Abstract
Uncorrected atrial septal defect undergoes right ventricle chronic volume overload which may lead to pulmonary hypertension and Eisenmenger Syndrome. The soluble suppression of tumorigenicity-2 is a left ventricle strain biomarker; however, its role in right ventricle strain is unclear. This study aimed to investigate the implication of serum soluble suppression of tumorigenicity-2 in adult uncorrected atrial septal defect. This was a cross-sectional study. We enrolled 81 adult uncorrected secundum atrial septal defect patients. Clinical and hemodynamic data were collected. Serum samples were withdrawn from the pulmonary artery during right heart catheterization. Serum soluble suppression of tumorigenicity-2 and NT-proBNP levels were measured. Subjects were divided into three groups based on clinical and hemodynamic severity. The correlation of soluble suppression of tumorigenicity-2 with patients' data and comparison among groups were analyzed. A p value <0.05 was considered statistically significant. Results showed that, there were significant correlations between serum soluble suppression of tumorigenicity-2 and mean pulmonary artery pressure (r = 0.203, p = 0.035) and right ventricle end-diastolic diameter (r = 0.203, p <0.05). Median serum soluble suppression of tumorigenicity-2 level was incrementally increased from group I (atrial septal defect and no-pulmonary hypertension), group II (left-to-right atrial septal defect and pulmonary hypertension), to group III (Eisenmenger Syndrome): (17.4 ng/mL, 21.8 ng/mL, and 29.4 ng/mL, respectively). A post-hoc analysis showed that serum soluble suppression of tumorigenicity-2 level was significantly different between groups I and III (p = 0.01). Serum N terminal pro brain natriuretic peptide (NT-proBNP) level was consistently associated with worse clinical and hemodynamic parameters. No correlation was found between serum soluble suppression of tumorigenicity-2 and NT-proBNP level. In conclusion, serum soluble suppression of tumorigenicity-2 level had significant positive correlation with mean pulmonary artery pressure and right ventricle end-diastolic diameter in uncorrected secundum atrial septal defect patients. Higher serum soluble suppression of tumorigenicity-2 level was associated with the presence of pulmonary hypertension and Eisenmenger Syndrome in uncorrected secundum atrial septal defect patients.
Keywords: uncorrected atrial septal defect, pulmonary hypertension, biomarker, soluble suppression of tumorigenicity-2 (sST2), NT-proBNP
Introduction
Uncorrected large atrial septal defect (ASD) sustains right ventricle (RV) volume overload. In the left-to-right shunt ASD, the RV undergoes chronic persistent volume overload and adapts to additional strain. Chronic blood overflow into the pulmonary blood vessels leads to pulmonary artery (PA) endothelial dysfunction, inflammation, and finally, remodeling.1 Pulmonary vascular remodeling develops into increased pulmonary vascular resistance (PVR) and pulmonary arterial pressure which lead to pulmonary hypertension (PH).1 The PH associated with uncorrected ASD usually occurs in the third to fifth decade of life.2,3 Its incidence is between 6% and 35% with an annual mortality rate of 15%.3 Unlike left ventricle pressure overload, the role of soluble biomarkers that predict the RV overload in uncorrected ASD is currently unavailable.
The suppression of tumorigenicity-2 (ST2) is a receptor protein belonging to the family of interleukin receptors.4 The ST2 expression, particularly membrane-bound ST2, increases when a ventricle undergoes mechanical strain as an adaptation mechanism to protect the myocardia under stress-induced strain.4 The serum soluble ST2 (sST2) level in the circulation also increases and gives an antagonistic effect on the protective mechanism, since sST2 acts as a decoy receptor and hinders protective pathways.5 In chronic left ventricle strain, sST2 level is increased and provides prognostic value in left heart failure.6 Current meta-analysis studies indicate the prognostic efficacy of sST2 in long-term follow-up of acute and chronic left heart failure.7,8 In RV volume and/or pressure overload due to PH-associated with uncorrected ASD, the role of sST2 measurement is not yet known. Current studies showed that increased serum sST2, along with other biomarkers, is associated with worsened outcome in patients with PH.9,10 However, PH-associated with congenital heart disease was underrepresented in this study due to the small number of subjects with congenital heart disease.10
This study aimed to investigate the implication of serum sST2 level, in comparison with the established biomarker N terminal pro brain natriuretic peptide (NT-proBNP), on PH associated with uncorrected ASD especially in relation with clinical and hemodynamic parameters.
