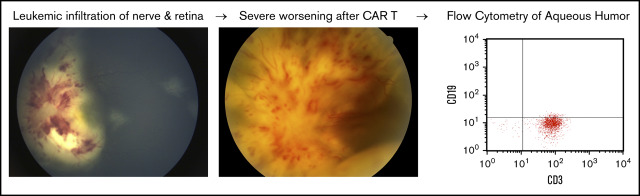
Key Points
CAR T-cell targeting of leukemic infiltrates in the optic nerve and retina caused retinal detachment as a presentation of pseudoprogression.
Treatment of this intraocular inflammation with intravitreal triamcinolone and orbital radiation led to marked improvement in visual acuity.
Visual Abstract
Introduction
Chimeric antigen receptor (CAR) T-cell treatment is an effective therapy for relapsed or refractory B-cell acute lymphoblastic leukemia with high rates of remission and durability.1,2 Achievement of remission is associated with systemic expansion of CAR+ T cells, accompanied by cytokine release syndrome (CRS) secondary to high levels of circulating interleukin-6 and other cytokines.3 Early treatment of CRS with the interleukin-6 antagonist tocilizumab has been described, with good outcomes.1,4-6 Central nervous system (CNS) complications have included confusion, aphasia, seizure, and rare cases of diffuse cerebral edema.7 Localized CNS complications affecting the retina and optic nerve have not been reported following CAR T-cell therapy.8-10
Case description
A 13-year-old girl presented with weight loss, night sweats, hair loss, and headaches and was found to have hyperleukocytosis with 86% blasts, anemia, and thrombocytopenia. Peripheral blood flow cytometry confirmed high-risk B-cell acute lymphoblastic leukemia, and lumbar puncture revealed CNS involvement. She was treated with 4-drug induction and twice-weekly intrathecal chemotherapy, achieving CNS-negative and minimal residual disease (MRD)–negative remission by the end of induction. Despite initial clearance of her CNS disease, she had intermittent and then sustained cerebrospinal fluid (CSF) cytology positive for blasts, consistent with CNS relapse, with detectable MRD of 0.07% of mononuclear cells in her marrow at the end of consolidation therapy. With CNS- and MRD-positive leukemic relapse, the patient was eligible for the phase 1/2 study PLAT-02 (NCT02028455) with CD19-targeted CAR T-cell therapy and elected to enroll.
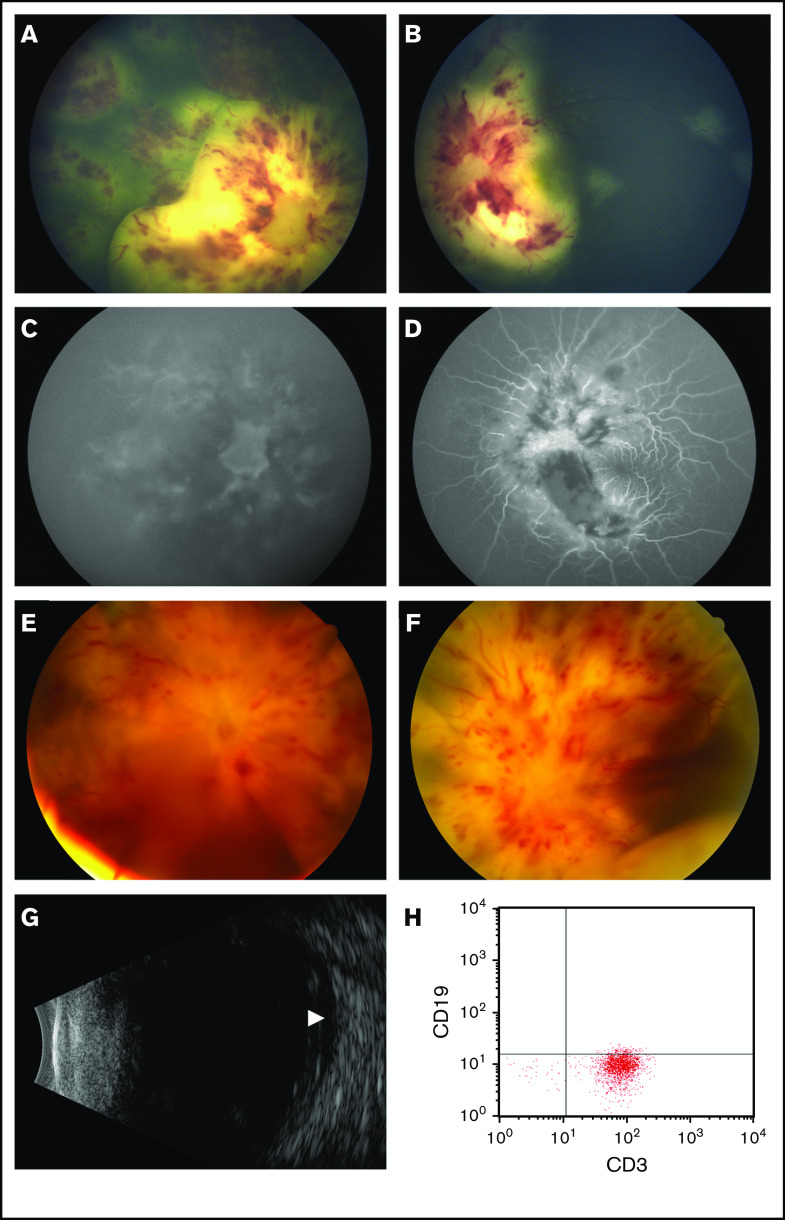
Following apheresis, the patient experienced sudden worsening of her vision, with visual acuity decreasing to count fingers in the left eye and no light perception (NLP) in the right eye (supplemental Table 1). Fundoscopic examination showed bilateral leukemic infiltration of the optic nerve and retina (Figure 1A-B). Fluorescein angiography demonstrated poor retinal and choroidal perfusion in the right eye corresponding to her NLP vision, whereas