Abstract
This study aimed to identify potential therapeutic targets in osteosarcoma (OS) through the network analysis of competing endogenous RNAs (ceRNAs). The differentially expressed miRNAs (DEMIs) and mRNAs (DEMs) were identified between OS cell lines and human mesenchymal stem cells (hMSCs) from the data deposited under GSE70415 using limma package. Functional analysis of DEMs was performed using DAVID and clusterProfiler to identify significantly enriched Gene Ontology biological processes and KEGG pathways, respectively. The DEMI-DEM interaction network was constructed using Cytoscape. LncRNA–miRNA interactions were predicted using starBase database. The ceRNA regulatory network was constructed by integrating mRNAs, miRNAs, and lncRNAs, and functional enrichment analysis was performed for the genes involved. The analysis revealed a total of 326 DEMs and 54 DEMIs between OS cells and hMSCs. We identified several novel therapeutic targets involved in the progression and metastasis of OS, such as CBX7, RAD9A, SNHG7 and miR-34a-5p. The miRNA, miR-543 (target gene: CBX7) was found to be associated with the pathway Mucin type O-glycan biosynthesis. Using the ceRNA network, we established the following regulatory interactions: NEAT1/miR-543/CBX7, SNHG7/miR-34a-5p/RAD9A, and XIST/miR-34a-5p/RAD9A. CBX7, RAD9A, lncRNA SNHG7, miR-543, and miR-34a-5p may be explored as novel therapeutic targets for treatment of OS.
Keywords: Osteosarcoma, differentially expression mRNA, differentially expression miRNA, competing endogenous RNAs network, lncRNA
Introduction
Osteosarcoma (OS) is the most commonly diagnosed primary malignant bone tumor in adolescents (He et al., 2014).The age-adjusted worldwide incidences per million of OS in the age group of 0-24 is 4.4, with the incidence rate in males being more than females in every race (male:female = 1.34:1) (Mirabello et al., 2009). Though the 5-year overall survival rate for OS is 68% (Ottaviani and Jaffe, 2009), it is highly metastatic (Marina et al., 2004), with lung being the most common site of metastasis (Fitzgerald et al., 1973). The complex molecular mechanisms associated with progression and metastasis of OS is not clear and is challenging to treat. Hence, it is important to identify the underlying molecular mechanisms associated with the development and metastasis of OS and identify better therapeutic targets for its treatment.
Long non-coding RNAs (lncRNAs), a class of nonprotein-coding RNA transcripts longer than 200 nucleotides (Morris and Mattick, 2014), are known to be involved in many biological processes, such as transcriptional regulation, cell proliferation, metastasis and tumorigenesis (Tang et al., 2016; Wei et al., 2016). Earlier studies have implicated that lncRNAs may be important factors in the malignant transformation of the tumors, and can be considered as novel biomarkers (Yarmishyn and Kurochkin, 2015). Similarly, micro RNAs (miRNAs), a class of short non-coding RNAs, play important regulatory roles in many biological processes, such as cell migration, apoptosis, cell differentiation and oncogenesis, by targeting the mRNA molecules (Wang et al., 2015). It is well known that lncRNAs act as competing endogenous RNA (ceRNA) and affect the level of mRNAs by negatively regulating miRNA expression (Salmena et al., 2011; Liu et al., 2014; Cao et al., 2015; Wang et al., 2016). For example, the lncRNA TUG1 acts as a ceRNA for miR-335-5p and promotes OS cells migration and invasion (Yong et al., 2017). Uzan et al. reported that high expression of the lncRNA HULC promotes metastasis and is associated with poor prognosis of OS (Uzan et al., 2016). The up-regulated lncRNA HNF1A-AS1 promotes proliferation and metastasis of OS cells by activating Wnt/catenin signaling pathway (Zhao H et al., 2016a). However, the complex mechanisms associated with metastasis of OS has not been fully understood. The integrative analysis can help us in better understanding of related genes, functions, and the complex mechanisms associated with the development and metastasis of OS.
The aim of the present study is to understand the molecular mechanisms by exploring the regulatory interactions through ceRNA regulatory network, and identify potential therapeutic targets in OS using bioinformatics analysis.
Material and Methods
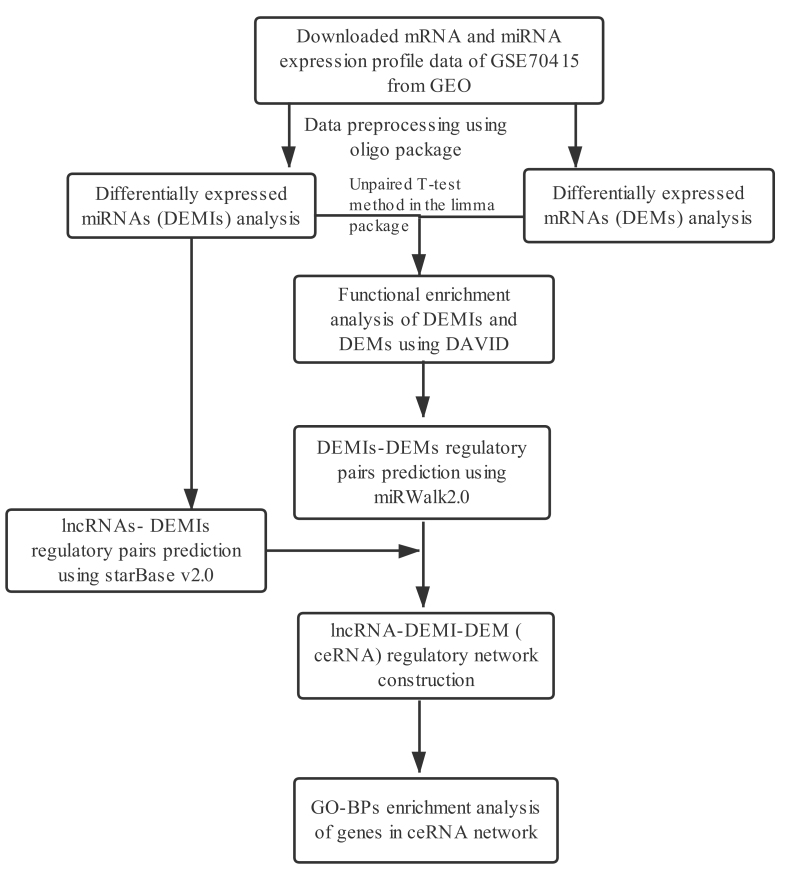
The flow of the analysis steps used in this study is shown in Figure 1.