Methods
The study design was a cross-sectional study. The data were retrieved from The COngenital HeARt Disease in adult and Pulmonary Hypertension (COHARD-PH) registry, Yogyakarta, Indonesia. The COHARD-PH registry recruited adults ( ≥18 years of age) with congenital heart diseases. The COHARD-PH registry is the first registry in Indonesia that performs the invasive hemodynamic examination. It is conducted in Dr. Sardjito Hospital, Yogyakarta, Indonesia, a tertiary referral hospital in the region. The COHARD-PH registry has been started from 2012 and is still currently recruiting patients.11
For this study, we enrolled patients who met the following inclusion criteria: (1) adult ASD patients (≥18 years of age) who were registered in the COHARD-PH registry, (2) no closure of the defect had been performed, (3) the hemodynamic assessment with right heart catheterization (RHC) had been performed, and (4) no previous PH-targeted therapy was taken. The exclusion criteria in this study were: (1) the presence of other major congenital heart disease, (2) left ventricular ejection fraction (LVEF) <50% or left ventricular hypertrophy presence on transthoracic echocardiography (TTE), (3) the presence of valvular heart disease, other than secondary tricuspid or pulmonary regurgitation, (4) the presence of lung disease other than PH, for example, bronchial asthma, chronic obstructive pulmonary disease, and active or previous lung tuberculosis, (5) the concomitant chronic kidney disease (eGFR <60 mL/min/1.73 m2), (6) a history of previous myocardial infarction at any time, and (7) any concomitant proven malignancy disease or pregnancy.
The TTE and transesophageal echocardiography (TEE) were performed in all subjects. The TTE examination (VIVID 7, (G.E. Healthcare, USA)) was performed by two blinded sonographers and confirmed by cardiologist consultants who had undergone the agreement test (Kappa test) for examination of ASD patients registered in COHARD-PH registry (agreement was >80%).12 The parameters measured were ASD diameter, shunt direction, right ventricular basal end diastolic (RVED) diameter, tricuspid annular plane systolic excursion (TAPSE), and LVEF, according to standard procedures.13 The RVED diameter was determined from apical four-chamber view in the end-diastolic phase by measuring the maximal dimension in one-third of RV basal distance as a minor axis and perpendicular to major axis.13 The TEE examination was performed by cardiologist consultants as part of the registry work-up.
The hemodynamic assessment was performed with RHC using standard procedures in non-sedated patients. The femoral access was performed in all subjects. The right ventricle systolic pressure (RVSP), mean aortic pressure, mean pulmonary artery pressure (mPAP), mean left atrial pressure (mLAP), and pulmonary artery wedge pressure (PAWP) values were measured directly using a Multi-Purpose or Swan-Ganz catheter. The cardiac output (Q) was determined by indirect Fick method, as per protocol for the COHARD-PH registry.11 The flow ratio was calculated with the formula: pulmonary blood flow (Qp)/systemic blood flow (Qs) = (Ao saturation – mixed vein (MV) saturation)/(pulmonary vein (PV) saturation – PA saturation). A MV saturation was calculated from: ((3 × superior vena cava saturation) + inferior vena cava saturation)/4. The PVR was derived from the formula: ((mPAP – mLAP (or PAWP)/Qp)/body surface area. A Qp was calculated from the formula: O2 consumption (mL/min)/(1.36 × 10 × hemoglobin level × ((PV saturation – PA saturation)/100). The diagnosis of PH (pre-capillary) was based on mPAP value ≥25 mmHg, and PAWP or mLAP value ≤15 mmHg.1 The Eisenmenger syndrome (ES) was diagnosed based on hemodynamics value of Qp/Qs ratio <1 and PVRi value > 8 WU.m2 in subjects with reduced peripheral O2 saturation.1
The blood samples were withdrawn during the RHC procedure; 2 mL of blood was drawn from the PA using an ethylenediaminetetraacetic acid tube. The sample was allowed to stand at room temperature and subsequently centrifuged at 4000 r/min for 10 min at 25 ℃. The supernatant was separated and serum was isolated in a polypropylene tube (2 mL) and stored in a –80 ℃ freezer until sST2 measurement. The serum sST2 level was measured using a cartridge-based immunofluorescence method using the principle of lateral flow immunoassay technology. This test used the ASPECT-PLUS™ ST2 TEST cassette and was analyzed with the ASPECT READER™ (Critical Diagnostics, USA) tool. The measured sST2 levels were expressed in concentration (ng/mL). The lower and upper limit of the measurement of sST2 according to the manufacturer was 12.5 ng/mL and 250 ng/mL, respectively. The serum NT-proBNP level was measured using an electrochemiluminescence immunoassay (Elecsys ProBNP II) and performed in Cobas e immunoassay analyzer (Roche Diagnostics, Germany). The measured NT-proBNP levels were expressed in concentration (pg/mL). The measurements of sST2 and NT-proBNP were performed by skilled technicians blinded to the clinical and hemodynamic data of the subjects.
All subjects gave an informed consent for this study. The Medical and Health Research Ethics Committee of Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia, had approved the study protocol.
