Abstract
Background
Children with sickle cell anemia (SCA) are at increased risk for invasive pneumococcal disease (IPD); antibiotic prophylaxis significantly reduces this risk. Our objective was to calculate the proportion of children with SCA who received ≥300 days of antibiotic prophylaxis and identify predictors of such receipt.
Methods
Children 3 months-5 years with SCA were identified by the presence of 3+ Medicaid claims with a diagnosis of SCA within a calendar year (2005–2012) in Florida, Illinois, Louisiana, Michigan, South Carolina, and Texas. Receipt of antibiotics was identified through Medicaid claims for filled prescriptions. The outcome, receipt of ≥300 days of antibiotics within each year, was assessed using varying classifications of antibiotics. Using logistic regression with generalized estimating equations, we estimated the odds of receiving ≥300 days of antibiotics, with potential predictors of age, sex, year, state, and health services utilization.
Results
A total of 2,821 children contributed 5,014 person-years throughout the study period. Overall, only 18% of children received ≥300 days of antibiotic prescriptions for penicillin, erythromycin, or amoxicillin. Each additional sickle cell disease-related outpatient visit (OR=1.01, 95% CI: 1.01, 1.02) and well-child visit (OR=1.08, 95%CI: 1.02, 1.13) was associated with incrementally increased odds of receiving ≥300 days of antibiotics.
Conclusions
Despite national recommendations and proven life-saving benefit, antibiotic prophylaxis rates are low among children with SCA. Numerous healthcare encounters may offer an opportunity for intervention; in addition, such interventions likely need to include social factors that may affect the ability for a child to receive and adhere to antibiotic prophylaxis.
Table of Contents Summary
Are children with sickle cell anemia adequately protected against invasive pneumococcal disease?
INTRODUCTION
Sickle cell disease affects predominately racial and ethnic minority populations in the US; 1 in 375 African American births are diagnosed with this recessive genetic condition.1–5 Children with sickle cell disease are affected by numerous morbidities, such as an increased risk of invasive pneumococcal disease (IPD), caused by Streptococcus pneumoniae.5,6
Although children and adults with sickle cell disease are at an increased risk of IPD, children with the sickle cell anemia (SCA) subtype (hemoglobin (Hb) SS) are at greatest risk for both the disease and related case fatality.7,8 Without intervention, children with SCA have up to 100 times the risk of IPD as compared to children with normal hemoglobin.7 Daily receipt of penicillin is an effective method to reduce the incidence of IPD among children with SCA. The Prophylactic Penicillin Study demonstrated an 84% reduction in the risk of infection among children that received daily penicillin as compared to those receiving placebo.9 More recently, the National Heart Lung and Blood Institute (NHLBI) reiterated the importance of penicillin prophylaxis in updated recommendations for the management of sickle cell disease indicating that children with SCA receive twice-daily oral penicillin until age 5.10,11
Although the effectiveness of daily penicillin prophylaxis has been known for decades, limited evidence indicates low rates of compliance among children.12,13 While these studies offer some insight into penicillin prophylaxis among children with sickle cell disease, they do not focus specifically on the NHLBI-specified target population of children with SCA. In addition, the classification of antibiotic prophylaxis varies between studies, making comparability difficult.12–14 To address these issues, we assessed rates of antibiotic prophylaxis among children with SCA using varying definitions of antibiotic prophylaxis. We also explored predictors of receipt of ≥300 days of antibiotics, with the goal of characterizing opportunities for intervention to increase rates of antibiotic prophylaxis among this population.
METHODS
We conducted a multi-state analysis of antibiotic prophylaxis among children with SCA using administrative claims data (University of Michigan IRB HUM00120422).
Data source
Our target population was drawn from the Medicaid programs for six states with average to high prevalence of SCA: Florida, Illinois, Louisiana, Michigan, South Carolina, and Texas. Medicaid Analytic eXtract (MAX) administrative data were acquired from the Centers for Medicare and Medicaid Services (CMS); at the time of the study, these states only contained valid data through 2012. Administrative data (2005–2012) included enrollment history and all paid claims for inpatient, outpatient, emergency department (ED), laboratory, and outpatient pharmacy services.15 As previous studies have indicated approximately 90% of children with SCA are enrolled in Medicaid at some point in time, we expect that Medicaid data will capture a large proportion of the children with SCA.16,17
Study Population
We identified children with SCA using a case definition of the presence of at least three claims for a child within a calendar year that were SCA-related (282.61, 282.62). This case definition was previously demonstrated to have a high level of sensitivity (91.4%) and specificity (80%) as compared to the “gold standard” of newborn screening records.17 Continuous enrollment in the Medicaid program for at least one calendar year within this time period was required. For each year a child was eligible for the study population, we restricted our analysis to children with no other forms of health insurance (i.e., private insurance) during the study period to maximize the completeness of claims available. Children were eligible to contribute multiple non-sequential years to the study population (e.g., 2005 and 2007). Children were <5 years throughout each contributed person-year, consistent with NHLBI recommendations for penicillin prophylaxis.11
Definitions of Antibiotic Prophylaxis
Oral penicillin is recommended by the NHLBI for prophylaxis against IPD. However, the American Academy of Pediatrics recommends erythromycin for children with suspected/proven penicillin allergy and amoxicillin is sometimes prescribed for practical reasons and is equally effective against S. pneumoniae. Therefore, we classified antibiotics using four definitions:
oral penicillin;
oral penicillin or erythromycin;
oral penicillin, erythromycin, or amoxicillin;
any antibiotic likely to protect against S. pneumoniae (including penicillin, erythromycin, amoxicillin).18–22
Antibiotics were identified in pharmacy claims using relevant national drug codes (NDCs) associated with an antibiotic (Appendix 1). An author with expertise in pediatric infectious diseases (A.T.) reviewed these records and classified them as described above.