the left eye had relatively preserved perfusion (Figure 1C-D). She was treated with 4 Gy of radiation to bilateral orbits in 2 fractions, along with 5 days of dexamethasone. She regained light perception in the right eye after the second fraction of radiation. She also received bridging chemotherapy with systemic cytarabine and etoposide, as well as intrathecal triple therapy with methotrexate, hydrocortisone, and cytarabine. Her vision subsequently improved to 20/800 in the right eye and 20/40 in the left eye, but she had persistent leukemic infiltration of the optic nerve and retina bilaterally on admission for CAR T-cell therapy.
Figure 1.
Ocular findings at presentation and following treatment with CAR T-cell therapy. (A-D) Color fundus photography and fluorescein angiography at presentation. (A) The right eye features optic disc infiltration and leukemic retinal infiltrates and hemorrhages. (B) The left eye has leukemic disc infiltration, as well as scattered retinal infiltrates sparing the macula. (C) Fluorescein angiography of the right eye shows minimal perfusion of the choroidal and retinal circulations. (D) The left eye has relatively maintained choroidal and retinal vascular filling, with blockage in areas of leukemic infiltration and hemorrhage. (E-F) Color fundus photography following CAR T-cell therapy. The right (E) and left (F) eyes demonstrate worsened retinal whitening, hemorrhages, and optic disc edema with total exudative retinal detachments. (G) Ocular ultrasound of the right eye demonstrates subretinal fluid (arrowhead) without evidence of choroidal involvement. (H) Flow cytometry of the right aqueous humor demonstrates a predominance of CD3+CD19− cells.
The patient received lymphodepleting chemotherapy with fludarabine and cyclophosphamide, followed by infusion of 0.5 × 106 CD19 CAR+ CD8+ cells per kilogram and 0.5 × 106 CD19 CAR+ CD4+ cells per kilogram.1 She developed grade 1 CRS with fever on day +6. On the following day, her vision worsened bilaterally: NLP in the right eye and light perception in the left eye. Fundoscopic examination revealed total bilateral exudative retinal detachments, intraretinal hemorrhage, retinal whitening, and 4+ optic disc edema (Figure 1E-F). Ultrasonography did not demonstrate choroidal effusion in either eye (Figure 1G). CSF showed leukocytosis but no malignancy, with 80% pleocytosis expressing T-cell–specific CD3 by flow cytometry. She did not develop any other neurologic symptoms or any signs of worsening CRS; therefore, systemic CRS treatment was not initiated.