For statistical analysis, the variables with numerical data were presented in the mean ± standard deviation or median (Q1–Q3) based on the normality distribution of data. The Kolmogorov–Smirnov test was performed to determine the normality distribution of the data. The variables with categorical data were presented in percentage. Subjects were divided into three groups, i.e. subjects with ASD and no PH (group I), subjects with left-to-right shunt-ASD and PH (group II), and subjects with ASD and ES (group III). The comparison of variables with numerical data among groups was analyzed using one-way ANOVA test or Kruskal–Wallis test and post-hoc analysis according to normality distribution of data. The comparison of variables with categorical data among groups was analyzed using chi-squared test and post-hoc analysis, accordingly. The correlation between two variables with numerical data was analyzed using Pearson or Spearman's correlation test, based on the normality distribution of data. An receiver-operating characteristic (ROC) curve and area under the curve (AUC) measurements were constructed to assess the prediction of two biomarkers (sST2 and NT-proBNP) for the presence of PH. A p <0.05 was deemed statistically significant. Statistical analysis was performed using SPSS version 23.0 for Windows (IBM Corp., Chicago, IL).
Results
The subjects were 81 patients who fulfilled the research criteria. The median age of subjects was 33.6 years old. The youngest subject was 18 years old and the oldest subject was 66 years old. The percentage of female subjects was 85.2%. The mean six minute walking distance was 345.5 ± 103.4 m and mean peripheral O2 saturation was 95.1 ± 6.7%. The TTE examination showed the mean ASD diameter was 2.7 cm. The mean TAPSE value was 24.5 mm. The median RVED diameter was 45 mm. The mean mPAP value was 44 mmHg and RVSP was 72.8 mmHg. The median PVR value was 4.5 Wood Unit. The median serum sST2 level was 22.2 ng/mL. The median NT-proBNP level was 706.8 pg/mL. Table 1 shows the characteristics of the subjects and their correlation with sST2 and NT-proBNP.
Table 1.
The characteristics of the subjects and their correlation with sST2 and NT-proBNP levels.
| The variables | The values (n = 81) | Correlation with sST2 levela |
Correlation with NT-pro BNP levela |
||
|---|---|---|---|---|---|
| r value | p value | r value | p value | ||
| Age (years), median (Q1–Q3) | 33.6 (22.5–43.5) | 0.196 | 0.08 | 0.185 | 0.11 |
| Female subjects, n (%) | 69 (85.2%) | NA | NA | NA | NA |
| Six minute walking distance (m), mean ± SD | 345.5 ± 103.4 | 0.078 | 0.50 | −0.255 | 0.03 |
| Peripheral O2 saturation (%), mean ± SD | 95.1 ± 6.7 | −0.213 | 0.06 | −0.525 | <0.001 |
| ASD diameter (cm), mean ± SD | 2.7 ± 0.7 | −0.119 | 0.29 | −0.062 | 0.59 |
| TAPSE (mm), mean ± SD | 24.5 ± 5.4 | −0.149 | 0.18 | −0.204 | 0.08 |
| RVED diameter (mm), median (quartiles) | 45 (41–50) | 0.315 | 0.004 | 0.565 | <0.001 |
| mPAP (mmHg), mean ± SD | 44.0 ± 20.7 | 0.203 | 0.04 | 0.542 | <0.001 |
| mAoP (mmHg), mean ± SD | 98.2 ± 13.7 | 0.061 | 0.59 | −0.107 | 0.36 |
| mLAP (mmHg), mean ± SD | 11.3 ± 5.5 | 0.159 | 0.16 | 0.084 | 0.48 |
| RVSP (mmHg), mean ± SD | 72.8 ± 30.3 | 0.109 | 0.33 | 0.513 | <0.001 |
| Flow ratio, median (quartiles) | 2.4 (1.6–3.6) | −0.078 | 0.11 | −0.239 | 0.04 |
| PVR (Wood Unit), median (quartiles) | 4.5 (1.8–12.8) | 0.172 | 0.12 | 0.561 | <0.001 |
| sST2 level (ng/mL), median (quartiles) | 22.2 (14.1–33.1) | NA | NA | 0.117 | 0.32 |
| NT-pro BNP level (pg/mL), median (quartiles) | 706.8 (160.7–2172.0) | 0.117 | 0.32 | NA | NA |
Spearman's correlation test for non-parametric variables.
ASD: atrial septal defect; TAPSE: tricuspid annular peak systolic excursion; RVED: right ventricle end diastolic; mPAP: mean pulmonary artery pressure; mAoP: mean aorta pressure; mLAP: mean left atrial pressure; RVSP: right ventricle systolic pressure; PVR: pulmonary vascular resistance; SD: standard deviation; Q1: first quartile; Q3: third quartile; NA: not applicable; NT-pro BNP: N terminal pro brain natriuretic peptide; sST2: soluble suppression of tumorigenicity-2.