Antibiotic Prophylaxis
The total number of days’ supply of antibiotics within a year was determined by summing the days’ supply reported within each filled prescription. Adequate antibiotic prophylaxis was defined as having filled antibiotic prescriptions that would cover ≥300 days of the year; this definition of adequate antibiotic adherence has been endorsed by the National Quality Forum.23 As such, this quality assessment should be viewed as a “best case” assessment as some children still would not have prophylaxis for all days in a given year.
Predictors of Antibiotic Adherence
We evaluated potential associations between receiving ≥300 days’ supply of antibiotics and the following predictors: age, sex, use of health services (sickle cell disease-related inpatient, outpatient, emergency department, or well-child visits), calendar year, and state of residence.24 Our approach adjusted for state of residence as a confounder to partially account for the unmeasured variation of these factors between states. Classification of healthcare encounters was expanded to include any mention of sickle cell disease to account for potential misclassification of sickle cell subtype within the encounter.
Statistical Analysis
Frequencies and percentages (or means, medians, and standard deviations) were determined for demographic characteristics obtained from the MAX enrollment files. The total number of days’ supply of antibiotics for each child within the study population was calculated by definition, as well as the proportion of children that received ≥300 days’ supply within the calendar year, for each year and state.
Means, standard deviations, and interquartile ranges (IQRs) of the number of annual health services visits were assessed. Logistic regression was used to estimate the bivariate associations between each potential predictor and receiving ≥300 days of antibiotics. For the purposes of this analysis, Definition 3 (penicillin/erythromycin/amoxicillin) was used, as this definition was permissive without being all-inclusive. Because multiple periods of enrollment were allowed for each child, generalized estimating equation models with robust standard errors accounted for correlation among children. Counts of health care services and age were modeled continuously; predictors showing an association (P < .20) were included in a final multivariable model. Odds ratios (ORs) with 95% CIs were used to assess the final associations. For all models, regression diagnostics were performed to assess normality of error variances.
We performed a sensitivity analysis to account for potential limitations of pharmacy claims data pertaining to the dispensed days’ supply reported on claims. If pharmacy claims for filled prescriptions are missing, our results would be an underestimate of the true proportion of children receiving antibiotic prophylaxis. First, we assessed the number of person-years that included zero fills for penicillin, erythromycin, or amoxicillin. Then, we explored how our results would be impacted if we assumed that observation with less than a 30 days’ supply of antibiotics was due to incomplete claims records. We excluded any person-years that had fewer than 30 days’ supply reported for penicillin, erythromycin, or amoxicillin within a calendar year. Among this restricted population, we calculated the proportion of children that had ≥300 days of antibiotics filled; these results were compared to those for the full study population for each year using two proportion z-tests.
RESULTS
A total of 2,821 children with SCA between the ages of 3 months and 5 years of age were identified from the MAX dataset from 2005–2012, contributing a total of 5,014 person-years. The number of person-years varied by state as follows: Florida (1,619, 32%), Texas (897, 18%), Louisiana (855, 17%), Illinois (622, 12%), Michigan (580, 12%), and South Carolina (441, 9%). The study population was comprised of 48% females (n=1,364) and 52% males (n=1,457). In 2005, the average age was 1.6 years (SD=1.1); this was consistent across each year of observation (Table 1). Across states, the median age was 2 years, with the exception of South Carolina, where the median age was 1 year.
Table 1.
