Abstract
Lolium arundinaceum [(Darbyshire) tall fescue] toxicosis is responsible for substantial beef production losses in the United States, due to its negative effects on reproduction, growth, and feed efficiency. These effects are consequences of toxic alkaloids within tall fescue. Interseeding legumes, such as Trifolium pratense (red clover), into pastures has been shown to mitigate a portion of these effects. Clovers contain isoflavones, which may play a role in tall fescue toxicosis mitigation. The present study utilized 36 Angus steers to determine the effects of daily supplementation with a red clover-isolated isoflavone feed additive on physiological symptoms of tall fescue toxicosis and the rumen microbial environment over a 21-d period. Angus steers were initially stratified based upon their single nucleotide polymorphism genotype at the DRD2 receptor. Treatments were then randomly assigned in a 2 × 2 factorial arrangement within a completely randomized design, where treatment factors consisted of tall fescue seed type (endophyte-infected tall fescue seed vs. endophyte-free tall fescue seed) supplemented with and without the isoflavone additive. Steers that consumed endophyte-infected tall fescue seed had lower serum prolactin concentrations (P = 0.0007), average daily gain (ADG; P = 0.003), final body weight (BW; P = 0.004), and feed efficiency (P = 0.018) when compared with steers that consumed endophyte-free tall fescue seed. Serum insulin-like growth factor-1 (IGF-1) tended to be reduced with supplementation of isoflavones (P = 0.06) but was unaffected by seed type (P ≥ 0.10) and seed by treatment interaction (P ≥ 0.10). Isoflavones reduced serum glucose levels (P = 0.023), but neither seed type, isoflavones, or their interaction affected serum urea nitrogen (SUN), nonesterified fatty acids (NEFA), or insulin (P ≥ 0.10). Volatile fatty acid concentrations, dry matter intake (DMI), ruminal pH, and overall feeding behaviors were also unaffected by seed type or isoflavone treatments (P ≥ 0.10). Twenty-eight ruminal bacteria taxa shifted as a result of seed type or isoflavone treatment (P < 0.05). In this experiment, feeding isoflavones to Angus cattle did not completely mitigate all symptoms of fescue toxicosis. However, dose–response trials may aid future research to determine if dietary supplementation with isoflavones alleviates fescue toxicosis symptoms and promotes livestock growth and performance.
Keywords: cattle, fescue, microbes, red clover, rumen
INTRODUCTION
The combined effects of fescue toxicosis contribute to an average annual loss of $2 billion for beef producers in the United States alone (Hancock and Andrae, 2009; Kallenbach, 2015). One of the most common strategies utilized to mitigate the effects of fescue toxicosis involves pasture forage diversification, most commonly performed through the interseeding of legumes, such as alfalfa and clovers. Legumes are relatively high-quality forages that contain phytoestrogenic compounds known as isoflavones. Among several isoflavones in clover tissue, biochanin A inhibits Gram-positive hyper-ammonia-producing bacteria (HAB; Flythe and Kagan, 2010) and reduces ammonia production from ruminal bacteria at a concentration of 30 ppm of rumen volume (Flythe et al., 2013). Biochanin A is also a selective inhibitor of certain amylolytic and cellulolytic bacteria (Harlow et al., 2017a, 2018; Melchior et al., 2018). Aiken et al. (2016) determined that when biochanin A was ruminally dosed into goats consuming endophyte-infected tall fescue seed, vasodilation, and return to normal blood flow rates was observed. This has been associated with agonist activity at β-adrenergic receptors within the endothelium of blood vessels, which stimulates synthesis of nitric oxide, and thus promotes vasodilation (Wu et al., 2010). These studies indicate changes in vasculature and rumen microbial communities in response to red clover-sourced isoflavones and suggest that red clover isoflavones may have a selective antimicrobial effect within the ruminal bacterial community.
Therefore, the objective of this study was to determine the short-term effects of red clover isoflavones on the entire rumen bacterial community and whole physiology in cattle. We hypothesized that supplementation of isoflavones to growing beef cattle consuming endophyte-infected tall fescue seed would mitigate a portion of the physiological symptoms of tall fescue toxicosis, driven in-part by altered rumen microbial populations.
MATERIALS AND METHODS
All animal handling and experimental procedures were conducted in accordance with guidelines set forth by the University of Tennessee Institutional Animal Care and Use Committee and were conducted at the Plateau Research and Education Center located in Crossville, TN.
DRD2 Receptor Genotyping
Before initiation of the study, 36 purebred Angus steers (~8 mo of age; 250 ± 20 kg) were blocked by genotype at the DRD2 receptor using methods similar to those established by Campbell et al. (2014). Briefly, genomic DNA was isolated from 5 to 10 tail hair follicles per steer using Quickextract (Epicentre, Cambridge, UK). Genomic amplification was then performed on the isolated DNA samples using the GenomiPhi V2 DNA amplification kit (GE Healthcare, Piscataway, NJ) followed by an ethanol precipitation and resuspension in 50 mL of water. Polymerase chain reaction (PCR) was utilized to amplify a 794 base pair (bp) portion of the DRD2 gene. Sequences of the primers used were 50-TATAGCCCCATTCCTGCTTC-30 and 50-GCCCATGCT CTACAACACACG-30. Cycling conditions were 2 min at 94 °C; 35 cycles for 30 s at 94 °C; 30 s at 58 °C; 30 s at 68 °C; followed by 10 min at 68 °C, and the product was held until further processing at 4 °C. The total reaction volume was 20 mL. Direct sequencing of the PCR product revealed an intronic A=G SNP which created a Tfi I restriction site (50-GAWTC-30) within the “A” allele. Following PCR, 5 mL of amplified product was subjected to a 2-h digestion reaction at 65 °C with 2.5 units Tfi I (USB Biolabs, Boston, MA) in a total reaction volume of 20 mL. Half of the reaction volume was used in agarose gel electrophoresis against a DNA size ladder (Promega Corporation, Madison, WI 53711), and genotypes were determined based on fragment size.
Experiment and Treatment Design
This study implemented a randomized complete block design, blocking on DRD2 genotype. Treatments were assigned in a 2 × 2 factorial arrangement, with two types of fescue seed (E+ or E−) and isoflavone treatment (Promensil or none). Promensil is a commercially available product that was chosen as the isoflavone source for the current study. This produced four treatment combinations: 1) endophyte-infected tall fescue seed alone (E+), 2) endophyte-free tall fescue seed alone (E−), 3) E+ with isoflavones (E+ Promensil), or 4) E- with isoflavones (E− Promensil). Within each genotype block, steers were randomly assigned to one of the four previously mentioned treatments.
Quantification of Ergot Alkaloids and Isoflavones
Before the study, quantities of ergovaline and its epimer ergovalinine in fescue seed were determined using HPLC with fluorescence detection as described by Aiken et al. (2009) with modifications described by Koontz et al. (2012).
The source of isoflavones utilized in this study were oral boluses of Promensil (PharmaCare Laboratories, Warriewood, NSW, Australia), an over-the-counter isoflavone supplement isolated from red clover, which was ground by mortar and pestle into a fine powder. Quantification of isoflavones in Promensil, including biochanin A, formononetin, genistein, and daidzein, was performed using methods similar to those previously described by Aiken et al. (2016), using LC-MS for detection. Briefly, isoflavone extracts were prepared by adding 7 mL of 85% methanol in 0.5% acetic acid to ground samples in 50-mL conical polypropylene tubes. Samples were vortexed briefly and sonicated for 30 min at ambient temperature. Three milliliters of deionized water was added to each sample before being vortexed and centrifuged for 8 min at 2,200 × g. The supernatant was filtered through a 0.45-μm GHP membrane syringe filter. Extracts were diluted and flavone added as internal standard. One portion of each sample was analyzed as-extracted and a second portion was heated at 85 °C for 5 h to hydrolyze isoflavone malonyl-glucosides to the corresponding isoflavone glucosides. Concentrations of biochanin a-malonyl-glucoside and formononetin-malonyl-glucoside were determined by the difference between hydrolyzed and un-hydrolyzed portions. Isoflavone extracts were analyzed by LC-MS on a Waters Acquity UPLC coupled to a Waters Synapt G2 (q-ToF) high-resolution mass spectrometer. Chromatographic separation was obtained using a Waters BEH C18 UPLC column (1.7 μm, 2.1 mm × 150 mm). The mobile phase employed a mixture of water containing 0.1% formic acid (solvent A) and acetonitrile containing 0.1% formic acid (solvent B) in a linear gradient from 20% B to 80% B at a flow rate of 0.35 mL × min−1. The high-resolution mass spectrometer was operated in positive ion electrospray mode with a resolving power of ~14,000 and scanned from 100 to 1,000 Da in 0.3 s. Leucine enkephalin was used to provide a lock mass (m/z 554.2615). Quantification of isoflavones was performed using QuanLynx software with a linear calibration curve and internal standard method. Extracted ion chromatograms with a mass window of 0.020 Da around the accurate mass of each analyte were used to calculate peak areas.
