Abstract
The recent outbreak of Covid-19 has represented a major challenge for the countries affected by the disease, not only in terms of loss of human life, economic downturn, and constraint on individual freedom, but also for the great pressure on the national health systems and hospitals. The 380 kDa virus has been a perfect storm, especially for those national health systems used to working with limited resources and high intensity rhythms, such as Italy. For the first time in the new century, a virtually unknown fast-spreading disease has caused a public health emergency thus forcing most countries to deal with an insurmountable logistic gap. Hence, every branch of Medicine, even though not directly involved in the treatment, has been called upon to provide its contribution to resolve the crisis. It is now becoming more apparent that Covid-19 is not solely a lung disease, but a complex systemic disease involving several organs and systems. This is due to an abnormal inflammatory response which eventually leads to multisystemic coagulopathy which mainly, but not uniquely, targets the lungs. Although the pathophysiology of this syndrome is still not fully understood, macrophages and their immune complex system seem to play a key role. It is not yet clear why some patients develop the violent immune response which results in pneumonitis while others do not. There are clues indicating that the systemic hyper-inflammation defined as macrophage activation syndrome (MAS), or cytokine storm, requires an increase in choline consumption to synthesize phosphatidylcholine and stimulate phagocytosis, organelle biogenesis, secretory functions, and endocytosis. 18F-Fluorocholine is a synthetic analog of the naturally occurring choline normally used for PET/CT imaging of prostate cancer patients. 18F-Fluorocholine could image and quantify the macrophage activity in pulmonary interstitial infiltrates of Covid-19 pneumonia. If the hypothesis is confirmed experimentally, 18F-Fluorocholine PET/CT could be used to in vivo image and quantify the degree of lung inflammation and potentially stratify the gravity of this disease.
Background
The global pandemic which originated in China and rapidly spread to Europe and the U.S. is caused by Coronavirus disease 19 (Covid-19) whose main clinical feature is a severe acute respiratory syndrome: up to 20% of the patients affected by Covid-19 develop respiratory symptoms which require oxygenation [1], [2], [3].
The urgency of finding a definition of the pathogenetic bases of the lung damage and establishing an effective therapy, together with insufficient medical information, has led clinicians to focus on alveolar injury characterized by altered alveolar lining cells, reactive type II pneumocyte hyperplasia, and intra-alveolar fibrinous exudates [4]. This vision posits the lung as the main, if not unique, organ affected by the infection and the altered alveolar cells as the consequent damage of the virus.
Following additional observations, a more complex mechanism underlying the pathogenesis of Covid-19 infection has been hypothesized. The alveolar cell damage is a consequence of a systemic hyper-inflammation defined as macrophage activation syndrome (MAS), or cytokine storm [5], [6], also known as secondary haemophagocytic lymphohistocytosis (sHLH) [7] which may lead to overt disseminated intravascular coagulation. Within this pathophysiologic scenario, the role of macrophages is pivotal. If resident alveolar macrophages initiating immune response succeed at containing the virus after Covid-19 infection, the evolution shifts towards a milder manifestation. On the contrary, if the integrity of the epithelial–endothelial lining is damaged, the alveolar macrophages produce proinflammatory cytokines and chemokines, thus resulting in a cytokine storm. The ability to discriminate between the alveolar macrophages responding positively to the Covid-19 infection and others which are failing provide clinicians with a useful and early parameter of the aggressiveness and prognosis of the disease, as well as potentially aid them in evaluating effective treatment (Fig. 1 ).
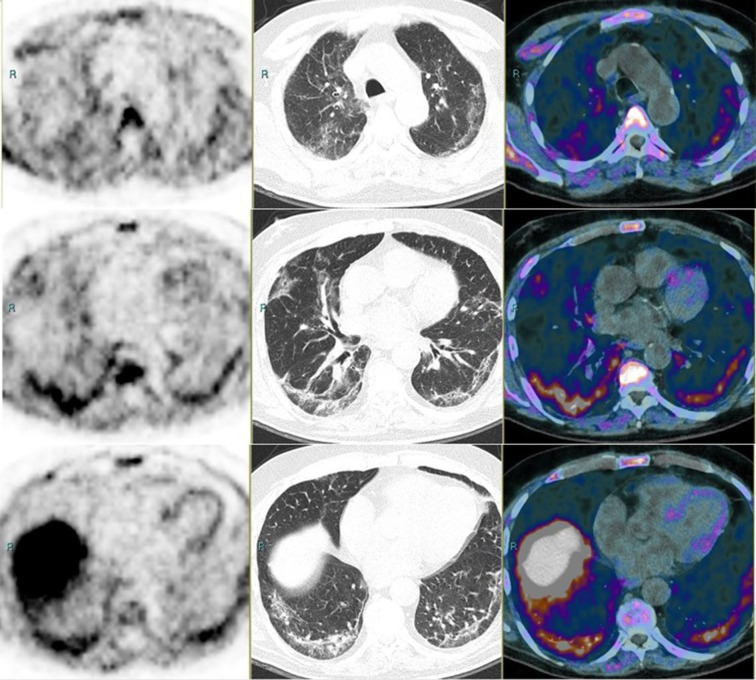
Fig. 1.