Demographic Characteristics of Children with Sickle Cell Anemia Enrolled in Medicaid by Year, 2005–2012
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | |
|---|---|---|---|---|---|---|---|---|
| n = 496 | n = 503 | n = 534 | n = 563 | n = 690 | n = 723 | n =756 | n = 749 | |
| Gender | ||||||||
| Female, n(%) | 231(47) | 248(49) | 267(50) | 276(49) | 336(49) | 349(48) | 348(46) | 353(47) |
| Male, n(%) | 265(53) | 255(51) | 267(50) | 287(51) | 354(51) | 374(52) | 408(54) | 396(53) |
| State | ||||||||
| Florida, n(%) | 170(34) | 160(32) | 122(23) | 145(26) | 233(34) | 270(37) | 266(35) | 253(34) |
| Illinois, n(%) | 53(11) | 75(15) | 69(13) | 69(12) | 96(14) | 75(10) | 82(11) | 103(14) |
| Louisiana, n(%) | 101(20) | 87(17) | 122(23) | 124(22) | 115(17) | 116(16) | 106(14) | 84(11) |
| Michigan, n(%) | 41(8) | 55(11) | 68(13) | 70(12) | 92(13) | 90(13) | 85(11) | 79(11) |
| South Carolina, n(%) | 49(10) | 52(10) | 57(11) | 33(6) | 35(5) | 41(6) | 64(9) | 110(15) |
| Texas, n(%) | 82(17) | 74(15) | 96(18) | 122(22) | 119(17) | 113(18) | 153(20) | 120(16) |
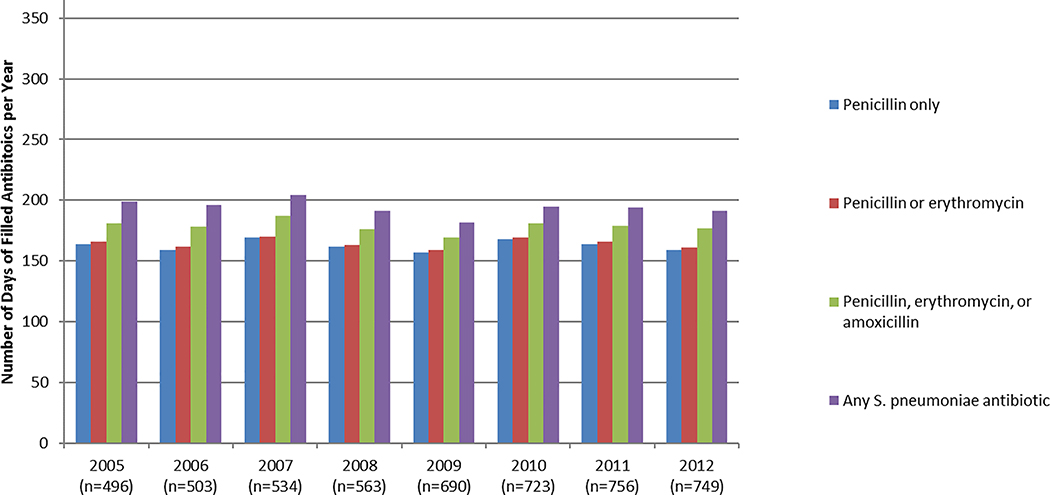
The mean number of days of filled antibiotic prescriptions varied by definition and by year (Figure 1A). The average number of days of filled prescriptions was as follows: penicillin: 162 (SD=117; median: 160); penicillin or erythromycin: 164 (SD=117; median: 160); penicillin, erythromycin, or amoxicillin: 178 (SD=113; median: 180); or any S. pneumoniae antibiotic: 193 (SD=116; median: 194).
Figure 1.
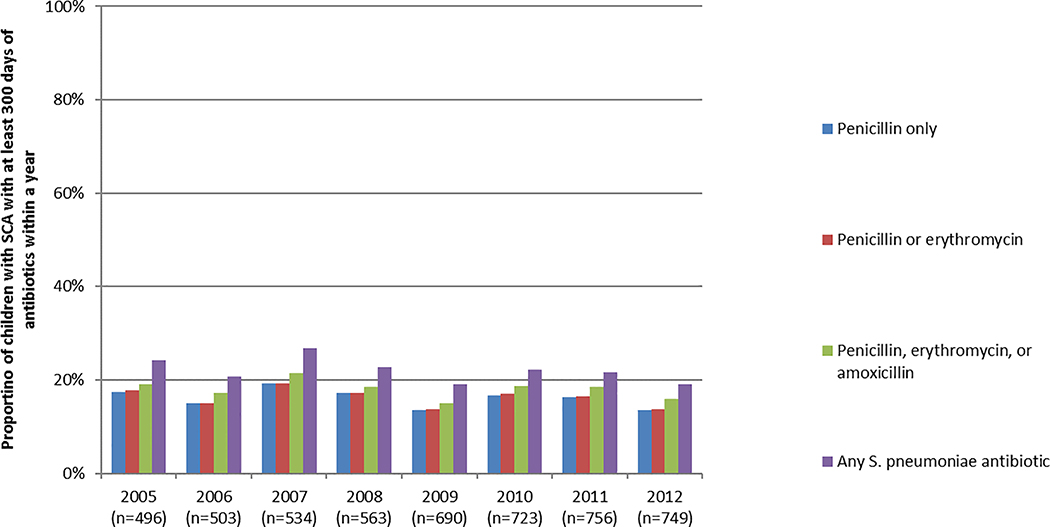
A, Mean number of days of dispensed antibiotics per child with SCA, by definition and year. B, Proportion of children with SCA dispensed ≥300 days of antibiotics within a year, by definition and year.