Figure 1. Overall strategy and analyses followed in the current study.
Data source and preprocessing
The mRNA and miRNA expression profiles were downloaded from the Gene Expression Omnibus (GEO) series GSE70415 (Wang H et al., 2017a). This series included two subseries: GSE70414 and GSE70367 corresponding to mRNA and miRNA expression data, respectively. The original study used five OS cell lines and t human mesenchymal stem cells, (hMSCs) as control. The mRNA expression data was generated using Affymetrix Human Genome U133 Plus 2.0 (HG-U133_Plus_2) array (GPL570), and the miRNA expression data was generated using Affymetrix Multispecies miRNA-3 (miRNA-3) array (GPL16384). The original study published by Wang et al. (2017a) corresponding to the data deposited in GSE70415, mainly focused on miRNA-mRNA regulatory interactions, however, the interactions between lncRNAs and miRNAs were studied.
The R-based oligo package (Irizarry et al., 2003) was used for data preprocessing, including background correction, normalization and summarization (median-polish). The Robust Multichip Average (RMA) method in oligo package was used to implement background correction with treating the perfect-match probe intensities as a convolution of noise and true signal, followed by normalization. The annotation file was used to map the probes in the mRNA matrix to gene symbols. When multiple probes corresponded to the same gene symbol, summarization at the probeset level was performed, and the mean expression values across multiple probe-sets were considered as the final expression value for that gene. Since the miRNA platform was composed of multi-species probes, the expression values of probes corresponding to human miRNA were selected for further analysis.
Identification of differentially expressed mRNAs and miRNAs between OS cell lines and hMSCs
The differentially expressed miRNAs (DEMIs) and mRNAs (DEMs) were identified using the unpaired T-test method implemented in limma package (Smyth, 2005). The DEMIs and DEMs with a P-value < 0.05 and log2 fold change (log2FC) > 1 were considered as statistically significant. The heatmaps and volcano plots of the significant DEMIs and DEMs were constructed using the R-based pheatmap (Wang et al., 2014) and ggplot2 (Warnes et al., 2009) software packages, respectively.
Regulatory pair prediction and functional enrichment analysis of DEMIs and DEMs
The DEMI probes were annotated using miRBase version 21 to identify the corresponding miRNAs (updated list of miRNAs using miRBase version 22.1 are shown in Table S1). The mRNA targets for the top (based on log2FC) 10 up- and down-regulated DEMIs were predicted using miRWalk2.0 tool (Dweep and Gretz, 2015). Further, the miRNA-target pairs showing consensus across five prediction methods integrated in miRWalk (miRanda, miRDB, miRMap, RNA22, and TargetScan) were considered as the for further analysis. Among the DEMI targets, only those that overlapped with the DEMs were considered for further analysis.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) (Ogata et al., 1999) pathways enriched by the miRNA targeted DEMs were analyzed using the cluster-profiler package in R software (Yu et al., 2012). The KEGG pathways with adjusted P-value < 0.05 were considered as statistically significant. Database for Annotation, Visualization and Integrated Discovery (DAVID, version 6.8) (Huang et al., 2008) was used to obtain enriched Gene Ontology biological processes (GO-BPs) (Ashburner et al., 2000) for the DEMs targeted by the DEMIs. The GO-BP terms with a gene count ≥ 2 and P-value < 0.05 were considered as significantly enriched.
Construction of DEMI-DEM network
The regulatory network between DEMIs and DEMs was constructed by using the Cytoscape software, version 3.2.0 (Shannon et al., 2003). The topological properties of the network were analyzed, and the results were presented with the Degree Centrality (DC). The nodes with higher DC scores were considered as hub proteins.
Construction and functional enrichment analysis of genes involved in lncRNA-miRNA-mRNA regulatory network
starBase database provides the most comprehensive CLIP-Seq experimentally supported lncRNA-miRNA interactions. It contains 35,459 human miRNA-lncRNA interactions (Li et al., 2014). The regulatory interactions between the lncRNAs and differentially expressed miRNAs were extracted from the starBase database based on the following criteria: medium stringency ≥ 2; number of cancer types ≥ 1; clade: mammal; genome: Human; assembly: hg19.
Using the lncRNA-DEMI and DEMI-DEM regulatory interactions, the lncRNA and DEM that were regulated by the same DEMI were identified, and were used for the construction of lncRNA-DEMI-DEM (lncRNA-miRNA-mRNA) regulatory network, also known as ceRNA regulatory network.
The enrichment analysis of the genes involved in ceRNA network was performed to obtain GO-BP and annotations with a P-value < 0.05 and gene count ≥ 2 were considered for further analysis.