Samples of red clover from various University of Tennessee pastures were procured and analyzed to characterize and quantify isoflavones as a comparison against the Promensil tablet. Whole plant samples of red clover were collected and freeze-dried before isoflavone quantification. Additionally, the protein supplement included in the diet was tested for isoflavone content using the previously described methods.
Management, Feeding, and Sampling
Beginning 10 d before the initiation of the trial, steers were fitted with EID tags. Steers were acclimated to a basal diet and fed ad libitum in a GrowSafe feed intake measurement system (GrowSafe Systems Ltd., Alberta, Canada). The basal diet was similar to Clemmons et al. (2017), consisting of 10% protein supplement, 10% cracked corn, and 80% corn silage (11.57% crude protein and 76.93% total digestible nutrients dry matter basis).
Tall fescue seed was ground with a Wiley Mill through a 5-mm screen before being included in the diet to provide a targeted minimum of 0.011 mg of total alkaloids (ergovaline + ergovalinine) × kg of BW−1 × d−1 to induce fescue toxicosis (Klotz, 2015). Daily seed administration was adjusted to achieve the inclusion amount. On d 0 of the trial, ground tall fescue seed was included in the diet to provide a minimum of 0.011 mg ergovaline plus ergovalinine × kg of body weight−1 (BW) × d−1. During the 21-d study, steers were orally dosed each d with 24.7 g of the ground isoflavone product via a 28.4-g bolus (Torpac, Inc., Fairfield, NJ) at 0700 h. Within the 24.7 g of isoflavone product, a total of 943 mg of isoflavones were provided daily to treatments receiving isoflavones, which was based on minimal supplementation estimates from previous work (Aiken et al., 2016). Feed intake was continually monitored throughout the study utilizing the GrowSafe feeding system. On d 0, rectal temperatures and un-shrunk BW measurements were taken. Rectal temperatures and un-shrunk BW were measured again on d 7, 14, and 21. Additionally, on d 21, ~9 mL of blood via coccygeal venipuncture (Corvac, Sherwood Medical., St. Louis, MO), and ~100 mL of rumen content were collected via gastric tubing (Guan et al., 2008). When content and fluid are collected via gastric tubing, studies have demonstrated the sampling method provides a representative rumen sample for microbiome analyses similar to a sample collected via a rumen cannula (Paz et al., 2016). Rumen samples were stored at −80 °C until further processing. Blood samples were cooled and centrifuged at 2,000 × g and 4 °C for 20 min. Serum was separated and stored at −80 °C until further processing and analysis.
Rumen Bacterial DNA Extraction, PCR, Sequencing, and Sequence Processing
The procedure used for the rumen bacterial DNA extraction method was similar to that described by Yu and Morrison (2004). After the chemical and physical cell lysis and isopropanol precipitation of nucleic acids, metagenomic DNA was purified with RNase and proteinase K treatment, followed by the use of QIAamp columns from the Qiagen DNA Stool Mini Kit (Qiagen, Hilden, Germany). Genomic DNA concentration was determined using a Nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE), and verified using the standard dsDNA quantification protocol for Picogreen (Thermo Fisher Scientific, Inc., Waltham, MA). Extractions were stored at −20 °C until sequencing library preparation. Bacterial 16S rRNA genes were PCR-amplified with dual-barcoded primers targeting the V4 region, as per the protocol of Kozich et al. (2013). Amplicons were sequenced with an Illumina MiSeq using the 250-bp paired-end kit (v.2). Sequences were denoised, taxonomically classified using Greengenes (v. 13_8) as the reference database, and clustered into 97%-similarity operational taxonomic units (OTUs) with the mothur software package (v. 1.39.5; Schloss et al., 2009), following the recommended procedure (https://www.mothur.org/wiki/MiSeq_SOP; accessed November 2017).
Volatile Fatty Acid Analysis and Rumen pH
A subsample of rumen contents was aliquoted from each rumen content sample for rumen pH and volatile fatty acid (VFA) analysis using HPLC, similar to the methods used by Harlow et al. (2017b). Samples were analyzed for concentrations of formate, acetate, propionate, butyrate, valerate, and isovalerate and methylbutyrate (IVMB) using a Summit HPLC (Dionex; Sunnyvale, CA) equipped with an anion exchange column (Aminex HP-87H; Bio-Rad, Hercules, CA) and UV detector. The eluting compounds were separated isocratically with an aqueous sulfuric acid solution (5 mM). The parameters included an injection volume of 0.1 mL, the flow rate of 0.4 mL × min−1, and a column temperature of 50 °C. Ruminal pH was measured using a pH meter (Denver Instruments, Bohemia, NY).
Serum Metabolites and Hormones
Serum samples were analyzed to quantify serum urea nitrogen (SUN), glucose, nonesterified fatty acid (NEFA), prolactin, insulin-like growth factor 1 (IGF-1), and insulin concentrations. Samples were analyzed using a 96-cell EPOCH 2 microplate reader (BioTek Instruments, Winooski, VT) with commercially available kits for NEFA (Wako Chemicals USA, Inc., Richmond, VA; sensitivity of 0.01 mmol × L−1), glucose (Thermo Electron Corp., Waltham, MA; sensitivity of 0.3 mg × dL−1) and SUN (Thermo Electron Corp.; sensitivity of 2.0 mg × dL−1). Serum prolactin concentrations were derived using the radioimmunoassay protocol established by Bernard et al. (1993). Serum insulin concentrations were derived via radioimmunoassay (Porcine Insulin RIA Kit PI-12K; Linco Research, Inc., St. Charles, MO). Serum IGF-1 was determined via commercially available ELISA kit (R&D Systems, Minneapolis, MN).
Behavior Monitoring
Animal movement within the pen was monitored throughout the duration of the study using a real-time location system (RTLS) technology (SmartBow, MKW Electronics, Weibern, Austria). Each pen within the facility was mapped along a two-dimensional grid that included physical structures, such as waterer and feed trough, as well as the pen perimeter. Each steer was outfitted with a radio frequency identification tag in the right ear. Distance traveled was recorded as average steps taken per day. Each tag continuously transmitted an individual signal that communicated with sensors located throughout the pen and triangulated the steer’s position. Spatio-temporal data were aggregated in hourly intervals at the individual level, then in 24-h intervals for comparison of behavior among treatments. If less than 16 h of data were reported, it was considered missing, and data were removed for that day. If data were reported for more than 16 h but less than 24 h of each day, data were extrapolated by each hour so that all steers could be equally compared across 24 h.
Statistical Analyses
Physiological data were analyzed using the MIXED procedure of SAS 9.4 (SAS Institute, Inc., Cary, NC) to determine the main effects of and interactions between seed type and isoflavone treatment on dry matter intake (DMI), average daily gain (ADG), VFA concentrations, and rectal temperatures, as well as serum metabolites hormones. Data were assessed for normality using the PROC UNIVARIATE procedure. Serum prolactin levels and rectal temperatures were non-normally distributed and subsequently log-transformed to establish normality. All other variables were normally distributed and thus were analyzed in their original state. As animals were blocked by genotype before the beginning of the study, the random effects of genotype and genotype × seed × treatment were included in the model. Least square means were compared using Fisher’s LSD. Effects were considered significant at P ≤ 0.05, with tendencies declared at 0.05 < P < 0.10. The PROC CORR procedure was used to determine correlations between glucose and insulin concentrations, as well as behavioral data and DMI or ADG. Initial BW was included in the model as a covariate for DMI and final BW. Analysis of rectal temperatures also included day as a main effect with subsequent interactions between seed type and isoflavone treatment.