The proportion of children that received ≥300 days of antibiotics also varied by definition and year (Figure 1B): penicillin: 16%; penicillin or erythromycin: 16%; penicillin, erythromycin, or amoxicillin: 18%; or any S. pneumoniae antibiotic: 22%.
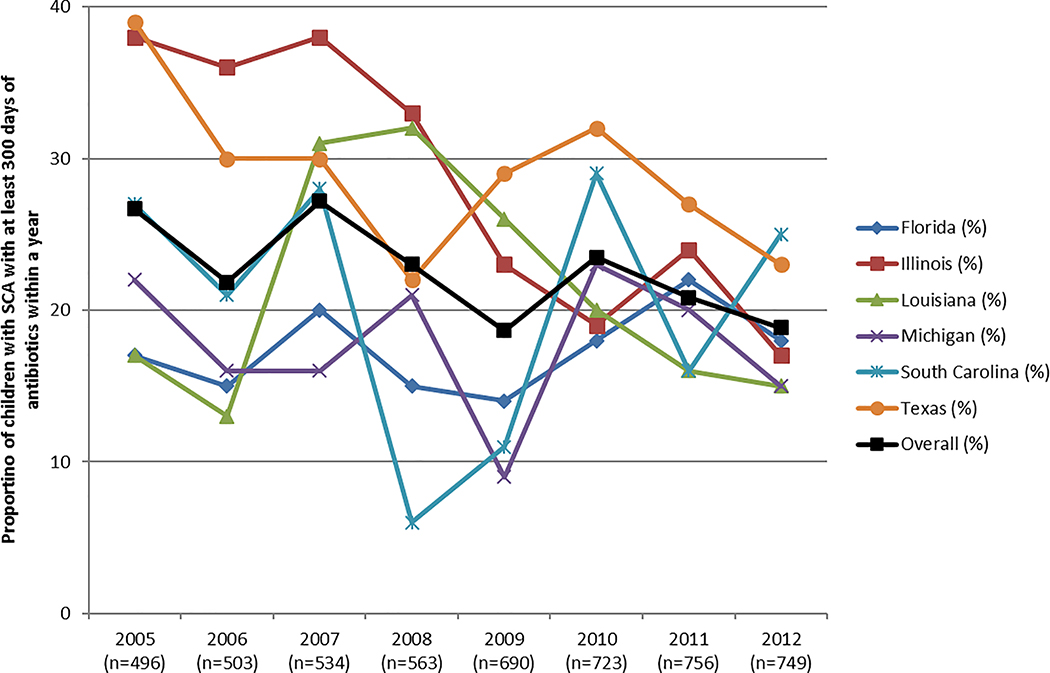
The proportion of children receiving ≥300 days of penicillin, erythromycin, or amoxicillin (Definition 3) varied by state (Figure 2). This proportion ranged from 19% (2009, 2012) to 27% (2005, 2007), with South Carolina having the lowest proportion of children with receiving ≥300 days of antibiotic prophylaxis at any time point (6% in 2009).
Figure 2.
Proportion of children with SCA with ≥300 days of filled antibiotics within a year. Antibiotics are defined as penicillin, erythromycin, or amoxicillin.
Overall, children in the study population had an annual mean of 1.7 SCD-related inpatient hospitalizations (SD=1.8), 13.2 SCD-related outpatient visits (SD=11.1), 3.8 ED visits (SD=3.4), and 1.6 well-child visits (SD=1.5) (Table 2). Bivariate analysis indicated that the number of sickle cell disease-related outpatient visits (OR=1.01, p<0.0001), well-child visits (OR=1.09, p=0.0008), ED visits (OR=1.05, p<0.0001), state of residence (ORs varied by state), and calendar year (ORs varied by year) were independently associated with receiving at least 300 days of antibiotics; age (OR=0.98, p=0.67) and number of SCD-related inpatient visits were not associated (OR=1.02, p=0.39). The final multivariable model indicated that the number of sickle cell disease-related outpatient visits, well-child visits, and state of residence continued to be associated with the outcome. Each additional well-child visit was associated with incrementally increased odds of receiving ≥300 days of antibiotics (OR=1.08, 95%CI: 1.02, 1.13), as was each additional sickle cell disease-related outpatient visit (OR=1.01, 95%CI: 1.01, 1.02). A child that was at the third quartile of sickle cell disease-related outpatient visits (17 annual visits) had 15% greater odds of receiving ≥300 days of antibiotics as a child in the first quartile of sickle cell disease-related outpatient visits (6 visits). The odds of receiving ≥300 days of antibiotics did not differ in any year as compared to 2005 (Table 3).
Table 2.