Results
Identification of DEMs and DEMIs between OS cell lines and hMSCs
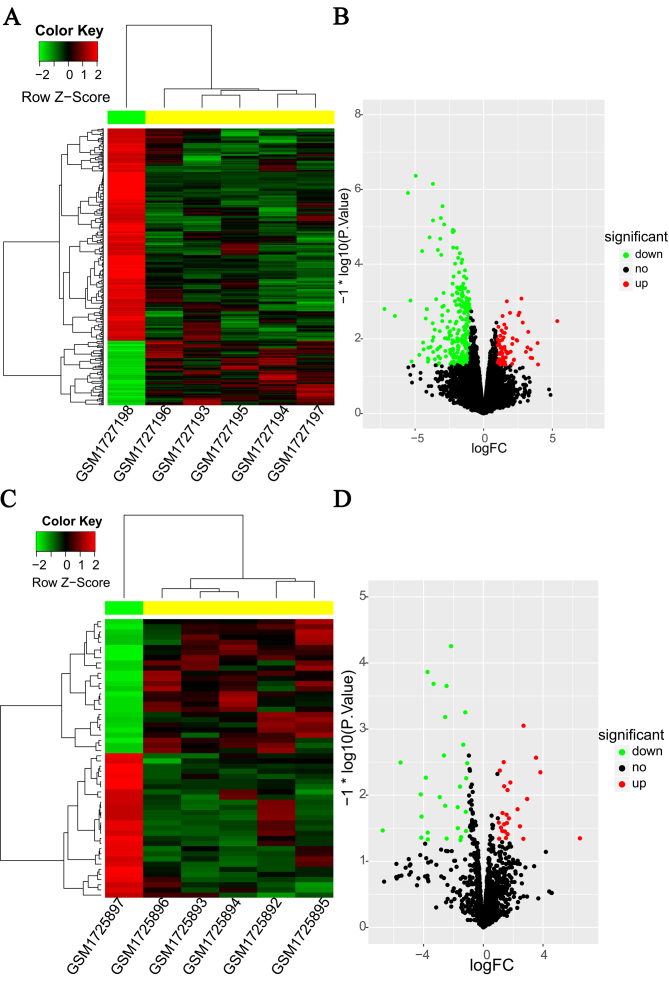
The DEMs and DEMIs between OS cell lines and hMSCs were derived using the microarray dataset GSE70414. A total of 326 DEMs (Table S2), including 76 up- and 250 down-regulated were identified, and are shown in the double-level clustering heatmap (Figure 2A) and volcano plot (Figure 2B). The heatmap shows independent clustering of OS cell lines and hMSCs. Similarly, a total of 54 DEMIs, including 26 up- and 28 down-regulated (Figure 2C & D) were identified. The complete list of DEMs and DEMIs can be found in Table S1 and Table S3, respectively.
Figure 2. Heatmap and volcano plot of differentially expressed mRNAs (A & B, respectively) and miRNAs (C & D, respectively) between osteosarcoma (OS) cell lines and human mesenchymal stem cells (hMSCs). In the heatmap, the red color indicates up-regulation, and the green color indicates down-regulation.
Functional enrichment analysis of DEMs and DEMIs
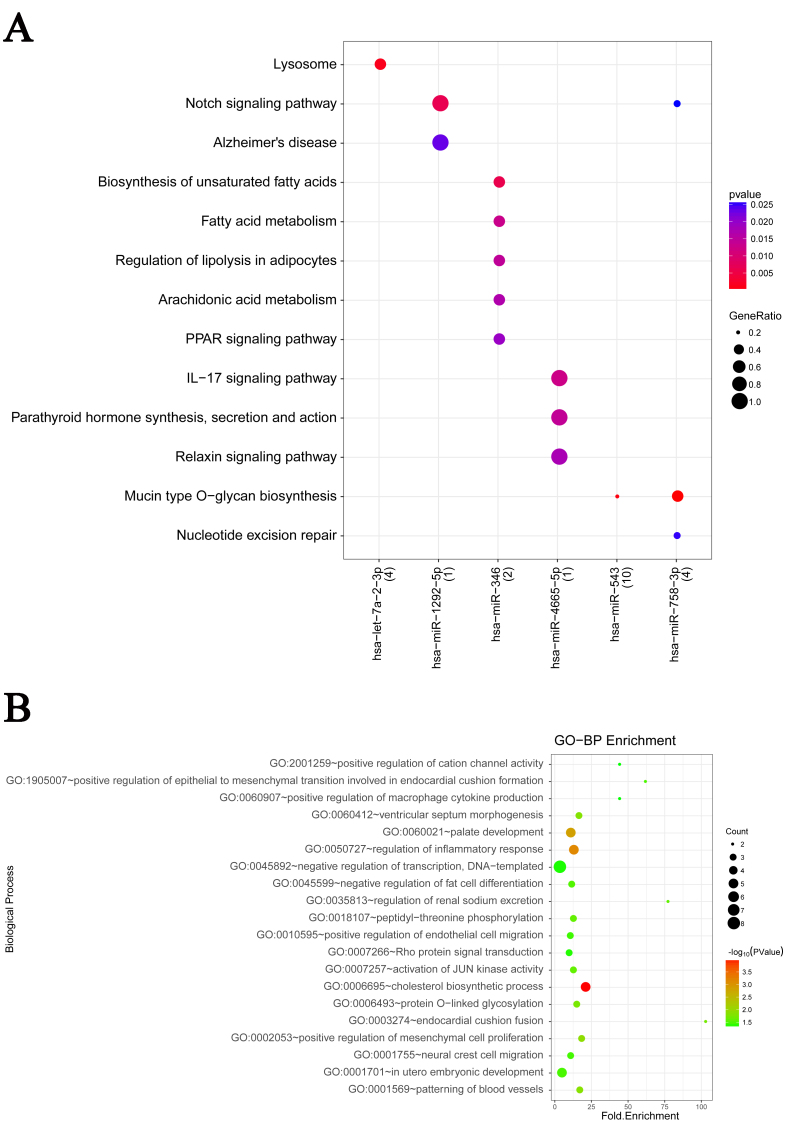
Functional enrichment analysis was performed to obtain GO-BPs and pathways influencing the development of OS. As shown in Figure 3A, a total of six miRNAs were found to be significantly enriched in 18 KEGG pathways. For example, has-miR-543 was found to be associated with “Mucin type O-glycan biosynthesis” pathway. The GO-BP enrichment analysis showed RPS6KA5 to be significantly related to “in utero embryonic development (GO: 0001701)”, EDNRA to be significantly related to “negative regulation of transcription, DNA-templated (GO: 0045892)” and TNFSF4 to be significantly related to “regulation of inflammatory response (GO: 0050727)”, Figure 3B.