Behavioral data was assessed for normality using the PROC UNIVARIATE procedure before analysis. Data needed to meet a minimum threshold of 1 for analysis. All outlying data in both the 1st and 99th percentiles were removed before analysis. Distance traveled each day was squared, minutes spent in the vicinity of the feed bunk was log transformed, and total water bouts was squared to achieve normality. Data were then compared on a weekly basis using the PROC MIXED procedure of SAS, with fixed effects of seed type, isoflavone treatment, and their interaction. Genotype and genotype × seed type × isoflavone treatment were included in the model as random variables. Effects were considered significant at P ≤ 0.05, with tendencies declared at 0.05 < P < 0.10.
Analysis of rumen bacterial communities was conducted in the R environment. Alpha diversity was estimated with the Shannon index on raw OTU abundance tables. The significance of diversity differences was tested using analysis of variance. To estimate beta diversity across ruminal fluid samples using computed Bray–Curtis indices, OTUs were excluded if occurring in fewer than 10% of the samples with a count of less than three. Beta diversity, emphasizing differences across samples, was visualized using nonmetric multidimensional (NMDS) ordination. Variation in community structure was assessed with permutational multivariate analysis of variance (PERMANOVA) with treatment combinations of seed type and isoflavone as the main fixed factor and using 4,999 permutations for significance testing.
RESULTS AND DISCUSSION
Alkaloid and Isoflavone Quantification
Kentucky 31 seed samples contained 2.94 mg of ergovaline plus ergovalinine × kg−1 of DM (1.85 and 1.09 mg × kg−1, respectively) and Kentucky 32 seed samples contained a total of 0 mg of ergovaline plus ergovalinine × kg−1 of DM. Additionally, both seed varieties tested negative for the presence of the alkaloid ergotamine and its epimer ergotaminine. The protein supplement utilized in this study also contained a total of 0 mg of ergovaline plus ergovalinine × kg−1 of DM.
BW, ADG, DMI, Feed Efficiency, and Rectal Temperature
Throughout the 21-d study, steers gained an average of 11.25 kg. At the completion of the study, mean BW was 293 ± 30 kg (Table 1). Average daily gain was associated with seed type (P = 0.003; Table 1). Steers fed endophyte-infected tall fescue seed gained less than cattle consuming endophyte-free tall fescue seed (0.29 ± 0.18 vs. 0.79 ± 0.18 kg × d−1, respectively), which is consistent with literature regarding growth performance. There was no effect of isoflavone treatment (P = 0.32) or the seed × treatment interaction (P = 0.11) on ADG. Characteristically, consumption of endophyte-infected tall fescue produces a marked reduction in both feed intake and ADG of growing cattle, which can be improved with the inclusion of clover (Burns et al., 1973; Lusby et al., 1990). Despite these characteristic observations of tall fescue toxicosis, only a reduced average daily gain was noted by consumption of endophyte-infected tall fescue seed, but this was not ameliorated with consumption of the clover isoflavones.
Table 1.
Effects of isoflavones and fescue seed type on final body weight (BW), average daily gain (ADG), dry matter intake (DMI), rumen pH, and feed efficiency in beef steers*
| Measurement | Isoflavones† | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 mg | 943 mg | |||||||
| Fescue seed type‡ | ||||||||
| E+ | E− | E+ | E− | Seed | Treatment | Seed × treatment | ||
| Initial BW,|| kg | 283.95 | 283.19 | 286.67 | 276.19 | 12.9 | 0.63 | 0.85 | 0.68 |
| Final BW,$ kg | 284.25 | 300.01 | 292.90 | 297.84 | 4.31 | 0.004 | 0.33 | 0.11 |
| ADG, kg × d−1 | 0.08 | 0.83 | 0.49 | 0.74 | 0.21 | 0.003 | 0.32 | 0.11 |
| DMI, kg × d−1 | 6.27 | 6.23 | 6.62 | 5.56 | 0.45 | 0.23 | 0.71 | 0.25 |
| Ruminal pH | 7.18 | 7.14 | 7.21 | 7.20 | 0.092 | 0.79 | 0.39 | 0.73 |
| Feed efficiency¶ | 0.013 | 0.133 | 0.074 | 0.133 | 0.039 | 0.018 | 0.29 | 0.39 |
*Data reported as LSMeans (n = 9/treatment combination group).
†Isoflavones administered at 0 mg × steer−1 × d−1 or 943 mg × steer−1 × d−1
‡Endophyte-infected (E+) tall fescue seed (0.011 mg ergot alkaloids × kg BW−1 × d−1) or endophyte-free (E−) tall fescue seed (0 mg ergot alkaloids × kg BW−1 × d−1) mixed in total ration, which was fed ad libitum.
||Measured on d 0 of the trial period before receiving treatments (kg).
$Measured on d 21 of the trial period at the end of treatments (kg).
¶Calculated as the ratio of ADG to DMI.
There were no effects of fescue seed type, isoflavone treatment, or their interaction on DMI (P > 0.10; Table 1). It is noteworthy that the present study observed no decreases in DMI with endophyte-infected tall fescue seed, which conflicts with previous observations (Hannah et al., 1990; Aldrich et al., 1993). As DMI was not reduced, but ADG was altered, it is possible the diet was fed for too short of a duration to observe changes in DMI.
Feed efficiency throughout the trial was measured as the ratio of BW gain to DMI. There was a difference in efficiency with respect to seed type, where steers consuming endophyte-infected tall fescue seed had lower feed efficiency compared with steers consuming endophyte-free tall fescue seed (0.04 ± 0.034 vs. 0.14 ± 0.034, respectively; P = 0.018; Table 1). However, feed efficiency was poor across all treatment groups, potentially due in part to the limited duration of the treatment administration and data collection period, and a small sample population.
Rectal temperatures were unaffected (P > 0.10) by seed type, isoflavones, or seed type × isoflavone interaction. The ambient temperature at the time of the study averaged 27.8 °C during the day (5:40–19:50) and 17.2 °C at night (20:00–5:40) and may not have been extreme enough to induce more pronounced symptoms of fescue toxicosis. The isoflavone formononetin has been shown to dilate arteries via nitric oxide synthase (Wu et al., 2010). In this way, isoflavones appear to act antagonistically to ergot alkaloids (Aiken et al., 2016), and may reduce heat stress. However, the ability of isoflavones to increase serum prolactin levels and mitigate other effects of ergotism have not been documented. Measurements of blood vessel diameter and blood flow dynamics will aid future research to better determine the physiological effects of isoflavone supplementation on cattle consuming endophyte-infected tall fescue.
The isoflavone dosage of 943 mg × d−1 in the present study may not have been sufficient to ameliorate all symptoms of fescue toxicosis, as reductions in ADG, DMI, and feed efficiency were not mitigated through isoflavone supplementation. Alternatively, isoflavones alone may not be capable of mitigating all the symptoms of fescue toxicosis.
Serum Metabolite and Hormone Concentrations
Glucose concentrations (Table 2) did not differ by seed type (P = 0.43; intra-assay CV = 5.12%; inter-assay CV = 6.59%) but were lower for steers supplemented with isoflavones compared with contemporaries that received no isoflavones (92.40 ± 13.91 vs. 124.46 ± 13.91 mg × dL−1, respectively; P = 0.023). Serum insulin concentrations were not altered by seed type or isoflavone treatment (P > 0.10; intra-assay CV = 5.30%; inter-assay CV = 5.87%), nor were any interactions observed (P > 0.10, Table 2). Additionally, there was no correlation between insulin and glucose concentrations (P > 0.10). Glucose concentrations may be reduced by endophyte level, as reported by Jackson et al. (2015), but this was not observed in the present study. Conversely to the study conducted by Jackson et al. (2015), steers consuming endophyte-infected tall fescue seed had numerically greater glucose concentrations. As there was no change in serum insulin concentrations across all treatments and only steers receiving isoflavone treatments had reduced glucose concentrations, this may suggest an improvement in insulin sensitivity when animals receive isoflavone treatment while consuming tall fescue seed. However, the insulin sensitivity observed by steers receiving isoflavone supplementation did not result in reduced or increased ADG or DMI. Cattle in the Southeastern United States benefit from several grazing seasons, wherein both cows and their calves have access to a higher nutritional plane than other locations in the United States dependent on limited moisture. Long et al. (2014) suggested that maternal overnutrition (when fed at 150% nutrient requirements) contributes to higher glucose concentrations in male offspring, and reduced insulin sensitivity compared with ewes on a lower nutritional plane.
Table 2.