Annual Healthcare Utilization among Children ages 3 months - 5 years with Sickle Cell Anemia (n=5,014 person-years)*
| Type of Visit | Mean Number of Encounters (Standard Deviation) | Interquartile Range (25th, 75th) |
|---|---|---|
| SCD-related Inpatient | 1.7 (1.8) | 2 (0, 2) |
| SCD-related Outpatient | 13.2 (11.1) | 11 (6, 17) |
| Emergency Department | 3.8 (3.4) | 4 (1, 5) |
| Well Child Visits | 1.6 (1.5) | 2 (0, 2) |
Abbreviations: SCD, Sickle Cell Disease.
Study consisted of 2,281 individual children that could contribute multiple person years to the study population.
Table 3.
Multivariable Model Predicting Receipt of ≥300 days of penicillin, erythromycin, or amoxicillin among children with sickle cell anemia (n=5,014 person-years)*
| Variable | OR | Lower 95% CI | Upper 95% CI |
|---|---|---|---|
| Type of visit | |||
| Emergency department | 1.02 | 0.99 | 1.04 |
| SCD-related outpatient | 1.01 | 1.01 | 1.02 |
| Well-child | 1.08 | 1.03 | 1.14 |
| State | |||
| Florida | 0.51 | 0.39 | 0.68 |
| Illinois | 1.00 | Reference | Reference |
| Louisiana | 0.57 | 0.41 | 0.78 |
| Michigan | 0.60 | 0.42 | 0.85 |
| South Carolina | 0.62 | 0.43 | 0.89 |
| Texas | 1.01 | 0.76 | 1.35 |
| Year | |||
| 2005 | 1.00 | Reference | Reference |
| 2006 | 0.92 | 0.69 | 1.23 |
| 2007 | 1.21 | 0.91 | 1.60 |
| 2008 | 0.98 | 0.72 | 1.33 |
| 2009 | 0.80 | 0.59 | 1.09 |
| 2010 | 0.99 | 0.73 | 1.33 |
| 2011 | 0.98 | 0.73 | 1.31 |
| 2012 | 0.85 | 0.63 | 1.15 |
Abbreviations: OR, odds ratio; CI, Confidence Interval; SCD, Sickle Cell Disease.
2,821 children
Sensitivity analysis
A total of 286 person years (5.7%) had zero fills for penicillin, erythromycin, or amoxicillin. Further, only 544 person-years (10.8%) had pharmacy claims for fewer than 30 days’ supply of penicillin, erythromycin, or amoxicillin. Upon exclusion of these children, there were 4,470 person-years (89.2%) in the restricted population; 20.1% of these person-years had ≥300 days filled, compared to 17.9% in the full study population. There was no statistically significant difference in the proportion of children receiving ≥300 days of antibiotics as compared to the full study population in any year.
DISCUSSION
In this multi-state analysis, receipt of antibiotic prophylaxis among children with SCA is persistently low, irrespective of year or state. We found that the majority of children with SCA do not receive ≥300 days of antibiotics within a year, even when broadened definitions of antibiotic prophylaxis were considered. These findings are particularly troubling given the elevated risk and case fatality rate of IPD among children with SCA, even with the introduction of the pneumococcal conjugate vaccine.7,8,25 The methods applied in this study establish a framework in which to assess the proportion of children adequately protected against IPD, providing an important first step toward identifying opportunities for improvement.
Our findings indicate that a substantial gap exists between use of prophylactic antibiotics among children with SCA and NHLBI recommendations, which indicate penicillin prophylaxis until age 5.10,11 Although the NHLBI guidelines indicate that oral penicillin is the most appropriate prophylaxis against IPD,11 we reasoned that it was important to understand if children were protected with alternative antibiotics, as intervention approaches to increase rates of prophylaxis would depend on if children were adequately protected (even if by non-recommended methods), or if the children were not receiving any antibiotics to protect against IPD. However, even considering the broadest definition of antibiotic prophylaxis, fewer than one-third of children received ≥300 days of these antibiotics across the study period. Although these results are remarkably low, they are consistent with other studies of medication adherence among young pediatric populations enrolled in Medicaid, such as hydroxyurea therapy among children with SCA or asthma controllers among children with persistent asthma.26,27
Other studies of antibiotic prophylaxis in children with sickle cell disease report similar results, even when varying definitions of antibiotic prophylaxis and study populations are taken into consideration.12–14 In the Wisconsin Medicaid program, only 23% of children with SCA received penicillin and/or amoxicillin for 80% of the year (292 days).13 Although our results do not differ substantially from these studies, this study provides an updated benchmark for receipt of antibiotic prophylaxis among children with SCA. Children with SCA receive suboptimal preventive care in other areas as well. For example, approximately 30% of children with sickle cell disease have not received recommended pneumococcal conjugate vaccine by 59 months of age.28 Transcranial Doppler ultrasonography, recommended to identify children at a high risk of stroke, also has low rates among these states with only 45% of children screened annually. However, unlike our rates of antibiotic prophylaxis, which did not increase over time, transcranial Doppler screening rates increased from 2005–2012.24 Although policy differences may exist across states within this study, all children benefited from the Early and Periodic Screening, Diagnostic, and Treatment (EPSDT) program, which are federally mandated to provide robust Medicaid benefits to children. Although state was included as an independent variable in our models, it is possible that differences between states in the availability and accessibility to healthcare services could contribute to the variation seen across time within states.
