided by Cello Health MedErgy (Europe), and funded by Janssen.
ORCID iDs: Pierre-Michel Llorca  https://orcid.org/0000-0001-7438-8990
https://orcid.org/0000-0001-7438-8990
Katalin Pungor  https://orcid.org/0000-0003-4679-2050
https://orcid.org/0000-0003-4679-2050
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Maria Paz Garcia-Portilla, Department of Psychiatry, University of Oviedo, CIBERSAM, Oviedo, Spain.
Pierre-Michel Llorca, CMP B, CHU Clermont-Ferrand, Clermont Auvergne University, Clermont-Ferrand, France.
Giuseppe Maina, SCDU Psichiatria, AOU San Luigi Gonzaga, Università degli Studi di Torino, Torino, Piemonte, Italy.
Vasilis P. Bozikas, Second Department of Psychiatry, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
Halise Devrimci-Ozguven, Department of Psychiatry, School of Medicine, Ankara University, Ankara, Turkey.
Sung-Wan Kim, Department of Psychiatry, Chonnam National University Medical School, Gwangju, South Korea.
Paul Bergmans, Janssen Cilag, Biostatistics, Breda, The Netherlands.
Irina Usankova, Johnson & Johnson, EMEA Medical Affairs Organization, Moscow, Russia.
Katalin Pungor, EMEA Medical Affairs, Janssen Cilag GmbH, Johnson & Johnson Platz 1, Neuss, 41470, Germany.
References
- 1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry 2015; 15: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale for schizophrenia. Schizophr Bull 1987; 13: 261–276. [DOI] [PubMed] [Google Scholar]
- 3. Lecrubier Y, Perry R, Milligan G, et al. Physician observations and perceptions of positive and negative symptoms of schizophrenia: a multinational, cross-sectional survey. Eur Psychiatry 2007; 22: 371–379. [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Portilla MP, Garcia-Alvarez L, Saiz PA, et al. Psychometric evaluation of the negative syndrome of schizophrenia. Eur Arch Psychiatry Clin Neurosci 2015; 265: 559–566. [DOI] [PubMed] [Google Scholar]
- 5. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 2012; 379: 2063–2071. [DOI] [PubMed] [Google Scholar]
- 6. Takeuchi H, Kantor N, Sanches M, et al. One-year symptom trajectories in patients with stable schizophrenia maintained on antipsychotics versus placebo: meta-analysis. Br J Psychiatry 2017; 211: 137–143. [DOI] [PubMed] [Google Scholar]
- 7. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res 2018; 197: 274–280. [DOI] [PubMed] [Google Scholar]
- 8. Andreasen NC, Carpenter WT, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005; 162: 441–449. [DOI] [PubMed] [Google Scholar]
- 9. Haynes VS, Zhu B, Stauffer VL, et al. Long-term healthcare costs and functional outcomes associated with lack of remission in schizophrenia: a post-hoc analysis of a prospective observational study. BMC Psychiatry 2012; 12: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haro JM, Novick D, Perrin E, et al. Symptomatic remission and patient quality of life in an observational study of schizophrenia: is there a relationship? Psychiatry Res 2014; 220: 163–169. [DOI] [PubMed] [Google Scholar]
- 11. Lambert M, De Marinis T, Pfeil J, et al. Establishing remission and good clinical functioning in schizophrenia: predictors of best outcome with long-term risperidone long-acting injectable treatment. Eur Psychiatry 2010; 25: 220–229. [DOI] [PubMed] [Google Scholar]
- 12. Alenius M, Hammarlund-Udenaes M, Hartvig P, et al. Treatment response in psychotic patients classified according to social and clinical needs, drug side effects, and previous treatment; a method to identify functional remission. Compr Psychiatry 2009; 50: 453–462. [DOI] [PubMed] [Google Scholar]
- 13. Lambert M, Naber D, Schacht A, et al. Rates and predictors of remission and recovery during 3 years in 392 never-treated patients with schizophrenia. Acta Psychiatr Scand 2008; 118: 220–229. [DOI] [PubMed] [Google Scholar]
- 14. Schooler NR, Buchanan RW, Laughren T, et al. Defining therapeutic benefit for people with schizophrenia: focus on negative symptoms. Schizophr Res 2015; 162: 169–174. [DOI] [PubMed] [Google Scholar]
- 15. Bhanji NH, Chouinard G, Margolese HC. A review of compliance, depot intramuscular antipsychotics and the new long-acting injectable atypical antipsychotic risperidone in schizophrenia. Eur Neuropsychopharmacol 2004; 14: 87–92. [DOI] [PubMed] [Google Scholar]
- 16. Ascher-Svanum H, Zhu B, Faries D, et al. A prospective study of risk factors for nonadherence with antipsychotic medication in the treatment of schizophrenia. J Clin Psychiatry 2006; 67: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 17. Olivares JM, Alptekin K, Azorin JM, et al. Psychiatrists’ awareness of adherence to antipsychotic medication in patients with schizophrenia: results from a survey conducted across Europe, the Middle East, and Africa. Patient Prefer Adherence 2013; 7: 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arango C, Baeza I, Bernardo M, et al. Long-acting injectable antipsychotics for the treatment of schizophrenia in Spain. Rev Psiquiatr Salud Ment. Epub ahead of print 25 June 2018. DOI: 10.1016/j.rpsm.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 19. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry 2017; 74: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California medicaid patients with schizophrenia. Psychiatr Serv 2004; 55: 886–891. [DOI] [PubMed] [Google Scholar]