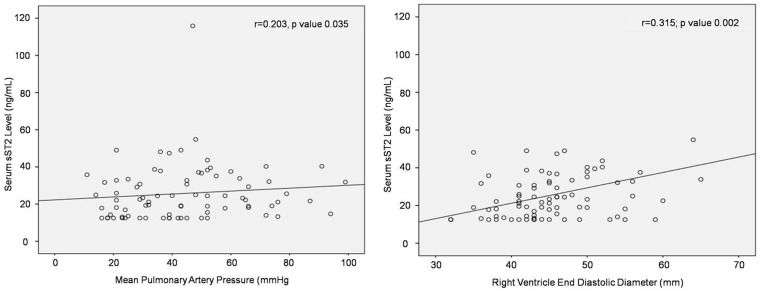
Serum sST2 level had significant positive correlation with mPAP (r = 0.203, p = 0.04) and with RVED diameter (r = 0.315; p = 0.002) (Fig. 1). The correlations between serum sST2 level and other variables were not statistically significant. Serum NT-proBNP level had significant correlation with six minute walking distance (r = –0255, p = 0.03), peripheral O2 saturation (r = –0.525; p <0.001), RVED diameter (r = 0.565, p <0.001), mPAP (r = 0.542, p <0.001), RVSP (r = 0.513, p <0.001), flow ratio (r = –0.239, p = 0.04), and PVR (r = 0.561, p <0.001). No significant correlation was detected between serum sST2 level and NT-proBNP level. The correlation test results are shown in Table 1.
Fig. 1.
The significant positive correlation between serum sST2 level with RVED diameter and mPAP.
sST2: soluble suppression of tumorigenicity-2.
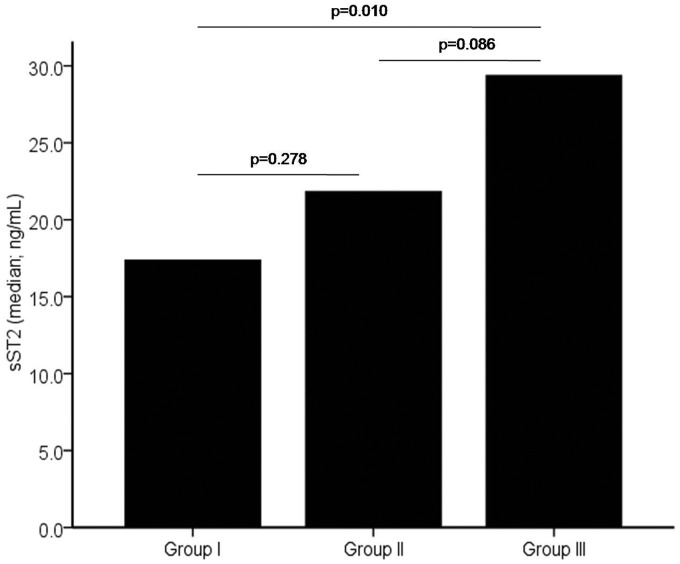
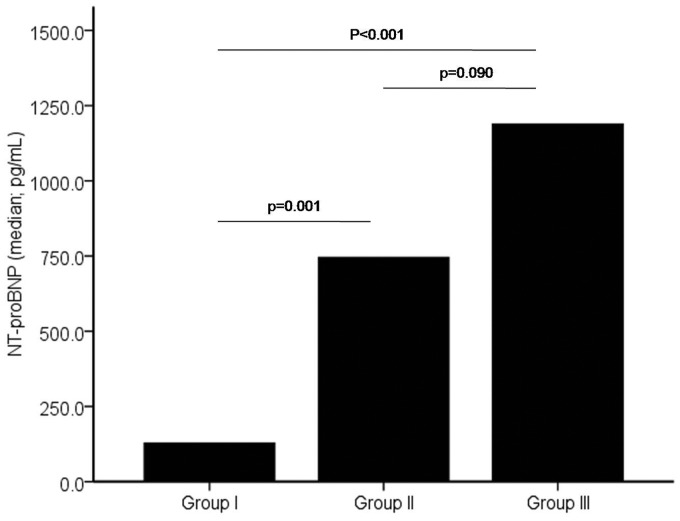
The subjects were divided into three groups based on clinical and hemodynamic parameters. Group I (n = 18) reflected the RV volume overload state, group II (n = 40) reflected the mixed volume and pressure overload state, and group III (n = 23) reflected the RV pressure overload. Table 2 shows the comparison of variables among groups. Group III had greatest mean RVED diameter as compared with other groups. The mean mPAP, RVSP, and PVR were significantly highest in group III. Median serum sST2 level was incrementally increased from group I to group III (17.4 ng/mL, 21.8 ng/mL, and 29.4 ng/mL, respectively) (as shown in Fig. 2). Post-hoc analysis showed that serum sST2 level was significantly different between groups I and III (p = 0.01). Median serum NT-proBNP level was also incrementally increased from group I to group III (128.5 pg/mL, 745.7 pg/mL, and 1189.0 pg/mL, respectively) (as shown in Fig. 3). Post-hoc analysis showed that serum NT-proBNP level was significantly different between groups I and II (p <0.001) and between groups I and III (p = 0.001).
Table 2.