Given the consistent finding that antibiotic prophylaxis rates are low among children with SCA, development of practical and effective interventions are key. These interventions should be focused at both the provider and the patient/parent level to ensure a more comprehensive approach to reducing the barriers associated with antibiotic prophylaxis among this population.25 Provider-focused strategies to increase adherence could capitalize on the numerous annual outpatient encounters with the healthcare system that children with SCA are already experiencing.24,29 For example, previously successful interventions to increase medication adherence within healthcare encounters have targeted physician prescribing habits and provider-led adherence promotion.30,31 Previous research has also indicated that primary care physicians have a lower level of self-efficacy and knowledge in the provision of preventive care of children with SCA. As such, education of primary care physicians in the importance of these preventive services is necessary.32 Patient/parent-focused interventions may be most effective when focused on family and social factors that may impact receipt of filled prescriptions at the pharmacy and administration of the antibiotics to the child. For example, the typical formulation of prophylactic antibiotics requires that refills be obtained frequently given the limited shelf life.33 Even though none of the state Medicaid programs included in this analysis require a co-payment for pediatric prescriptions, social factors such as the availability of transportation to pharmacies, and the time required to pick up medications, may be a significant barrier to families since numerous trips to the pharmacy each year are required for refills.34,35 Families with SCA already face a substantial burden of care, which is coupled with challenges of administering daily antibiotics to a young child that may by all outward signs appear quite healthy. Therefore, assessment of the knowledge and perceptions regarding the risk of IPD among caregivers, particularly after introduction of the pneumococcal conjugate vaccine, may provide key information for focused interventions to increase administration of antibiotics.36,37
There are several limitations to this study. First, the presence of a filled antibiotic prescription does not necessarily indicate that the medication was actually administered to the patient for whom it was prescribed. This limitation suggests that our results may be an overestimate of the true proportion of children with SCA protected against IPD. Second, our study population consisted of children with at least 3 annual claims for SCA; although this definition had a high sensitivity and specificity for identifying cases, children with less interaction with the healthcare system would not be included. We anticipate these children would also be less likely to receive ≥300 days of antibiotics within a year, indicating another overestimation of the true rates. Third, the use of administrative data to assess quality of care among children with SCA is advantageous given the broad potential for application and low cost. As with other administrative claims methods that are commonly used in quality of care assessments, these methods are subject to the limitations of coding accuracy and claims completeness.38 However, our administrative claims-based SCA case definition was previously validated using newborn screening as a gold standard, demonstrating a high degree of accuracy in Michigan.17 We would expect this definition to perform similarly across the US due to similar claims-based definitions to identify children with SCA in other states, as well as similarly high levels of healthcare utilization across states.17,24,39–42 Fourth, although our data was only complete through 2012, we do not expect that care has improved markedly since that time, absent a coordinated and directed quality improvement program. Finally, we were unable to ascertain pneumococcal vaccination coverage among our study population, which may provide additional protection against IPD among this population.
Conclusion
Despite long-standing national recommendations, antibiotic prophylaxis against IPD remains low among children with SCA, and efforts aimed at increasing adherence are urgently needed. It is unknown which mechanisms will be the most effective; however, numerous healthcare encounters may offer an opportunity for intervention. In addition, such interventions likely need to include social factors that may affect the ability for a child to receive and adhere to antibiotic prophylaxis.
Supplementary Material
What’s Known on This Subject
Children with sickle cell anemia are at substantially increased risk for invasive pneumococcal disease; daily antibiotic prophylaxis until the age of 5 significantly reduces this risk.
What This Study Adds
We assessed rates and predictors of antibiotic prophylaxis among children with sickle cell anemia. In doing so, our goal was to characterize opportunities for intervention to increase rates of antibiotic prophylaxis among this high-risk population.
Acknowledgments
Funding Source: All phases of this study were supported by the Agency for Healthcare Research and Quality (AHRQ) and the Centers for Medicare & Medicaid Services (CMS) under the CHIPRA Pediatric Quality Measures Program Centers of Excellence grant number U18 HS020516.
Abbreviations
- ED
Emergency Department
- GEE
Generalized Estimating Equations
- Hb
Hemoglobin
- IPD
Invasive Pneumococcal Disease
- IQR
Interquartile Range
- MAX
Medicaid Analytic eXtract
- NDC
National Drug Code
- NHLBI
National Heart, Lung, and Blood Institute
- OR
Odds Ratio
- SCA
Sickle Cell Anemia
- SD
Standard Deviation
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
Financial Disclosure: All authors have no financial relationships relevant to this article to disclose.