Figure 3. Functional enrichment analysis of differentially expressed mRNAs and miRNAs between osteosarcoma (OS) cell lines and human mesenchymal stem cells (hMSCs). A: representative enriched pathways by DEMIs; B: representative enriched biological processes by DEMs.
Analysis of DEMI-DEM regulatory network
For the 54 DEMIs obtained from the microarray analysis, potential target genes consistent across multiple target prediction tools. The interactions between the target DEMIs and DEMs were used to construct a miRNA-gene regulatory network (e.g., has-miR-543-CBX7, miR-34a-5p-RAD9A and has-miR-495-3p-DGKD. The DEMI-DEM regulatory network (Figure 4), had a total of 139 nodes and 238 interactions (edges). Top five miRNAs (miR-495-3p, miR-543, miR-34a-5p, miR-182-5p and miR-760) and genes (EPG5, ADAMTSL1, CDC42EP3, NEDD4L, and PTGS1) identified based on the degree ranking are shown in Table 1.
Figure 4. Regulatory network showing mRNA-miRNA interactions. Pink circles: Up-regulated mRNA;Light green diamonds: Down-regulated mRNA; the Red triangles: Up-regulated miRNA; Green arrows: Down-regulated miRNA.
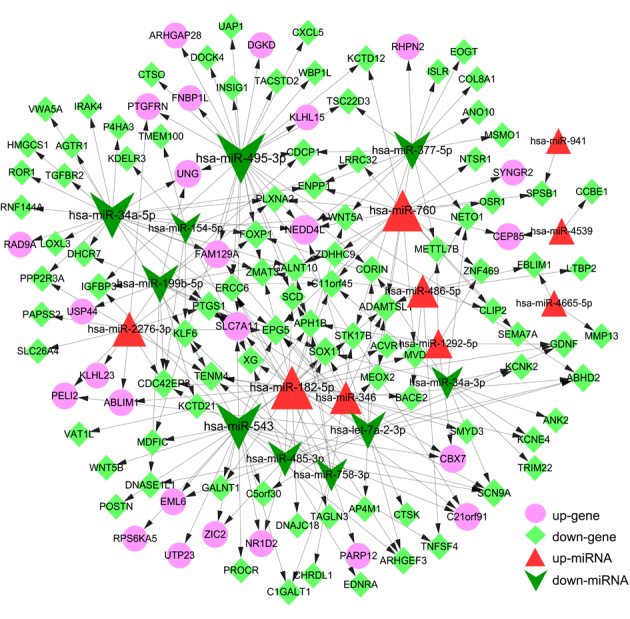
Table 1. The top 5 differentially expressed miRNAs and differentially expressed genes between osteosarcoma cells and human mesenchymal stem cells .
| miRNA | Description | Degree | Gene | Description | Degree |
|---|---|---|---|---|---|
| hsa-miR-495-3p | down | 29 | EPG5 | down | 6 |
| hsa-miR-543 | down | 25 | NEDD4L | up | 5 |
| hsa-miR-34a-5p | down | 24 | ADAMTSL1 | down | 5 |
| hsa-miR-182-5p | up | 21 | CDC42EP3 | down | 5 |
| hsa-miR-760 | up | 19 | PTGS1 | down | 5 |
miRNAs, microRNAs.
Analysis of ceRNA regulatory network
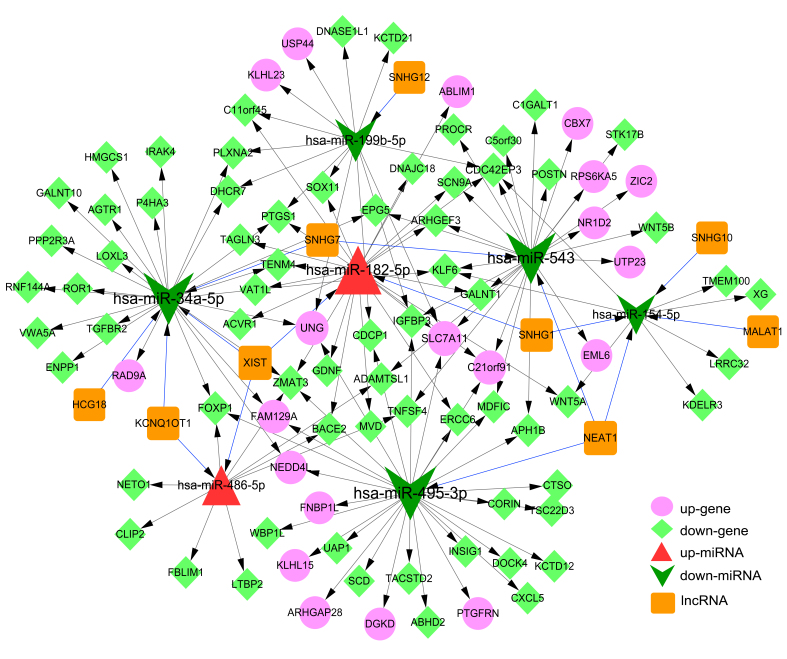
To obtain miRNA and lncRNA interactions, the lncRNAs regulating 19 miRNAs present in the DEMI-DEM network were predicted (Supplemental File S1). The miRNA-lncRNA interaction network consisted of 16 nodes, including seven miRNAs and nine lncRNAs. Examples of lncRNA-miRNA interactions include NEAT1-has-miR-543, SNHG7-has-miR-34a, and XIST-miR-34a-5p. Finally, the lncRNA-miRNA and miRNA-gene regulatory networks were merged to obtain the integrated ceRNA regulatory network. The ceRNA network had 145 pair-wise interactions among 105 nodes, consisting of two up-regulated and five down-regulated miRNAs, 20 up-regulated and 69 down-regulated DEMs, and nine lncRNAs (Figure 5 and Table S4). Top five miRNAs, mRNAs and lncRNAs in the network are listed in Table 2.