PET (left panel), CT (middle), and PET/CT (right). First row: upper lung. Second row: middle lung. Third roe: lower lung. The patient underwent examination within his routine follow-up for a prostate adenocarcinoma. PSA was 1.97 ng/ml and unchanged compared with the previous evaluation. The CT scan shows diffuse wide areas of solid-subsolid ground-glass opacities, bilateral and subpleural crazy-paving bronchovascular thickening. The patient referred mild fever (>38 °C), cough, fatigue, shortness of breath, ageusia-dysgeusia and anosmia, all strongly suggestive symptoms for a Covid-19 infection. PET shows some mild-intense areas of uptake which follow, with irregular shape and intensity, the CT alterations.
Hypotheses
Phospholipids are the major component of macrophage cell membranes. The main phospholipid is phosphatidylcholine (PC), a chemical compound which incorporates choline. Once activated by an external noxa, the macrophages undergo the so-called polarization through the production of inflammatory cytokine and the onset of the pro-inflammatory phase [8]. The activation, coupled with changes in membrane composition and dynamics due to the induction of phospholipid biosynthesis (mainly PC), has been largely attributed to a demand for processes such as phagocytosis, organelle biogenesis, secretory functions, and endocytosis [9]. In summary, the activated macrophages therefore require more choline to adapt to the altered situation.
Fluorocholine (18F) chloride (18F-FCH) is an analog of choline in which a hydrogen atom has been replaced by fluorine (18F). After crossing the cell membrane by a carrier-mediated mechanism, choline is phosphorylated by choline kinase to produce phosphorylcholine. This, in turn, is converted into cytidinediphosphatecholine and subsequently incorporated into PC, which is the main component of the cell membrane. 18F-FCH PET/CT is widely used to stage and restage patients affected by prostate cancer with good sensitivity and less specificity, especially in the evaluation of the prostate. Indeed, non-neoplastic conditions, such as benign prostate hyperplasia and inflammatory changes, are associated with an increased choline uptake and false positivity [10], [11]. More recently, the link between choline phospholipid metabolism and macrophage immune responsiveness has been identified. In activated polarized macrophages present in pneumonia-affected lungs, there is an increase of the choline transporter-like protein-1 (CTL1) which enables the uptake of choline. Therefore, 18F-FCH uptakes in lungs affected by Covid-19 interstitial pneumonitis is proportional with the local activity of activated macrophages in the immune response to the viral attack. This could be of paramount importance in a clinical setting where most of the parameters used to stratify the patient and evaluate the treatment (partial pressure of oxygen in arterial blood, oxygen saturation, CT scan) are the consequences of macrophage activation and not a direct measure of it.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.109885.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H., Zhou P., Wei Y. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient With COVID-19. Ann Intern Med. 2020 doi: 10.7326/M20-0533. [Epub ahead of print] PubMed PMID: 32163542; PubMed Central PMCID: PMC7081173. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of Cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020:102537. doi: 10.1016/j.autrev.2020.102537. Epub ahead of print] Review. PubMed PMID: 32251717; PubMed Central PMCID: PMC7195002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. Review. PubMed PMID: 16322748. [DOI] [PubMed] [Google Scholar]
- 9.Ecker J., Liebisch G., Englmaier M., Grandl M., Robenek H., Schmitz G. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc Natl Acad Sci USA. 2010;107:7817–7822. doi: 10.1073/pnas.0912059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souvatzoglou M., Weirich G., Schwarzenboeck S. The sensitivity of [11C] choline PET/CT to localize prostate cancer depends on the tumor configuration. Clin Cancer Res. 2011;17:3751–3759. doi: 10.1158/1078-0432.CCR-10-2093. [DOI] [PubMed] [Google Scholar]
- 11.Beheshti M., Imamovic L., Broinger G. 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology. 2010;254:925–933. doi: 10.1148/radiol.09090413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.