Effects of isoflavones and fescue seed type on serum hormone and metabolite concentrations in beef steers*
| Measurement | Isoflavones† | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 mg | 943 mg | |||||||
| Fescue seed type‡ | ||||||||
| E+ | E− | E+ | E− | Seed | Treatment | Seed × treatment | ||
| Insulin,* ng × mL−1 | 0.63 | 0.47 | 0.59 | 0.66 | 0.13 | 0.71 | 0.59 | 0.41 |
| Glucose, mg × dL−1 | 134.8 | 114.12 | 92.42 | 92.38 | 15.82 | 0.36 | 0.023 | 0.37 |
| NEFA, ng × mL−1 | 81.84 | 104.79 | 80.96 | 122.31 | 17.23 | 0.11 | 0.64 | 0.62 |
| SUN, mg × dL−1 | 4.32 | 5.06 | 4.46 | 5.46 | 0.48 | 0.12 | 0.59 | 0.79 |
| IGF-1, ng × mL−1 | 171.40 | 207.34 | 111.19 | 136.53 | 30.24 | 0.33 | 0.06 | 0.86 |
| Prolactin, ng × mL−1 | 1.32 | 15.67 | 2.22 | 20.65 | 1.44 | 0.0007 | 0.32 | 0.75 |
*Data reported as LSMeans (n = 9/treatment group).
†Isoflavone treatment administered at 0 mg × steer−1 × d−1 or 943 mg × steer−1 × d−1.
‡Endophyte-infected (E+) tall fescue seed (0.011 mg ergovaline × kg BW−1 × d−1) or endophyte-free (E−) tall fescue seed (0 mg ergot alkaloids × kg BW−1 × d−1) mixed in total ration, which was fed ad libitum.
High serum NEFA concentrations are often indicators of nutritional stress and are juxtaposed with lower glucose concentrations. In the present study, no differences were observed in serum NEFA concentrations (P > 0.1; intra-assay CV = 6.95%; inter-assay CV = 7.67%). We did not detect any effects of seed type (P = 0.12), isoflavone treatment (P = 0.57) or interaction between seed type and isoflavone treatment (P = 0.78; intra-assay CV = 1.21%; inter-assay CV = 2.21%) on SUN concentrations.
Receptor ER-β is most widely expressed on nonreproductive tissues such as bone and blood vasculature, which mediates some of the growth-promoting effects of estrogen on nonreproductive tissues (Sunita and Pattanayak 2011). Klotz et al. (2000) suggested that IGF-1 may help regulate estrogen-mediated growth of tissues, and thus elevated concentrations may indicate estrogenic activity throughout tissues. Ren et al. (2001) fed the isoflavone daidzein to late gestating sows and observed higher fetal growth in male piglets associated with higher IGF-1R gene expression in skeletal muscle. Daidzein, as well as other isoflavones, are commonly found in clover tissue. In ruminants, formononetin is metabolized to daidzein and further metabolized to equol which can be found throughout other tissues in the body and in milk (Andersen et al., 2009). In the present study, IGF-1 concentrations were not affected by seed type (Table 2) but tended to be reduced with isoflavone treatment (P = 0.08; intra-assay CV= 8.54; inter-assay CV= 12.5%). Contrary to previous studies where the estrogenic activity of isoflavones increased IGF-1 concentrations, this increased response was not observed in the present study, possibly due to the limited dose of isoflavones received. The dosage of isoflavones in the present study was not enough to increase circulating IGF-1 concentrations and mimic an estrogenic effect.
Serum prolactin concentrations (Table 2) were reduced in steers consuming endophyte-infected tall fescue seed compared with steers consuming endophyte-free tall fescue seed (1.71 ± 1.29 vs. 17.98 ± 1.29 ng × mL−1, respectively; P = 0.0007; intra-assay CV = 4.88%; inter-assay CV = 4.79%). Symptoms of fescue toxicosis include reduced serum prolactin concentrations and subsequent failure to shed or regrow the winter hair coat (Hurley et al., 1980; Hoveland et al., 1983; Goetsch et al., 1987; Porter and Thompson, 1992; Aiken et al., 2011). Campbell et al. (2014) determined a single nucleotide polymorphism at the DRD2 receptor indicating reduced susceptibility to fescue toxicosis that was correlated with serum prolactin levels. In comparison to steers that consumed the endophyte-free tall fescue seed, steers consuming endophyte-infected tall fescue seed had significantly reduced prolactin levels, providing evidence that the animals were likely experiencing at least a moderate level of fescue toxicosis. However, there was not an effect of treatment with isoflavones (P = 0.34), nor was a seed type × isoflavone treatment interaction observed (P = 0.78) in serum prolactin concentrations.
Animal Behavioral Patterns
No differences were observed in overall distance traveled among treatment groups (P ≥ 0.10; Table 3). Overall number of feed bouts tended to be increased among steers consuming endophyte-free tall fescue seed when compared with those consuming endophyte-infected tall fescue seed (P = 0.09). Minutes spent in the feedbunk each day differed by seed type (P = 0.002). Steers consuming endophyte-infected tall fescue seed, regardless of isoflavone treatment, spent ~45 min more in the feedbunk per day than steers consuming endophyte-free tall fescue seed (106.81 ± 1.1 vs. 60.64 ± 1.1, respectively). With respect to activity around the automatic waterers, no differences between isoflavone treatment or fescue seed type were observed (P ≥ 0.10). Steers spent an average of 9.5 min each day at the waterer regardless of treatment.
Table 3.
Effects of isoflavones and fescue seed type on feeding behavior of beef steers*
| Behavior metric | Isoflavones† | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 mg | 943 mg | |||||||
| Fescue seed type‡ | ||||||||
| E+ | E− | E+ | E− | Seed | Treatment | Seed × treatment | ||
| Distance traveled, steps × d−1 | 5230.9 | 5495.8 | 5617.6 | 5258.1 | 1980.5 | 0.53 | 0.47 | 0.92 |
| Number of feed bouts, per day | 272.06 | 196.44 | 277.10 | 212.78 | 23.63 | 0.09 | 0.66 | 0.80 |
| Number of water bouts, per day | 10.02 | 7.6 | 10.17 | 9.73 | 4.45 | 0.53 | 0.54 | 0.89 |
| Feed bout duration, minutes | 108.67 | 65.71 | 104.97 | 55.97 | 1.12 | 0.002 | 0.41 | 0.59 |
*Data reported as LSMeans (n = 9/treatment group).
†Isoflavone treatment administered daily at 0 mg × steer−1 × d−1 or 943 mg × steer−1 × d−1.
‡Endophyte-infected (E+) tall fescue seed (0.011 mg ergot alkaloids × kg BW−1 × d−1) or endophyte-free (E−) tall fescue seed (0 mg ergot alkaloids × kg BW−1 × d−1) mixed in total ration, which was fed ad libitum.
Lauriault et al. (1990) observed ingestive behaviors (rate of intake, rate of biting, and intake per bite) of cows consuming endophyte-infected tall fescue with thiamin supplementation compared with endophyte-free tall fescue but did not indicate differences in grazing time or intake. The present study indicated a tendency for higher numbers of daily feed bouts and significantly greater time spent in the feed bunk for animals consuming endophyte-infected tall fescue seed when compared with animals consuming endophyte-free tall fescue seed. However, this was not reflected in increased DMI between animals consuming endophyte-infected tall fescue seed when compared with animals consuming endophyte-free tall fescue seed (P = 0.23). Stuedemann and Hoveland (1988) reviewed several concerns with fescue toxicosis including animal behaviors such as reduced grazing time and increased time wallowing in mud or laying in the shade to avert heat stress that may result in reduced performance. However, additional feeding behaviors such as feeding time or aggression have rarely been characterized with incidences of fescue toxicosis.
Ruminal VFA Concentrations, pH, and Bacterial Populations
Ruminal concentrations of formate, acetate, propionate, butyrate, valerate, and IVMB can be found in Table 4. There were no differences between seed types, isoflavone treatments, or their interactions, for acetate, propionate, butyrate, formate, IVMB, or valerate, or the ratio of acetate:propionate. In addition, seed type, isoflavone treatment, or their interaction did not affect mean rumen pH (P > 0.10; Table 1), which overall averaged 7.17 ± 0.04.
Table 4.