Figure 5. Competing-endogenous RNA regulatory network. Pink circles: Up-regulated mRNA; Light green diamonds: Down-regulated mRNA; Red triangles: Up-regulated miRNA; Green arrows: Down-regulated miRNA; Orange squares: LncRNA. Blue lines (edges): LncRNA-miRNA interactions; Gray lines (edges): miRNA-mRNA interactions.
Table 2. The top 5 miRNAs, lncRNAs, and mRNAs in ceRNA regulatory network .
| miRNA | Description | Degree | Gene | Description | Degree | LncRNA | Degree |
|---|---|---|---|---|---|---|---|
| hsa-miR-495-3p | down | 30 | CDC42EP3 | down | 4 | NEAT1 | 3 |
| hsa-miR-34a-5p | down | 28 | SLC7A11 | up | 4 | XIST | 3 |
| hsa-miR-543 | down | 27 | ZMAT3 | down | 4 | SNHG1 | 2 |
| hsa-miR-182-5p | up | 23 | KLF6 | down | 3 | SNHG7 | 2 |
| hsa-miR-199b-5p | down | 15 | C2lorf91 | up | 3 | KCNQ1OT1 | 2 |
lncRNA, long non-coding RNA; ceRNA, competing endogenous RNAs.
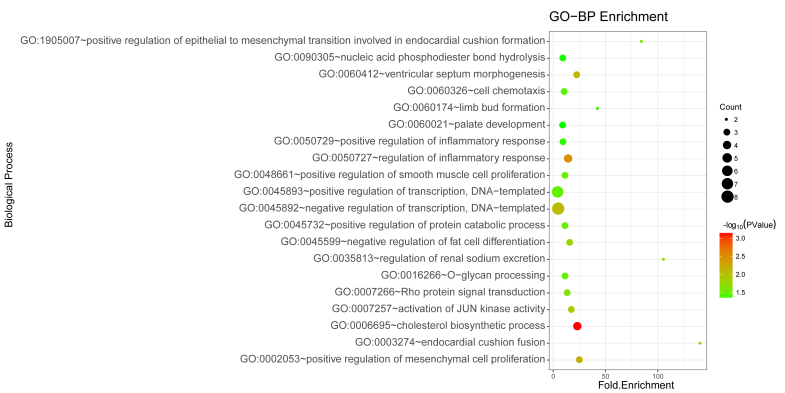
Function enrichment analysis of the network identified 20 significant GO-BP annotations. As shown in Figure 6, the DEMs were found to be significantly related to “positive regulation of transcription, DNA-templated (GO: 0045893)” as well as “negative regulation of transcription, DNA-templated (GO: 0045892)”.
Figure 6. Enrichment of Gene ontology biological processes by differentially expressed mRNAs involved in the ceRNA regulatory network. Count represents the number of genes associated with the annotation .
Discussion
In the current study, a total of 326 DEMs and 54 DEMIs were identified by re-analyzing the dataset, GSE70415 using limma package. Several potential targets that may be involved in the progression and metastasis of OS were identified, e.g., CBX7, RAD9A, SNHG7 (lncRNA) and has-miR-34a-5p. In addition, few potential regulatory interactions, such as NEAT1/ miR-543, miR-543/CBX7, SNHG7/ miR-34a-5p, miR-34a-5p/RAD9A, and XIST/miR-34a-5p were identified in the OS cells through the ceRNA network. The dataset GSE70415 had been analyzed in a previous study (Wang H et al., 2017a); and a total of 3,856 DEMs and 250 DEMIs were identified by using GCBI. When compared, the results were found to be similar between the earlier and current study. For example, the key gene POSTN discussed in the earlier study was also found to be highly downregulated in our study. Similarly, the miRNAs, miR-34a, miR-182, miR-493 and miR-29 were found to be differentially expressed in the previous study. However, there were significant differences observed between the current and the previous study. For instance, the GO annotations including extracellular matrix organization, small molecule metabolic process, cell adhesion, and KEGG pathways including PI3K-Akt signaling pathway and metabolic pathways were found to be significantly enriched in the previous study, however not in the current study. One of the reasons behind this could be the method used for analysis. Additionally, we performed lncRNA and ceRNA network analysis, which was not performed by the previous study.