Comparison of variables among subjects divided in Group I (ASD and no PH group) (n = 18), group II (persistent left-to-right shunt ASD and PH group) (n = 40) and group III (ASD and ES group) (n = 23).
| The variables | Group I (n = 18) | Group II (n = 40) | Group III (n = 23) | p Values |
|---|---|---|---|---|
| Age (years), mean ± SD | 30.1 ± 10.6 | 36.7 ± 13.9 | 30.9 ± 10.2 | 0.079 |
| Female gender, n (%) | 14 (78) | 35 (88) | 20 (87) | 0.604 |
| Six minute walking distance (m), mean ± SD | 390.69 ± 75.9 | 342.46 ± 111.81 | 317.86 ± 98.84 | 0.096a |
| NYHA functional class, n (%) | 6 (33.3) | 10 (25.0) | 6 (26.1) | 0.872 |
| Class I | 11 (61.1) | 27 (67.5) | 14 (60.9) | |
| Class II | 1 (5.6) | 3 (7.5) | 3 (13.0) | |
| Class III | ||||
| Peripheral O2 saturation (%), mean ± SD | 98.5 ± 0.7 | 97.2 ± 3.9 | 89.6 ± 8.6 | < 0.001b |
| ASD diameter (cm), mean ± SD | 2.7 ± 0.9 | 2.7 ± 0.7 | 2.5 ± 0.5 | 0.630 |
| TAPSE (mm), mean ± SD | 27.5 ± 5.1 | 24.7 ± 5.4 | 21.7 ± 4.2 | 0.002c |
| RVED diameter (mm), mean ± SD | 40.1 ± 3.9 | 46.7 ± 7.4 | 48.0 ± 6.1 | < 0.001d |
| mPAP (mmHg), mean ± SD | 19.3 ± 3.5 | 41.1 ± 10.3 | 68.4 ± 15.0 | < 0.001e |
| Flow ratio, mean ± SD | 2.8 ± 1.2 | 3.6 ± 1.7 | 1.2 ± 0.5 | < 0.001f |
| RVSP (mmHg), mean ± SD | 40.7 ± 8.6 | 69.6 ± 23.8 | 103.3 ± 20.9 | < 0.001g |
| PVR (Wood Unit), mean ± SD | 1.8 ± 1.0 | 4.2 ± 2.6 | 25.9 ± 11.6 | < 0.001h |
| mAoP (mmHg), mean ± SD | 95.6 ± 12.2 | 100.9 ± 13.9 | 95.5 ± 14.1 | 0.209 |
| mLAP (mmHg), mean ± SD | 8.8 ± 2.6 | 13.3 ± 6.3 | 9.6 ± 4.5 | 0.004i |
| sST2 level (ng/mL), median (quartiles)l | 17.4 (12.5–27.3) | 21.8 (12.7–32.3) | 29.4 (19.1–37.6) | 0.038j |
| NT-pro BNP level (pg/mL), median (quartiles)l | 128.5 (48.2–280.7) | 745.7 (181.2–2178.3) | 1189.0 (674.6–2721.0) | < 0.001k |
SD: standard deviation; ASD: atrial septal defect; TAPSE: tricuspid annular peak systolic excursion; RVED: right ventricle end diastolic; mPAP: mean pulmonary artery pressure; RVSP: right ventricle systolic pressure; PVR: pulmonary vascular resistance; NT-pro BNP: N terminal pro brain natriuretic peptide; mAoP: mean aorta pressure; mLAP: mean left atrial pressure; sST2: soluble suppression of tumorigenicity-2.
Post-Hoc analysis:
Group I vs. group III (p = 0.032).
b,hGroup I vs. group III (p < 0.001), group II vs. group III (p < 0.001).
Group I vs. group III (p < 0.001), group II vs. group III (p = 0.028).
d,kGroup I vs. group II (p = 0.001), group I vs. group III (p < 0.001).
e,gGroup I vs. groupII (p < 0.001), group I vs. group III (p < 0.001), group II vs. group III (p < 0.001).
Group I vs. group II (p = 0.030), group I vs. group III (p < 0.001), group II vs. group III (p < 0.001).
Group I vs. group II (p = 0.004), group II vs. group III (p = 0.009).
Group I vs. group III (p = 0.010).
Non-parametric test: Kruskal–Wallis, with post-hoc Mann–Whitney test.
Fig. 2.
The comparison of sST2 median level among subjects with ASD and no PH (group I), subjects with left-to-right shunt ASD and PH (group II), and subjects with ASD and Eisenmenger syndrome (group III).
sST2: soluble suppression of tumorigenicity-2.
Fig. 3.
The comparison of NT-proBNP median level among subjects with ASD and no PH (group I), subjects with left-to-right shunt ASD and PH (group II), and subjects with ASD and ES (group III).