Effects of isoflavones and fescue seed type on rumen VFA concentrations in beef steers*
| VFA concentration, mmol × L−1 | Isoflavones† | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 943 | |||||||
| Fescue seed type‡ | ||||||||
| E+ | E− | E+ | E− | Seed | Treatment | Seed × treatment | ||
| Formate | 0.34 | 0.75 | 0.62 | 1.11 | 0.22 | 0.21 | 0.08 | 0.86 |
| Acetate | 25.4 | 24.4 | 24.5 | 24.6 | 2.92 | 0.87 | 0.91 | 0.84 |
| Propionate | 15.0 | 14.5 | 16.0 | 15.2 | 1.82 | 0.75 | 0.67 | 0.93 |
| A:P|| | 1.76 | 1.73 | 1.57 | 1.63 | 0.10 | 0.92 | 0.21 | 0.69 |
| Butyrate | 5.33 | 4.72 | 4.33 | 4.44 | 0.60 | 0.67 | 0.29 | 0.55 |
| IVMB$ | 1.14 | 0.98 | 0.87 | 0.99 | 0.23 | 0.86 | 0.43 | 0.39 |
| Valerate | 0.06 | 0.16 | 0.07 | 0.44 | 0.13 | 0.13 | 0.29 | 0.32 |
*Data reported as LSMeans (n = 9/treatment group).
†Isoflavone treatment administered daily at 0 mg × steer−1 × d−1 (control) or 943 mg × steer−1 × d−1 (isoflavones).
‡Endophyte-infected (E+) tall fescue seed (0.011 mg ergot alkaloids × kg BW−1 × d−1) or endophyte-free (E−) tall fescue seed (0 mg ergot alkaloids × kg BW−1 × d−1) mixed in total ration, which was fed ad libitum.
||Ratio of acetate:propionate.
$Isovalerate and methylbutyrate.
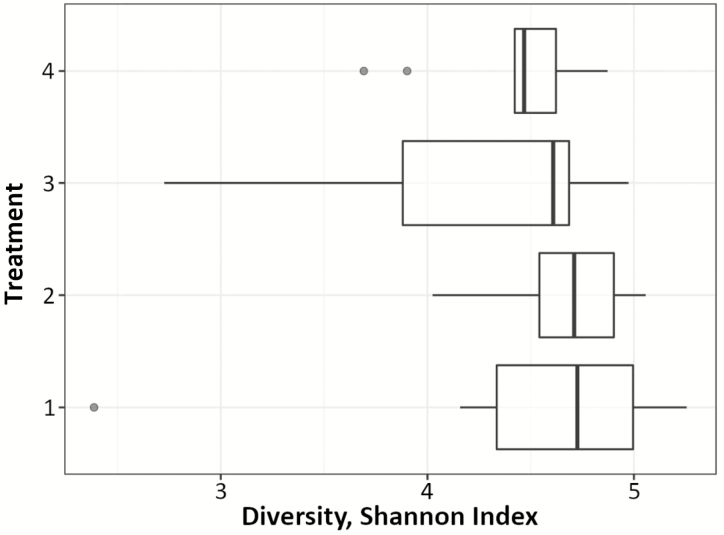
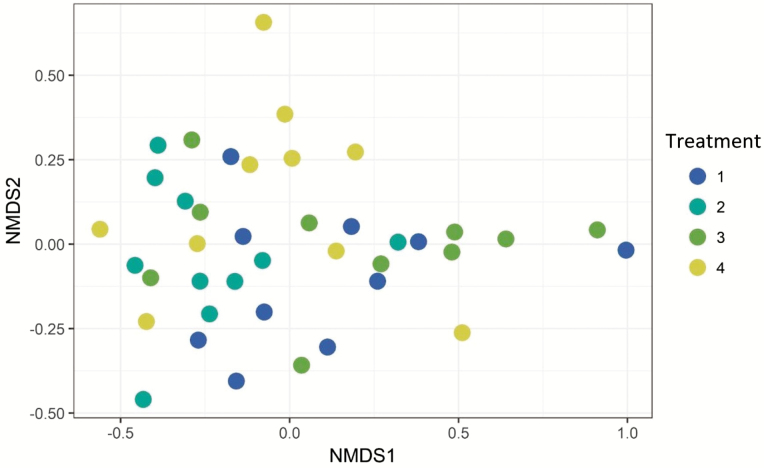
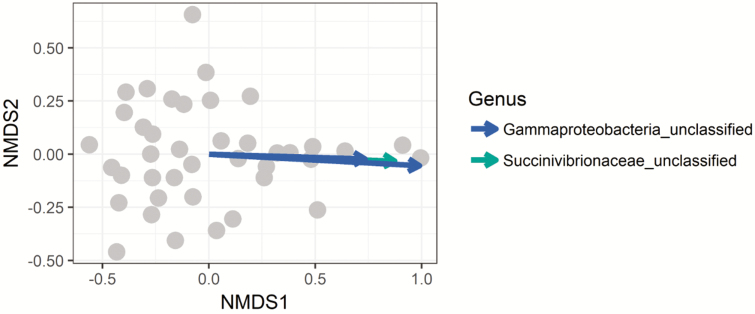
After stringent sequence processing, a total of 365,195 high-quality reads were obtained and averaged 9,242 ± 465 per sample, which is a consistent sequencing analysis depth for ruminal samples (Myer et al., 2015). Number of observed operational taxonomic units (OTUs) totaled 4,033 and averaged 1,008 ± 60 per sample. Shannon’s Diversity Index by treatment was not different (P > 0.05, Figure 1). Nonmetric multidimensional scaling (NMDS) was utilized to analyze beta diversity (Figures 2 and 3), where clusters of samples represent similarity of bacterial genera by treatment based on rank. At the genus level, bacterial populations were overall different between endophyte-free and endophyte-infected seed with isoflavone treatment groups, and differences caused a shift in overall rumen bacterial composition (Figure 4; P < 0.05; R2 = 0.12). Shifts at the genus level between groups were dominated by class Gammaproteobacteria and family Succinivibrionaceae (Figure 3). Both of these bacteria are known acetate and succinate producers in the rumen (Hespell, 1992) and may alter VFA profiles favoring acetate production.
Figure 1.
Shannon’s Diversity Index box plot of bacterial species diversity on d 21, grouped by treatment combination. Treatment 1: endophyte-infected tall fescue seed. Treatment 2: endophyte-free tall fescue seed. Treatment 3: endophyte-infected tall fescue seed with isoflavones. Treatment 4: endophyte-free tall fescue seed with isoflavones.
Figure 2.
NMDS ordination plot grouped by treatment combination. Treatment 1: endophyte-infected tall fescue seed. Treatment 2: endophyte-free tall fescue seed. Treatment 3: endophyte-infected tall fescue seed with isoflavones. Treatment 4: endophyte-free tall fescue seed with isoflavones. No defined clustering of rumen bacterial communities among treatments was identified in the present study.
Figure 3.
NMDS ordination plot of two significant taxa differing across all four treatment groups. Both Gammaproteobacteria and Succinivibrionaceae were significant at the genus level in influencing bacterial shifts. Gammaproteobacteria and Succinivibrionaceae were increased (P < 0.05) in animals fed endophyte-infected tall fescue seed compared with animals fed endophyte-free tall fescue seed.
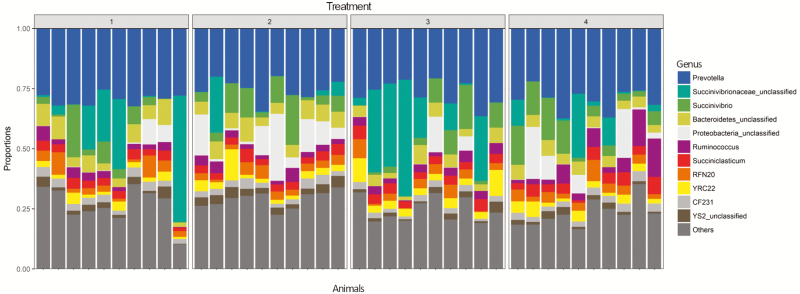
Figure 4.
Taxonomic profiles of the relative proportions of bacterial communities by genus, grouped by treatment combination. Treatment 1: endophyte-infected tall fescue seed. Treatment 2: endophyte-free tall fescue seed. Treatment 3: endophyte-infected tall fescue seed with isoflavones. Treatment 4: endophyte-free tall fescue seed with isoflavones.
When individual populations within the bacterial communities were analyzed, differences were observed at d 21 in 28 different genus classifications, either by seed, treatment, or seed × treatment interaction (Table 5). Relative proportions of genera grouped according to treatments are depicted in Figure 4. Of these, 13 taxa were reduced with supplementation of isoflavones in the diet, 8 were reduced with consumption of endophyte-free tall fescue seed, and 5 were increased with consumption of endophyte-free tall fescue seed relative to endophyte-infected tall fescue seed (P < 0.05).