Nuclear enriched abundant transcript 1 (NEAT1) is a lncRNA transcribed from the multiple endocrine neoplasia locus. Study by Zhao et al., have shown that NEAT1 acts as an oncogene and is associated with the development of OS (Zhao H et al., 2016b). Knockdown of NEAT1 is known to inhibit tumor cells proliferation and metastases and induce cell apoptosis (Wang H et al., 2017b). In addition, it promotes oncogenic proliferation by affecting the epigenetic structure of target gene promoters and driving their transcription in prostate cancer (Chakravarty et al., 2014). In the current study, miR-543 was predicted to be a target of NEAT1. The molecular mechanism of this interaction has not been characterized in the progression of OS. The expression of miR-543 inhibits epithelial-mesenchymal transition during tumor metastasis (Haga and Phinney, 2012). Over-expression of miR-543 inhibits tumor cell proliferation and has been reported to reduce the migration and invasion of tumor cells in endometrial cancer (Bing et al., 2014). Based on the published studies and findings from the current study, it can be hypothesized that the lncRNA NEAT1 promotes proliferation and metastasis of OS cells by sponging the miRNA, miR-543.
Chromobox 7 (CBX7, also known as chromobox homolog 7), a polycomb family protein, and a component of the polycomb repressive complex 1, is known to extend the lifespan of some normal human cells. Reportedly, the over-expressed CBX7 acts as an oncogene in the gastric cancer (Zhang et al., 2010). Further, studies have shown high expression of CBX7 in various prostate cancer cell lines (Bernard et al., 2005) and clear cell adenocarcinoma of the ovary (Shinjo et al., 2014). In addition, another member of the polycomb family, chromobox 4 (CBX4, also known as chromobox homolog 4) is known to be involved in progression of OS, and over-expression of CBX4 is associated with advanced clinical stage of OS (Yang et al., 2016). However, the role of CBX7 in OS progression has been not reported. Based on the existing evidences for CBX4, we presume that CBX7 may act as an oncogene during the development of OS. In addition, based on lncRNA regulatory interactions, we believe that the lncRNA NEAT1 may affect the expression of CBX7 by competing for miR-543 during the progression of OS.
Small nucleolar RNA host gene 7 (SNHG7) belongs to the long non-coding RNA class. Reportedly, SNHG7 contributes to the growth and metastasis of glioblastoma by suppressing miR-5059 and activating the wnt/β-catenin signaling pathway (Ren et al., 2018). Additionally, in prostate cancer, SNHG7 promotes cell proliferation via cyclin D1 by modulating miR-503 (Qi et al., 2018). Further, SNHG7 sponges miR-34a and induces over-expression of GALNT7, which in turn results in the progression of colo-rectal cancer (Li et al., 2018). In our study, miR-34a-5p was predicted to be a target of SNHG7, for the first time in OS cell lines. It was reported as a tumor suppressor in for the first time in neuroblastoma (Welch et al., 2007). The over-expression of miR-34a has been reported to inhibit cell growth and induce differentiation of glioma stem cells in human glioma tumors (Guessous et al., 2010). The, lncRNA C2dat1 has been shown to promote cell proliferation, metastasis and infiltration of OS cells by targeting miR-34a-5p (Jia et al., 2018). Thus, our results suggest that lncRNA SNHG7 may promote cell proliferation and metastasis of OS cells by suppressing miR-34a-5p.
RAD9 checkpoint clamp component A (RAD9A, also known as RAD9) is a cell cycle checkpoint protein associated with cell cycle arrest and DNA damage repair. It is an oncogene and is known to be regulated by DNA methylation, and its chromosome locus 11q13 has been reported to be amplified in breast cancer (Cheng et al., 2005). A study by Lieberman et al., has shown abnormal over-expression of RAD9 in approximately 45% of clinically detected prostate tumors, and its significant correlation with tumor stage (Lieberman et al., 2018). However, the role of RAD9A in OS is has not been explored. In the current study, RAD9A was predicted to be a target of miR-34a-5p. A study by Wang et al. has shown that over-expression of miR-34a-5p inhibits cell proliferation and metastasis of cervical cancer cells and promotes cell apoptosis (Wang X et al., 2017c). In colon cancer cells (HCT116), the expression of miR-34a-5p has been reported to induce apoptosis, cell cycle arrest at G1 stage and transcription of P53 (Gao et al., 2015). Hence, based on previous reports and the results from the current study, it can be implicated that the RAD9A regulation by miR-34a-5p may affect the progression of OS through cell cycle regulation. In summary, the lncRNA SNHG7 and the protein coding gene RAD9A may play an important role in the progression of OS by competing with each other for miR-34a-5p.
X inactivate-specific transcript (XIST) is a well-known lncRNA. The high expression of XIST is associated with cell proliferation and poor prognosis of OS (Li et al., 2017). In our ceRNA network, miR-34a-5p was predicted to be a target of XIST. This regulatory interaction has been reported in multiple cancers, such as pancreatic cancer (Sun Z et al., 2018), colon cancer (Sun N et al., 2018), and human nasopharyngeal carcinoma (Song et al., 2016). However, the underlying regulatory mechanisms of XIST have been hardly reported in the progression of OS. The current study indicates that interaction of XIST and miR-34a-5p may regulate downstream mRNA levels associated with progression of OS.
The interaction of different RNA molecules explored in the current study may provide insights into novel targets and underlying molecular mechanisms associated with development and metastasis of OS. However, the current study has limitations. The results obtained in the current study including, genes and their interactions need to be validated using in vivo and in vitro experimental approaches to confirm their regulatory roles. Further, the number of samples considered by the dataset used in the current study is less.