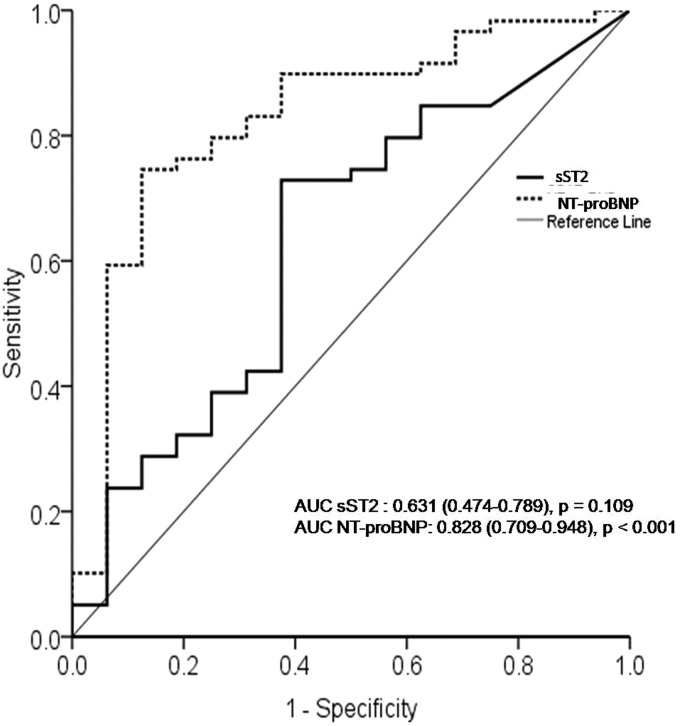
The ROC curve for prediction of the presence of PH indicated that AUC of NT-proBNP level was 0.828 and statistically significant. The AUC of sST2 level was 0.631 with no statistical significance for the presence of PH. The ROC curve and AUC analysis are shown in Fig. 4.
Fig. 4.
The receiver-operating characteristic (ROC) curves comparing sensitivity and specificity of sST2 and NT-proBNP levels in predicting the presence of PH among ASD patients.
sST2: soluble suppression of tumorigenicity-2; NT-proBNP : N terminal pro brain natriuretic peptide; AUC: area under the curve.
Discussion
The results of this study show that serum sST2 level correlated with the presence and severity of PH in adult patients with uncorrected secundum ASD. Serum sST2 level had significantly weak positive correlation with mPAP and RVED diameter. In patients with PH associated with left-to-right shunt ASD, the serum sST2 level tended to be higher as compared to ASD with no PH. Furthermore, in the group with ES, the serum sST2 level was higher as compared with the other groups of patients. Serum NT-proBNP level, the accepted biomarker for prognosis indicator in PH patients, consistently showed significant correlation with clinical severity (reduced six minute walking distance, increased NYHA functional class, and decreased peripheral O2 saturation) and PH hemodynamic parameters (increased mPAP, RVSP, PVR, and reduced flow ratio). It had moderate strong positive correlation with RVED diameter, which was stronger than serum sST2 correlation. The prediction for PH was also better by NT-proBNP level than by sST2 level.
Secundum ASD comprises 75% of all types of ASD. The majority of subjects in this study still had a normal RV function measured by TAPSE value. Even in ES, the TAPSE was within normal limits. In contrast, most subjects had undergone RV dilatation indicated by increased RVED diameter size.
The pathophysiology of PH associated with uncorrected ASD involves the blood overflow in pulmonary vessels and volume overload in the RVs due to the persistent shunt from left to right heart, which result in RV cardiomyocyte stretching. The strained cardiomyocytes upregulate ST2 expression, both membrane-bound and serum sST2 levels.9 Further study indicated that pulmonary endothelial cells constitutively express ST2. The disrupted PA endothelial cells due to hypoxia-induced PH overexpress IL-33 and ST2.14 The activation of IL-33/ST2 axis on PA endothelial cells initiates pulmonary vascular smooth muscle and cardiomyocyte remodeling which eventually result in PH.14
The expression of ST2 is most prevalent in the lungs, heart, kidneys, intestines, and vessel walls.15–17 The previous research showed that there was an increase in the expression of ST2 in the pulmonary vasculatures of idiopathic PAH patients, which was associated with endothelial dysfunction and inflammation.15 Therefore, serum sST2 was suggested as a biomarker of pulmonary vascular remodeling as well as a predictor of the severity and clinical outcome of patients with PAH.10,15 It has a higher sensitivity as a PAH biomarker than NT-proBNP, a previously known prognostic biomarker.18
Serum sST2 level in this study had a median value of 22.2 ng/mL. In subjects with PH associated with uncorrected ASD and ES, median serum sST2 level was higher as compared to subjects with no PH. The previous studies showed that mean serum sST2 level was significantly higher in the subjects in the group with PAH compared with healthy control.10,15,19 Similar to our data, previous study obtained mean serum sST2 levels in subjects with PAH below 35 ng/mL. It has been identified as the clinically relevant cut-off level for left heart failure.17 In our study, median serum sST2 level in subjects with PH associated with left-to-right shunt ASD was 21.8 ng/mL and in ES it was 29.4 ng/mL, whereas in subjects with idiopathic PAH, mean serum sST2 was 28.9 ng/mL.10 Meanwhile, the mean serum sST2 level of PAH subjects was beyond 35 ng/mL in other studies and correlated with the RV fibrosis parameter.19,20 It should be noted that, these studies utilized different sST2 assays and from different blood sources which may contribute to the different results obtained.
Our study indicated that RV dilatation, indicated by increased RVED diameter, had a significantly positive correlation with serum sST2. This finding was in line with the previous study, in which sST2 significantly correlated with RVED dimension.6 Our study finding suggested that sST2 level underwent dynamic alteration in the volume overload phase in uncorrected ASD, which chronically occurred. The elevated mPAP and PVR at a further stage of disease, i.e. mixed volume–pressure overload and pressure overload stages, leads to an incremental increase in RVED diameter and serum sST2 level.