Table 5.
Effects of isoflavones and fescue seed type on relative abundance of significant bacterial taxa*
| Classification, % of total sequences | Isoflavones† | Effect | SEM | P-value | |||
|---|---|---|---|---|---|---|---|
| 0 mg | 943 mg | ||||||
| Fescue seed type‡ | |||||||
| E+ | E− | E+ | E− | ||||
| Acholeplasma | 0.00571b | 0.0216a | 0.00883ab | 0.00192b | Seed × Treatment | 0.0005 | 0.0265 |
| Anaeroplasma | 0.0269ab | 0.0563a | 0.0422ab | 0.0124b | Seed × Treatment | 0.0001 | 0.0268 |
| Anaerostipes | 0.0195 | 0.0144 | 0.0492 | 0.0370 | Treatment | 0.0001 | 0.0362 |
| Bacillales | 0.0279a | 0.00411b | 0.00231b | 0.00931b | Seed × Treatment | 0.0001 | 0.0026 |
| Bacillus_unclassified | 0.000469 | 0.0301 | 3.13 | 0.0155 | Seed | 0.0001 | 0.0462 |
| Bacteriodetes_unclassified | 5.25 | 5.54 | 4.48 | 4.79 | Treatment | 0.0044 | 0.0216 |
| Blautia | 0.0255 | 0.00571 | 0.00243 | 0.00213 | Treatment | 0.0001 | 0.0101 |
| Campylobacter | 0.00592 | 0.0211 | 0.00411 | 0.0183 | Seed | 0.0001 | 0.0005 |
| Clostridium | 0.883 | 0.377 | 0.212 | 0.189 | Treatment | 0.0019 | 0.0261 |
| Coprococcus | 0.343 | 0.311 | 0.161 | 0.102 | Treatment | 0.0008 | 0.0245 |
| Gammaproteobacteria_unclassified | 0.444 | 0.184 | 0.527 | 0.276 | Seed | 0.0011 | 0.0314 |
| Lachnospiraceae | 1.60 | 1.81 | 1.27 | 1.17 | Treatment | 0.0021 | 0.0227 |
| Lactobacillus | 0.157 | 0.0735 | 0.277 | 0.0941 | Seed | 0.0005 | 0.0152 |
| Mogibactericeae | 0.121 | 0.133 | 0.0962 | 0.0888 | Treatment | 0.0001 | 0.0315 |
| Oceanobacillus | 0.0395 | 0.00661 | 0.0287 | 0.0157 | Seed | 0.0001 | 0.0045 |
| Olsenella | 0.0157 | 0.00882 | 0.0476 | 0.0329 | Treatment | 0.0001 | 0.0301 |
| Oscillospira | 1.024 | 1.51 | 0.831 | 1.18 | Seed | 0.0019 | 0.0417 |
| PL11B10 | 0.0118b | 0.0568a | 0.00984b | 0.00362b | Seed × Treatment | 0.0001 | 0.0153 |
| Prevotella | 29.7a | 24.5b | 26.6ab | 28.9a | Seed × Treatment | 0.0138 | 0.0095 |
| Proteobacteria | 0.183 | 8.66 | 1.99 | 6.05 | Seed | 0.0201 | 0.0101 |
| Ruminobacter | 0.246 | 1.03 | 0.00882 | 0.121 | Treatment | 0.0021 | 0.0102 |
| Succinimonas | 0.0528 | 0.522 | 0.0675 | 0.272 | Seed | 0.0009 | 0.0006 |
| Succinivibrionaceae_unclassified | 11.7 | 2.97 | 16.7 | 5.27 | Seed | 0.0406 | 0.0108 |
| TG5 | 0.0256 | 0.0274 | 0.00893 | 0.00812 | Treatment | 0.0001 | 0.0135 |
| Veillonellaceae | 0.289 | 0.415 | 0.252 | 0.393 | Seed | 0.0004 | 0.0323 |
| YS2 | 2.19 | 3.25 | 1.88 | 1.78 | Treatment | 0.0036 | 0.0184 |
a,b,cWithin a column, means with different superscripts differ (P < 0.05).
*Data shown as LSMeans (n = 9/treatment group).
†Isoflavone treatment administered daily at 0 mg × steer−1 × d−1 or 943 mg × steer−1 × d−1.
‡Endophyte-infected (E+) tall fescue seed (0.011 mg ergot alkaloids × kg BW−1 × d−1) or endophyte-free (E−) tall fescue seed (0 mg ergot alkaloids × kg BW−1 × d−1) mixed in total ration, which was fed ad libitum.
It is noteworthy that in previous studies the isoflavones were effective in reducing the number of Clostridium species (Flythe and Kagan 2010), and this was also consistent in the current study in animals that were supplemented with isoflavones. Rather than causing overall bacterial shifts due to supplementation with or without isoflavones, finer bacterial OTUs were altered through both seed type and isoflavone treatment (Table 5). Recent studies targeted specific amylolytic or cellulolytic bacterial species and their sensitivities to biochanin A (Harlow et al. 2017a, 2018). Three cellulolytic bacteria (Fibrobacter succinogenes S85, Ruminococcus flavefaciens 8, and Ruminococcus albus 8) were reduced with biochanin A, and four amylolytic bacteria (Streptococcus bovis JB1, S. bovis HC5, Lactobacillus reuteri, Selenemonas ruminatium) were inhibited with biochanin A. Compared with the present study, those conducted by Harlow et al. (2018) utilized only biochanin A and observed shifts in the bacteria. The present study identified nine taxa that were reduced due to isoflavones; all of which were not described in the previous studies (Table 5). The gram-negative bacterial class Gammaproteobacteria and family Succinivibrionaceae were not identified among those inhibited by biochanin A in previous studies but were two of the main shifts in the present study. These ruminal bacteria shifts may be influenced by other isoflavones, as well as by seed type. Indeed, similar bacterial profile shifts were observed among seed type, isoflavone treatment, and the interaction in vitro (Melchior et al., 2018). These data may provide future insight to using additional culturable strains of microorganisms to determine the influence of isoflavones on ruminal microorganisms. The isoflavone dosage used in this study drove several rumen microbiological shifts that may provide beneficial targeting in the future, as the bacterial family Succinivibrionaceae and class Gammaproteobacteria are both known for acetate production. Improving VFA production through a higher dosage of isoflavones should be considered in future studies to promote growth performance.
In the study conducted by Aiken et al. (2016), the quantity of Promensil utilized was based on the amount required to inhibit the growth and ammonia production of several hyper-ammonia-producing bacteria using an in vitro model using previously cultured strains of bacteria from caprine and bovine rumen fluid (Flythe and Kagan, 2010; Flythe et al., 2013; Harlow et al., 2017b). The dose used by Aiken et al. (2016) aimed to provide a minimum of 30 mg × L−1 of biochanin A in the rumen. A total of 38.17 mg of isoflavones × g−1 of Promensil tablet was determined using the method by Aiken et al. (2016) for the current study. As the purpose of the present study was to determine mitigation of fescue toxicosis, not reduction of ammonia, the previously used dose of 30 mg of biochanin A × L−1 of rumen volume may not be satisfactory for the current study’s objectives. Subclinical symptoms of fescue toxicosis were evident in the current study and the isoflavone dosage used began to reduce some, but not all, of the symptoms, including decreased serum glucose and IGF-1. Based on the current findings, future studies may benefit from a dose–response trial using this or similar isoflavone products with tall fescue.