In conclusion, CBX7, RAD9A, SNHG7 lncRNA and has-miR-34a-5p may be explored as novel therapeutic targets for the treatment of OS. Further, the lncRNA NEAT1 may be involved in promoting the proliferation and metastasis of OS cells by sponging miR-543. The lncRNA SNHG7 and the protein coding gene RAD9A may play a role in the progression of OS through their competitive interaction with miR-34a-5p. Finally, the interaction between the lncRNA XIST and miR-34a-5p may be important in understanding molecular mechanisms associated with OS.
Acknowledgments
This work was supported by the Science and Technology Research Project for Colleges and Universities in Hebei Province (grant number: QN2016060, QN2015044) and Medical Scientific Research Project for Hebei Provincial Health and Family Planning Commission (grant number: 20160313, 20160310).
Supplementary material.
References
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25 doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard D, Martinezleal JF, Rizzo S, Martinez D, Hudson D, Visakorpi T, Peters G, Carnero A, Beach D, and Gil. CBX7 controls the growth of normal and tumor-derived prostate cells by repressing the Ink4a/Arf locus. Oncogene. 2005;24 doi: 10.1038/sj.onc.1208735. [DOI] [PubMed] [Google Scholar]
- Bing L, Hong C, Li-Xin S, Wei G. MicroRNA-543 suppresses endometrial cancer oncogenicity via targeting FAK and TWIST1 expression. Arch Gynecol Obstet. 2014;290 doi: 10.1007/s00404-014-3219-3. [DOI] [PubMed] [Google Scholar]
- Cao SE, Tian J, Chen S, Zhang X, Zhang Y. Role of miR-34c in ketamine-induced neurotoxicity in neonatal mice hippocampus. Cell Biol Int. 2015;39 doi: 10.1002/cbin.10349. [DOI] [PubMed] [Google Scholar]
- Chakravarty D, Sboner A, Nair SS, Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K, Kossai M. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun. 2014;5 doi: 10.1038/ncomms6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CK, Chow LW, Loo WT, Chan TK, Chan V. The cell cycle checkpoint gene Rad9 is a novel oncogene activated by 11q13 amplification and DNA methylation in breast cancer. Cancer Research. 2005;65 doi: 10.1158/0008-5472.CAN-04-4243. [DOI] [PubMed] [Google Scholar]
- Dweep H, Gretz N. miRWalk2. Nat Methods. 2015;0 doi: 10.1038/nmeth.3485. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RJ, Dahlin D, Sim F. Multiple metachronous osteogenic sarcoma. J Bone Joint Surg Am. 1973;55 [PubMed] [Google Scholar]
- Gao J, Li N, Dong Y, Li S, Xu L, Li X, Li Y, Li Z, Ng SS, Sung JJ. miR-34a-5p suppresses colorectal cancer metastasis and predicts recurrence in patients with stage II/III colorectal cancer. Oncogene. 2015;34 doi: 10.1038/onc.2014.348. [DOI] [PubMed] [Google Scholar]
- Guessous F, Zhang Y, Kofman A, Catania A, Li Y, Schiff D, Purow B, Abounader R. microRNA-34a is tumor suppressive in brain tumors and glioma stem cells. Cell Cycle. 2010;9 doi: 10.4161/cc.9.6.10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga CL, Phinney DG. MicroRNAs in the imprinted DLK1-DIO3 region repress the epithelial-to-mesenchymal transition by targeting the TWIST1 protein signaling network. J Biol Chem. 2012;287 doi: 10.1074/jbc.M112.387761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JP, Hao Y, Wang XL, Yang XJ, Shao JF, Guo FJ, Feng JX. Review of the molecular pathogenesis of osteosarcoma. Asian Pac J Cancer Prev. 2014;15 doi: 10.7314/apjcp.2014.15.15.5967. [DOI] [PubMed] [Google Scholar]
- Huang da, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2008;4 doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4 doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Jia D, Niu Y, Li D, Liu Z. LncRNA C2dat1 promotes cell proliferation, migration, and Invasion by targeting MiR-34a-5p in osteosarcoma cells. Oncol Res. 2018;26 doi: 10.3727/096504017X15024946480113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GL, Wu YX, Li YM, Li J. High expression of long non-coding RNA XIST in osteosarcoma is associated with cell proliferation and poor prognosis. Eur Rev Med Pharmacol Sci. 2017;21 [PubMed] [Google Scholar]
- Li W, Ma H, Sun J. MicroRNA-34a/c function as tumor suppressors in Hep-2 laryngeal carcinoma cells and may reduce GALNT7 expression. Mol Med Rep. 2014;9 doi: 10.3892/mmr.2014.1929. [DOI] [PubMed] [Google Scholar]
- Li Y, Zeng C, Hu J, Pan Y, Shan Y, Liu B, Jia L. Long non-coding RNA-SNHG7 acts as a target of miR-34a to increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in colorectal cancer progression. J Hematol Oncol. 2018;11 doi: 10.1186/s13045-018-0632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman HB, Rai AJ, Friedman RA, Hopkins KM, Broustas CG. Prostate cancer: unmet clinical needs and RAD9 as a candidate biomarker for patient management. Transl Cancer Res. 2018;6 doi: 10.21037/tcr.2018.01.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB, Yin DD, Kong R, Xia R, Lu KH, Li JH. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13 doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9 doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115 doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet. 2014;15 doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research. 1999;27 doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152 doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- Qi H, Wen B, Wu Q, Cheng W, Lou J, Wei J, Huang J, Yao X, Weng G. Long noncoding RNA SNHG7 accelerates prostate cancer proliferation and cycle progression through cyclin D1 by sponging miR-503. Biomed Pharmacother. 2018;102 doi: 10.1016/j.biopha.2018.03.011. [DOI] [PubMed] [Google Scholar]