This result also suggested that in the patients with PH associated with uncorrected ASD, an increase in mPAP had a significant association with serum sST2. However, sST2 level did not significantly correlate with mean PVR. These findings were in contrast with the previous research which showed a significant positive correlation between serum sST2 and PVR but not between sST2 and mPAP in patients with idiopathic PAH.10 This difference may be due to the natural history that PH associated with uncorrected ASD patients had experienced chronic volume overload mixed by pressure overload and longer RV strain phase, resulting in a higher correlation between serum sST2 and mPAP. Furthermore, incremental increases of sST2 levels based on severity of disease and hemodynamic parameters confirmed this possibility.
The ES is terminal form of PH associated with uncorrected ASD. The higher PVR may reverse the direction of shunt from left-to-right heart to bidirectional or right-to-left heart shunt.1,21 This reversal results in reactive PH that causes PVR to be higher than systemic vascular resistance,21 which leads to a much greater pressure overload on the RV.17 In this study, we found that serum sST2 levels were the highest in patients with ES. It is associated with increased RVED diameter, mPAP, PVR, and RV systolic pressure, which in turn develop into increased RV strain and elevated serum sST2 levels. The higher levels of serum sST2 may be predictors of the fully developed ES.
The level of NT-proBNP is a parameter for risk stratification and prognostic prediction in PH. NT-proBNP is known to have prognostic significance in predicting worsening events and mortality in patients with PH associated with congenital heart disease.1,22 Our study confirmed that NT-proBNP level was consistently associated with worse clinical severity and PH hemodynamic parameters. The ROC curve indicated that NT-proBNP levels have better predictive value for PH than sST2 level. This biomarker had better correlation with disease severity variables compared with serum sST2. We also observed there was no significant correlation between serum sST2 and NT-proBNP level. This finding indicates that there is a distinctive pathophysiological process of sST2 and NT-proBNP in the right-sided heart volume and pressure overload and pulmonary circulation in the left-to-right shunt heart defect. Another study found only weak correlation between sST2 and NT-proBNP from peripheral veins sample in subjects with complex congenital heart disease.23 In myocardial function, sST2 is a biomarker of strain and fibrosis, whereas NT-proBNP is a biomarker of myocardial stretch due to overloading. In the PA level, right-sided chronic overloading is the main source of NT-proBNP which is independent from sST2 production.
Several limitations of this study should be addressed. First, the sample size should be increased to further corroborate our findings. Second, the measurement of RV dimension and function should be validated by other means of measurement, such as magnetic resonance imaging. Third, the healthy control group should be added to assess the discriminative value of sST2 between healthy and ASD patients. Finally, the measurement of sST2 by using ST2 rapid test should be performed by using fresh serum samples, other than thawed samples, so that the results could be more applicable in daily practice.
Conclusion
Serum sST2 level had significant positive correlation with mPAP and RVED diameter in adult uncorrected secundum ASD patients. Higher serum sST2 level associated with the presence of PH and ES in adult uncorrected secundum ASDs. However, in comparison with NT-proBNP, the sST2 value did not outperform the earlier established biomarker.
Acknowledgement
Authors express gratitude to the following persons: Monika Setiawan, MD, Reza Pandu Aji, MD, Zaki Horison Islami, MD, Aditya Doni Pradana, MD, and Dimas Setiaji, MD, who have managed the database of COHARD-PH registry. Authors are indebted to Ms. Sri MardilahWuryani AMK for her assistance during echocardiography examination, Mr. Farid Abdullah for his technical assistance on blood sample handling and analysis, Ira Puspitawati, MD (Clinical Pathologist) for her assistance during NT-proBNP measurement, and Kurniasari Endah, MD from UBC Medical Indonesia for kindly providing Aspect plus ST2 reader and Aspect plus ST2 rapid test cassette. Authors are grateful to Klinik Bahasa Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada for English language editing of this manuscript. Authors express appreciation to all of the cardiologist staff of Dr. Sardjito Hospital, Yogyakarta, Indonesia, for their assistance and cooperation during this study.
Author contributions
All authors contributed sufficiently in data collection (R.S.P., A.B.H., and V.C.D.), data interpretation (R.S.P., A.B.H., D.W.A., and L.K.D.), manuscript writing (R.S.P., A.B.H., D.W.A., V.C.D., and L.K.D.), scientific manuscript contents (R.S.P., A.B.H., D.W.A., and L.K.D.), and final paper submission approval (R.S.P., A.B.H., D.W.A., V.C.D., and L.K.D.).
Conflict of interest
The author(s) declare that there is no conflict of interest.
Ethical approval
Approved by Medical and Health Research Ethic Committee of Faculty of Medicine, Public Health and Nursing Universitas Gadjah Mada and Dr. Sardjito Hospital, Yogyakarta, Indonesia.