We postulated that local CAR T-cell expansion and cytokine release led to this sudden deterioration in the patient’s ocular status and, therefore, attempted local control. An additional 10 Gy of radiation to bilateral orbits was given in 5 fractions, and on day +12 she received bilateral intravitreal triamcinolone injections. Anterior chamber paracentesis was also performed on the right eye for flow cytometry of the aqueous fluid (Figure 1H), which contained white blood cells, with 35% expressing CD3 consistent with T cells, 22% expressing CD45 and side scatter patterns consistent with monocytes, and the remainder characteristic of debris. No blasts were noted. There was insufficient aqueous fluid for specific detection of the CAR T-cell receptor. She had marked improvement in her retinal detachments within 1 week after intravitreal triamcinolone. Bone marrow aspirate and CSF showed complete remission on day +20, with additional findings of CAR+ T cells in the peripheral blood, bone marrow, and CSF with ongoing B-cell aplasia.
Unfortunately, bone marrow evaluation on day +60 demonstrated loss of CAR+ T cells and recovery of B cells. She presented on day +105 with vomiting and headache and was found to have isolated CNS relapse. Visual changes were not noted. Following reinduction with systemic dexamethasone and intrathecal triple therapy, she underwent a haploidentical peripheral blood stem cell transplant and has remained in remission for 21 months posttransplant and 27 months post–CAR T-cell therapy at this time.
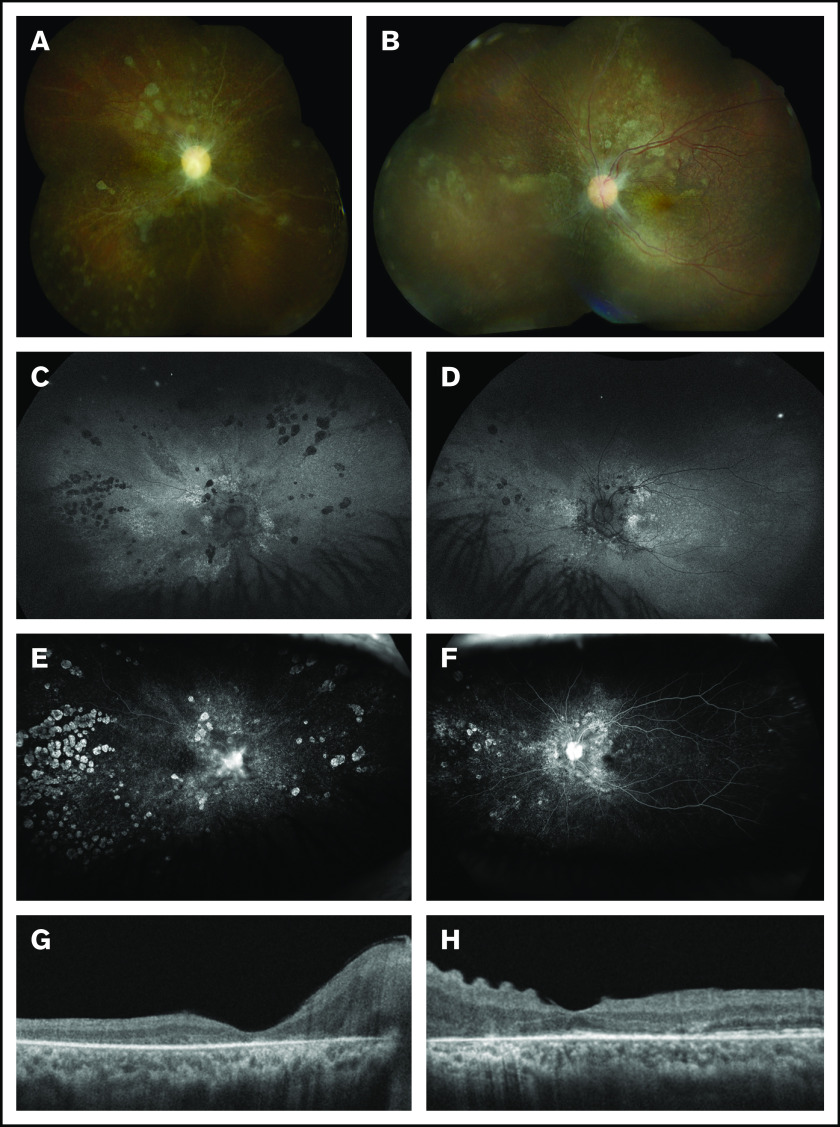
With regard to the patient’s ocular findings, the subretinal fluid in both eyes resolved by 6 weeks postinjection with associated optic nerve pallor and photoreceptor loss. By 6 months postinjection, her visual acuity had improved to 20/200 in the right eye and 20/60 in the left eye, but she subsequently developed visually significant posterior subcapsular cataracts. After undergoing cataract surgery with intraocular lens placement in both eyes (18 months after CAR T-cell therapy), her visual acuity was 20/350 in the right eye and 20/40 in the left eye with stable retinal findings (Figure 2).