The isoflavone tablet utilized in this study was fortified to have a greater concentration of the isoflavone biochanin A compared with most red clover stands. Due to the potential differences between the isoflavone concentrations in the product and isoflavone concentrations in red clover pastures, samples were collected across the state of Tennessee and analyzed using the previously mentioned LC-MS methodology for content of the four major isoflavones and several glucosides (Table 6). The present study supplemented 24.7 g of tablet per d over the course of the 21-d study and provided ~943 mg of isoflavones × bolus × d−1 (~3.29 mg isoflavones × kg BW−1). Across a sample of red clover stands in Tennessee (n = 5, Table 6), an average isoflavone concentration of 20.227 mg × g−1 (DM basis) of red clover whole-plant tissue was determined. Cattle entering a stocker or backgrounding program may consume 2%–3% of their BW each day in DM (NRC, 2000) from mixed forages. Given that most pastures in the mid-South have mixed forages, red clover may occupy 25%–35% of each pasture. Cattle weighing 200–300 kg may consume 4–6 kg DM each day, with roughly 0.5–1.0 kg of that consisting of clover varieties. Using this estimate, cattle in a pasture setting may consume 10–20 g of isoflavones per d (~33–60 mg of isoflavones × kg of BW−1) based on collected pasture samples, which can vary depending on forage maturity, intake, and composition of pasture. At d 21 of the present study, steers weighed an average of 291 kg and were provided a dose of 3.29 mg of isoflavones × kg of BW−1. Thus, for the present study, the amount of isoflavones supplemented was substantially less than expected consumption rates on pasture. As both physiological and microbial changes were observed at a fraction of the isoflavones found in expected forage consumption in the present study and by Aiken et al. (2016), this could provide insight to future experiments and dosing levels.
Table 6.
Isoflavone quantities in red clover stands across Tennessee,* and in Promensil
| Sample, μg × g−1 DM | Isoflavone† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biochanin A | Formononetin | Genistein | Daidzein | BG | FG | GG | DG | BMG‡ | FMG‡ | |
| Red clover A | 1,701 | 3,819 | 42 | 20 | 462 | 1,268 | 365 | 309 | 3,421 | 10,367 |
| Red clover B | 1,258 | 3,346 | 37 | 18 | 606 | 1,223 | 356 | 265 | 5,238 | 9,745 |
| Red clover C | 889 | 2,361 | 25 | 1 | 576 | 1,192 | 423 | 159 | 4,957 | 9,883 |
| Red clover D | 1,242 | 3,067 | 31 | 9 | 524 | 1,217 | 559 | 282 | 3,871 | 8,667 |
| Red clover E | 906 | 2,158 | 25 | 9 | 489 | 1,132 | 342 | 479 | 3,541 | 8,256 |
| Average of pasture samples | 1,199 | 2,950 | 32 | 11 | 531 | 1,206 | 409 | 299 | 4,205 | 9,383 |
| Promensil|| | 7,346 | 5,997 | 449 | 169 | 3658 | 5,572 | 0 | 0 | 0 | 0 |
*Red clover samples were collected from five different locations across University of Tennessee research centers.
†BG = biochanin A glucoside (sissotrin), FG = formononetin glucoside (ononin), GG = genistein glucoside (genistin), DG = daidzein glucoside (daidzin), BMG = biochanin A malonyl glucoside, and FMG = formononetin malonyl glucoside.
‡Corrected for hydrolysis.
||Measured in μg × 600 mg tablet−1.
The combined physiological, biochemical, behavioral, and microbiological results from the present study indicate more direct influences of fescue toxicosis on the animal’s rumen microbial populations and physiology than have previously been identified, including rumen bacteria profile shifts resulting from endophyte-infected tall fescue seed consumption. In an effort to combat the detrimental effects of fescue toxicosis, the use of isoflavones at the dose used in the present study does not appear to be a viable alternative as a top-dressed supplement to cattle grazing endophyte-infected tall fescue pastures. Thus, further research discerning beneficial isoflavone dosage, and specific species of red clovers that may provide increased concentrations of isoflavones for mitigation of tall fescue toxicosis is warranted.
Conflict of interest statement. None declared.
Footnotes
Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA and does not imply approval to the exclusion of other products that may be suitable.
We acknowledge the University of Tennessee CVM, Center for Excellence in Livestock and Human Diseases for funding this project, PharmaCare, Inc. for providing the Promensil isoflavone product, and the USDA-NIFA Hatch/Multistate Project W2010-TEN00493—Integrated Approach to Enhance Efficiency of Feed Utilization in Beef Production Systems. We thank Ames Plantation, UT Plateau Research and Education Center, Dr Rebecca Payton, Lezek Wojakiewicz, and Gloria Gellin (USDA-ARS) for project assistance.
LITERATURE CITED
- Aiken G. E., M. D. Flythe I. A. Kagan H. Ji, and Bush L. P.. 2016. Mitigation of ergot vasoconstriction by clover isoflavones in goats (Capra hircus). Front. Vet. Sci. 3:17. doi:10.3389/fvets.2016.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken G. E., Klotz J. L., Looper M. L., Tabler S. F., and Schrick F. N.. . 2011. Disrupted hair follicle activity in cattle grazing endophyte-infected tall fescue in the summer insulates core body temperatures. Prof. Anim. Sci. 27(4):336–343. doi:10.15232/S1080-7446(15)30497-6 [Google Scholar]
- Aiken G. E., J. R. Strickland M. L. Looper L. P. Bush, and Schrick F. N.. 2009. Hemodynamics are altered in the caudal artery of beef heifers fed different ergot alkaloid concentrations. J. Anim. Sci. 87:2142–2150. doi:10.2527/jas.2008-1562 [DOI] [PubMed] [Google Scholar]
- Aldrich C. G., J. A. Paterson J. L. Tate, and Kerley M. S.. 1993. The effects of endophyte-infected tall fescue consumption on diet utilization and thermal regulation in cattle. J. Anim. Sci. 71:164–170. doi:10.2527/1993.711164x [DOI] [PubMed] [Google Scholar]
- Andersen C., T. S. Nielsen S. Purup T. Kristensen J. Eriksen K. Søegaard J. Sørensen, and Fretté X. C.. 2009. Phyto-oestrogens in herbage and milk from cows grazing white clover, red clover, lucerne or chicory-rich pastures. Animal. 3:1189–1195. doi:10.1017/S1751731109004613 [DOI] [PubMed] [Google Scholar]
- Bernard J. K., Chestnut A. B., Erickson B. H., and Kelly F. M. 1993. Effects of prepartum consumption of endophyte-infested tall fescue on serum prolactin and subsequent milk production of Holstein cows. J. Dairy Sci. 76: 1928-1933. doi:10.3168/jds.S0022-0302(93)77526-8 [Google Scholar]
- Burns J., Goode L., Gross H., and Linnerud A.. . 1973. Cow and calf gains on Ladino clover-tall fescue and tall fescue, grazed alone and with coastal bermudagrass. Agron. J. 65(6):877–880. doi:10.2134/agronj1973.00021962006500060009x [Google Scholar]
- Campbell B. T., C. J. Kojima T. A. Cooper B. C. Bastin L. Wojakiewicz R. L. Kallenbach F. N. Schrick, and Waller J. C.. 2014. A single nucleotide polymorphism in the dopamine receptor D2 gene may be informative for resistance to fescue toxicosis in angus-based cattle. Anim. Biotechnol. 25:1–12. doi:10.1080/10495398.2013.796960 [DOI] [PubMed] [Google Scholar]
- Clemmons B. A., Mihelic R. I., Beckford R. C., Powers J. B., Melchior E. A., McFarlane Z. D., Cope E. R., Embree M. M., Mulliniks J. T., Campagna S. R., . et al. 2017. Serum metabolites associated with feed efficiency in black angus steers. Metabolomics. 13(12): 147. doi:10.1007/s11306-017-1282-z [Google Scholar]
- Flythe M., Harrison B., Kagan I., Klotz J., Gellin G., Goff B., and Aiken G.. . 2013. Antimicrobial activity of red clover (Trifolium pratense L.) extract on caprine hyper ammonia-producing bacteria. Agric Food Anal Bacteriol. 3:176–185. [Google Scholar]
- Flythe M., and Kagan I.. . 2010. Antimicrobial effect of red clover (Trifolium pratense) phenolic extract on the ruminal hyper ammonia-producing bacterium, clostridium sticklandii. Curr. Microbiol. 61:125–131. doi:10.1007/s00284-010-9586-5 [DOI] [PubMed] [Google Scholar]