References
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512–521. [DOI] [PubMed] [Google Scholar]
- 2.Berg AO. Sickle cell disease: screening, diagnosis, management, and counseling in newborns and infants. The Agency for Health Care Policy and Research. J Am Board Fam Pract. 1994;7(2):134–140. [PubMed] [Google Scholar]
- 3.Lorey FW, Arnopp J, Cunningham GC. Distribution of hemoglobinopathy variants by ethnicity in a multiethnic state. Genet Epidemiol. 1996;13(5):501–512. [DOI] [PubMed] [Google Scholar]
- 4.Michlitsch J, Azimi M, Hoppe C, et al. Newborn screening for hemoglobinopathies in California. Pediatr Blood Cancer. 2009;52(4):486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364(9442):1343–1360. [DOI] [PubMed] [Google Scholar]
- 6.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359(21):2254–2265. [DOI] [PubMed] [Google Scholar]
- 7.Overturf GD, Powars D, Baraff LJ. Bacterial meningitis and septicemia in sickle cell disease. Am J Dis Child. 1977;131(7):784–787. [DOI] [PubMed] [Google Scholar]
- 8.Sabarense AP, Lima GO, Silva LM, Viana MB. Characterization of mortality in children with sickle cell disease diagnosed through the Newborn Screening Program. J Pediatr (Rio J). 2015;91(3):242–247. [DOI] [PubMed] [Google Scholar]
- 9.Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314(25):1593–1599. [DOI] [PubMed] [Google Scholar]
- 10.National Heart Lung and Blood Institute. The Management of Sickle Cell Disease. 2002; http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf. Accessed 11/19, 2014.
- 11.National Heart Lung and Blood Institute. Evidence Based Management of Sickle Cell Disease. 2014; http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/sickle-cell-disease-report.pdf. Accessed 11/11, 2014.
- 12.Sox CM, Cooper WO, Koepsell TD, DiGiuseppe DL, Christakis DA. Provision of pneumococcal prophylaxis for publicly insured children with sickle cell disease. JAMA. 2003;290(8):1057–1061. [DOI] [PubMed] [Google Scholar]
- 13.Beverung LM, Brousseau D, Hoffmann RG, Yan K, Panepinto JA. Ambulatory quality indicators to prevent infection in sickle cell disease. Am J Hematol. 2014;89(3):256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witherspoon D, Drotar D. Correlates of Adherence to Prophylactic Penicillin Therapy in Children With Sickle Cell Disease. Child Health Care. 2006;35(4):281–296. [Google Scholar]
- 15.Centers for Medicare and Medicaid Services. http://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/MAXGeneralInformation.html. Accessed 10/22, 2013.
- 16.Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol.85(1):77–78. [DOI] [PubMed] [Google Scholar]
- 17.Reeves S, Garcia E, Kleyn M, et al. Identifying sickle cell disease cases using administrative claims. Acad Pediatr. 2014;14(5 Suppl):S61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Critchley IA, Brown Sd Fau - Traczewski MM, Traczewski Mm Fau - Tillotson GS, Tillotson Gs Fau - Janjic N, Janjic N. National and regional assessment of antimicrobial resistance among community-acquired respiratory tract pathogens identified in a 2005–2006 U.S. Faropenem surveillance study. (0066–4804 (Print)). [DOI] [PMC free article] [PubMed]
- 19.Doern GV, Heilmann Kp Fau - Huynh HK, Huynh Hk Fau - Rhomberg PR, Rhomberg Pr Fau - Coffman SL, Coffman Sl Fau - Brueggemann AB, Brueggemann AB. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999−-2000, including a comparison of resistance rates since 1994−-1995. (0066–4804 (Print)). [DOI] [PMC free article] [PubMed]
- 20.Fritsche TR, Biedenbach Dj Fau - Jones RN, Jones RN. Update of the activity of cefditoren and comparator oral beta-lactam agents tested against community-acquired Streptococcus pneumoniae isolates (USA, 2004–2006). (1973–9478 (Electronic; )). [DOI] [PubMed] [Google Scholar]
- 21.Harrison CJ, Woods C Fau - Stout G, Stout G Fau - Martin B, Martin B Fau - Selvarangan R, Selvarangan R. Susceptibilities of Haemophilus influenzae, Streptococcus pneumoniae, including serotype 19A, and Moraxella catarrhalis paediatric isolates from 2005 to 2007 to commonly used antibiotics. (1460–2091 (Electronic; )). [DOI] [PubMed] [Google Scholar]
- 22.Jacobs MR, Good Ce Fau - Windau AR, Windau Ar Fau - Bajaksouzian S, et al. Activity of ceftaroline against recent emerging serotypes of Streptococcus pneumoniae in the United States. (1098–6596 (Electronic; )). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves SLMB, Shevrin CA, McCormick J, Freed GL, Dombkowski KJ. Anticipatory Guidance Regarding Hydroxyurea Treatment for Children with Sickle Cell Disease.. 2017; www.qualityforum.org.