Funding
Dana Masyarakat Faculty of Medicine, Public Health and Nursing Universitas Gadjah Mada Fiscal Year 2017 to L.K.D (as P.I.).
ORCID iD
Anggoro B. Hartopo https://orcid.org/0000-0002-6373-1033
References
- 1.Galie N, Humbert M, Vachiery KL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. EurHeart J 2016; 37: 67–119. [Google Scholar]
- 2.Martin SS, Shapiro EP, Mukherjee M. Atrial septal defects – clinical manifestations, echo assessment, and intervention. Clin Med Insights Cardiol 2014; 8: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diller GP, Gatzoulis MA. Pulmonary vascular disease in adults with congenital heart disease. Circulation 2007; 115: 1039–1050. [DOI] [PubMed] [Google Scholar]
- 4.Sanada S, Hakuno D, Higgins LJ, et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007; 117: 1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov 2008; 7: 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail 2011; 4: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aimo A, Vergaro G, Passino C, et al. Prognostic value of soluble suppression of tumorigenicity-2 in chronic heart failure: a meta-analysis. JACC Heart Fail 2017; 5: 280–286. [DOI] [PubMed] [Google Scholar]
- 8.Aimo A, Vergaro G, Ripoli A, et al. Meta-analysis of soluble suppression of tumorigenicity-2 and prognosis in acute heart failure. JACC Heart Fail 2017; 5: 287–296. [DOI] [PubMed] [Google Scholar]
- 9.Shimpo M, Morrow DA, Weinberg EO, et al. Serum levels of the interleukin-1 receptor family member ST2 predicts mortality and clinical outcome in acute myocardial infarction. Circulation 2004; 109: 2186–2190. [DOI] [PubMed] [Google Scholar]
- 10.Zheng YG, Yang T, He JG, et al. Plasma soluble ST2 levels correlate with disease severity and predict clinical worsening in patients with pulmonary arterial hypertension. Clin Cardiol 2014; 37: 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinarti LK, Hartopo AB, Kusuma AD, et al. The COngenital HeARt Disease in adult and Pulmonary Hypertension (COHARD-PH) registry: a descriptive study from single-center hospital registry of adult congenital heart disease and pulmonary hypertension in Indonesia. BMC Cardiovasc Disord 2020; 20: 163. [DOI] [PMC free article] [PubMed]
- 12.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 13.Dinarti LK, Hartopo AB, Anggrahini DW, et al. Profile of endothelin-1, nitric oxide, and prostacyclin levels in pulmonary arterial hypertension related to uncorrected atrial septal defect: result from a single center study in Indonesia. Cardiol Res Pract 2020. DOI: 10.1155/2020/7526508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Wang W, Wang L, et al. IL-33 initiates vascular remodelling in hypoxic pulmonary hypertension by up-regulating HIF-1α and VEGF expression in vascular endothelial cells. EBioMedicine 2018; 33: 196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao D, Perros F, Humbert M, et al. Is there a role for IL-33in the pathogenesis of pulmonary arterial hypertension?. Thorax 2010; 65: A70. [Google Scholar]
- 16.Villacorta H, Maisel AS. Soluble ST2 testing: a promising biomarker in the management of heart failure. Arq Bras Cardiol 2016; 106: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller T, Dieplinger B. The Presage® ST2 assay: analytical considerations and clinical applications for a high-sensitivity assay for measurement of soluble ST2. Expert Rev MolDiagn 2013; 13: 13–30. [DOI] [PubMed] [Google Scholar]
- 18.Chida A, Sato H, Shintani M. Soluble ST2 and N-terminal pro-brain natriuretic peptide combination. Useful biomarker for predicting outcome of childhood pulmonary arterial hypertension. Circ J 2014; 78: 436–442. . [DOI] [PubMed] [Google Scholar]
- 19.Carlomagno G, Messalli G, Melillo RM. Serum soluble ST2 and interleukin-33 levels in patients with pulmonary arterial hypertension. Int J Cardiol 2013; 168: 1545–1547. [DOI] [PubMed] [Google Scholar]
- 20.Plácido R, Cortez-Dias N, Robalo Martins S, et al. Prognostic stratification in pulmonary hypertension: a multi-biomarker approach. Rev Port Cardiol 2017; 36: 111–125. [DOI] [PubMed] [Google Scholar]
- 21.Kaemmerer H, Mebus S, Neick IS, et al. The adult patient with Eisenmenger syndrome: a medical update after Dana Point. Curr Cardiol Rev 2010; 6: 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuijt MTU, Blok IM, Zwinderman AH, et al. Mortality in pulmonary arterial hypertension due to congenital heart disease: serial changes improve prognostication. Int J Cardiol 2017; 243: 449–453. [DOI] [PubMed] [Google Scholar]
- 23.Laqqan M, Schwaighofer C, Graeber S, et al. Predictive value of soluble ST2 in adolescent and adult patients with complex congenital heart disease. PLoS One 2018; 13: e0202406. [DOI] [PMC free article] [PubMed] [Google Scholar]