- Goetsch A. L., A. L. Jones S. R. Stokes K. W. Beers, and Piper E. L.. 1987. Intake, digestion, passage rate and serum prolactin in growing dairy steers fed endophyte-infected fescue with noninfected fescue, clover or wheat straw. J. Anim. Sci. 64:1759–1768. doi: 10.2527/jas1987.6461759x [DOI] [PubMed] [Google Scholar]
- Guan L. L., J. D. Nkrumah J. A. Basarab, and Moore S. S.. 2008. Linkage of microbial ecology to phenotype: correlation of rumen microbial ecology to cattle’s feed efficiency. FEMS Microbiol. Lett. 288:85–91. doi:10.1111/j.1574-6968.2008.01343.x [DOI] [PubMed] [Google Scholar]
- Hancock D. W., and Andrae J. G.. . 2009. Novel endophyte-infected tall fescue. Univ. Georgia Extension Circular. 861:1–8. [Google Scholar]
- Hannah S. M., J. A. Paterson J. E. Williams M. S. Kerley, and Miner J. L.. 1990. Effects of increasing dietary levels of endophyte-infected tall fescue seed on diet digestibility and ruminal kinetics in sheep. J. Anim. Sci. 68:1693–1701. doi:10.2527/1990.6861693x [DOI] [PubMed] [Google Scholar]
- Harlow B. E., Flythe M. D., and Aiken G. E.. . 2017a. Effect of biochanin A on corn grain (Zea mays) fermentation by bovine rumen amylolytic bacteria. J. Appl. Microbiol. 122:870–880. doi:10.1111/jam.13397 [DOI] [PubMed] [Google Scholar]
- Harlow B. E., M. D. Flythe, and Aiken G. E.. 2018. Biochanin A improves fibre fermentation by cellulolytic bacteria. J. Appl. Microbiol. 124:58–66. doi:10.1111/jam.13632 [DOI] [PubMed] [Google Scholar]
- Harlow B. E., Goodman J. P., Lynn B. C., Flythe M. D., Ji H., and Aiken G. E.. . 2017c. Ruminal tryptophan-utilizing bacteria degrade ergovaline from tall fescue seed extract1. J. Anim. Sci. 95: 980–988. doi:10.2527/jas.2016.1128 [DOI] [PubMed] [Google Scholar]
- Hespell R. B. 1992. The Genera Succinivibrio and Succinimonas. In: Balows, A., Trüper, H.G., Dworkin, M., Harder, W., Schleifer, K.H., editors. The prokaryotes. New York (NY): Springer; p. 3979–3982. doi:10.1007/978-1-4757-2191-1_60 [Google Scholar]
- Hoveland C. S., Schmidt S. P., King C. C., Odom J. W., Clark E. M., McGuire J. A., Smith L. A., Grimes H. W., and Holliman J. L.. . 1983. Steer performance and association of Acremonium coenophialum fungal endophyte on tall fescue pasture1. Agron. J. 75(5):821–824. doi:10.2134/agronj1983.00021962007500050021x [Google Scholar]
- Hurley W. L., E. M. Convey K. Leung L. A. Edgerton, and Hemken R. W.. 1980. Bovine prolactin, TSH, T and T concentrations as affected by tall fescue summer toxicosis and temperature. J. Anim. Sci. 51:374–379. doi:10.2527/jas1980.512374x [DOI] [PubMed] [Google Scholar]
- Jackson J. J., M. D. Lindemann J. A. Boling, and Matthews J. C.. 2015. Summer-long grazing of high vs. low endophyte (Neotyphodium coenophialum)-infected tall fescue by growing beef steers results in distinct temporal blood analyte response patterns, with poor correlation to serum prolactin levels. Front. Vet. Sci. 2:77. doi:10.3389/fvets.2015.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenbach R. L. 2015. BILL E. KUNKLE INTERDISCIPLINARY BEEF SYMPOSIUM: coping with tall fescue toxicosis: solutions and realities. J. Anim. Sci. 93:5487–5495. doi:10.2527/jas.2015-9229 [DOI] [PubMed] [Google Scholar]
- Klotz D.M., Hewitt S.C., Ciana P., Raviscioni M., Lindzey J.K., Foley J., Maggi A., DiAugustine R.P. and Korach K.S., 2002. Requirement of estrogen receptor-α in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J. Biol. Chem. 277:8531-8537. doi:10.1074/jbc.M109592200 [DOI] [PubMed] [Google Scholar]
- Klotz J. L. 2015. Activities and effects of ergot alkaloids on livestock physiology and production. Toxins (Basel). 7:2801–2821. doi:10.3390/toxins7082801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz A. F., L. P. Bush J. L. Klotz K. R. McLeod F. N. Schrick, and Harmon D. L.. 2012. Evaluation of a ruminally dosed tall fescue seed extract as a model for fescue toxicosis in steers. J. Anim. Sci. 90:914–921. doi:10.2527/jas.2011-4292 [DOI] [PubMed] [Google Scholar]
- Kozich J. J., Westcott S. L., Baxter N. T., Highlander S. K., Schloss P. D.. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. p. AEM. 01043-13. doi:10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauriault L. M., C. T. Dougherty N. W. Bradley, and Cornelius P. L.. 1990. Thiamin supplementation and the ingestive behavior of beef cattle grazing endophyte-infected tall fescue. J. Anim. Sci. 68(5):1245–1253. doi:10.2527/1990.6851245x [DOI] [PubMed] [Google Scholar]
- Long N.M., Rule D. C., Tuersunjiang N., Nathanielsz P. W., and Ford S. P.. . 2014. Maternal obesity in sheep increases fatty acid synthesis, upregulates nutrient transporters and increases adiposity in adult male offspring after a feeding challenge. PLoS One. 10(4): e0122152. doi:10.1371/journal.pone.0122152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusby K. S., McMurphy W. E., Strasia C. A., Smith S. C., and Muntz S. H.. . 1990. Effects of fescue endophyte and interseeded clovers on subsequent finishing performance of steers. J. Prod. Agri. 3(1):103–105. doi:10.2134/jpa1990.0103 [Google Scholar]
- Melchior E. A., J. K., Smith L. G., Schneider J. T., Mulliniks G. E., Bates Z. D., McFarlane M. D., Flythe J. L., Klotz J. P., Goodman H., Ji, et al. 2018. Effects of red clover isoflavones on tall fescue seed fermentation and microbial populations in vitro. PLoS One. 13:e0201866. doi:10.1371/journal.pone.0201866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer P. R., T. P. Smith J. E. Wells L. A. Kuehn, and Freetly H. C.. 2015. Rumen microbiome from steers differing in feed efficiency. PLoS One. 10:e0129174. doi:10.1371/journal.pone.0129174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC 2000. Nutrient requirements of beef cattle. 7th revised ed Washington, DC: National Academies Press. [Google Scholar]
- Paz H. A., C. L. Anderson M. J. Muller P. J. Kononoff, and Fernando S. C.. 2016. Rumen bacterial community composition in holstein and jersey cows is different under same dietary condition and is not affected by sampling method. Front. Microbiol. 7:1206. doi:10.3389/fmicb.2016.01206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. K., and Thompson F. N. Jr. 1992. Effects of fescue toxicosis on reproduction in livestock. J. Anim. Sci. 70:1594–1603. doi:10.2527/1992.7051594x [DOI] [PubMed] [Google Scholar]
- Ren M. Q., G. Kuhn J. Wegner G. Nürnberg J. Chen, and Ender K.. 2001. Feeding daidzein to late pregnant sows influences the estrogen receptor beta and type 1 insulin-like growth factor receptor mrna expression in newborn piglets. J. Endocrinol. 170:129–135. doi:10.1677/joe.0.1700129 [DOI] [PubMed] [Google Scholar]
- Schloss P. D., S. L., Westcott T., Ryabin J. R., Hall M., Hartmann E. B., Hollister R. A., Lesniewski B. B., Oakley D. H., Parks C. J., Robinson, et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75:7537–7541. doi:10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuedemann J. A., and Hoveland C. S.. . 1988. Fescue endophyte: history and impact on animal agriculture. J. Prod. Agri. 1(1):39–44. doi:10.2134/jpa1988.0039 [Google Scholar]
- Sunita P., and Pattanayak S. P.. . 2011. Phytoestrogens in postmenopausal indications: a theoretical perspective. Pharmacogn. Rev. 5(9):41–47. doi:10.4103/0973-7847.79098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. H., Q., Li M. Y., Wu D. J., Guo H. L., Chen S. L., Chen S. W., Seto A. L., Au C. C., Poon G. P., Leung, et al. 2010. Formononetin, an isoflavone, relaxes rat isolated aorta through endothelium-dependent and endothelium-independent pathways. J. Nutr. Biochem. 21:613–620. doi:10.1016/j.jnutbio.2009.03.010 [DOI] [PubMed] [Google Scholar]
- Yu Z., and Morrison M.. . 2004. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques. 36:808–812. doi:10.2144/04365ST04 [DOI] [PubMed] [Google Scholar]