Figure 2.
Multimodal retinal imaging at 18 months post–CAR T-cell therapy. (A) The right eye has severe optic disc pallor, diffuse retinal atrophic changes, and sclerotic vessels. (B) The left eye has moderate-severe optic disc pallor and retinal atrophic changes. (C-D) The retina is entirely attached in both eyes. Fundus autofluorescence shows bilateral stippled and nummular areas of hyper- and hypoautofluorescence representing areas of photoreceptor and retinal pigment epithelium damage. Late-phase fluorescein angiography reveals peripheral window defects in the right eye (E) that are more common than in the left eye (F) with staining of the disc bilaterally. (G) Spectral-domain optical coherence tomography shows diffuse photoreceptor loss in the right macula. (H) The left macula has relative preservation of the photoreceptor bands and corrugation of the inner retina.
Methods
This study (NCT02028455) was approved by the Institutional Review Boards of Children’s Hospital Los Angeles and Seattle Children's Hospital. Retinal imaging was performed on the following devices: fundus photography on Retcam 3 (Natus, Pleasanton, CA), Canon 50D (Canon Medical Systems, Tustin, CA), and Optos California (Optos, Dunfermline, Scotland); fluorescein angiography on the Retcam 3 and Optos California; optical coherence tomography on Cirrus HD-OCT 5000 (Carl Zeiss Meditec, Dublin, CA); and ultrasound on Eye Cubed (Ellex, Minneapolis, MN). Flow cytometry data were acquired using CellQuest Pro software on a BD FACSCalibur instrument (BD Biosciences, San Jose, CA).
Results and discussion
Leukemic infiltration of the optic nerve has been reported in 1.4% of cases of pediatric acute lymphoblastic leukemia.11 Infiltration of the retina is extremely rare, occurring in <1% of relapses of acute lymphoblastic leukemia.12,13 Management typically includes radiation to the orbits with doses ≥20 Gy over multiple fractions.12,14,15
After her initial visual loss, this patient received a suboptimal dose of orbital radiation. Although she experienced a remarkable improvement in vision bilaterally, she still had evidence of ocular leukemic infiltrate just prior to CAR T-cell therapy. She then developed severe optic nerve swelling and exudative retinal detachment secondary to local inflammation vs leukemic progression. Our sampling of aqueous fluid showed no evidence of leukemic cells and a clear presence of T cells, likely CAR T cells. This local inflammation produced a transient increase in perceived disease burden after immune-targeted treatment, or pseudoprogression, which has been described in CAR T-cell therapy for non-Hodgkin lymphoma.16 This case emphasizes the danger associated with CAR T-cell expansion and tumor targeting in the setting of active retinal disease.
Without severe CRS or neurologic toxicity necessitating systemic therapy, and with concern for losing CAR T-cell efficacy, we chose to deliver local intravitreal steroids and sample the aqueous humor of the right eye, which showed T cells and no evidence of lymphoblasts. Along with radiation, we were able to rapidly control the local inflammation and improve vision while preserving systemic CAR T-cell function. Studies published after this patient was treated have shown that tocilizumab intervention for symptomatic CRS does not impact the long-term presence of CAR T cells17; therefore, in retrospect, systemic therapy may have been appropriate.