- 21. de Bartolomeis A, Fagiolini A, Vaggi M, et al. , Targets, attitudes, and goals of psychiatrists treating patients with schizophrenia: key outcome drivers, role of quality of life, and place of long-acting antipsychotics. Neuropsychiatr Dis Treat 2016; 12: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiden PJ, Kim E, Bermak J, et al. Does half-life matter after antipsychotic discontinuation? A relapse comparison in schizophrenia with 3 different formulations of paliperidone. J Clin Psychiatry 2017; 78: e813–e820. [DOI] [PubMed] [Google Scholar]
- 23. Bera R, Offord S, Zubek D, et al. Impact on healthcare resource usage and costs among medicaid-insured schizophrenia patients after initiation of treatment with long-acting injectable antipsychotics. J Med Econ 2013; 16: 522–528. [DOI] [PubMed] [Google Scholar]
- 24. Pilon D, Tandon N, Lafeuille MH, et al. Treatment patterns, health care resource utilization, and spending in medicaid beneficiaries initiating second-generation long-acting injectable agents versus oral atypical antipsychotics. Clin Ther 2017; 39: 1972–1985e2. [DOI] [PubMed] [Google Scholar]
- 25. Nielsen RE, Hessellund KB, Valentin JB, et al. Second-generation LAI are associated to favorable outcome in a cohort of incident patients diagnosed with schizophrenia. Schizophr Res 2018; 202: 234–240. [DOI] [PubMed] [Google Scholar]
- 26. Lloyd K, Latif M, Simpson S, et al. Switching stable patients with schizophrenia from depot and oral antipsychotics to long-acting injectable risperidone: efficacy, quality of life and functional outcome. Hum Psychopharmacol 2010; 25: 243–252. [DOI] [PubMed] [Google Scholar]
- 27. Macfadden W, DeSouza C, Crivera C, et al. Assessment of effectiveness measures in patients with schizophrenia initiated on risperidone long-acting therapy: the SOURCE study results. BMC Psychiatry 2011; 11: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pietrini F, D’Anna G, Tatini L, et al. Changes in attitude towards LAI antipsychotic maintenance treatment: a two-year follow-up study. Eur Psychiatry 2018; 53: 58–65. [DOI] [PubMed] [Google Scholar]
- 29. TREVICTA®. TREVICTA® 175/263/350/525 mg prolonged release suspension for injection. Summary of product characteristics. England: Janssen-Cilag Ltd., https://www.ema.europa.eu/en/documents/product-information/trevicta-epar-product-information_en.pdf. (2018, accessed 21 January 2020).
- 30. INVEGA TRINZA® (paliperidone palmitate). Prescribing information: INVEGA TRINZA® 273/410/546/819 mg extended release injectable suspension for intramuscular use. Titusville, NJ: Janssen Pharmaceuticals Inc., https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207946s008lbl.pdf. (2019, accessed 21 January 2020).
- 31. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia a randomized clinical trial. JAMA Psychiatry 2015; 72: 830–839. [DOI] [PubMed] [Google Scholar]
- 32. Savitz AJ, Xu H, Gopal S, et al. Efficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter, double-blind, noninferiority study. Int J Neuropsychopharmacol 2016; 19: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. AlAqeel B, Margolese HC. Remission in schizophrenia: critical and systematic review. Harv Rev Psychiatry 2012; 20: 281–297. [DOI] [PubMed] [Google Scholar]
- 34. Gorwood P, Bouju S, Deal C, et al. Predictive factors of functional remission in patients with early to mid-stage schizophrenia treated by long acting antipsychotics and the specific role of clinical remission. Psychiatry Res 2019; 281: 112560. [DOI] [PubMed] [Google Scholar]
- 35. DerSarkissian M, Lefebvre P, Joshi K, et al. Health care resource utilization and costs associated with transitioning to 3-month paliperidone palmitate among US veterans. Clin Ther 2018; 40: 1496–1508. [DOI] [PubMed] [Google Scholar]
- 36. Einarson TR, Hemels ME, Nuamah I, et al. An analysis of potentially prolactin-related adverse events and abnormal prolactin values in randomized clinical trials with paliperidone palmitate. Ann Pharmacother 2012; 46: 1322–1330. [DOI] [PubMed] [Google Scholar]
- 37. McEvoy JP, Byerly M, Hamer RM, et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA 2014; 311: 1978–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cortesi PA, Mencacci C, Luigi F, et al. Compliance, persistence, costs and quality of life in young patients treated with antipsychotic drugs: results from the COMETA study. BMC Psychiatry 2013; 13: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joshi K, Mao L, Biondi DM, et al. The research and evaluation of antipsychotic treatment in community behavioral health organizations, outcomes (REACH-OUT) study: real-world clinical practice in schizophrenia. BMC Psychiatry 2018; 18: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ratner Y, Gibel A, Yorkov V, et al. Effectiveness, safety, and tolerability of ziprasidone for treating schizophrenia patients undergoing usual care: a 12-month, open-label, flexible-dose, naturalistic observational trial. Prog Neuropsychopharmacology Biol Psychiatry 2007; 31: 1401–1409. [DOI] [PubMed] [Google Scholar]
- 41. Takaesu Y, Kishimoto T, Murakoshi A, et al. Factors associated with discontinuation of aripiprazole treatment after switching from other antipsychotics in patients with chronic schizophrenia: a prospective observational study. Psychiatry Res 2016; 236: 71–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Tables for Symptomatic and functional outcomes after treatment with paliperidone palmitate 3-month formulation for 52 weeks in patients with clinically stable schizophrenia by Maria Paz Garcia-Portilla, Pierre-Michel Llorca, Giuseppe Maina, Vasilis P. Bozikas, Halise Devrimci-Ozguven, Sung-Wan Kim, Paul Bergmans, Irina Usankova and Katalin Pungor in Therapeutic Advances in Psychopharmacology