- 24.Reeves SL, Madden B, Freed GL, Dombkowski KJ. Transcranial Doppler Screening Among Children and Adolescents With Sickle Cell Anemia. JAMA pediatrics. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yildirim I, Shea KM, Little BA, Silverio AL, Pelton SI. Vaccination, underlying comorbidities, and risk of invasive pneumococcal disease. Pediatrics. 2015;135(3):495–503. [DOI] [PubMed] [Google Scholar]
- 26.Anders DG, Tang F, Ledneva T, et al. Hydroxyurea Use in Young Children With Sickle Cell Anemia in New York State. Am J Prev Med. 2016;51(1 Suppl 1):S31–38. [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein JA, Lozano P, Farber HJ, Miroshnik I, Lieu TA. Underuse of controller medications among Medicaid-insured children with asthma. Arch Pediatr Adolesc Med. 2002;156(6):562–567. [DOI] [PubMed] [Google Scholar]
- 28.Nero AC, Akuete K, Reeves SL, Dombkowski KJ. Pneumococcal Vaccination Rates in Children With Sickle Cell Disease. J Public Health Manag Pract. 2014;20(6):587–590. [DOI] [PubMed] [Google Scholar]
- 29.Raphael JL, Dietrich CL, Whitmire D, Mahoney DH, Mueller BU, Giardino AP. Healthcare utilization and expenditures for low income children with sickle cell disease. Pediatr Blood Cancer. 2009;52(2):263–267. [DOI] [PubMed] [Google Scholar]
- 30.Shah S, Sawyer SM, Toelle BG, et al. Improving paediatric asthma outcomes in primary health care: a randomised controlled trial. Med J Aust. 2011;195(7):405–409. [DOI] [PubMed] [Google Scholar]
- 31.Wu YP, Pai AL. Health care provider-delivered adherence promotion interventions: a meta-analysis. Pediatrics. 2014;133(6):e1698–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeves SL, Fullerton HJ, Dombkowski KJ, Boulton ML, Braun TM, Lisabeth LD. Physician Attitude, Awareness, and Knowledge Regarding Guidelines for Transcranial Doppler Screening in Sickle Cell Disease. Clin Pediatr (Phila). 2014. [DOI] [PubMed] [Google Scholar]
- 33.US National Libraries of Medicine. Penicillin (Oral route, Injection route, Intravenous route, Intramuscular route). 2017; https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0011640/?report=details. Accessed 10/15, 2017.
- 34.Elliott V, Morgan S, Day S, Mollerup LS, Wang W. Parental health beliefs and compliance with prophylactic penicillin administration in children with sickle cell disease. J Pediatr Hematol Oncol. 2001;23(2):112–116. [DOI] [PubMed] [Google Scholar]
- 35.Patel NG, Lindsey T, Strunk RC, DeBaun MR. Prevalence of daily medication adherence among children with sickle cell disease: a 1-year retrospective cohort analysis. Pediatr Blood Cancer. 2010;55(3):554–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treadwell MJ, McClough L, Vichinsky E. Using qualitative and quantitative strategies to evaluate knowledge and perceptions about sickle cell disease and sickle cell trait. J Natl Med Assoc. 2006;98(5):704–710. [PMC free article] [PubMed] [Google Scholar]
- 37.Reeves SL, Braun TM, Dombkowski KJ, Fullerton HJ, Boulton ML, Lisabeth LD. The Role of Neighborhoods in the Receipt of Transcranial Doppler Screening Among Children With Sickle Cell Disease. J Pediatr Hematol Oncol. 2015;37(4):269–273. [DOI] [PubMed] [Google Scholar]
- 38.Grosse SD, Boulet SL, Amendah DD, Oyeku SO. Administrative Data Sets and Health Services Research on Hemoglobinopathies: A Review of the Literature. Am J Prev Med. 2010;38(4, Supplement):S557–S567. [DOI] [PubMed] [Google Scholar]
- 39.Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44(11):1428–1433. [DOI] [PubMed] [Google Scholar]
- 40.Raphael JL, Dietrich CL, Whitmire D, Mahoney DH, Mueller BU, Giardino AP. Healthcare utilization and expenditures for low income children with sickle cell disease. Pediatr Blood Cancer. 2009;52(2):263–267. [DOI] [PubMed] [Google Scholar]
- 41.Amendah DD, Mvundura M, Kavanagh PL, Sprinz PG, Grosse SD. Sickle cell disease-related pediatric medical expenditures in the U.S. Am J Prev Med. 2010;38(4 Suppl):S550–556. [DOI] [PubMed] [Google Scholar]
- 42.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288–1294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.