This patient subsequently developed cataracts, a complication of intravitreal triamcinolone,18 which was likely exacerbated by several courses of systemic steroids, local radiation, and total body irradiation.19,20 Whether radiation alone would have been adequate to control the retinal inflammation remains unknown, but she has enjoyed sustained remission in the retina and optic nerve, despite progression elsewhere prior to her hematopoietic stem cell transplantation. This is the first report, to our knowledge, of vision loss due to local inflammation in CAR T-cell therapy, highlighting the importance of recognizing and aggressively managing less common toxicities with this novel anticancer therapy.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by an unrestricted grant to the University of Southern California Department of Ophthalmology from Research to Prevent Blindness, a Knights Templar Eye Foundation career starter grant, and the Las Madrinas Endowment for Experimental Therapeutics in Ophthalmology (all A.N.).
Footnotes
Data sharing requests should be sent to Aaron Nagiel (anagiel@chla.usc.edu).
Authorship
Contribution: C.C.D., W.S.G., M.A.P., and A.N. conducted research and wrote the manuscript; H.A.-A., S.J., N.K., M.J.O., K.W., J.K., A.V., P.M., K.V.C., D.S., and A.N. participated in the patient’s care; and H.A.-A., S.J., N.K., M.J.O., K.W., J.K., A.V., P.M., K.V.C., D.S., R.G., and M.C.J. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Aaron Nagiel, Children’s Hospital Los Angeles, 4650 Sunset Blvd, MS #88, Los Angeles, CA 90027; e-mail: anagiel@chla.usc.edu.
References
- 1.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a therapeutic target. Clin Cancer Res. 2015;21(6):1248-1257. [DOI] [PubMed] [Google Scholar]
- 5.Mueller KT, Waldron E, Grupp SA, et al. Clinical pharmacology of tisagenlecleucel in B-cell acute lymphoblastic leukemia. Clin Cancer Res. 2018;24(24):6175-6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald JC, Weiss SL, Maude SL, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45(2):e124-e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs. 2018;32(12):1091-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camera A, Piccirillo G, Cennamo G, et al. Optic nerve involvement in acute lymphoblastic leukemia. Leuk Lymphoma. 1993;11(1-2):153-155. [DOI] [PubMed] [Google Scholar]
- 12.Somervaille TC, Hann IM, Harrison G, et al. ; MRC Childhood Leukaemia Working Party . Intraocular relapse of childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;121(2):280-288. [DOI] [PubMed] [Google Scholar]
- 13.Gillette TB, Cabrera MT, Tarlock K, Murphy CE, Chisholm KM, Stacey AW. Rapidly progressive, isolated subretinal leukemic relapse: a case report. Ocul Oncol Pathol. 2018;4(4):220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaikov Y. Optic nerve head infiltration in acute leukemia in children: an indication for emergency optic nerve radiation therapy. Med Pediatr Oncol. 1996;26(2):101-104. [DOI] [PubMed] [Google Scholar]
- 15.Nagpal MP, Mehrotra NS, Mehta RC, Shukla CK. Leukemic optic nerve infiltration in a patient with acute lymphoblastic leukemia. Retin Cases Brief Rep. 2016;10(2):127-130. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Hu Y, Yang S, et al. Role of fluorodeoxyglucose positron emission tomography/computed tomography in predicting the adverse effects of chimeric antigen receptor T cell therapy in patients with non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2019;25(6):1092-1098. [DOI] [PubMed] [Google Scholar]
- 17.Stein AM, Grupp SA, Levine JE, et al. Tisagenlecleucel model-based cellular kinetic analysis of chimeric antigen receptor-T cells. CPT Pharmacometrics Syst Pharmacol. 2019;8(5):285-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson JT. Cataract formation and other complications of intravitreal triamcinolone for macular edema. Am J Ophthalmol. 2006;141(4):629-637. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen SM, Sison J, Jones M, et al. Lens dose-response prediction modeling and cataract incidence in patients with retinoblastoma after lens-sparing or whole-eye radiation therapy. Int J Radiat Oncol Biol Phys. 2019;103(5):1143-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall MD, Schultheiss TE, Smith DD, Nguyen KH, Wong JY. Dose response for radiation cataractogenesis: a meta-regression of hematopoietic stem cell transplantation regimens. Int J Radiat Oncol Biol Phys. 2015;91(1):22-29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.