- Ren J, Yang Y, Xue J, Xi Z, Hu L, Pan SJ, Sun Q. Long noncoding RNA SNHG7 promotes the progression and growth of glioblastoma via inhibition of miR-5095. Biochem Biophys Res Commun. 2018;496 doi: 10.1016/j.bbrc.2018.01.109. [DOI] [PubMed] [Google Scholar]
- Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146 doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A siftware environment for integrated models of biomolecular interation networks. Genome Res. 2003;13 doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinjo K, Yamashita Y, Yamamoto E, Akatsuka S, Uno N, Kamiya A, Niimi K, Sakaguchi Y, Nagasaka T, Takahashi T. Expression of chromobox homolog 7 (CBX7) is associated with poor prognosis in ovarian clear cell adenocarcinoma via TRAIL-induced apoptotic pathway regulation. Int J Cancer. 2014;135 doi: 10.1002/ijc.28692. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Bioinformatics and computational biology solutions using R and bioconductor. New York: Springer; 2005. Limma: Linear models for microarray data. [Google Scholar]
- Song P, Ye LF, Zhang C, Peng T, Zhou XH. Long non-coding RNA XIST exerts oncogenic functions in human nasopharyngeal carcinoma by targeting miR-34a-5p. Gene. 2016;592 doi: 10.1016/j.gene.2016.07.055. [DOI] [PubMed] [Google Scholar]
- Sun N, Zhang G, Liu Y. Gene. 2018;665 doi: 10.1016/j.gene.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhang B, Cui T. Oncology Reports. 2018;39 doi: 10.3892/or.2018.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Cui Y, Li Z, Jiao Z, Yong Z, Yan H, Chen G, Zhou Q, Wang W, Zhou X. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. J Exp Clin Cancer Res. 2016;35 doi: 10.1186/s13046-016-0285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzan VR, Lengert Av, Boldrini É, Penna V, Scapulatempo-Neto C, Scrideli CA, Filho AP, Cavalcante CE, de Oliveira CZ, Lopes LF. High expression of HULC is associated with poor prognosis in osteosarcoma patients. Plos One. 2016;11 doi: 10.1371/journal.pone.0156774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tang M, Ou L, Hou M, Feng T, Huang YE, Jin Y, Zhang H, Zuo G. Sci Rep. 2017;7 doi: 10.1038/s41598-017-05819-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu Y, Fan S, Luo L. Yonsei Med J. 2017;58 doi: 10.3349/ymj.2017.58.6.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cao C, Ma Q, Zeng Q, Wang H, Cheng Z, Zhu G, Qi J, Ma H, Nian H. RNA-seq analyses of multiple meristems of soybean: novel and alternative transcripts, evolutionary and functional implications. BMC Plant Biol. 2014;14 doi: 10.1186/1471-2229-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Zhang WJ, Wu XC, Weng MZ, Zhang MD, Cai Q, Zhou D, Wang JD, Quan ZW. The lncRNA MALAT1 functions as a competing endogenous RNA to regulate MCL-1 expression by sponging miR-363-3p in gallbladder cancer. J Cell Mol Med. 2016;20 doi: 10.1111/jcmm.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xie Y, Wang J. Oncol Res. 2017;26 [Google Scholar]
- Wang W, Zhang E, Lin C. MicroRNAs in tumor angiogenesis. Life Sci. 2015;136 doi: 10.1016/j.lfs.2015.06.025. [DOI] [PubMed] [Google Scholar]
- Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A, Lumley T, Mächler M, Magnusson A, Möller S. gplots: Various R programming tools for plotting data. R Package Version. 2009;2 [Google Scholar]
- Wei S, Min D, Jiang Z, Hausman GJ, Zhang L, Dodson MV. Long noncoding RNAs in regulating adipogenesis: new RNAs shed lights on obesity. Cell Mol Life Sci. 2016;73 doi: 10.1007/s00018-016-2169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26 doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- Yang J, Cheng D, Zhu B, Zhou S, Ying T, Yang Q. Chromobox homolog 4 is positively correlated to tumor growth, survival and activation of HIF-1α Signaling in human osteosarcoma under normoxic condition. J Cancer. 2016;7 doi: 10.7150/jca.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmishyn AA, Kurochkin IV. Long noncoding RNAs: a potential novel class of cancer biomarkers. Front Genet. 2015;6 doi: 10.3389/fgene.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong W, Tao Y, Zhen Z, Ming L, Wei Z, Zeng X, Zhang W. Long non-coding RNA TUG1 promotes migration and invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells. Cancer Sci. 2017;108 doi: 10.1111/cas.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16 doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XW, Zhang L, Qin W, Yao XH, Zheng LZ, Liu X, Li J, Guo WJ. Oncogenic role of the chromobox protein CBX7 in gastric cancer. J Exp Clin Cancer Res. 2010;29 doi: 10.1186/1756-9966-29-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Hou W, Tao J, Zhao Y, Wan G, Ma C, Xu H. Am J Transl Res. 2016;8 [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhao Y, Tao J, Ma C, Zhang J, Xu H, Dong Y. Int J Clin Exp Pathol. 2016;9 [Google Scholar]
Internet Resources
- Gene Expression Omnibus (GEO) https://www.ncbi.nlm.nih.gov/geo/
- Clusterprofiler. http://www.bioconductor.org/packages/release/bioc/html/clusterProfiler.html.
- Database for Annotation, Visualization and Integrated Discovery (DAVID) https://david-d.ncifcrf.gov/starBase, http://starbase.sysu.edu.cn/ [PubMed]
- miRBase. http://www.mirbase.org/.
- Cytoscape. https://cytoscape.org.
- miRWalk2.0. http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.